Evaluation of Antithrombogenic pHPC on CoCr Substrates for Biomedical Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimens and Coating
2.2. Coating Efficiency—Wettability
2.3. Atomic Force Microscopy
2.4. In Vitro Blood Contact
2.5. CD61 Immunohistochemistry
2.6. Semi-Quantitative Phase Analyses
2.7. Scanning Electron Microscopy
2.8. Statistical Analysis
3. Results
3.1. Surface Analysis—Wettability and Surface Topography
3.2. Thrombogenicity—Fluorescence and SEM Imaging
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bundesamt, S. Gesundheit—Todesursachen in Deutschland; Statistisches Bundesamt: Wiesbaden, Germany, 2015. [Google Scholar]
- Leon, M.B.; Baim, D.S.; Popma, J.J.; Gordon, P.C.; Cutlip, D.E.; Ho, K.K.; Giambartolomei, A.; Diver, D.J.; Lasorda, D.M.; Williams, D.O.; et al. A clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting. Stent anticoagulation restenosis study investigators. N. Engl. J. Med. 1998, 339, 1665–1671. [Google Scholar] [CrossRef] [PubMed]
- Dotter, C.T. Transluminally-placed coilspring endarterial tuve grafts: Long term patency in canine popliteal artery. Investig. Radiol. 1969, 4, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Machraoui, A.; Grewe, P.; Fischer, A. Koronarstenting. Werkstofftechnik, Pathomorphologie, Therapie; Steinkopff: Heidelberg, Germany, 2001; ISBN 978-3-642-57637-9. [Google Scholar]
- Gorbet, M.B.; Sefton, M.V. Biomaterial-associated thrombosis: Roles of coagulation factors, complement, platelets and leukocytes. Biomaterials 2004, 25, 5681–5703. [Google Scholar] [CrossRef] [PubMed]
- Mikhalovska, L.I.; Santin, M.; Denyer, S.P.; Lloyd, A.W.; Teer, D.G.; Field, S.; Mikhalovsky, S.V. Fibrinogen adsorption and platelet adhesion to metal and carbon coatings. Thromb. Haemost. 2004, 92, 1032–1039. [Google Scholar] [CrossRef]
- Versteeg, H.H.; Heemskerk, J.W.M.; Levi, M.; Reitsma, P.H. New fundamentals in hemostasis. Physiol. Rev. 2013, 93, 327–358. [Google Scholar] [CrossRef] [PubMed]
- Reininger, A.J. Function of von Willebrand factor in haemostasis and thrombosis. Haemophilia 2008, 14, 11–26. [Google Scholar] [CrossRef]
- Honda, Y.; Fitzgerald, P.J. Stent thrombosis: An issue revisited in a changing world. Circulation 2003, 108, 2–5. [Google Scholar] [CrossRef][Green Version]
- Kastrati, A.; Schühlen, H.; Hausleiter, J.; Zitzmann-Roth, E. Restenosis after coronary stent placement and randomization to a 4-week combined antiplatelet or anticoagulant therapy: Six-month angiographic follow-up of the intracoronary stenting and antithrombotic regimen (ISAR) trial. Circulation 1997, 96, 462–467. [Google Scholar]
- Martinez-Moreno, R.; Aguilar, M.; Wendl, C.; Bäzner, H.; Ganslandt, O.; Henkes, H. Fatal thrombosis of a flow diverter due to ibuprofen-related antagonization of acetylsalicylic acid. Clin. Neuroradiol. 2016, 26, 355–358. [Google Scholar] [CrossRef][Green Version]
- Berger, P.B.; Bhatt, D.L.; Fuster, V.; Steg, P.G.; Fox, K.A.A.; Shao, M.; Brennan, D.M.; Hacke, W.; Montalescot, G.; Steinhubl, S.R.; et al. Bleeding complications with dual antiplatelet therapy among patients with stable vascular disease or risk factors for vascular disease: Results from the clopidogrel for high atherothrombotic risk and ischemic stabilization, management, and avoidance (CHARISMA) trial. Circulation 2010, 121, 2575–2583. [Google Scholar] [CrossRef]
- Hankey, G.J.; Eikelboom, J.W. Aspirin resistance. Lancet 2006, 367, 606–617. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Diodati, J.G.; Pharand, C. Resistance to clopidogrel: A review of the evidence. J. Am. Coll. Cardiol. 2005, 45, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Farid, N.A.; Kurihara, A.; Wrighton, S.A. Metabolism and disposition of the thienopyridine antiplatelet drugs ticlopidine, clopidogrel, and prasugrel in humans. J. Clin. Pharmacol. 2010, 50, 126–142. [Google Scholar] [CrossRef] [PubMed]
- Dobesh, P.P.; Oestreich, J.H. Ticagrelor: Pharmacokinetics, pharmacodynamics, clinical efficacy, and safety. Pharmacotherapy 2014, 34, 1077–1090. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, H.; Kaiser, A.F.C.; Endres, H.G.; Krüger, J.C.; Engelhardt, A.; Lask, S.; Pepinghege, F.; Kusber, A.; Mügge, A. Tailored antiplatelet therapy can overcome clopidogrel and aspirin resistance--the BOchum CLopidogrel and Aspirin Plan (BOCLA-Plan) to improve antiplatelet therapy. BMC Med. 2011, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Steiner, T.; Juvela, S.; Unterberg, A.; Jung, C.; Forsting, M.; Rinkel, G. European Stroke Organization guidelines for the management of intracranial aneurysms and subarachnoid haemorrhage. Cerebrovasc. Dis. 2013, 35, 93–112. [Google Scholar] [CrossRef]
- Pan, C.J.; Tang, J.J.; Weng, Y.J.; Wang, J.; Huang, N. Preparation, characterization and anticoagulation of curcumin-eluting controlled biodegradable coating stents. J. Control. Release 2006, 116, 42–49. [Google Scholar] [CrossRef]
- Kufner, S.; Joner, M.; Thannheimer, A.; Hoppmann, P.; Ibrahim, T.; Mayer, K.; Cassese, S.; Laugwitz, K.-L.; Schunkert, H.; Kastrati, A.; et al. Ten-year clinical outcomes from a trial of three limus-eluting stents with different polymer coatings in patients with coronary artery disease. Circulation 2019, 139, 325–333. [Google Scholar] [CrossRef]
- Lopez-Donaire, M.L.; Santerre, J.P. Surface modifying oligomers used to functionalize polymeric surfaces: Consideration of blood contact applications. J. Appl. Polym. Sci. 2014, 131, 40328:1–40328:15. [Google Scholar] [CrossRef]
- Muramatsu, T.; Onuma, Y.; Zhang, Y.-J.; Bourantas, C.V.; Kharlamov, A.; Diletti, R.; Farooq, V.; Gogas, B.D.; Garg, S.; García-García, H.M.; et al. Progress in treatment by percutaneous coronary intervention: The stent of the future. Rev. Esp. Cardiol. 2013, 66, 483–496. [Google Scholar] [CrossRef]
- Kaplan, O.; Hierlemann, T.; Krajewski, S.; Kurz, J.; Nevoralová, M.; Houska, M.; Riedel, T.; Riedelová, Z.; Zárubová, J.; Wendel, H.P.; et al. Low-thrombogenic fibrin-heparin coating promotes in vitro endothelialization. J. Biomed. Mater. Res. A 2017, 105, 2995–3005. [Google Scholar] [CrossRef]
- Shuvalova, Y.A.; Shirokov, R.O.; Kaminnaya, V.I.; Samko, A.N.; Kaminnyi, A.I. Two-year follow-up of percutaneous coronary intervention using EucaTax or Cypher. Cardiovasc. Revasc. Med. 2013, 14, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Cutlip, D.E.; Garratt, K.N.; Novack, V.; Barakat, M.; Meraj, P.; Maillard, L.; Erglis, A.; Jauhar, R.; Popma, J.J.; Stoler, R.; et al. 9-month clinical and angiographic outcomes of the COBRA polyzene-f nanocoated coronary stent system. JACC Cardiovasc. Interv. 2017, 10, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Girdhar, G.; Li, J.; Kostousov, L.; Wainwright, J.; Chandler, W.L. In-vitro thrombogenicity assessment of flow diversion and aneurysm bridging devices. J. Thromb. Thrombolysis 2015, 40, 437–443. [Google Scholar] [CrossRef]
- Martínez-Galdámez, M.; Lamin, S.M.; Lagios, K.G.; Liebig, T.; Ciceri, E.F.; Chapot, R.; Stockx, L.; Chavda, S.; Kabbasch, C.; Farago, G.; et al. Periprocedural outcomes and early safety with the use of the Pipeline Flex Embolization Device with Shield Technology for unruptured intracranial aneurysms: Preliminary results from a prospective clinical study. J. Neurointerv. Surg. 2017, 9, 772–776. [Google Scholar] [CrossRef]
- Girdhar, G.; Ubl, S.; Jahanbekam, R.; Thinamany, S.; Belu, A.; Wainwright, J.; Wolf, M.F. Thrombogenicity assessment of Pipeline, Pipeline Shield, Derivo and P64 flow diverters in an in vitro pulsatile flow human blood loop model. eNeurologicalSci 2019, 14, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Galdámez, M.; Gil, A.; Caniego, J.L.; Gonzalez, E.; Bárcena, E.; Perez, S.; Garcia-Bermejo, P.; Ortega-Gutierrez, S. Preliminary experience with the Pipeline Flex Embolization Device: Technical note. J. Neurointerv. Surg. 2015, 7, 748–751. [Google Scholar] [CrossRef]
- Hanel, R.A.; Aguilar-Salinas, P.; Brasiliense, L.B.; Sauvageau, E. First US experience with Pipeline Flex with Shield Technology using aspirin as antiplatelet monotherapy. BMJ Case Rep. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Lenz-Habijan, T.; Bhogal, P.; Peters, M.; Bufe, A.; Martinez Moreno, R.; Bannewitz, C.; Monstadt, H.; Henkes, H. Hydrophilic stent coating inhibits platelet adhesion on stent surfaces: Initial results in vitro. Cardiovasc. Intervent. Radiol. 2018, 41, 1779–1785. [Google Scholar] [CrossRef]
- Colgan, F.; Aguilar Pérez, M.; Hellstern, V.; Reinhard, M.; Krämer, S.; Bäzner, H.; Henkes, H. Vertebral Artery Aneurysm: Ruptured Dissecting Aneurysm, Implantation of Telescoping p48_HPC Flow Diverter Stents under Antiaggregation with ASA only: The Aneurysm Casebook: A Guide to Treatment Selection and Technique; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–16. [Google Scholar]
- Roos, E.; Maile, K.; Seidenfuß, M. Werkstoffkunde für Ingenieure. Grundlagen, Anwendung, Prüfung, 6., Ergänzte und bearbeitete Auflage; Springer Vieweg: Berlin/Heidelberg, Germany, 2017; ISBN 9783662495322. [Google Scholar]
- Murphy, W.; Black, J.; Hastings, G. Handbook of Biomaterial Properties; Springer: New York, NY, USA, 2016; ISBN 978-1-4939-3303-7. [Google Scholar]
- Williams, D.F. On the mechanisms of biocompatibility. Biomaterials 2008, 29, 2941–2953. [Google Scholar] [CrossRef]
- Wintermantel, E.; Ha, S.-W. Medizintechnik. Life Science Engineering; Interdisziplinarität, Biokompatibilität, Technologien, Implantate, Diagnostik, Werkstoffe, Zertifizierung, Business, 5., überarb. und erw. Aufl.; Springer: Berlin/Heidelberg, Germany, 2009; ISBN 9783540939351. [Google Scholar]
- Henkes, H.; Bhogal, P.; Aguilar Pérez, M.; Lenz-Habijan, T.; Bannewitz, C.; Peters, M.; Sengstock, C.; Ganslandt, O.; Lylyk, P.; Monstadt, H. Anti-thrombogenic coatings for devices in neurointerventional surgery: Case report and review of the literature. Interv. Neuroradiol. 2019, 25, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Kaps, M. Sonografie in der Neurologie; 3. Auflage; Georg Thieme Verlag: New York, NY, USA, 2017. [Google Scholar]
- Horbett, T.A. The role of adsorbed proteins in animal cell adhesion. Colloids Surf. B Biointerfaces 1994, 2, 225–240. [Google Scholar] [CrossRef]
- Xu, L.-C.; Bauer, J.W.; Siedlecki, C.A. Proteins, platelets, and blood coagulation at biomaterial interfaces. Colloids Surf. B Biointerfaces 2014, 124, 49–68. [Google Scholar] [CrossRef] [PubMed]
- Maxisch, M.; Ebbert, C.; Torun, B.; Fink, N.; de los Arcos, T.; Lackmann, J.; Maier, H.J.; Grundmeier, G. PM-IRRAS studies of the adsorption and stability of organophosphonate monolayers on passivated NiTi surfaces. Appl. Surf. Sci. 2011, 257, 2011–2018. [Google Scholar] [CrossRef]
- de Scheerder, I.; Verbeken, E.; van Humbeeck, J. Metallic surface modification. Semin. Interv. Cardiol. 1998, 3, 139–144. [Google Scholar] [PubMed]
- Sojitra, P.; Engineer, C.; Kothwala, D.; Raval, A.; Kotadia, H.; Mehta, G. Investigation of material removal, surface roughnessand corrosion behaviour: Surface roughnessand corrosion behaviour. Trends Biomater. Artif. Organs 2010, 23, 115–121. [Google Scholar]
- Bracco, G.; Holst, B. Surface Science Techniques; Springer: Berlin/Heidelberg, Germany, 2013; ISBN 9783642342431. [Google Scholar]
- Faucheux, N.; Schweiss, R.; Lützow, K.; Werner, C.; Groth, T. Self-assembled monolayers with different terminating groups as model substrates for cell adhesion studies. Biomaterials 2004, 25, 2721–2730. [Google Scholar] [CrossRef]
- Harnett, E.M.; Alderman, J.; Wood, T. The surface energy of various biomaterials coated with adhesion molecules used in cell culture. Colloids Surf. B Biointerfaces 2007, 55, 90–97. [Google Scholar] [CrossRef]
- Wan, G.J.; Huang, N.; Yang, P.; Fu, R.K.Y.; Ho, J.P.Y.; Xie, X.; Zhou, H.F.; Chu, P.K. Platelet activation behavior on nitrogen plasma-implanted silicon. Mater. Sci. Eng. C 2007, 27, 928–932. [Google Scholar] [CrossRef]
- Yang, P.; Huang, N.; Leng, Y.X.; Yao, Z.Q.; Zhou, H.F.; Maitz, M.; Leng, Y.; Chu, P.K. Wettability and biocompatibility of nitrogen-doped hydrogenated amorphous carbon films: Effect of nitrogen. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2006, 242, 22–25. [Google Scholar] [CrossRef]
- Tzoneva, R.; Groth, T.; Altankov, G.; Paul, D. Remodeling of fibrinogen by endothelial cells in dependence on fibronectin matrix assembly. Effect of substratum wettability. J. Mater. Sci. Mater. Med. 2002, 13, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
- Arima, Y.; Iwata, H. Effect of wettability and surface functional groups on protein adsorption and cell adhesion using well-defined mixed self-assembled monolayers. Biomaterials 2007, 28, 3074–3082. [Google Scholar] [CrossRef] [PubMed]
- Bhogal, P.; Lenz-Habijan, T.; Bannewitz, C.; Hannes, R.; Monstadt, H.; Simgen, A.; Mühl-Benninghaus, R.; Reith, W.; Henkes, H. The pCONUS HPC: 30-day and 180-day in vivo biocompatibility results. Cardiovasc. Intervent. Radiol. 2019, 42, 1008–1015. [Google Scholar] [CrossRef]
- Lenz-Habijan, T.; Bhogal, P.; Bannewitz, C.; Hannes, R.; Monstadt, H.; Simgen, A.; Mühl-Benninghaus, R.; Reith, W.; Henkes, H. Prospective study to assess the tissue response to HPC-coated p48 flow diverter stents compared to uncoated devices in the rabbit carotid artery model. Eur. Radiol. Exp. 2019, 3, 47. [Google Scholar] [CrossRef] [PubMed]
- Beythien, C.; Terres, W.; Hamm, C.W. In vitro model to test the thrombogenicity of coronary stents. Thromb. Res. 1994, 75, 581–590. [Google Scholar] [CrossRef]
- Miyazaki, Y.; Nomura, S.; Miyake, T.; Kagawa, H.; Kitada, C.; Taniguchi, H.; Komiyama, Y.; Fujimura, Y.; Ikeda, Y.; Fukuhara, S. High shear stress can initiate both platelet aggregation and shedding of procoagulant containing microparticles. Blood 1996, 88, 3456–3464. [Google Scholar] [CrossRef] [PubMed]
- van Beusekom, H.M.M.; van der Giessen, W.J.; van Suylen, R.J.; Bos, E.; Bosman, F.; Serruys, P.W. Histology after stenting of human saphenous vein bypass grafts: Observations from surgically excised grafts 3 to 320 days after stent implantation. J. Am. Coll. Cardiol. 1993, 21, 45–54. [Google Scholar] [CrossRef][Green Version]

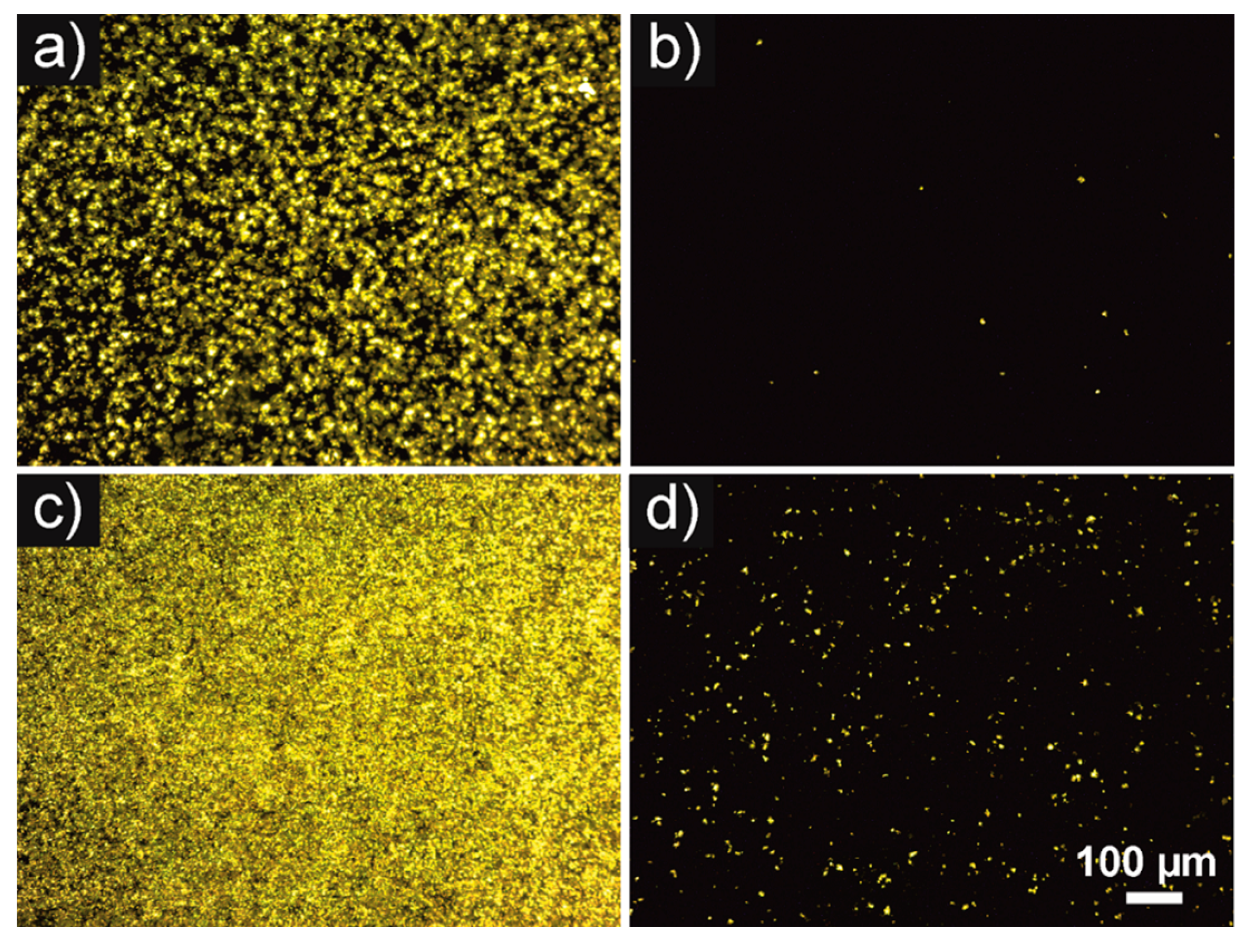

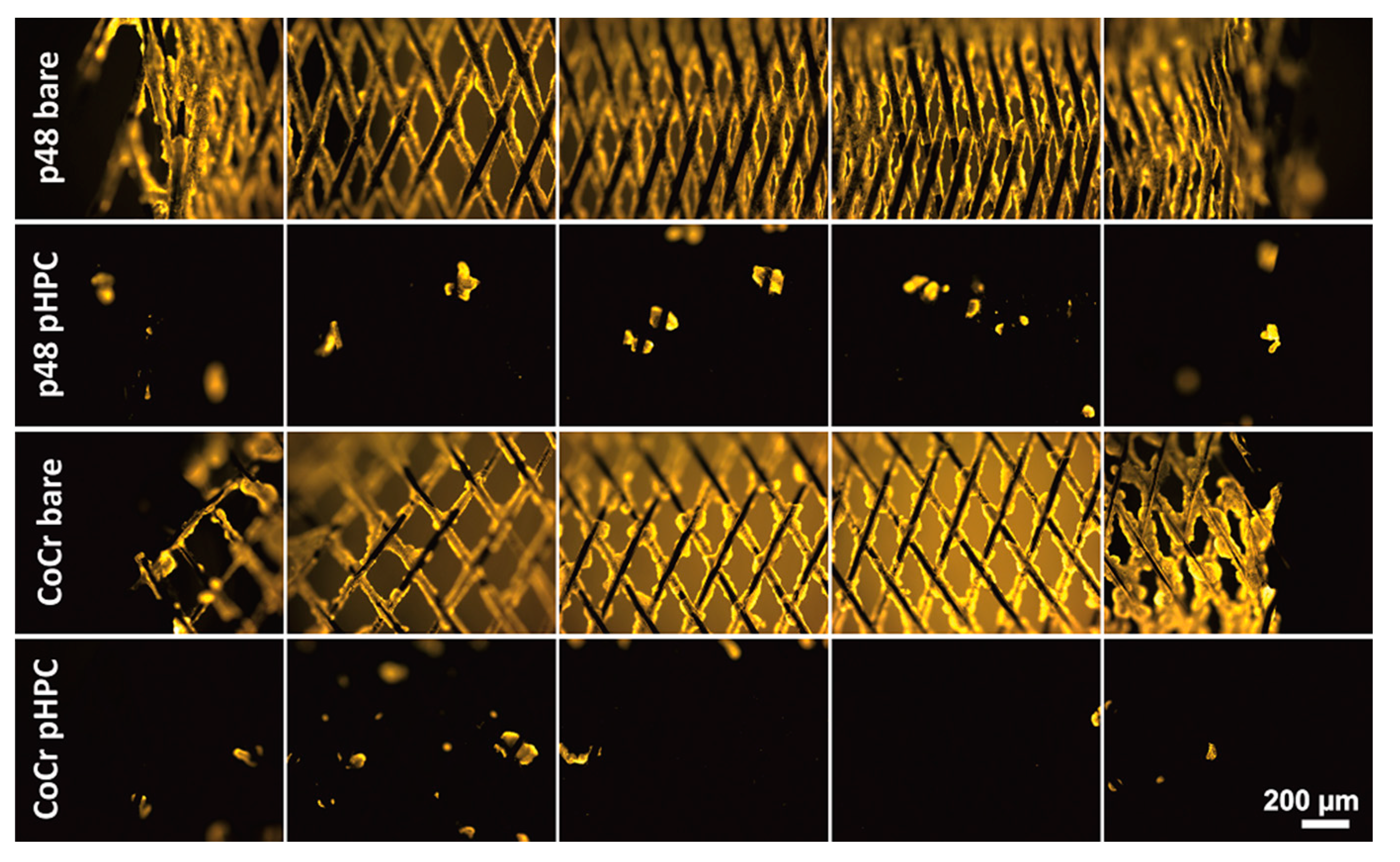
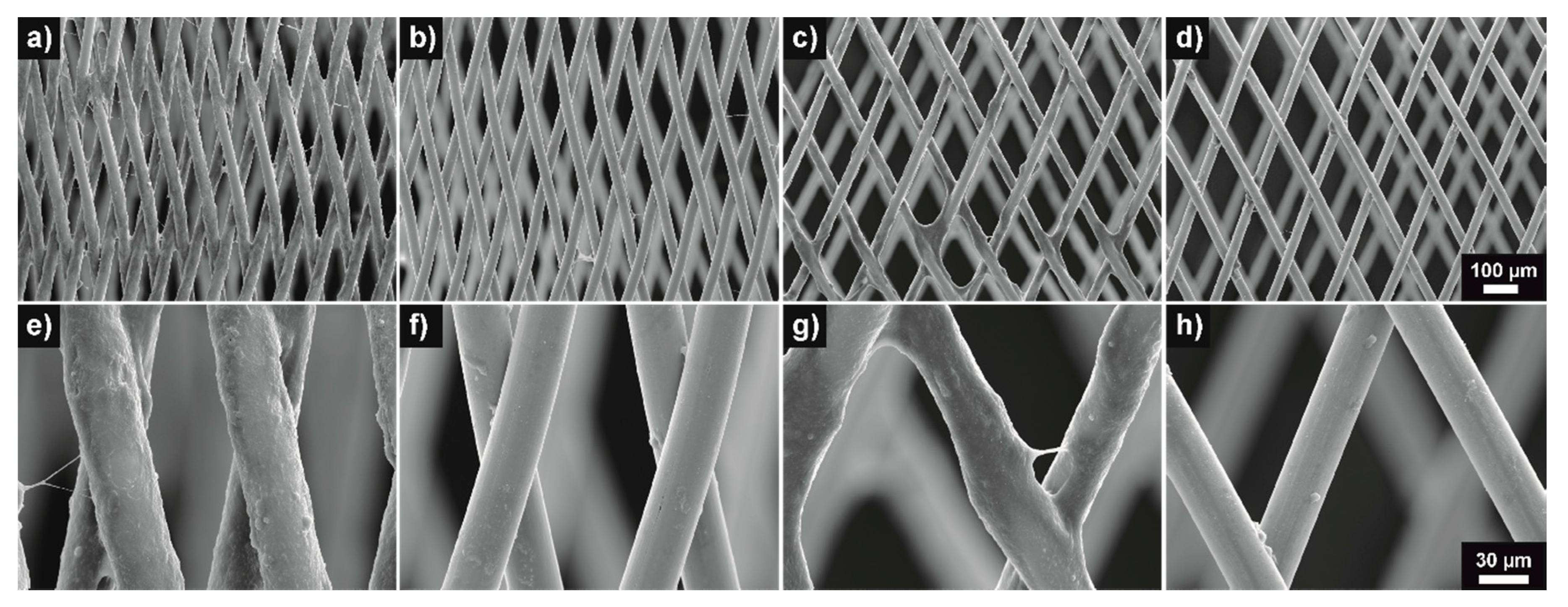
| Specimen | NiTi Plate Uncoated | NiTi Plate pHPC Coated | CoCr Plate Uncoated | CoCr Plate pHPC Coated |
|---|---|---|---|---|
| Contact Angle (CA) [°] | 78.1 ± 4.8 | 31.6 ± 2.2 | 84.4 ± 5.1 | 36.2 ± 5.2 |
| Average roughness (Ra) [nm] | 0.383 ± 0.094 | 0.214 ± 0.020 | 0.029 ± 0.002 | 0.036 ± 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bannewitz, C.; Lenz-Habijan, T.; Lentz, J.; Peters, M.; Trösken, V.; Siebert, S.; Weber, S.; Theisen, W.; Henkes, H.; Monstadt, H. Evaluation of Antithrombogenic pHPC on CoCr Substrates for Biomedical Applications. Coatings 2021, 11, 93. https://doi.org/10.3390/coatings11010093
Bannewitz C, Lenz-Habijan T, Lentz J, Peters M, Trösken V, Siebert S, Weber S, Theisen W, Henkes H, Monstadt H. Evaluation of Antithrombogenic pHPC on CoCr Substrates for Biomedical Applications. Coatings. 2021; 11(1):93. https://doi.org/10.3390/coatings11010093
Chicago/Turabian StyleBannewitz, Catrin, Tim Lenz-Habijan, Jonathan Lentz, Marcus Peters, Volker Trösken, Sabine Siebert, Sebastian Weber, Werner Theisen, Hans Henkes, and Hermann Monstadt. 2021. "Evaluation of Antithrombogenic pHPC on CoCr Substrates for Biomedical Applications" Coatings 11, no. 1: 93. https://doi.org/10.3390/coatings11010093
APA StyleBannewitz, C., Lenz-Habijan, T., Lentz, J., Peters, M., Trösken, V., Siebert, S., Weber, S., Theisen, W., Henkes, H., & Monstadt, H. (2021). Evaluation of Antithrombogenic pHPC on CoCr Substrates for Biomedical Applications. Coatings, 11(1), 93. https://doi.org/10.3390/coatings11010093






