Volatile Iridium and Platinum MOCVD Precursors: Chemistry, Thermal Properties, Materials and Prospects for Their Application in Medicine
Abstract
1. Introduction
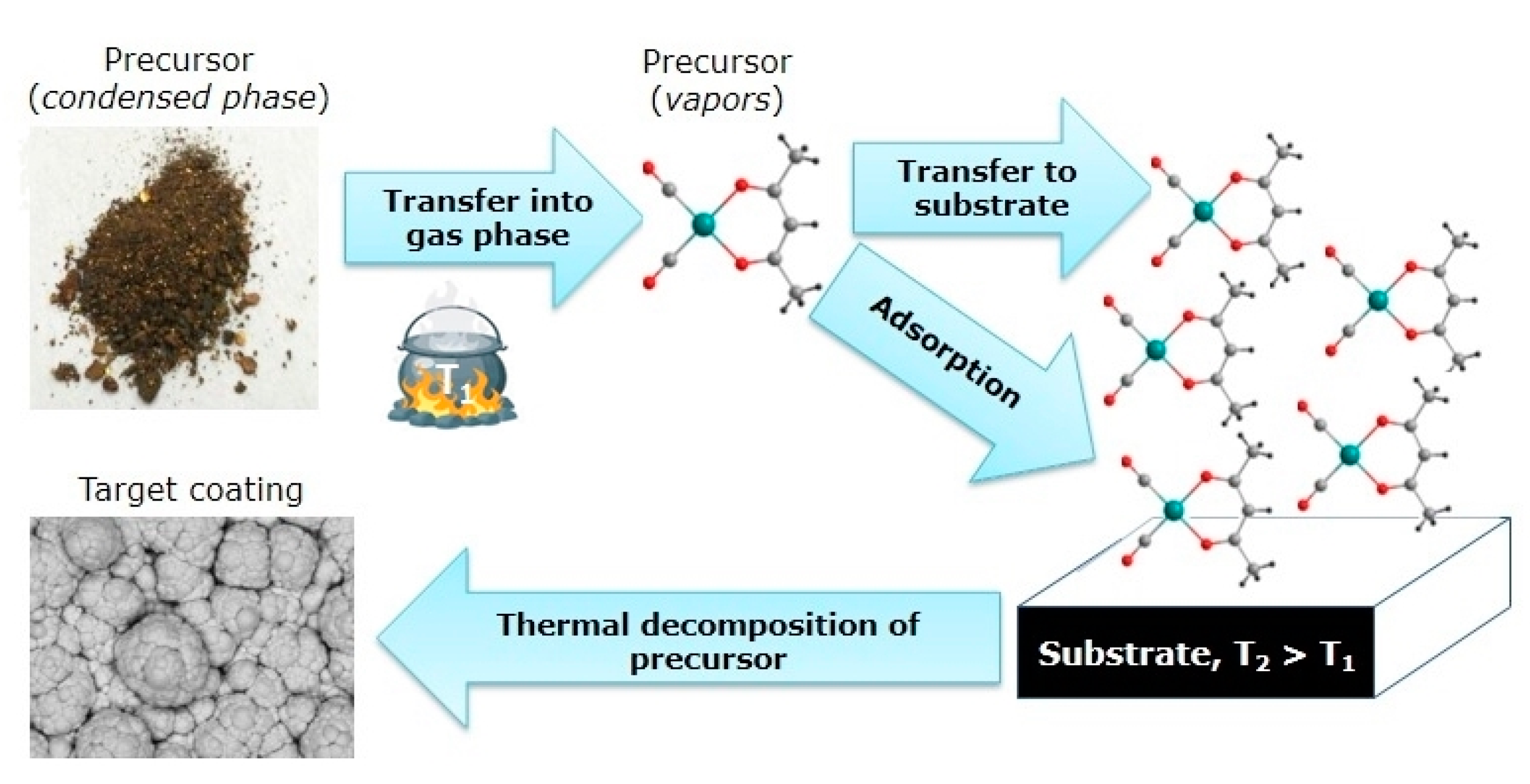
2. Volatile Precursors
2.1. Volatile Iridium Precursors
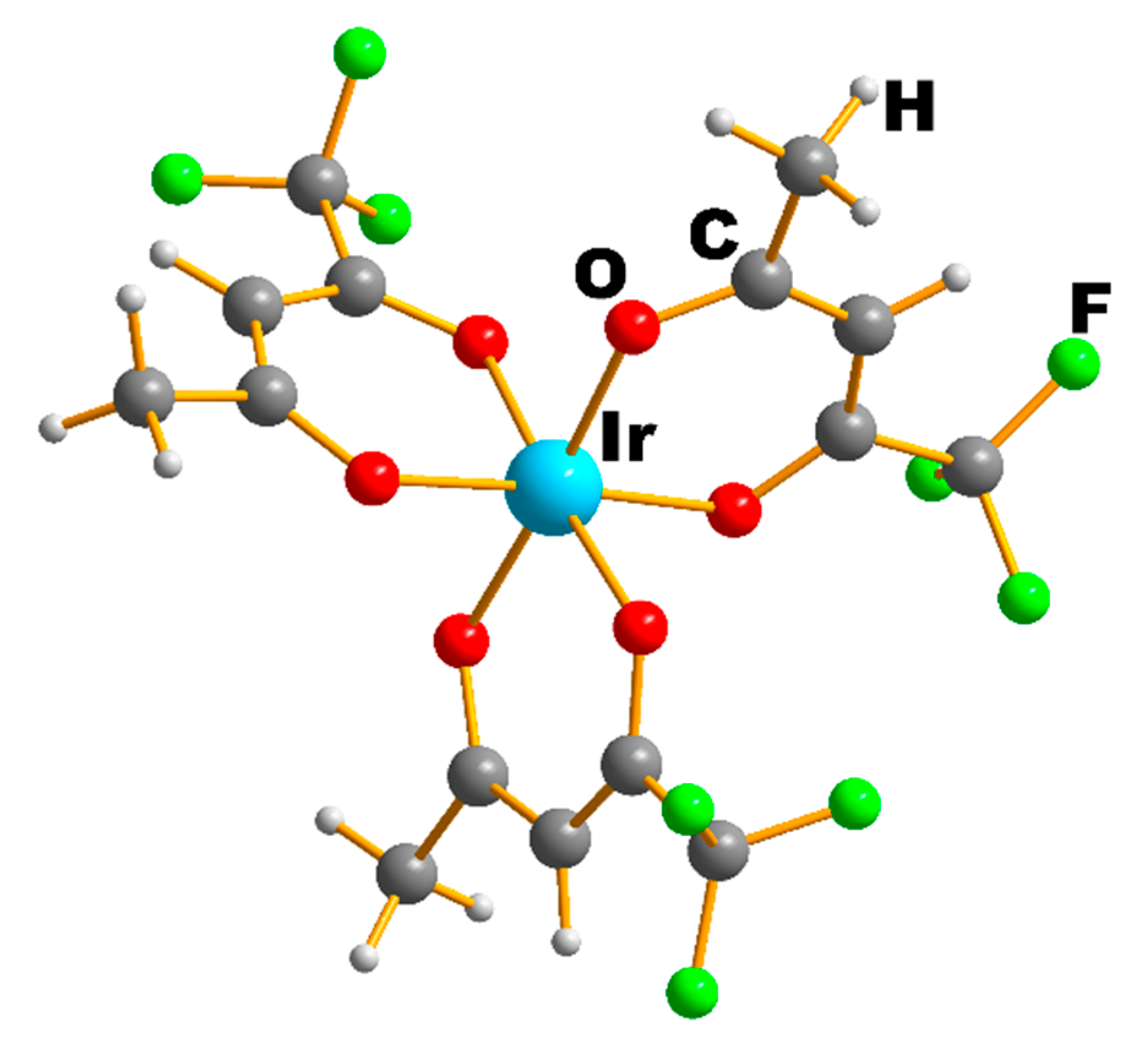
2.1.1. Iridium(III) Precursors
2.1.2. Iridium(I) Precursors
- β-heteroarylketonate [Ir(cod)(ThTFP)] [41];
- complexes with carbon monooxide [Ir(CO)2Cp*] [58].
2.1.3. Application of Iridium Precursors in MOCVD Processes
| Ref. | Precursor | Reagent Gas | Source/Deposition Temperature, °C | Substrate | Thickness, nm | Growth Rate, nm/min | Composition (XRD) | Purity and Other Details |
|---|---|---|---|---|---|---|---|---|
| [26] | [Ir(cod)(OMe)]2 | H2 | –/550 | – | – | – | Ir | 5–10 mass.% impurities |
| [Ir(cod)(acac)] | H2 | –/600 | – | – | – | 1 mass.% C and O | ||
| [29] | [Ir(cod)(OAc)]2 | no | 130/250 | Si (100) | 40 | 13.3 | Ir | 1 mass.% C and O |
| [34] | [Ir(cod)(hfac)] | H2 | 60–65/250–350 | SiO2/Si, Pt/Si, Cu/Si | – | 15 | Ir | 0.1–0.3 μm agglomerates, 1 mass.% C |
| [32] | [Ir(cod)(thd)] | H2 + iPrOH | 130/350 | Quartz glass | 25 | 4.1 | Ir | 1 mass.% C and O, islands |
| H2 | 130/350–550 | – | <0.7 | Ir | – | |||
| [25] | [Ir(CO)2(acac)] | H2 or O2 | –/240–300 | Si (100) | – | – | Ir | Crystallite size 4–20 nm |
| [42] | [Ir(cod)(Eti-hfac)] | O2 | –/400 | Si (100) | 278 | 7.0 | Ir | 2 mass.% O |
| [Ir(cod)(nPri-hfda)] | H2 | –/500 | Si (100) | 322 | 1.3 | 30–90 nm agglomerates, 41 mass.% C | ||
| O2 | –/375 | Si (100) | 320 | 3.8 | 1 mass.% O | |||
| [Ir(cod)(amakN(Me)2)] | O2 | –/350 | Si (100) | 264 | 4.4 | 2 mass.% O | ||
| [43] | [Ir(CO)2(nPri-hfda)] | O2 | 50/400 | Si (100) | 290 | 4.8 | Ir | 60–90 nm agglomerates, 2 mass.% impurities |
| 75/425 | 400 | 1.7 | IrO2 | Polycrystalline | ||||
| 75/425 | LiTaO3 (012) | 2000 | 8.3 | tilted needles: 15–25 nm in diameter, 1.5 μm in length | ||||
| 75/425 | LiNbO3 (100) | 3000 | 12.5 | vertical needles | ||||
| 95/425 | LiNbO3 (100) | 430 | 7.1 | vertical pillars | ||||
| [41] | [Ir(cod)(ThTFP)] | no | 110–130/700–800 | Si (100), SiO2, Al2O3 | 30–140 | 0.5–0.6 | Ir | Uniform, crystallites < 30 nm |
| [65] | [Ir(cod)CpMe] | H2 | 100/300–400 | W | 700–1500 | 11.7–25 | Ir | Untextured constituted of small grains |
| [29] | O2 | 80/270 | Si (100) | 95 | 0.42 | <1 mass.% C and O | ||
| [67] | C = 0.05M(THF) 200/300–450 | Si(100), SiO2/Si, TiN/SiO2/Si, Pt/Ti/Si, IrOx/poly-Si/SiO2/Si | 420 | 70 | 0.9 mass.% C, 0.4 mass.% O | |||
| [73] | [Ir(cod)CpEt] | O2 | 150–180/300–500 | Si, SiO2/Si, TiO2/SiO2/Si | 1.2–300 | 0.02–5 | Ir | 0.4 mass.% O |
| [57] | [Ir(C2H4)2CpEt] | O2 | 40/400 | SiO2/Si | – | – | Ir | Low O2 flow (0–2 sccm) |
| IrO2 | Higher O2 flow (10–160 sccm) | |||||||
| [55] | [Ir(chd)CpEt] | O2 | 70/250, 350 | SiO2 | 65, 120 | 1.6, 30 | Ir | Uniform crystallites, roughness—1.2 nm |
2.2. Volatile Platinum Precursors
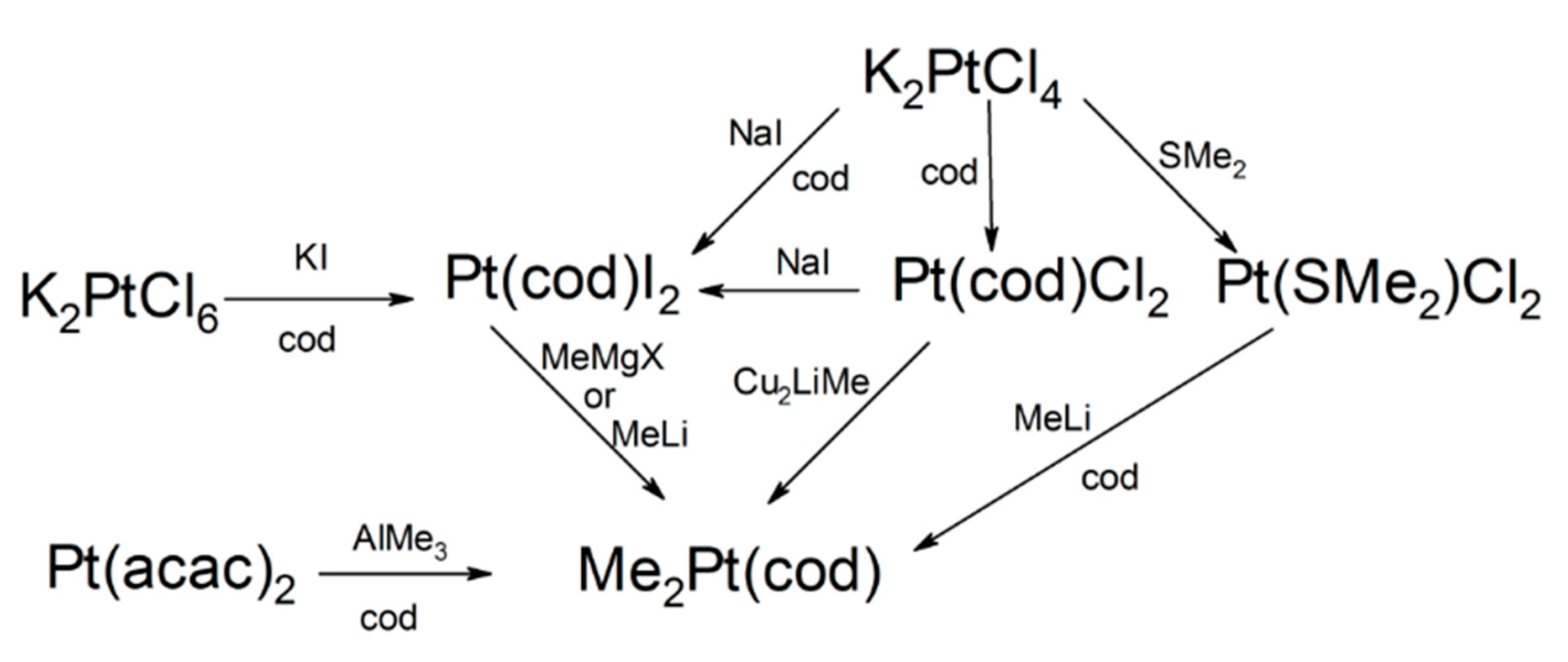
2.2.1. Platinum(0) Precursors
2.2.2. Platinum(II) Precursors
2.2.3. Platinum(IV) Precursors
2.2.4. Application of Platinum Precursors in MOCVD Processes
3. Precursors for the Preparation of Bimetallic Platinum- and Iridium-Containing Materials by MOCVD
4. MOCVD of Platinum and Iridium for Medical Application
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rao, C.R.; Trivedi, D. Chemical and electrochemical depositions of platinum group metals and their applications. Coord. Chem. Rev. 2005, 249, 613–631. [Google Scholar] [CrossRef]
- Hübert, T.; Boon-Brett, L.; Black, G.; Banach, U. Hydrogen sensors—A review. Sens. Actuators B Chem. 2011, 157, 329–352. [Google Scholar] [CrossRef]
- Wu, W.-P.; Chen, Z.-F. Iridium coating: Processes, properties and application. Part I. Johns. Matthey Technol. Rev. 2017, 61, 16–28. [Google Scholar] [CrossRef]
- Padamata, S.K.; Yasinskiy, A.S.; Polyakov, P.V.; Pavlov, E.A.; Varyukhin, D.Y. Recovery of noble metals from spent catalysts: A review. Met. Mater. Trans. B 2020, 51, 2413–2435. [Google Scholar] [CrossRef]
- Jang, H.; Lee, J. Iridium oxide fabrication and application: A review. J. Energy Chem. 2020, 46, 152–172. [Google Scholar] [CrossRef]
- Cogan, S.F. Neural stimulation and recording electrodes. Annu. Rev. Biomed. Eng. 2008, 10, 275–309. [Google Scholar] [CrossRef]
- Cowley, A.; Woodward, B. A Healthy future: Platinum in medical applications. Platin. Met. Rev. 2011, 55, 98–107. [Google Scholar] [CrossRef]
- Norlin, A.; Pan, J.; Leygraf, C. Investigation of interfacial capacitance of Pt, Ti and TiN coated electrodes by electrochemical impedance spectroscopy. Biomol. Eng. 2002, 19, 67–71. [Google Scholar] [CrossRef]
- Suska, F.; Svensson, S.; Johansson, A.; Emanuelsson, L.; Karlholm, H.; Ohrlander, M.; Thomsen, P. In vivo evaluation of noble metal coatings. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 92, 86–94. [Google Scholar] [CrossRef]
- Raphel, J.; Holodniy, M.; Goodman, S.B.; Heilshorn, S.C. Multifunctional coatings to simultaneously promote osseointegration and prevent infection of orthopaedic implants. Biomaterials 2016, 84, 301–314. [Google Scholar] [CrossRef]
- Svensson, S.; Suska, F.; Emanuelsson, L.; Palmquist, A.; Norlindh, B.; Trobos, M.; Bäckros, H.; Persson, L.; Rydja, G.; Ohrlander, M.; et al. Osseointegration of titanium with an antimicrobial nanostructured noble metal coating. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Köller, M.; Bellova, P.; Javid, S.M.; Motemani, Y.; Khare, C.; Sengstock, C.; Tschulik, K.; Schildhauer, T.A.; Ludwig, A. Antibacterial activity of microstructured sacrificial anode thin films by combination of silver with platinum group elements (platinum, palladium, iridium). Mater. Sci. Eng. C 2017, 74, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Abuayyash, A.; Ziegler, N.; Gessmann, J.; Sengstock, C.; Schildhauer, T.A.; Ludwig, A.; Köller, M. Antibacterial efficacy of sacrifical anode thin films combining silver with platinum group elements within a bacteria-containing human plasma clot. Adv. Eng. Mater. 2017, 20. [Google Scholar] [CrossRef]
- Garcia, J.R.V.; Goto, T. Chemical vapor deposition of iridium, platinum, rhodium and palladium. Mater. Trans. 2003, 44, 1717–1728. [Google Scholar] [CrossRef]
- Thurier, C.; Doppelt, P. Platinum OMCVD processes and precursor chemistry. Co-ord. Chem. Rev. 2008, 252, 155–169. [Google Scholar] [CrossRef]
- Hatanpää, T.; Ritala, M.; Leskelä, M. Precursors as enablers of ALD technology: Contributions from university of helsinki. Co-ord. Chem. Rev. 2013, 257, 3297–3322. [Google Scholar] [CrossRef]
- Hämäläinen, J.; Ritala, M.; Leskela, M. Atomic layer deposition of noble metals and their oxides. Chem. Mater. 2014, 26, 786–801. [Google Scholar] [CrossRef]
- Vasilyev, V.Y.; Morozova, N.B.; Basova, T.; Igumenov, I.K.; Hassan, A. Chemical vapour deposition of Ir-based coatings: Chemistry, processes and applications. RSC Adv. 2015, 5, 32034–32063. [Google Scholar] [CrossRef]
- Bennett, M.A.; Mitchell, T.R.B. .gamma.-Carbon-bonded 2,4-pentanedionato complexes of trivalent iridium. Inorg. Chem. 1976, 15, 2936–2938. [Google Scholar] [CrossRef]
- Yan, X.; Ai, T.; Su, X.; Wang, Z.; Sun, G.; Zhao, P. Synthesis and thermal decomposition mechanism study of a novel iridium precursor. MATEC Web Conf. 2016, 43, 1002. [Google Scholar] [CrossRef]
- Isakova, V.G.; Semyannikov, P.P.; Grankin, V.M.; Igumenov, I.K. Thermal stability of rhodium (III) and iridium (III) be-ta-diketonates. Koord. Khim. 1988, 14, 57–62. (In Russian) [Google Scholar]
- Zherikova, K.V.; Kuratieva, N.V.; Baidina, I.A.; Morozova, N.B. Crystal Structure of Iridium(III) trans-Trifluoroacetylacetonate. J. Struct. Chem. 2010, 51, 769–772. [Google Scholar] [CrossRef]
- Isakova, V.G.; Baidina, I.A.; Morozova, N.B.; Igumenov, I.K. y-Halogenated iridium (III) acetylacetonates. Polyhedron 2000, 19, 1097–1103. [Google Scholar] [CrossRef]
- Morozova, N.B.; Semyannikov, P.P.; Sysoev, S.V.; Grankin, V.M.; Igumenov, I.K. Saturated vapor pressure of iridium(iii) acetylacetonate. J. Therm. Anal. Calorim. 2000, 60, 489–495. [Google Scholar] [CrossRef]
- Gelfond, N.V.; Morozova, N.B.; Semyannikov, P.P.; Trubin, S.V.; Igumenov, I.K.; Gutakovskii, A.K.; Latyshev, A.V. Preparation of thin films of platinum group metals by pulsed MOCVD. I. Deposition of Ir layers. J. Struct. Chem. 2012, 53, 715–724. [Google Scholar] [CrossRef]
- Papke, J.A.; Stevenson, R.D. Evaluation of metal-organic compounds as materials for chemical vapor deposition. In Proceedings of the Conference on Chemical Vapor Deposition of Refractory Metals, Alloys, and Compounds, Gatlinburg, TN, USA, 12–14 September 1967; pp. 193–204. [Google Scholar]
- Uson, R.; Oro, L.A.; Cabeza, J.A.; Bryndza, H.E.; Stepro, M.P. Dinuclear methoxy, cyclooctadiene, and barrelene complexes of rhodium(i) and iridium(i). Inorg. Synth. 1985, 23, 126–130. [Google Scholar] [CrossRef]
- Tabrizi, D.; Pannetier, G. Etude radiocristallographique du di-μ-méthoxy-bis(π-cyclooctadiéne-1,5)diiridium [(COD-1,5)IrOCH3]2. J. Less Common Met. 1971, 23, 443–445. [Google Scholar] [CrossRef]
- Hoke, J.B.; Stern, E.W.; Murray, H.H. Low-temperature vapour deposition of high-purity iridium coatings from cyclooctadiene complexes of iridium. Synthesis of a novel liquid iridium chemical vapour deposition precursor. J. Mater. Chem. 1991, 1, 551–554. [Google Scholar] [CrossRef]
- Haszeldine, R.N.; Lunt, R.J.; Parish, R.V. Organic reactions involving transition metals. Part IV. Carboxylatodiene complexes of rhodium(I) and iridium(I). J. Chem. Soc. A 1971, 3696–3698. [Google Scholar] [CrossRef]
- Davignon, L.; Dereigne, A.; Bonnaire, R.; Manoli, J. Complexes β-dicétonato-(π-cyclooctadiène-1,5) iridium synthèses et étude cristallographique. J. Less Common Met. 1971, 25, 75–81. [Google Scholar] [CrossRef]
- Gerfin, T.; Hälg, W.J.; Atamny, F.; Dahmen, K.-H. Growth of iridium films by metal organic chemical vapour deposition. Thin Solid Film. 1993, 241, 352–355. [Google Scholar] [CrossRef]
- Vikulova, E.S.; Ilyin, I.Y.; Karakovskaya, K.I.; Piryazev, D.A.; Turgambaeva, A.E.; Morozova, N.B. Volatile iridium(I) complexes with β-diketones and cyclooctadiene: Syntheses, structures and thermal properties. J. Co-ord. Chem. 2016, 69, 2281–2290. [Google Scholar] [CrossRef]
- Xu, C.; Baum, T.H.; Rheingold, A.L. New precursors for chemical vapor deposition of iridium. Chem. Mater. 1998, 10, 2329–2331. [Google Scholar] [CrossRef]
- Karakovskaya, K.I.; Vikulova, E.S.; Ilyin, I.Y.; Piryazev, D.A.; Sysoev, S.V.; Morozova, N.B. Synthesis, structure and thermal investigation of a new volatile iridium (I) complex with cyclooctadiene and methoxy-substituted β-diketonate. J. Therm. Anal. Calorim. 2019, 137, 931–940. [Google Scholar] [CrossRef]
- Zherikova, K.V.; Kuratieva, N.V.; Morozova, N.B. Crystal structure of (acetylacetonato)(dicarbonyl)iridium(I). J. Struct. Chem. 2009, 50, 574–576. [Google Scholar] [CrossRef]
- Bonati, F.; Ugo, R. Iridium dicarbonyl β-diketonates. J. Organomet. Chem. 1968, 11, 341–352. [Google Scholar] [CrossRef]
- Vikulova, E.S.; Ilyin, I.Y.; Karakovskaya, K.I.; Piryazev, D.A.; Morozova, N.B. Crystal structure and thermal properties of (1,1,1,5,5,5-hexafluoropentanoato-4)··(dicarbonyl)iridium(I). J. Struct. Chem. 2015, 56, 1212–1214. [Google Scholar] [CrossRef]
- Bhirud, V.A.; Uzun, A.; Kletnieks, P.W.; Craciun, R.; Haw, J.F.; Dixon, D.A.; Olmstead, M.M.; Gates, B.C. Synthesis and crystal structure of Ir(C2H4)2(C5H7O2). J. Organomet. Chem. 2007, 692, 2107–2113. [Google Scholar] [CrossRef]
- Tucker, P.A. Acetylacetonato(1,5-cyclooctadiene)iridium(I). Acta Crystallogr. Sect. B 1981, 37, 1113–1115. [Google Scholar] [CrossRef]
- Jürgensen, L.; Frank, M.; Pyeon, M.; Czympiel, L.; Mathur, S. Subvalent iridium precursors for atom-efficient chemical vapor deposition of Ir and IrO2 thin films. Organometallics 2017, 36, 2331–2337. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Liu, C.-S.; Chi, Y.; Carty, A.J.; Peng, S.-M.; Lee, G.-H. Deposition of iridium thin films using new IrI CVD pre-cursors. Adv. Mater. 2002, 14, 17–20. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Hsu, C.-C.; Song, Y.-H.; Chi, Y.; Carty, A.J.; Peng, S.-M.; Lee, G.-H. Iridium metal thin films and patterned IrO2 nanowires deposited using iridium(I) carbonyl precursors. Chem. Vap. Depos. 2006, 12, 442–447. [Google Scholar] [CrossRef]
- Karakovskaya, K.I.; Vikulova, E.S.; Piryazev, D.A.; Morozova, N.B. Structure and thermal properties of (1,1,1-trifluoro-4-methyliminopentanoato-2) (cyclooctadiene-1,5)iridium(I). J. Struct. Chem. 2017, 58, 1427–1431. [Google Scholar] [CrossRef]
- Vikulova, E.S.; Karakovskaya, K.I.; Ilyin, I.Y.; Zelenina, L.N.; Sysoev, S.V.; Morozova, N.B. Thermodynamic study of volatile iridium (I) complexes with 1,5-cyclooctadiene and acetylacetonato derivatives: Effect of (O,O) and (O,N) coordination sites. J. Chem. Thermodyn. 2019, 133, 194–201. [Google Scholar] [CrossRef]
- Jürgensen, L.; Frank, M.; Graf, D.; Gessner, I.; Fischer, T.; Welter, K.; Jägermann, W.; Mathur, S. Nanostructured IrOx coatings for efficient oxygen evolution reactions in PV-EC setup. Z. Phys. Chem. 2020, 234, 911–924. [Google Scholar] [CrossRef]
- Sowa, J.R.; Angelici, R.J. Calorimetric determination of the heats of protonation of the metal in (methyl-substituted cyclopentadienyl)iridium complexes, Cp’Ir(1,5-COD). J. Am. Chem. Soc. 1991, 113, 2537–2544. [Google Scholar] [CrossRef]
- Zherikova, K.V.; Morozova, N.B.; Baidina, I.A. Crystal structure of (5-methylcyclopentadienyl)-(1,5-cyclooctadiene) iridium(I). J. Struct. Chem. 2009, 50, 570–573. [Google Scholar] [CrossRef]
- Bonegardt, D.V.; Il’In, I.Y.; Sukhikh, T.S.; Ilyina, E.V.; Morozova, N.B. Crystal structure and properties of Iridium(I)(1,5-Cyclooctadiene) (η5-Tetramethylcyclopentadiene) [Ir(cod)CpMe4]. J. Struct. Chem. 2020, 61, 456–465. [Google Scholar] [CrossRef]
- Bonegardt, D.V.; Il’In, I.Y.; Sukhikh, T.S.; Morozova, N.B. Crystal structure and properties of (1,5-cyclooctadiene) (η5-pentamethylcyclopentadienyl) iridium(I) [Ir(cod)Cp*]. J. Struct. Chem. 2017, 58, 983–988. [Google Scholar] [CrossRef]
- Herrmann, C.; Kehr, G.; Fröhlich, R.; Erker, G. Reactions of pendant boryl groups in Cp–metal complexes: Heterocyclic ring annelation in a CpIr system. Eur. J. Inorg. Chem. 2008, 2008, 2273–2277. [Google Scholar] [CrossRef]
- Müller, J.; Stock, R.; Pickardt, J. π-oefin iridium complexes, VII [1] Complex formation of iridium with fulvenes. Z. Nat. B 1981, 36, 1219–1224. [Google Scholar] [CrossRef]
- Johnson, B.F.G.; Lewis, J.; Yarrow, D.J. Reactivity of co-ordinated ligands. Part XIII. Electrophilic substitution reactions of cyclohexa-1,3-diene complexes of rhodium(I) and iridium(I). J. Chem. Soc. Dalton Trans. 1972, 2084–2089. [Google Scholar] [CrossRef]
- Hämäläinen, J.; Hatanpää, T.; Puukilainen, E.; Costelle, L.; Pilvi, T.; Ritala, M.; Leskelä, M. (MeCp)Ir(CHD) and molecular oxygen as precursors in atomic layer deposition of iridium. J. Mater. Chem. 2010, 20, 7669–7675. [Google Scholar] [CrossRef]
- Kawano, K.; Takamori, M.; Yamakawa, T.; Watari, S.; Fujisawa, H.; Shimizu, M.; Niu, H.; Oshima, N. A novel iridium precursor for MOCVD. MRS Proc. 2003, 784, C3.30. [Google Scholar] [CrossRef]
- Dziallas, M.; Hohn, A.; Werner, H. Basische metalle: LXII. Darstellung und reaktionen der metall-basen C5H5Ir(OLEFIN)PPri3 und C5H5Ir(OLEFIN)2. J. Organomet. Chem. 1987, 330, 207–219. [Google Scholar] [CrossRef]
- Kawano, K.; Furukawa, T.; Takamori, M.; Tada, K.-I.; Yamakawa, T.; Oshima, N.; Fujisawa, H.; Shimizu, M. A novel iridium precursor for MOCVD. ECS Trans. 2019, 1, 133–138. [Google Scholar] [CrossRef]
- Morozova, N.B.; Semyannikov, P.P.; Trubin, S.V.; Stabnikov, P.P.; Bessonov, A.A.; Zherikova, K.V.; Igumenov, I.K. Vapor pressure of some volatile iridium(I) compounds with carbonyl, acetylacetonate and cyclopentadienyl ligands. J. Therm. Anal. Calorim. 2009, 96, 261–266. [Google Scholar] [CrossRef]
- Ahmed, T.S.; Tonks, I.A.; Labinger, J.A.; Bercaw, J.E. Kinetics and mechanism of indene C–H bond activation by [(COD)Ir(μ2-OH)]2. Organometallics 2013, 32, 3322–3326. [Google Scholar] [CrossRef]
- Lee, H.-B.; Moseley, K.; White, C.; Maitlis, P.M. Pentamethylcyclopentadienyl-rhodium and -iridium complexes. Part X. The kinetics of the reactions of some cyclic and acyclic dienes with µ-hydrido-compounds. J. Chem. Soc. Dalton Trans. 1975, 2322–2329. [Google Scholar] [CrossRef]
- Sun, Y.-M.; Yan, X.-M.; Mettlach, N.; Endle, J.P.; Kirsch, P.D.; Ekerdt, J.; Madhukar, S.; Hance, R.L.; White, J.M. Precursor chemistry and film growth with (methylcyclopentadienyl) (1,5-cyclooctadiene)iridium. J. Vac. Sci. Technol. A 2000, 18, 10–16. [Google Scholar] [CrossRef]
- Endle, J.; Sun, Y.-M.; Nguyen, N.; Madhukar, S.; Hance, R.; White, J.; Ekerdt, J.G. Iridium precursor pyrolysis and oxidation reactions and direct liquid injection chemical vapor deposition of iridium films. Thin Solid Film. 2001, 388, 126–133. [Google Scholar] [CrossRef]
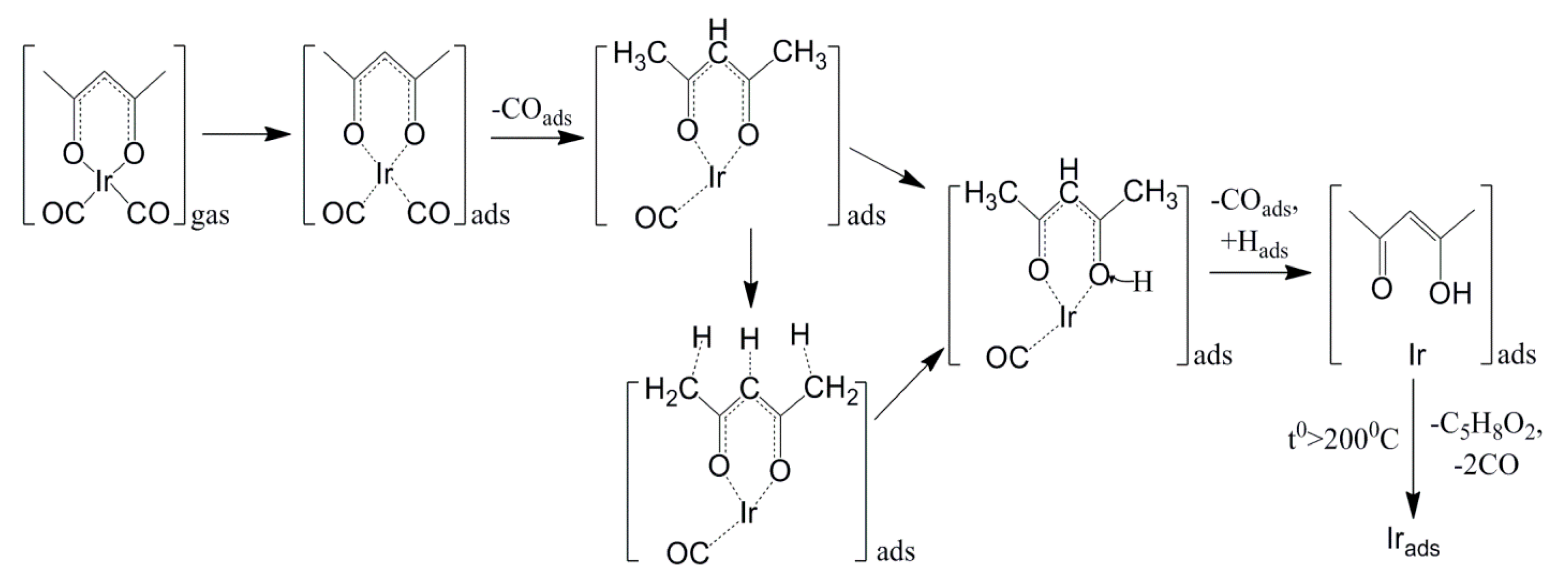
- Kovaleva, E.A.; Kuzubov, A.A.; Vikulova, E.S.; Basova, T.; Morozova, N.B. Mechanism of dicarbonyl(2,4-pentanedionato)iridium(I) decomposition on iron surface and in gas phase: Complex experimental and theoretical study. J. Mol. Struct. 2017, 1146, 677–683. [Google Scholar] [CrossRef][Green Version]
- Yang, S.; Yu, X.; Tan, C.; Wang, Y.; Ma, H.; Liu, K.; Cai, H. Growth kinetics and microstructure of MOCVD iridium coating from iridium(III) acetylacetonate with hydrogen. Appl. Surf. Sci. 2015, 329, 248–255. [Google Scholar] [CrossRef]
- Maury, F.; Senocq, F. Iridium coatings grown by metal–organic chemical vapor deposition in a hot-wall CVD reactor. Surf. Coatings Technol. 2003, 163–164, 208–213. [Google Scholar] [CrossRef]
- Winter, C.H.; Knisley, T.J.; Kalutarage, L.C.; Zavada, M.A.; Klesko, J.P.; Hiran Perera, T. Metallic Materials Deposition: Metal-Organic Precursors. In Encyclopedia of Inorganic and Bioinorganic Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011. [Google Scholar] [CrossRef]
- Dey, S.K.; Goswami, J.; Wang, C.-G.; Majhi, P. Preparation of iridium films by liquid source metalorganic chemical vapor deposition. Jpn. J. Appl. Phys. 1999, 38, L1052–L1054. [Google Scholar] [CrossRef]
- Goswami, J.; Wang, C.-G.; Majhi, P.; Shin, Y.-W.; Dey, S.K. Highly (111)-oriented and conformal iridium films by liquid source metalorganic chemical vapor deposition. J. Mater. Res. 2001, 16, 2192–2195. [Google Scholar] [CrossRef]
- Kim, S.-W.; Kwon, S.-H.; Kwak, D.-K.; Kang, S.-W. Phase control of iridium and iridium oxide thin films in atomic layer deposition. J. Appl. Phys. 2008, 103, 23517. [Google Scholar] [CrossRef]
- Lim, Y.H.; Yoo, H.; Choi, B.H.; Lee, J.H.; Lee, H.-N.; Lee, H.K. Atomic-layer-deposited Ir thin film as a novel diffusion barrier layer in Cu interconnection. Phys. Status Solidi C 2011, 8, 891–894. [Google Scholar] [CrossRef]
- Schlicht, S.; Haschke, S.; Mikhailovskii, V.; Manshina, A.; Bachmann, J. Highly reversible water oxidation at ordered nanoporous iridium electrodes based on an original atomic layer deposition. ChemElectroChem 2018, 5, 1259–1264. [Google Scholar] [CrossRef]
- Lisker, M.; Ritterhaus, Y.; Hur’Yeva, T.; Burte, E. Iridium electrodes for ferroelectric capacitors deposited by liquid-delivery MOCVD and PVD. ECS Trans. 2019, 2, 67–78. [Google Scholar] [CrossRef]
- Ritterhaus, Y.; Hur’Yeva, T.; Lisker, M.; Burte, E.P. Iridium thin films deposited by liquid delivery MOCVD using Ir(EtCp)(1,5-COD) with toluene solvent. Chem. Vap. Depos. 2007, 13, 698–704. [Google Scholar] [CrossRef]
- Kumar, R.; Roy, S.; Rashidi, M.; Puddephatt, R. New precursors for chemical vapour deposition of platinum and the hydrogen effect on cvd. Polyhedron 1989, 8, 551–553. [Google Scholar] [CrossRef]
- Dryden, N.H.; Kumar, R.; Ou, E.; Rashidi, M.; Roy, S.; Norton, P.R.; Puddephatt, R.J.; Scott, J.D. Chemical vapor deposition of platinum: New precursors and their properties. Chem. Mater. 1991, 3, 677–685. [Google Scholar] [CrossRef]
- Kruck, T. Trifluorophosphine complexes of transition metals. Angew. Chem. Int. Ed. Engl. 1967, 6, 53–67. [Google Scholar] [CrossRef]
- Nabeya, S.; Saito, M.; Application, F.; Data, P. Chemical Vapor Deposition Methods Using an Organoplatinum Compound. U.S. Patent 8,911,827, 16 December 2014. [Google Scholar]
- Maudez, W.; Roy, C.; Tran, P.D.; Thurier, C.; Karmous, F.; Doppelt, P. New dimethyl(norbornadienyl)platinum(II) precursors for platinum MOCVD. Chem. Vap. Depos. 2014, 20, 59–68. [Google Scholar] [CrossRef]
- Faust, M.; Enders, M.; Gao, K.; Reichenbach, L.; Muller, T.; Gerlinger, W.; Sachweh, B.; Kasper, G.; Bruns, M.; Bräse, S.; et al. Synthesis of Pt/SiO2 catalyst nanoparticles from a continuous aerosol process using novel cyclo-octadienylplatinum precursors. Chem. Vap. Depos. 2013, 19, 274–283. [Google Scholar] [CrossRef]
- Hughes, R.P.; Overby, J.S.; Lam, K.-C.; Incarvito, C.D.; Rheingold, A.L. Oxidative addition reaction of perfluoro-n-butyl iodide to (COD)PtMe2 to give (COD)PtMe(nC4F9): The molecular structure of (COD)PtMe2. Polyhedron 2002, 21, 2357–2360. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, M.; Li, C.; Williams, C.T.; Liang, C. CVD of Pt(C5H9)2 to synthesize highly dispersed Pt/SBA-15 catalysts for hydrogenation of chloronitrobenzene. Chem. Vap. Depos. 2014, 20, 146–151. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Z.; Gray, D.L.; Zhu, L.; Abelson, J.R.; Girolami, G.S. Platinum ω-alkenyl compounds as chemical vapor deposition precursors: Synthesis and characterization of Pt[CH2CMe2CH2CH═CH2]2 and the impact of ligand design on the deposition process. Chem. Mater. 2020, 32, 9316–9334. [Google Scholar] [CrossRef]
- Baidina, I.A.; Zharkova, G.I.; Piryazev, D.A. Structure and properties of the volatile isomers of bis(1,1,1-trifluoro-5,5-dimethylhexane-2,4-dionato) of platinum(II). J. Struct. Chem. 2016, 57, 1188–1194. [Google Scholar] [CrossRef]
- Lashdaf, M.; Hatanpaa, T.; Tiitta, M. Volatile β-diketonato complexes of ruthenium, palladium and platinum. Preparation and thermal characterization. J. Therm. Anal. Calorim. 2001, 64, 1171–1182. [Google Scholar] [CrossRef]
- Zharkova, G.I.; Sysoev, S.V.; Baidina, I.A.; Stabnikov, P.A.; Igumenov, I.K. Vapor pressure and crystal structure of platinum(II) trans-bis-(trifluoroketoiminate). J. Struct. Chem. 2005, 46, 494–500. [Google Scholar] [CrossRef]
- Baidina, I.A.; Gromilov, S.A.; Zharkova, G.I. Crystal and molecular structures ofcis-bis-(1,1,1-trifluoro-5-methoxy-5-methyl-2,4-hexanedionato)palladium(II) and -platinum(II). J. Struct. Chem. 1999, 40, 633–639. [Google Scholar] [CrossRef]
- Zharkova, G.I.; Baidina, I.A.; Smolentsev, A.I.; Stabnikov, P.A.; Morozova, N.B. Structure and thermal properties of a novel volatile bis(1,1,1-trifluoro-5,5-dimethyl-3-hexene-4-imino-2-onate) platinum(II) compound. J. Struct. Chem. 2017, 58, 970–974. [Google Scholar] [CrossRef]
- Baidina, I.A.; Zharkova, G.I.; Gromilov, S.A. Crystal and molecular structure of platinum(ii) trans-Bis-(1,1,1-trifluoro-4-iminopentan-2-onate). J. Struct. Chem. 2001, 42, 114–119. [Google Scholar] [CrossRef]
- Brückmann, L.; Tyrra, W.; Stucky, S.; Mathur, S. Novel air-stable and volatile Bis(pyridylalkenolato)palladium(II) and -platinum(II) derivatives. Inorg. Chem. 2012, 51, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Lee, H.; Park, M.; An, K.; Kim, J.; Lee, J.; Chung, T.; Kim, C.G. Synthesis of novel platinum precursor and its application to metal organic chemical vapor deposition of platinum thin films. Bull. Korean Chem. Soc. 2008, 29, 1491–1494. [Google Scholar] [CrossRef][Green Version]
- Musetha, P.L.; Revaprasadu, N.; Kolawole, G.A.; Pullabhotla, R.V.; Ramasamy, K.; O’Brien, P. Homoleptic single molecular precursors for the deposition of platinum and palladium chalcogenide thin films. Thin Solid Film. 2010, 519, 197–202. [Google Scholar] [CrossRef]
- Zeng, D.; Linda, Y.; Jeffery, S.; Beach, H.; King, K. Process for the preparation of platinum acetylacetonato complexes. U.S. Patent 7,442,820, 28 October 2008. [Google Scholar]
- Zharkova, G.I.; Baidina, I.A.; Stabnikov, P.A.; Igumenov, I.K. Trans-, cis-isomers of (1,1,1-trifluoro-2,4-pentandionato)Pt(II): Volatility, structure and crystal lattice energy. J. Struct. Chem. 2006, 47, 716–725. [Google Scholar] [CrossRef]
- Hierso, J.; Feurer, R.; Kalck, P. Platinum and palladium films obtained by low-temperature MOCVD for the formation of small particles on divided supports as catalytic materials. Chem. Mater. 2000, 12, 390–399. [Google Scholar] [CrossRef]
- Igor, K.; Tamara, V.; Vladimir, R. Volatile Precursors for films deposition: Vapor pressure, structure and thermodynamics. Appl. Thermodyn. Biol. Mater. Sci. 2011, 521–546. [Google Scholar] [CrossRef]
- Salazar, B.; Liu, H.; Walker, A.V.; McElwee-White, L. Low temperature platinum chemical vapor deposition on functionalized self-assembled monolayers. J. Vac. Sci. Technol. A 2020, 38, 033404. [Google Scholar] [CrossRef]
- Taguchi, A.; Inoue, M.; Hiromi, C.; Tanizawa, M.; Kitami, T.; Abe, T. Study of the surface morphology of platinum thin films on powdery substrates prepared by the barrel sputtering system. Vacuum 2008, 83, 575–578. [Google Scholar] [CrossRef]
- Crane, E.L.; You, Y.; Nuzzo, R.G.; Girolami, G.S. Mechanistic studies of CVD metallization processes: Reactions of rhodium and platinumβ-diketonate complexes on copper surfaces. J. Am. Chem. Soc. 2000, 122, 3422–3435. [Google Scholar] [CrossRef]
- Meiere, S.H.; Hoover, C.A. Method for Producing Organometallic Compounds. U.S. Patent 6,809,212, 24 October 2004. [Google Scholar]
- Dorovskikh, S.I.; Zharkova, G.I.; Turgambaeva, A.E.; Krisyuk, V.V.; Morozova, N.B. Chemical vapour deposition of platinum films on electrodes for pacemakers: Novel precursors and their thermal properties. Appl. Organomet. Chem. 2017, 31, e3654. [Google Scholar] [CrossRef]
- Zharkova, G.I.; Baidina, I.; Turgambaeva, A.; Romanenko, G.V.; Igumenov, I. Volatile fluorinated trimethylplatinum(IV) β-diketonates: Synthesis, properties, and structure. Polyhedron 2012, 40, 40–45. [Google Scholar] [CrossRef]
- Dorovskikh, S.I.; Krisyuk, V.V.; Mirzaeva, I.V.; Komarov, V.Y.; Trubin, S.V.; Turgambaeva, A.E.; Morozova, N.B. The volatile trimethylplatinum(IV) complexes: Effect of β-diketonate substituents on thermal properties. Polyhedron 2020, 182, 114475. [Google Scholar] [CrossRef]
- Xue, Z.; Strouse, M.J.; Shuh, D.K.; Knobler, C.B.; Kaesz, H.D.; Hicks, R.F.; Williamst, R.S. Characterization of (methylcyclopentadienyl)trimethylplatinum and low-temperature organometallic chemical vapor deposition of platinum metal. J. Am. Chem. Soc. 1989, 111, 8779–8784. [Google Scholar] [CrossRef]
- Dorovskikh, S.I.; Zharkova, G.I.; Zelenina, L.N.; Asanov, I.P.; Kal’Nii, D.B.; Kokovkin, V.V.; Shubin, Y.V.; Basova, T.; Morozova, N.B. MOCVD growth of Pt films using a novel Pt(IV) compound as a precursor. Phys. Status Solidi 2015, 12, 1053–1059. [Google Scholar] [CrossRef]
- Kessels, W.M.M.; Knoops, H.C.M.; Dielissen, S.A.F.; Mackus, A.J.M.; Van De Sanden, M.C.M. Surface reactions during atomic layer deposition of Pt derived from gas phase infrared spectroscopy. Appl. Phys. Lett. 2009, 95, 013114. [Google Scholar] [CrossRef]
- Mohlala, L.M.; Jen, T.-C.; Olubambi, P.A. Initial reaction mechanism on the atomic layer deposition of platinum on a graphene surface: A density functional theory study. Procedia Manuf. 2019, 35, 1250–1255. [Google Scholar] [CrossRef]
- Jackson, C.; Smith, G.T.; Mpofu, N.; Dawson, J.M.S.; Khoza, T.; September, C.; Taylor, S.M.; Inwood, D.W.; Leach, A.S.; Kramer, D.; et al. A quick and versatile one step metal-organic chemical deposition method for supported Pt and Pt-alloy catalysts. RSC Adv. 2020, 10, 19982–19996. [Google Scholar] [CrossRef]
- Igumenov, I.; Gelfond, N.; Galkin, P.; Morozova, N.; Fedotova, N.; Zharkova, G.; Shipachev, V.; Reznikova, E.; Ryabtsev, A.; Kotsupalo, N.; et al. Corrosion testing of platinum metals CVD coated titanium anodes in seawater-simulated solutions. Desalination 2001, 136, 273–280. [Google Scholar] [CrossRef]
- Daniele, S.; Battistel, D.; Gerbasi, R.; Benetollo, F.; Battiston, S. Titania-coated platinum thin films by MOCVD: Electrochemical and photoelectrochemical properties. Chem. Vap. Depos. 2007, 13, 644–650. [Google Scholar] [CrossRef]
- Goswami, J.; Wang, C.-G.; Cao, W.; Dey, S. MOCVD of platinum films from (CH3)3CH3CpPt and Pt(acac)2: Nanostructure, conformality, and electrical resistivity. Chem. Vap. Depos. 2003, 9, 213–220. [Google Scholar] [CrossRef]
- Barison, S.; Fabrizio, M.; Carta, G.; Rossetto, G.; Zanella, P.; Barreca, D.; Tondello, E. Nanocrystalline Pt thin films obtained via metal organic chemical vapor deposition on quartz and CaF2 substrates: An investigation of their chemico-physical properties. Thin Solid Film. 2002, 405, 81–86. [Google Scholar] [CrossRef]
- Battiston, G.A.; Gerbasi, R.; Rodriguez, A. A Novel study of the growth and resistivity of nanocrystalline pt films obtained from pt(acac)2 in the presence of oxygen or water vapor. Chem. Vap. Depos. 2005, 11, 130–135. [Google Scholar] [CrossRef]
- Hiratani, M.; Nabatame, T.; Matsui, Y.; Kimura, S. Crystallographic and electrical properties of platinum film grown by chemical vapor deposition using (methylcyclopentadienyl)trimethylplatinum. Thin Solid Film. 2002, 410, 200–204. [Google Scholar] [CrossRef]
- Hämäläinen, J.; Munnik, F.; Ritala, M.; Leskela, M. Atomic layer deposition of platinum oxide and metallic platinum thin films from Pt(acac)2 and ozone. Chem. Mater. 2008, 20, 6840–6846. [Google Scholar] [CrossRef]
- Choi, W.-G.; Choi, E.-S.; Yoon, S.-G. Pt thin film collectors prepared by liquid-delivery metal–organic CVD using Pt(C2H5C5H4)(CH3)3 for LiCoO2 thin film cathodes. Chem. Vap. Depos. 2003, 9, 321–325. [Google Scholar] [CrossRef]
- Dorovskikh, S.I.; Klyamer, D.D.; Koretskaya, T.P.; Kal′nyi, D.B.; Morozova, N.B. Studying the microstructure of Pt layers prepared by chemical vapor deposition in the presence of hydrogen. J. Struct. Chem. 2020, 61, 1211–1218. [Google Scholar] [CrossRef]
- Martin, T.P.; Tripp, C.P.; DeSisto, W.J. Composite platinum/silicon dioxide films deposited using CVD. Chem. Vap. Depos. 2005, 11, 170–174. [Google Scholar] [CrossRef]
- Faust, M.; Seipenbusch, M. Highly controlled structuring of Pt nanoparticles on TiO2 and on ZrO2 by a modified MOCVD process. Surf. Coat. Technol. 2014, 259, 577–584. [Google Scholar] [CrossRef]
- Valet, O.; Doppelt, P.; Baumann, P.; Schumacher, M.; Balnois, E.; Bonnet, F.; Guillon, H. Study of platinum thin films deposited by MOCVD as electrodes for oxide applications. Microelectron. Eng. 2002, 64, 457–463. [Google Scholar] [CrossRef]
- Huang, S.-F.; Chi, Y.; Liu, C.-S.; Carty, A.; Mast, K.; Bock, C.; MacDougall, B.; Peng, S.-M.; Lee, G.-H. Preparation of Pt–Ru alloyed thin films using a single-source CVD precursor. Chem. Vap. Depos. 2003, 9, 157–161. [Google Scholar] [CrossRef]
- Contreras, M.A.G.; Fernández-Valverde, S.; García, J.R.V. Pt, PtNi and PtCoNi film electrocatalysts prepared by chemical vapor deposition for the oxygen reduction reaction in 0.5 M KOH. J. Alloys Compd. 2010, 504, S425–S428. [Google Scholar] [CrossRef]
- Colindres, S.C.; García, J.V.; Antonio, J.T.; Chavez, C.A. Preparation of platinum-iridium nanoparticles on titania nanotubes by MOCVD and their catalytic evaluation. J. Alloys Compd. 2009, 483, 406–409. [Google Scholar] [CrossRef]
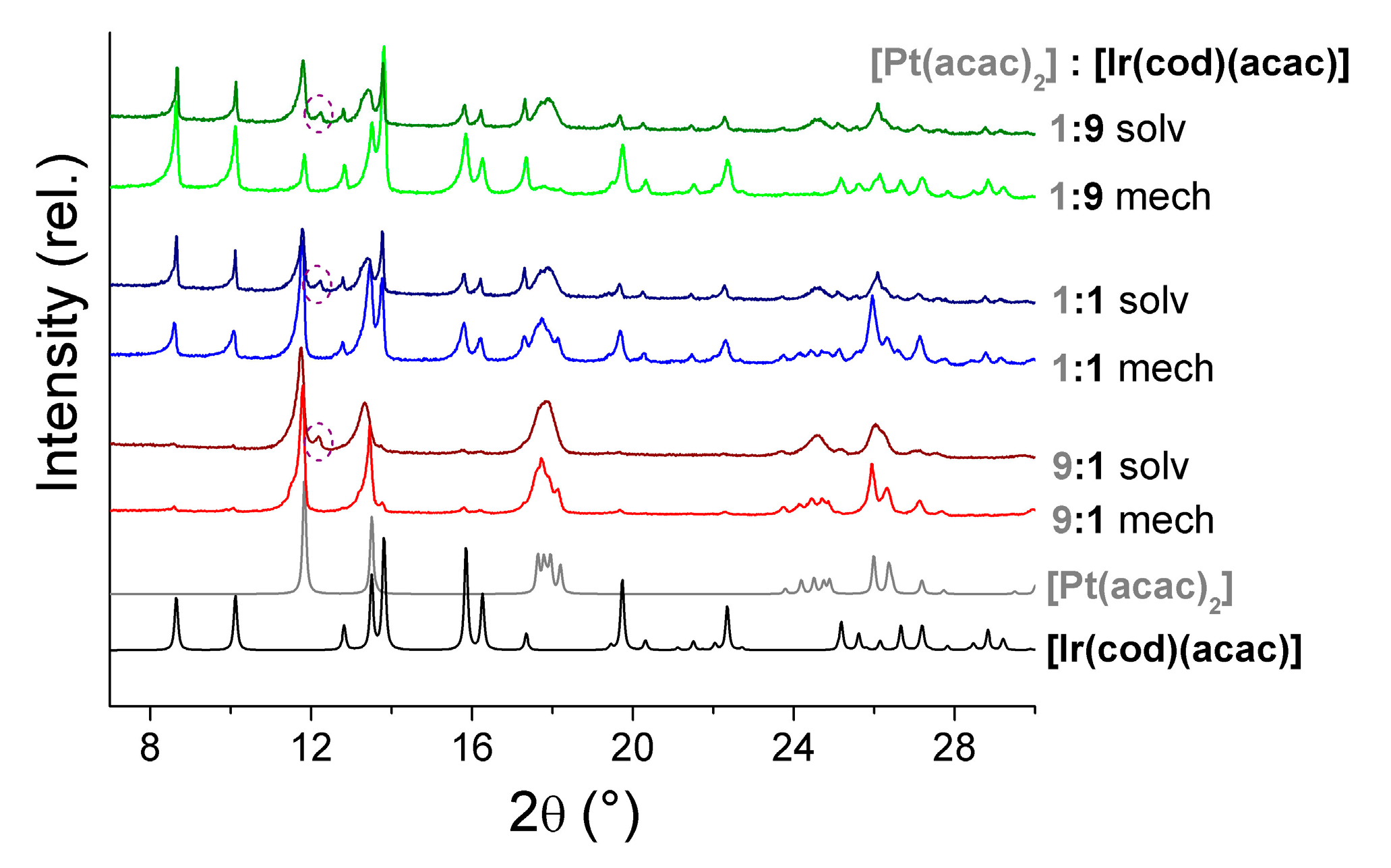
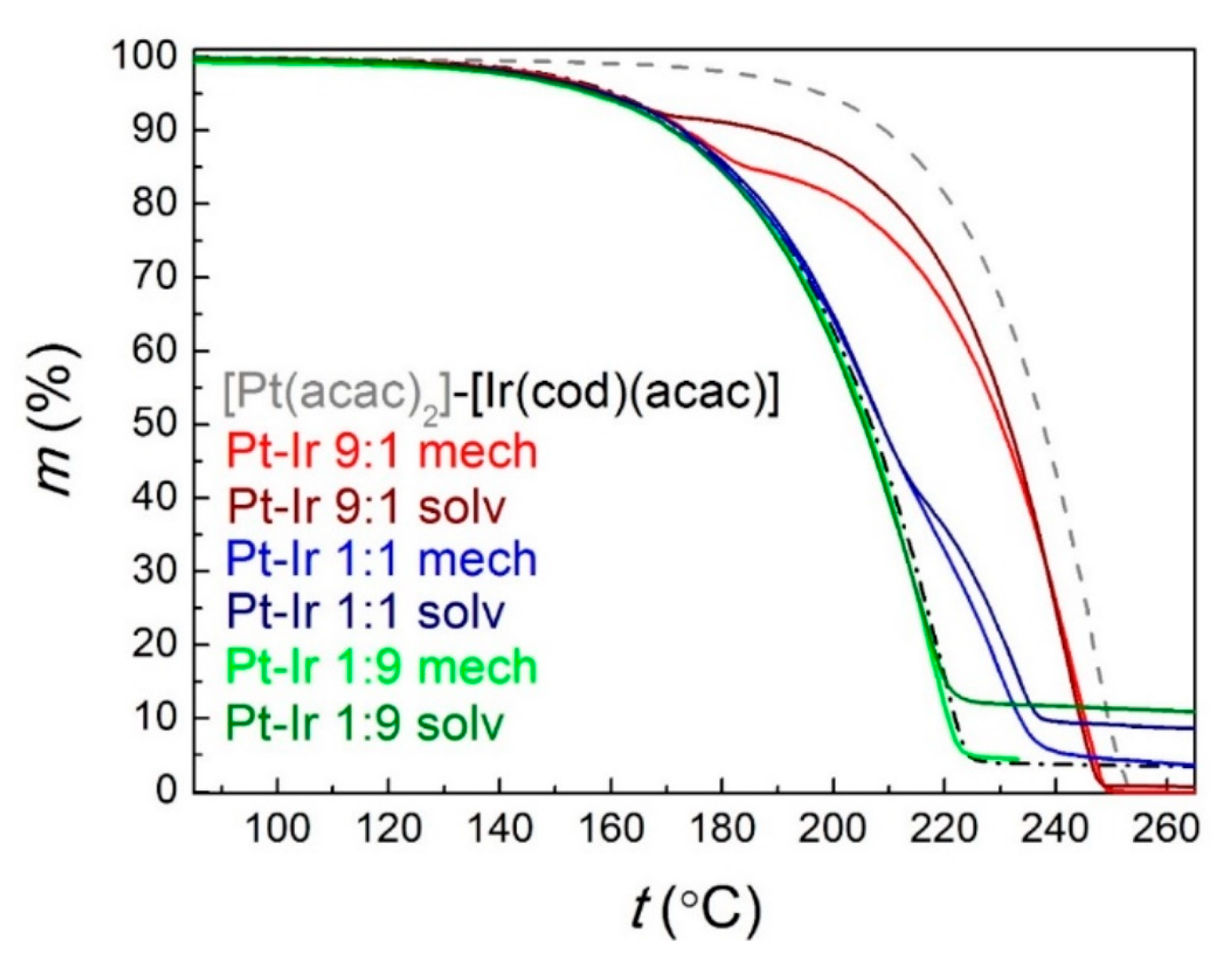
- Dorovskikh, S.I.; Vikulova, E.S.; Kal’Nyi, D.B.; Shubin, Y.V.; Asanov, I.P.; Maximovskiy, E.A.; Gutakovskii, A.K.; Morozova, N.B.; Basova, T.V. Bimetallic Pt,Ir-containing coatings formed by MOCVD for medical applications. J. Mater. Sci. Mater. Med. 2019, 30, 69. [Google Scholar] [CrossRef]
- Turgambaeva, A.E.; Zherikova, K.V.; Mosyagina, S.A.; Igumenov, I.K. A study of the thermal behavior of the system of zirconium and neodymium dipyvaloylmethanates. Russ. J. Appl. Chem. 2017, 90, 1062–1067. [Google Scholar] [CrossRef]
- Onuma, S.; Horioka, K.; Inoue, H.; Shibata, S. The crystal structure of Bis(acetylacetonato)platinum(II). Bull. Chem. Soc. Jpn. 1980, 53, 2679–2680. [Google Scholar] [CrossRef]
- Sykaras, N.; Iacopino, A.M.; Marker, V.A.; Triplett, R.G.; Woody, R.D. Implant materials, designs, and surface topographies: Their effect on osseointegration. A literature review. Int. J. Oral Maxillofac. Implant. 2000, 15, 675–690. [Google Scholar]
- Liu, Y.; Rath, B.; Tingart, M.; Eschweiler, J. Role of implants surface modification in osseointegration: A systematic review. J. Biomed. Mater. Res. Part A 2020, 108, 470–484. [Google Scholar] [CrossRef] [PubMed]
- Vikulova, E.S.; Kal’Nyi, D.B.; Shubin, Y.V.; Kokovkin, V.V.; Morozova, N.B.; Hassan, A.; Basova, T. Metal Ir coatings on endocardial electrode tips, obtained by MOCVD. Appl. Surf. Sci. 2017, 425, 1052–1058. [Google Scholar] [CrossRef]
- Gelfond, N.V.; Krisyk, V.V.; Dorovskikh, S.I.; Kal’Nyi, D.B.; Maksimovskii, E.A.; Shubin, Y.V.; Trubin, S.V.; Morozova, N.B. Structure of platinum coatings obtained by chemical vapor deposition. J. Struct. Chem. 2015, 56, 1215–1219. [Google Scholar] [CrossRef]
- Dorovskikh, S.I.; Kal’Nui, D.B.; Maximovskyi, E.; Morozova, N.B. MOCVD of Pt coatings with high surface areas on the contacts of electrophysiological diagnostic electrodes. Mater. Today Proc. 2019, 19, 924–930. [Google Scholar] [CrossRef]
- Chen, C.; Ruan, S.; Bai, X.; Lin, C.; Xie, C.; Lee, I.-S. Patterned iridium oxide film as neural electrode interface: Biocompatibility and improved neurite outgrowth with electrical stimulation. Mater. Sci. Eng. C 2019, 103, 109865. [Google Scholar] [CrossRef]
- Carnicer-Lombarte, A.; Lancashire, H.T.; Vanhoestenberghe, A. In vitrobiocompatibility and electrical stability of thick-film platinum/gold alloy electrodes printed on alumina. J. Neural Eng. 2017, 14, 036012. [Google Scholar] [CrossRef]
- Breisch, M.; Grasmik, V.; Schildhauer, T.A.; Köller, M.; Sengstock, C.; Loza, K.; Pappert, K.; Rostek, A.; Ziegler, N.; Ludwig, A.; et al. Bimetallic silver–platinum nanoparticles with combined osteo-promotive and antimicrobial activity. Nanotechnology 2019, 30, 305101. [Google Scholar] [CrossRef]
- Krumdieck, S.P.; Reyngoud, B.P.; Barnett, A.D.; Clearwater, D.J.; Hartshorn, R.M.; Bishop, C.M.; Redwood, B.P.; Palmer, E.L. Deposition of bio-integration ceramic hydroxyapatite by pulsed-pressure MOCVD using a single liquid precursor solution. Chem. Vap. Depos. 2010, 16, 55–63. [Google Scholar] [CrossRef]
- Cimpean, A.; Popescu, S.; Ciofrangeanu, C.M.; Gleizes, A.N. Effects of LP-MOCVD prepared TiO2 thin films on the in vitro behavior of gingival fibroblasts. Mater. Chem. Phys. 2011, 125, 485–492. [Google Scholar] [CrossRef]
- Popescu, S.; Demetrescu, I.; Sarantopoulos, C.; Gleizes, A.N.; Iordachescu, D. The biocompatibility of titanium in a buffer solution: Compared effects of a thin film of TiO2 deposited by MOCVD and of collagen deposited from a gel. J. Mater. Sci. Mater. Med. 2007, 18, 2075–2083. [Google Scholar] [CrossRef]
- Nazarov, D.V.; Zemtsova, E.G.; Valiev, R.Z.; Smirnov, V.M. Formation of micro- and nanostructures on the nanotitanium surface by chemical etching and deposition of titania films by atomic layer deposition (ALD). Materials 2015, 8, 8366–8377. [Google Scholar] [CrossRef] [PubMed]
- Visentin, F.; Galenda, A.; Fabrizio, M.; Battiston, S.; Brianese, N.; Gerbasi, R.; Zin, V.; El Habra, N. Assessment of synergistic effects of LP-MOCVD TiO2 and Ti surface finish for dental implant purposes. Appl. Surf. Sci. 2019, 490, 568–579. [Google Scholar] [CrossRef]
- Chouirfa, H.; Bouloussa, H.; Migonney, V.; Falentin-Daudré, C. Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomater. 2019, 83, 37–54. [Google Scholar] [CrossRef] [PubMed]
- Piszczek, P.; Radtke, A. Silver nanoparticles fabricated using chemical vapor deposition and atomic layer deposition techniques: Properties, applications and perspectives: Review. Noble Precious Met. Prop. Nanoscale Eff. Appl. 2018, 9, 187–213. [Google Scholar] [CrossRef]
- Basova, T.; Hassan, A.; Morozova, N. Chemistry of gold(I, III) complexes with organic ligands as potential MOCVD precursors for fabrication of thin metallic films and nanoparticles. Coord. Chem. Rev. 2019, 380, 58–82. [Google Scholar] [CrossRef]
- Jeyaraj, M.; Gurunathan, S.; Qasim, M.; Kang, M.-H.; Kim, J.-H. A comprehensive review on the synthesis, characterization, and biomedical application of platinum nanoparticles. Nanomaterials 2019, 9, 1719. [Google Scholar] [CrossRef]
- Zhao, Y.; Ye, C.; Liu, W.; Chen, R.; Jiang, X. Tuning the composition of AuPt bimetallic nanoparticles for antibacterial application. Angew. Chem. Int. Ed. 2014, 53, 8127–8131. [Google Scholar] [CrossRef]
- Rice, K.M.; Ginjupalli, G.K.; Manne, N.D.P.K.; Jones, C.B.; Blough, E.R. A review of the antimicrobial potential of precious metal derived nanoparticle constructs. Nanotechnology 2019, 30, 372001. [Google Scholar] [CrossRef]
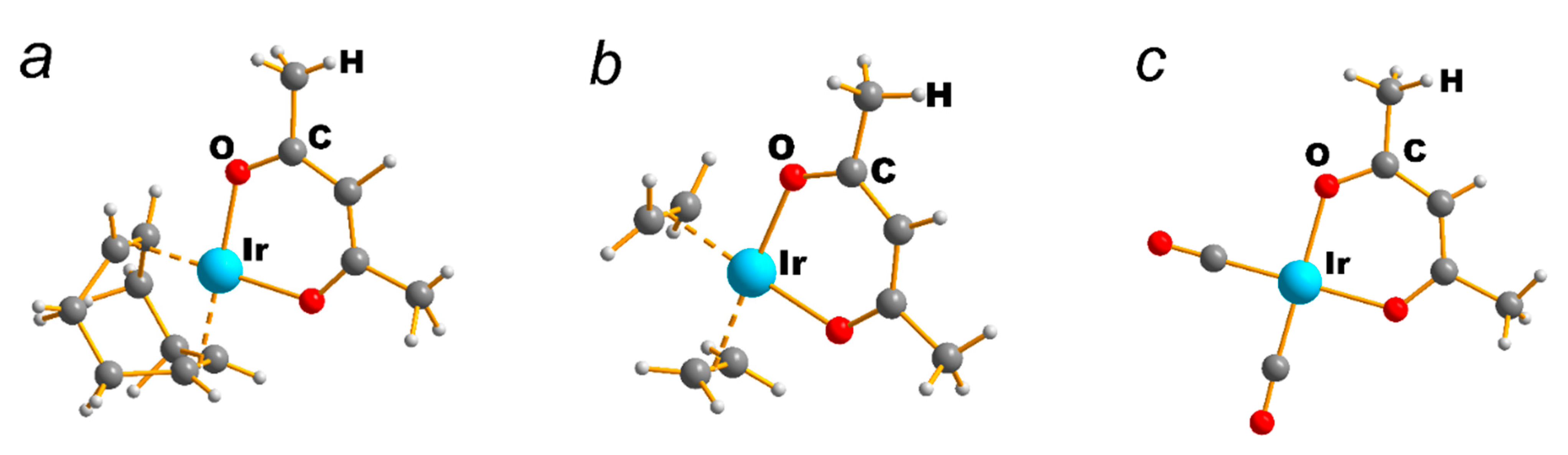

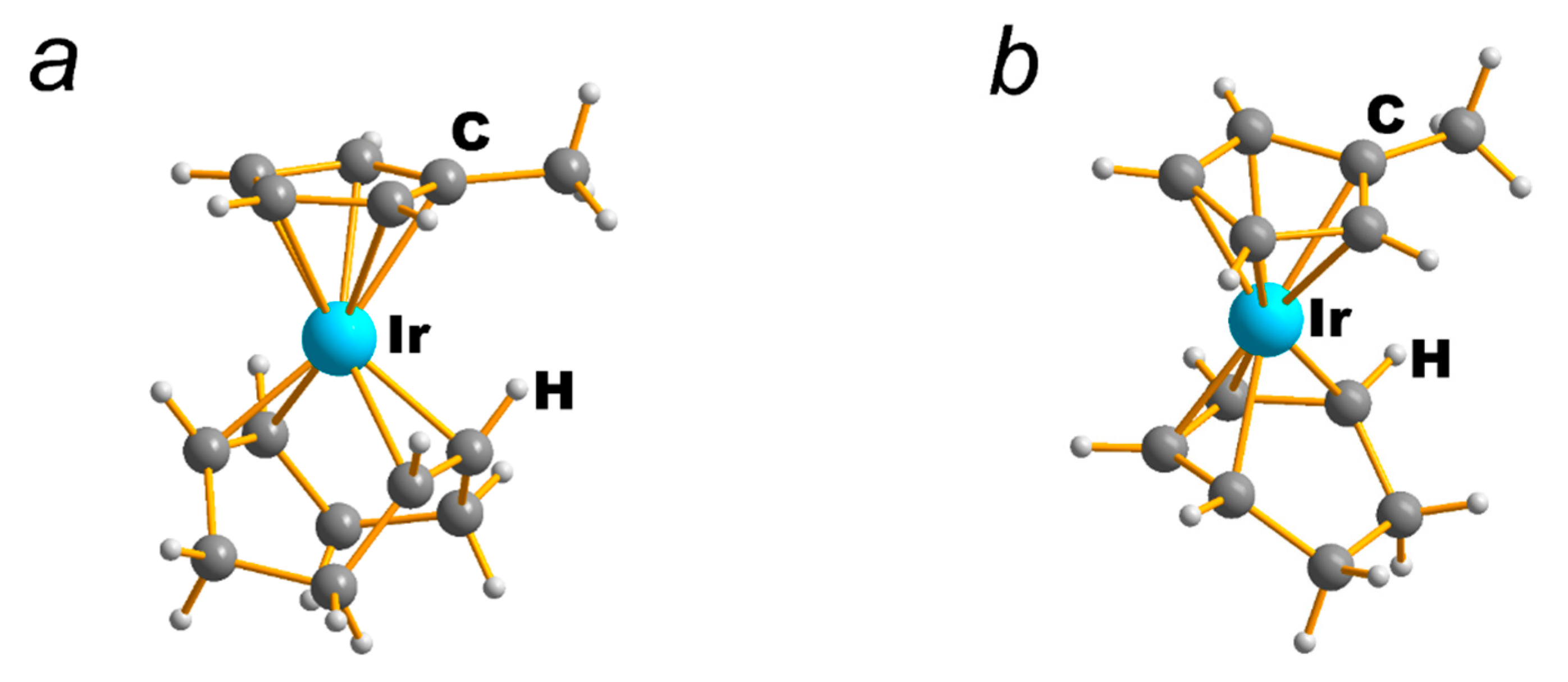

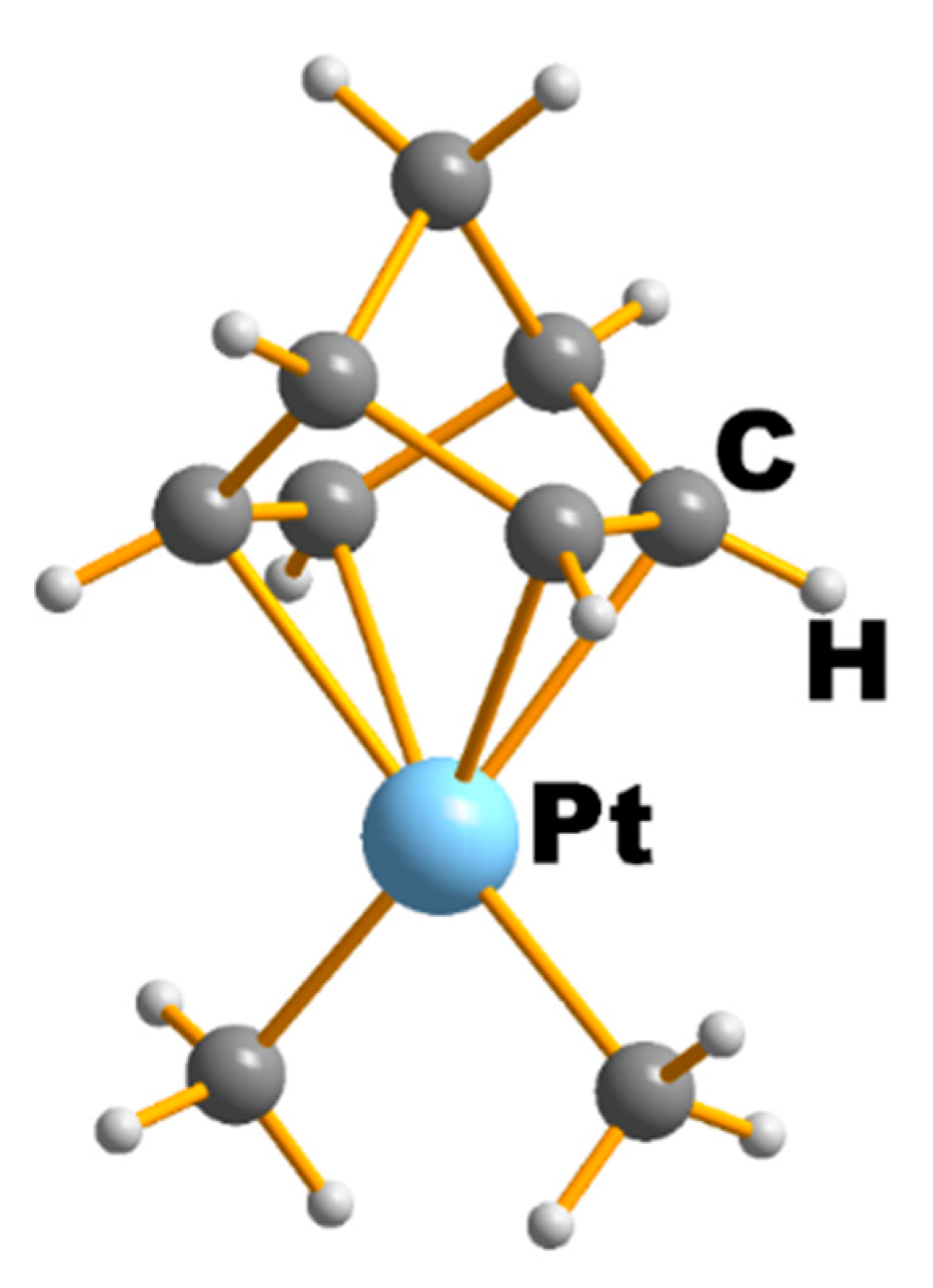
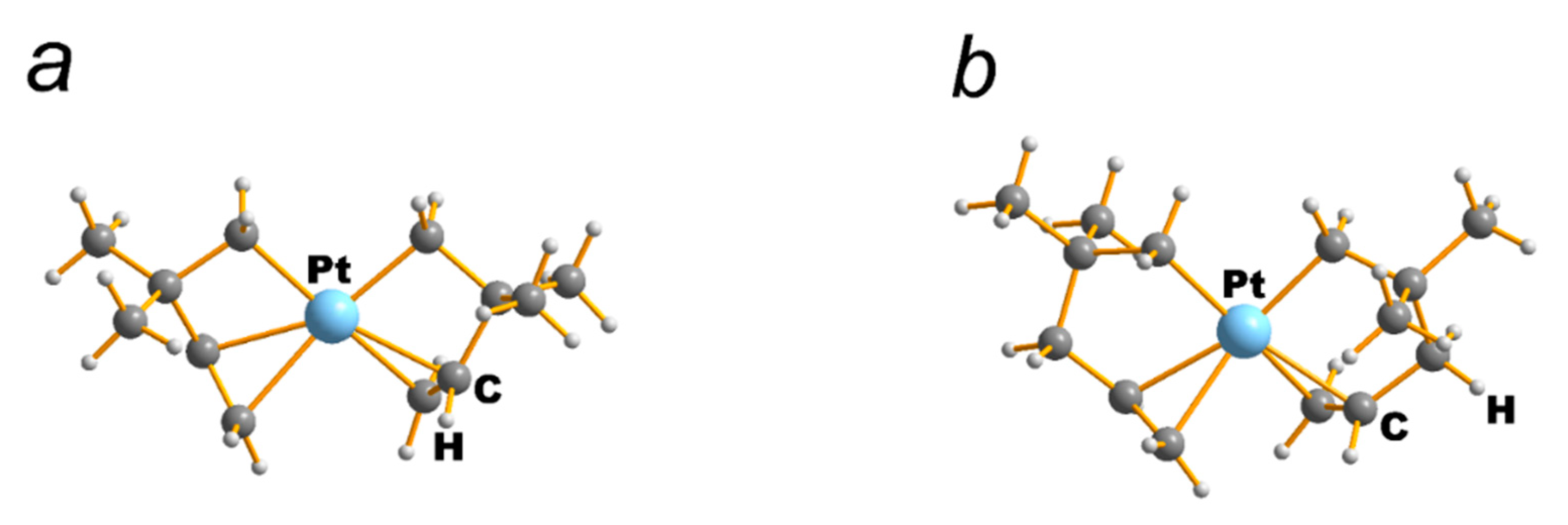
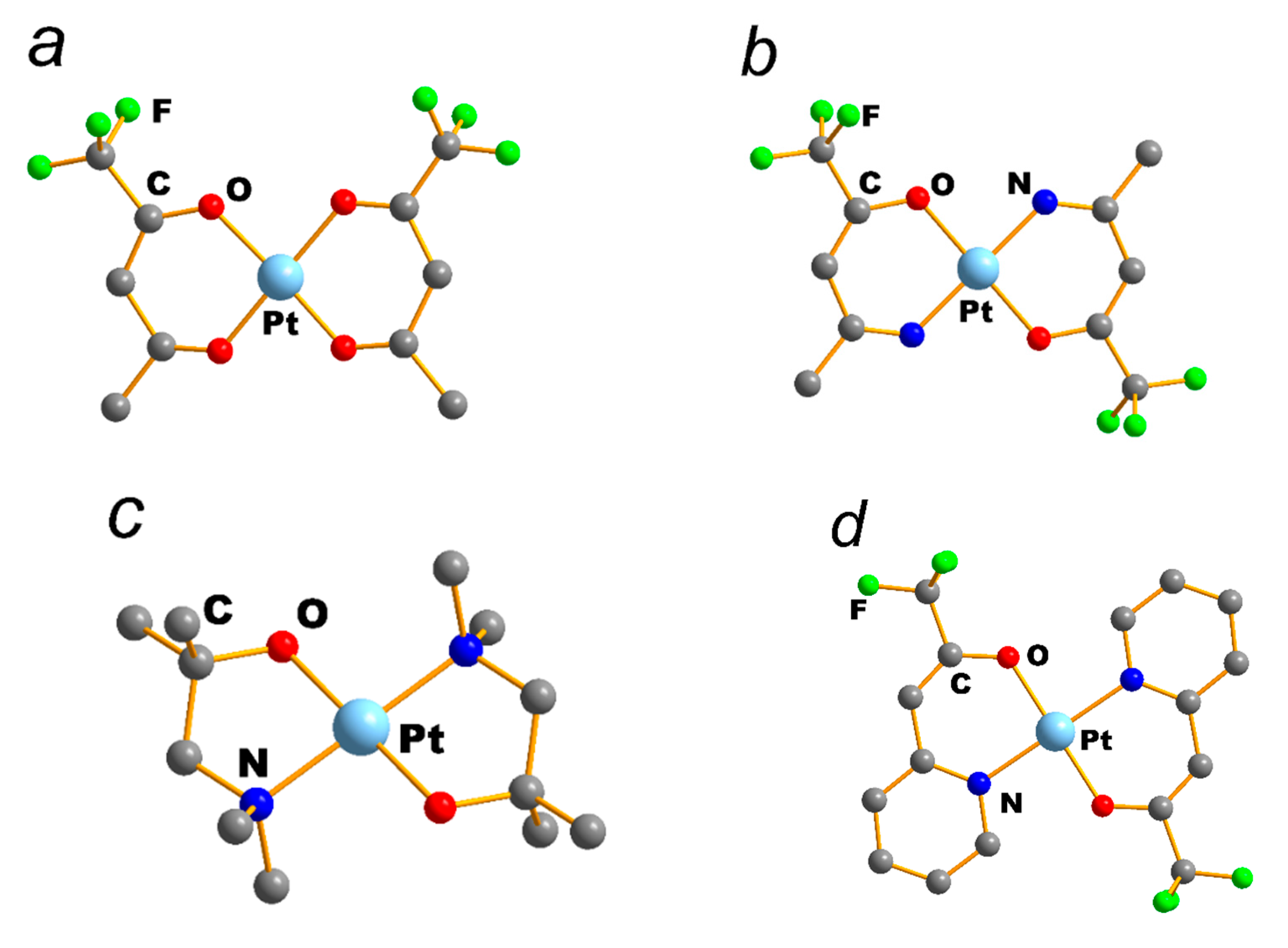
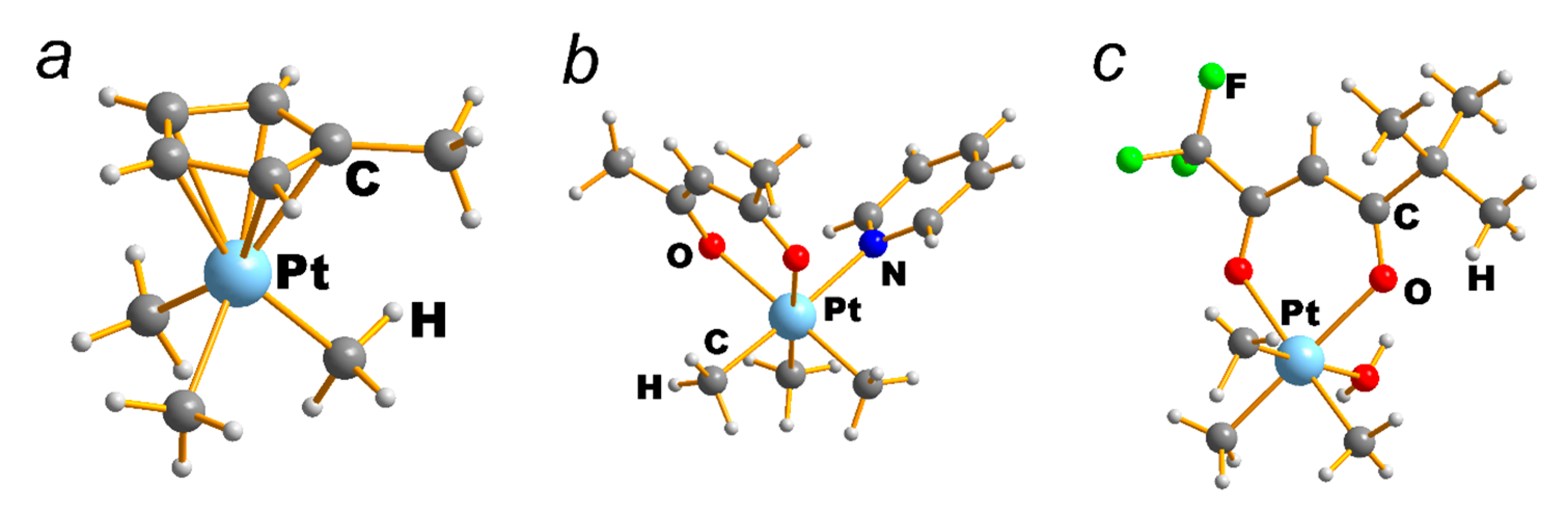

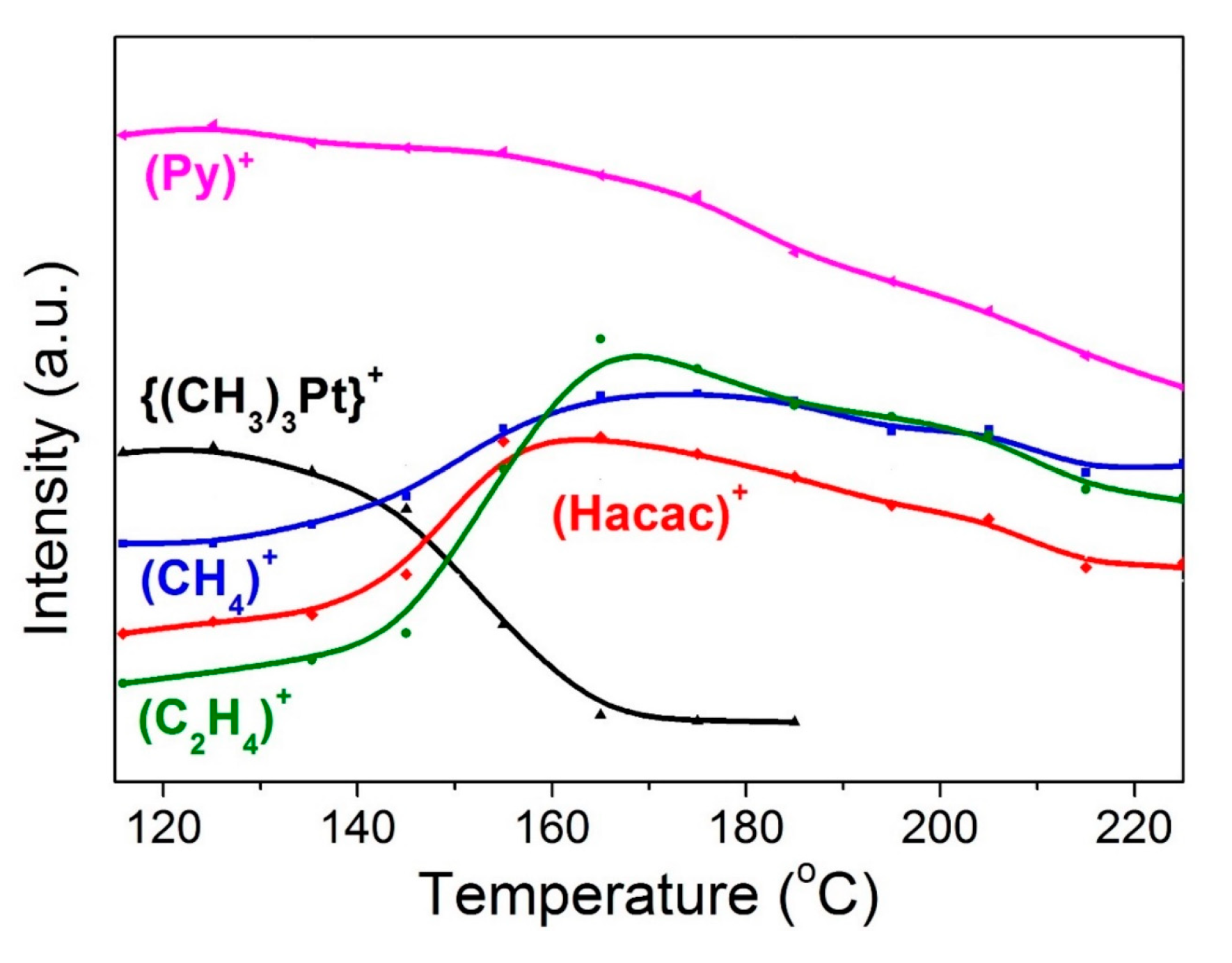
| NEUTRAL LIGANDS | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| hexadiens | norbornadiens | cyclooctadiens | ||||||||||
| Structure | R1 | R2 | Abbreviation | Structure | R | Abbreviation | Structure | R | Abbreviation | |||
 | H | H | hd | 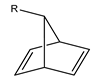 | H | nbd | 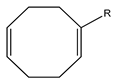 | H | cod | |||
| H | Me | hd-Me | Me | cod-Me | Et | cod-Et | ||||||
| Me | Me | hd-Me2 | Et | nbd-Et | Bu | cod-Bu | ||||||
| iPr | nbd-iPr | iBu | cod-iBu | |||||||||
| ANIONIC LIGANDS | ||||||||||||
| β-diketonates | cyclopentadienyls | |||||||||||
| Structure | R1 | R2 | Abbreviation | Structure | R1 | R2 | R3 | R4 | R5 | Abbreviation | ||
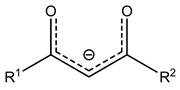 | Me | acac |  | H | H | H | H | H | Cp | |||
| tBu | thd | Me | H | H | H | H | CpMe | |||||
| tBu/CF3 | C(OMe)Me2 | zis/zif | Et | H | H | H | H | CpEt | ||||
| tBu | CF3 | ptac | allyl | H | H | H | H | Cp-allyl | ||||
| Me | CF3 | tfac | nPr | H | H | H | H | Cp-propenyl | ||||
| CF3 | hfac | iPr | H | H | H | H | Cp-ipropenyl | |||||
| CF3 | Ph | btfac | nBu | H | H | H | H | CpBu | ||||
| Ph | dbac | Me | H | Me | Me | H | CpMe3 | |||||
| Me | Ph | bac | Me | Me | Me | Me | H | CpMe4 | ||||
| CF3 | C4H4S | ttfac | Me | Me | Me | Me | Me | Cp* | ||||
| β-iminoketonates | β-iminoalcoholates | ω-Alkenyls | ||||||||||
| Structure | R1 | R2 | R3 | Abbreviation | Structure | R | Abbreviation | Structure | Abbreviation | |||
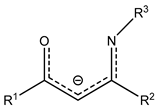 | CF3 | Et | Eti-hfac | 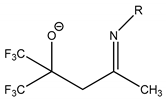 | Me | Mei-hfda | 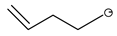 | C5H9 | ||||
| nPr | nPri-hfac | |||||||||||
| CF3 | Me | Me | Mei-tfac | Et | Eti-hfda | Structure | n | Abbreviation | ||||
| CF3 | H | CH2CF3 | TFB-TFEA | 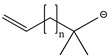 | 0 | C6H11 | ||||||
| Me | H | i-acac | nPr | nPri-hfda | 1 | C7H13 | ||||||
| Me | Mei-acac | 2 | C8H15 | |||||||||
| α-aminoalcoholates | β-heteroarylketonate | β-alkenols | dithio/diselenoimidodiphosphinato | |||||||||
| Structure | R | Abbreviation | Structure | Abbreviation | Structure | R | Abbreviation | Structure | Hal | Abbreviation | ||
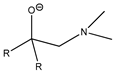 | Me | amN(Me)2 | 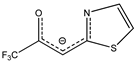 | ThTFP |  | Me | alk(Me) | 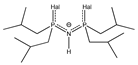 | S | S/S-idpp | ||
| CF3 | amakN(Me)2 | CF3 | alk(CF3) | Se | Se/Se-idpp | |||||||
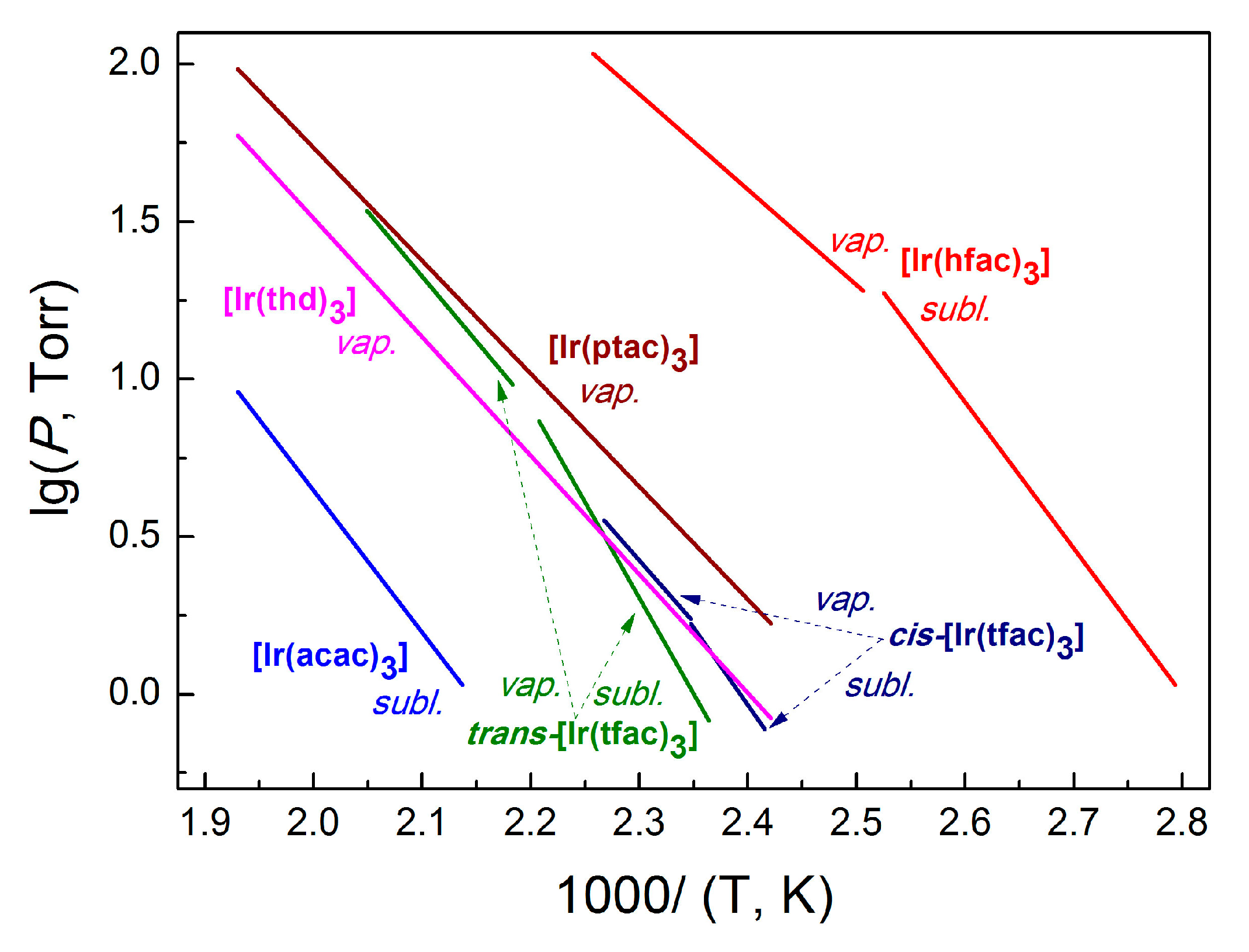
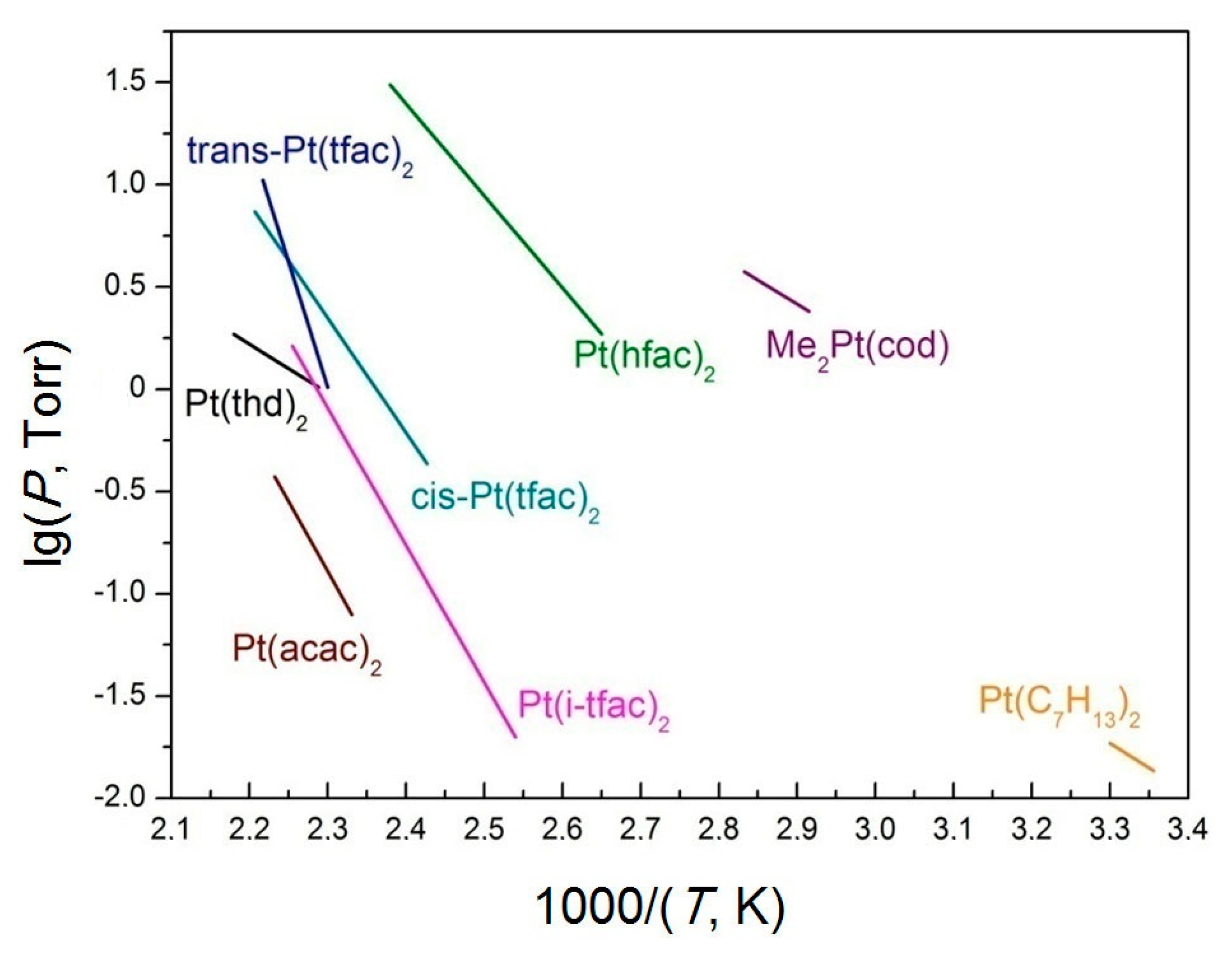
| Ref. | Complex | Process | ΔT, K | ln(p/p0) = A − B/(T/K) | ΔHT*, kJ·mol−1 | ΔS0T*, J·(mol·K)−1 | |
|---|---|---|---|---|---|---|---|
| A | B | ||||||
| [24] | Ir(acac)3 | Subl. | 468–518 | 15.65 | 10,397 | 86 ± 2 | 130 ± 4 |
| [21] | trans-Ir(tfac)3 | Subl. | 423–453 | 26.30 | 14,013 | 116 ± 3 | 218 ± 17 |
| Vap. | 458–488 | 16.29 | 9462 | 79 ± 3 | 136 ± 6 | ||
| cis-Ir(tfac)3 | Subl. | 414–426 | 20.62 | 11,389 | 95 ± 8 | 171 ± 18 | |
| Vap. | 426–441 | 15.15 | 9045 | 75 ± 3 | 126 ± 6 | ||
| Ir(hfac)3 | Subl. | 358–396 | 23.29 | 10,688 | 88 ± 6 | 193 ± 9 | |
| Vap. | 401–443 | 13.79 | 6973 | 56 ± 1 | 115 ± 2 | ||
| Ir(thd)3 | Vap. | 418–522 | 14.55 | 8927 | 74 ± 2 | 116 ± 4 | |
| Ir(ptac)3 | Vap. | 413–518 | 13.88 | 8259 | 68 ± 1 | 116 ± 2 | |
| Ref. | Complex | Process | ΔT, K | ln(p/p0) = A − B/(T,K) | ΔHT*, kJ·mol−1 | ΔS0T*, J·(mol·K)−1 | |
|---|---|---|---|---|---|---|---|
| A | B | ||||||
| [45] | [Ir(cod)(acac)] | Subl. | 363–423 | 23.03 | 12,817 | 106.6 ± 0.7 | 191 ± 2 |
| [35] | [Ir(cod)(zis)] | Subl. | 353–376 | 28.04 | 14,886 | 124 ± 4 | 233 ± 10 |
| Vap. | 381–403 | 18.95 | 11,477 | 96 ± 5 | 158 ± 13 | ||
| [45] | [Ir(cod)(i-acac)] | Subl. | 383–431 | 25.31 | 14,312 | 119 ± 2 | 210 ± 5 |
| [Ir(cod)(Mei-acac)] | Subl. | 383–420 | 22.56 | 13,398 | 111 ± 3 | 188 ± 7 | |
| [58] | [Ir(cod)CpMe] | Subl. | 304–310 | 33.43 | 14,990 | 124.6 ± 5.0 | 279 ± 16 |
| Vap. | 310–330 | 19.39 | 10,592 | 88.1 ± 1.3 | 161.2 ± 4.2 | ||
| [Ir(CO)2Cp*] | Subl. | 297–332 | 27.91 | 12,641 | 105.0 ± 3.4 | 232 ± 11 | |
| [Ir(CO)2(acac)] | Subl. | 306–333 | 22.40 | 11,293 | 94 ± 2 | 186 ± 8 | |
| Ref. | Compounds | Process | ΔT, K | ln(p/p0) = A − B/(T,K) | ΔHT*, kJ·mol−1 | ΔS0T*, J·(mol·K)−1 | |
|---|---|---|---|---|---|---|---|
| A | B | ||||||
| [94] | Me2Pt(cod) | Subl. | 343–353 | 17.2 | 5441 | 45.2 | 142.9 |
| [82] | Pt(C7H13)2 | Subl. | – | 14.2 | 5520 | 44.1 | 118.2 |
| [94] | Pt(hfac)2 | Subl. | 323–358 | 29.30 | 9320 | 77 ± 2 | 243.5 |
| [95] | Subl. | 377–419 | 21.9 | 10,520 | 83.6 ± 0.4 | 183 | |
| Vap. | 419–439 | 12.9 | 6670 | 52.8 ± 1.5 | 107.2 | ||
| [85] | cis-Pt(tfac)2 | Subl. | 412–461 | 23.8 | 12,900 | 106.6 ± 0.4 | 196.46 ± 4.59 |
| trans-Pt(tfac)2 | Subl. | 437–506 | 23.14 | 13,210 | 109.93 ± 2.92 | 192.28 ± 5.43 | |
| [95] | Pt(thd)2 | Vap. | 447–467 | 16.2 | 9280 | 77.1 ± 0.7 | 134.3 ± 1.6 |
| [85] | Pt(acac)2 | Subl. | 393–453 | 23.40 | 14,136 | 117.52 ± 1.38 | 117.52 ± 1.38 |
| Subl. | 332–399 | 22.15 | 13,391 | 111.33 ± 0.81 | 184.18 ± 2.20 | ||
| Pt(i-tfac)2 | Subl. | 393–453 | 23.62 | 13,429 | 111.54 ± 4.17 | 196.20 ± 9.82 | |
| Ref. | Compounds | Process | ΔT, K | ln(p/p0) = A − B/(T,K) | ΔHT*, kJ·mol−1 | ΔS0T*, J·(mol·K)−1 | |
|---|---|---|---|---|---|---|---|
| A | B | ||||||
| [15] | Me3Pt(Cp) | Subl. | – | 25.9 | 8590 | 71.4 | 215 |
| Me3Pt(CpMe) | Subl. | – | 26.1 | 8690 | 72.21 | 217 | |
| [95] | Me3Pt(hfac)H2O | Subl. | 338–365 | 32.78 | 14,103 | 117.2 ± 0.5 | 272.4 ± 1.5 |
| [100] | Me3Pt(hfac)Py | Vap. | 343–393 | 11.23 | 6787 | 56.4 ± 0.5 | 93.3 ± 1.5 |
| Me3Pt(tfac)Py | Vap. | 348–393 | 18.51 | 10,060 | 83.6 ± 0.3 | 153.8 ± 0.8 | |
| Me3Pt(thd)Py | Vap. | 366–403 | 12.72 | 8147 | 67.7 ± 0.7 | 105.7 ± 1.8 | |
| [102] | Me3Pt(ptac)Py | Subl. | 309–343 | 34.17 | 15,631 | 129.9 ± 2.5 | 284.2 ± 7.7 |
| Me3Pt(zis)Py | Subl. | 326–353 | 32.79 | 15,896 | 132.1 ± 1.8 | 272.5 ± 5.3 | |
| [104] | Me3Pt(acac)Py | Subl. * | – | 12407 | 23.46 | 103.1 ± 0.7 | 195.0 ± 1.3 |
| Vap. | 390–414 | 9699 | 16.47 | 80.6 ± 0.5 | 136.9 ± 1.2 | ||
| Ref. | Precursor | Reagent Gas | Source/Deposition Temperature, °C | Substrate | Thickness, nm | Growth Rate, nm/min | Composition (XRD) | Purity and Other Details |
|---|---|---|---|---|---|---|---|---|
| [94] | Me2Pt(cod) | H2 | –/90–120 | SiO2 + TEOS | – | – | Pt | FB-MOCVD, PtNPs with average d = 1–3 nm Pt0, 2.3–3.8 mass.%; C, 4 mass.% |
| [79] | Me2Pt(cod-Et) | O2 | 100/380 | Aerosil®200 SiO2 | – | – | CVS/MOCVD, PtNPs with average d = 2–2.2 nm Pt0, 4–5.7 mass.%; C, 4 mass.% PtO, up to 8.6 mass.%; PtO2 up to 0.7 mass.% | |
| Me2Pt(cod-Bu) | 100/380 | |||||||
| Me2Pt(cod-iBu) | 110/380 | |||||||
| [77] | Me2Pt(hd) | H2 | 40/200–300 | SiO2 + TEOS | 100 | – | Smoothed films (Ra~1 nm), 25 μΩ·cm or less | |
| Me2Pt(hd-Me) | ||||||||
| Me2Pt(hd-Me2) | ||||||||
| [78] | Me2Pt(nbd) | N2 + O2 | C = 0.1 M(tolulene)/300 | Si/SiO2 | 60 | 2 | LD-MOCVD, 1 mass.% C and O | |
| [81] | Pt(C5H9)2 | no | 25/500 | mesoporous silica | – | – | PtNPs with average d = 4.6 ± 1.1nm, Pt0, 2.27–4.95 mass.%, | |
| [82] | Pt(C7H13)2 | O2 | 75–80/250–330 | Al2O3 SiO2, Si, Au | 12 (PACVD) | – | PA-MOCVD (Ra~1.7–2.2 nm) or MOCVD 47–56% Pt, 38–40% C, and 5–13% O | |
| 300 (MOCVD) | ||||||||
| [90] | Pt(amN(Me)2)2 | H2 | 80/400–500 | TaN/Ta/Si | – | 0.35 | Smoothed films (Ra~1 nm), 13.6 μΩ·cm 9 mass.% C and 1 mass.% O | |
| [91] | Pt(S/S-idpp)2 Pt(Se/Se-idpp)2 | no | 150/200–600 | glass | 250–300 | – | PtS2 PtSe2 | Large particles of PtS2 with d = 2–3 μm PtSe2 films with a worm-like morphology |
| [108] | Pt(acac)2 | O2 | 180–220/260–380 | Ti | 200–1700 | – | Pt | Electrode life up to 698 hrs |
| [109] | H2O | 140/280–380 | glass rods, TiO2 | 10–200 | 0.1–1.1 | Pt or Pt-TiO2 | 100 nm Pt film behaves like a bulk Pt electrode | |
| [110] | O2 | C = 0.04 M(THF) 200/350 | Si—trench structures | 200–600 | 1.8 | Pt | LD-MOCVD, Ra = 5 nm 27.6 μΩ·cm, 2 mass.% C | |
| [111] | O2, H2O | 140/260–300(O2) vs. 280–400(H2O) | Soda glass | – | 0.7–2.4(O2) vs. 0.1–1.1(H2O) | 110 vs. 91 kJ/mol activation energy | ||
| [112] | N2–O2, N2–O2-H2O, N2–H2 | 160–170/420 | quartz CaF2 | 50–80 280–310 | 15 | 53–16 mass.% C | ||
| [117] | O2 | 130/300–440 | sappfire | – | 1.3–21.2 | Pt-SiO2 | 11–18 mass.% C | |
| [110] | O2 | C = 0.06 M(THF) 70/350 | Si—trench structures | 12–140 | 2 | Pt | (111)-oriented films, Ra = 15 nm 23.6 μΩ·cm, 6 mass.% C | |
| [113] | Me3Pt(CpMe) | O2 | 35/200–400 | Si | 20–600 | – | (111)-oriented films, >15 μΩ·cm | |
| [118] | O2 | 35/150 | TiO2, ZrO2 | – | – | FB-MOCVD, PtNPs with average d = 2.1 nm Pt0, 3.4–5.7 mass.%; C, 4 mass.% | ||
| [119] | O2 | 48/350–400 | TiO2/SiO2/Si, SiO2/Si | 60–100 | 0.5 | LD-MOCVD, Ra = 3.1–4.5 nm(TiO2/SiO2/Si), Ra = 4.9–7.6 nm(SiO2/Si), 8–10 mass.% C | ||
| [115] | Me3Pt(CpEt) | O2 | C = 0.05 M(THF) 130/300–450 | Si—trench structures, LiCoO2 | – | – | LD-MOCVD, (111)-oriented films, Ra = 5.4 nm, 11.8–25 μΩ·cm | |
| [100] | Me3Pt(acac)Py | H2/O2 | 85/280–310 | Si(100), Ti electrodes | 110–270 800–850 | 1.1(H2) 3.6 (O2) | 15 mass.% C and 1 mass.% O (H2) 5 mass.% C and 1 mass.% O (O2) | |
| [116] | Me3Pt(hfac)Py | H2 | 50/200–270 | Si(100), Ti electrodes | – | 0.7–3.2 | UV-MOCVD, fractal Pt films were deposited at a high hydrogen concentration |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karakovskaya, K.I.; Dorovskikh, S.I.; Vikulova, E.S.; Ilyin, I.Y.; Zherikova, K.V.; Basova, T.V.; Morozova, N.B. Volatile Iridium and Platinum MOCVD Precursors: Chemistry, Thermal Properties, Materials and Prospects for Their Application in Medicine. Coatings 2021, 11, 78. https://doi.org/10.3390/coatings11010078
Karakovskaya KI, Dorovskikh SI, Vikulova ES, Ilyin IY, Zherikova KV, Basova TV, Morozova NB. Volatile Iridium and Platinum MOCVD Precursors: Chemistry, Thermal Properties, Materials and Prospects for Their Application in Medicine. Coatings. 2021; 11(1):78. https://doi.org/10.3390/coatings11010078
Chicago/Turabian StyleKarakovskaya, Ksenya I., Svetlana I. Dorovskikh, Evgeniia S. Vikulova, Igor Yu. Ilyin, Kseniya V. Zherikova, Tamara V. Basova, and Natalya B. Morozova. 2021. "Volatile Iridium and Platinum MOCVD Precursors: Chemistry, Thermal Properties, Materials and Prospects for Their Application in Medicine" Coatings 11, no. 1: 78. https://doi.org/10.3390/coatings11010078
APA StyleKarakovskaya, K. I., Dorovskikh, S. I., Vikulova, E. S., Ilyin, I. Y., Zherikova, K. V., Basova, T. V., & Morozova, N. B. (2021). Volatile Iridium and Platinum MOCVD Precursors: Chemistry, Thermal Properties, Materials and Prospects for Their Application in Medicine. Coatings, 11(1), 78. https://doi.org/10.3390/coatings11010078