Biomechanical Interfaces of Corticotomies on Periodontal Tissue Remodeling during Orthodontic Tooth Movement
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Corticotomy and Tooth Movement
2.3. Sample Preparation, Micro-CT Test and Tissue Processing
2.4. Histology and Cellular Analyses
2.5. Immunohistochemistry and Immunofluorescence
2.6. Quantitative Real-Time Polymerase Chain Reaction(qRT-PCR)
2.7. Statistical Analyses
3. Results
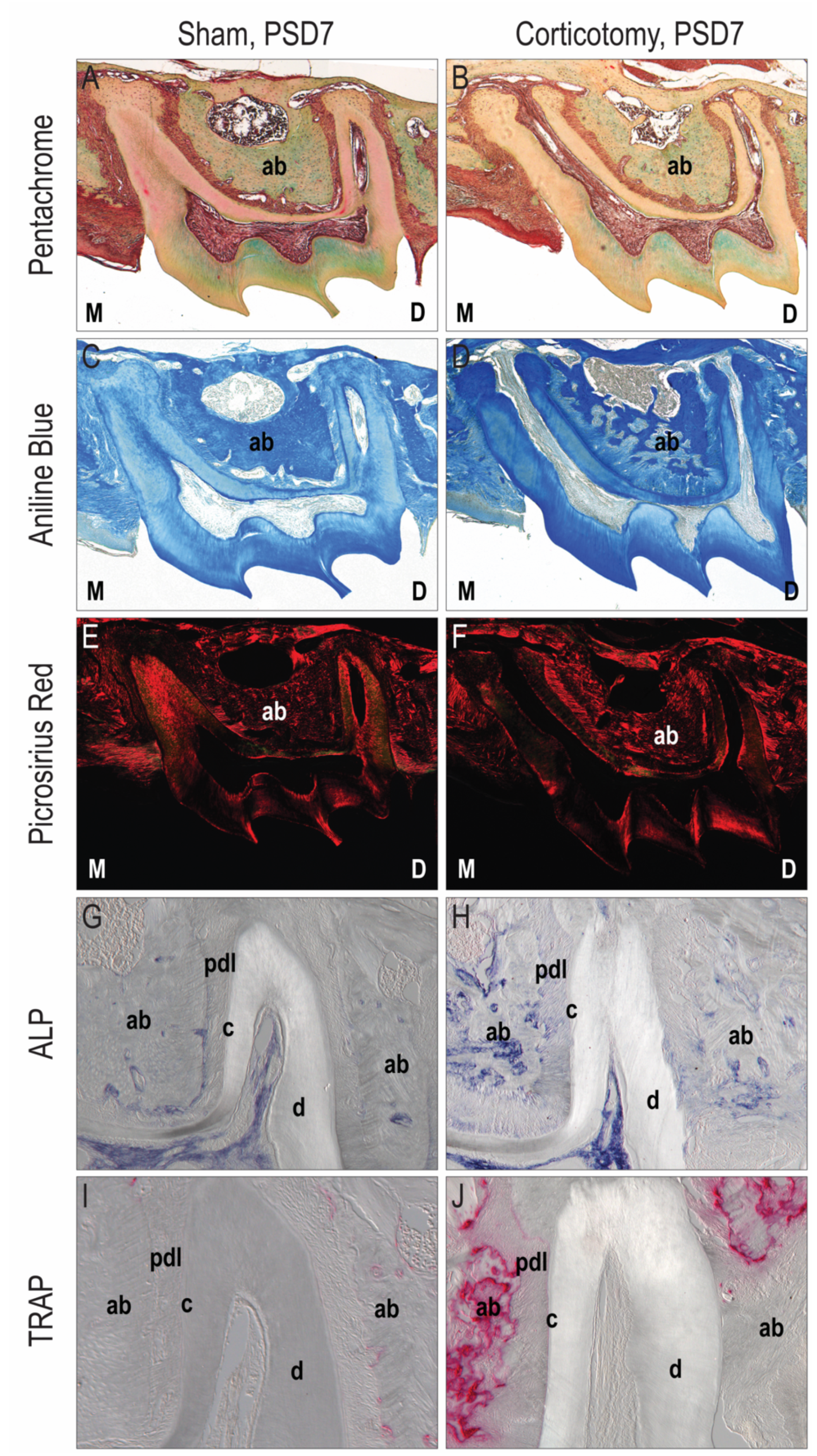
3.1. Corticotomy Promoted Local Alveolar Bone Remodeling
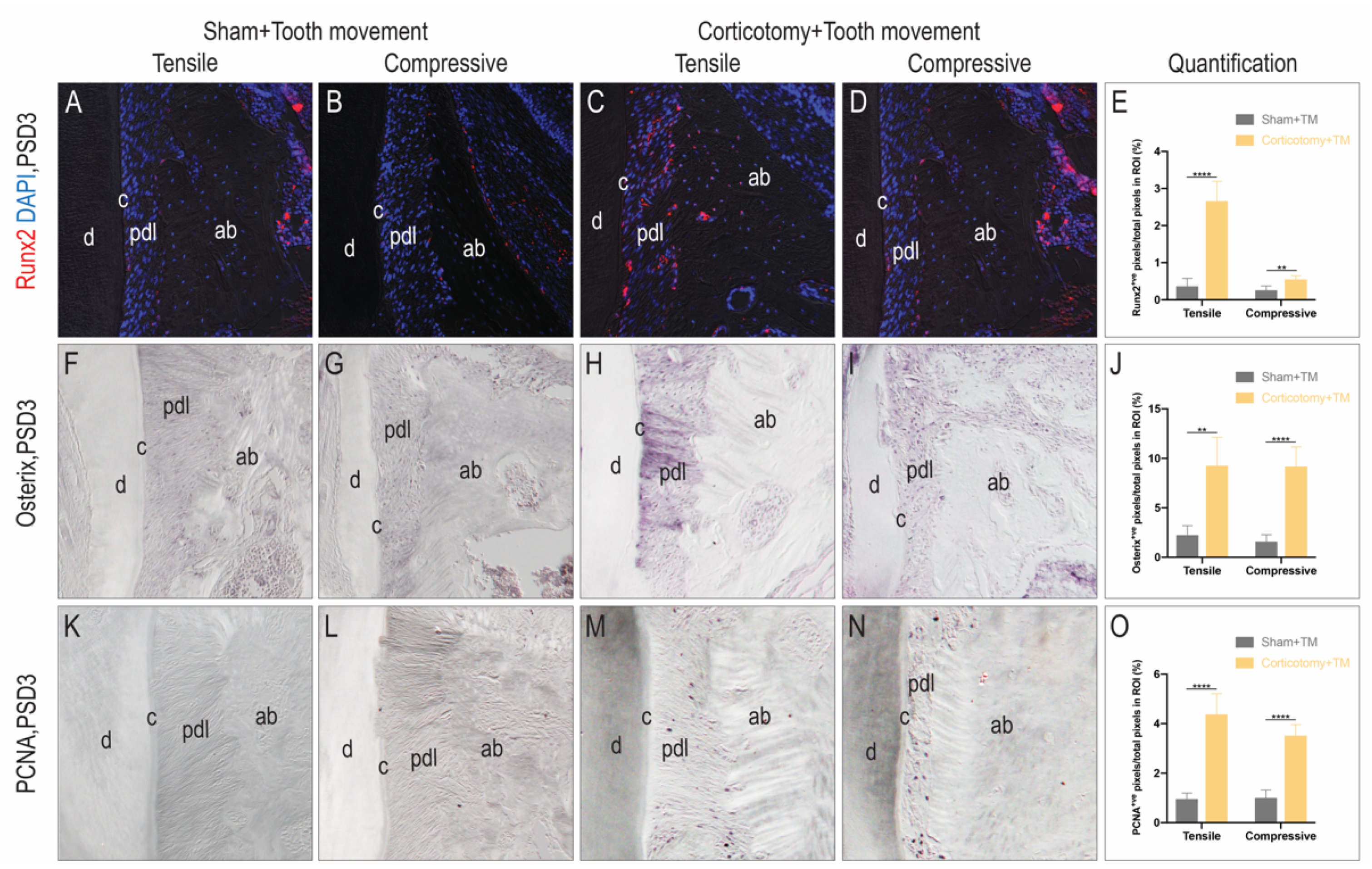
3.2. Corticotomy Promoted Cell Proliferation and Osteogenic Mineralization during Tooth Movement in the Alveolar Bone
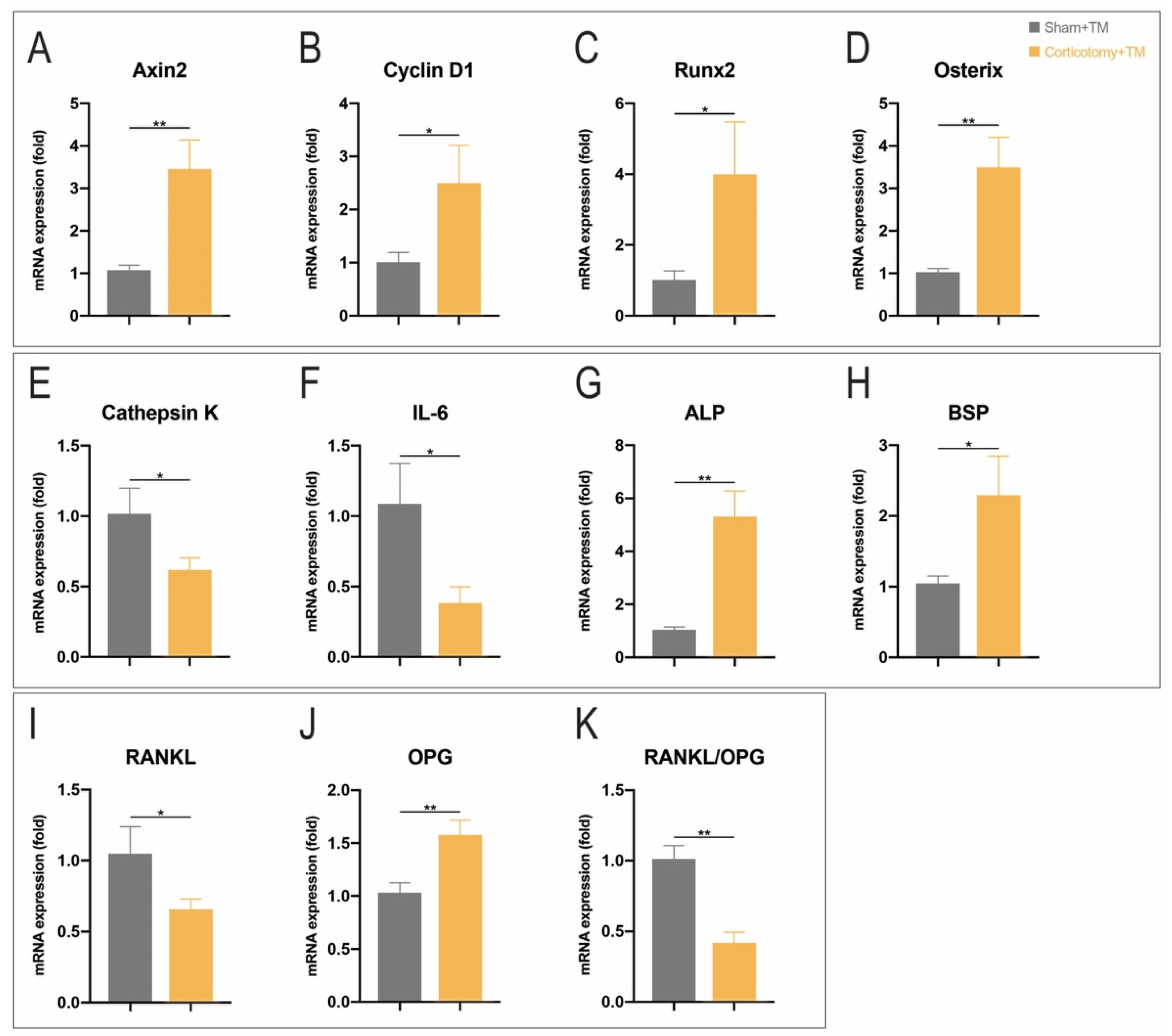
3.3. Corticotomy Promoted Osteogenesis and Inhibits Inflammation by Upregulating Wnt Signaling Pathway in Periodontal Tissue
3.4. Corticotomy Eliminated Root Resorption during Tooth Movement
3.5. Corticotomy Led to Transient Osteoporosis in Alveolar Bone and Accelerated Orthodontic Tooth Movement
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gil, A.P.S.; Haas, O.L., Jr.; Méndez-Manjón, I.; Masiá-Gridilla, J.; Valls-Ontañón, A.; Hernández-Alfaro, F.; Guijarro-Martínez, R. Alveolar corticotomies for accelerated orthodontics: A systematic review. J. Craniomaxillofac. Surg. 2018, 46, 438–445. [Google Scholar] [PubMed]
- Jawad, Z.; Bates, C.; Hodge, T. Who needs orthodontic treatment? Who gets it? And who wants it? Br. Dent. J. 2015, 218, 99. [Google Scholar] [PubMed]
- Gkantidis, N.; Mistakidis, I.; Kouskoura, T.; Pandis, N. Effectiveness of non-conventional methods for accelerated orthodontic tooth movement: A systematic review and meta-analysis. J. Dent. 2014, 42, 1300–1319. [Google Scholar] [PubMed]
- Nimeri, G.; Kau, C.H.; Abou-Kheir, N.S.; Corona, R. Acceleration of tooth movement during orthodontic treatment—A frontier in Orthodontics. Prog. Orthod. 2013, 14, 42. [Google Scholar]
- Al-Naoum, F.; Hajeer, M.Y.; Al-Jundi, A. Does Alveolar Corticotomy Accelerate Orthodontic Tooth Movement When Retracting Upper Canines? A Split-Mouth Design Randomized Controlled Trial. J. Oral Maxillofac. Surg. 2014, 72, 1880–1889. [Google Scholar]
- Bhattacharya, P.; Bhattacharya, H.; Anjum, A.; Bhandari, R.; Agarwal, D.K.; Gupta, A.; Ansar, J. Assessment of Corticotomy Facilitated Tooth Movement and Changes in Alveolar Bone Thickness—A CT Scan Study. J. Clin. Diagn. Res. 2014, 8, 26–30. [Google Scholar]
- Wilcko, M.T.; Ferguson, D.J.; Makki, L.; Wilcko, W.M. Keratinized Gingiva Height Increases After Alveolar Corticotomy and Augmentation Bone Grafting. J. Periodontol. 2015, 86, 1107–1115. [Google Scholar]
- Frost, H.M. The regional acceleratory phenomenon: A review. Henry Ford Hosp. Med. J. 1983, 31, 3–9. [Google Scholar]
- Umar, R.; Queiroz, C.R.; Prasad, G.M. Surgically accelerated orthodontic techniques and periodontal response: A systematic review. Eur. J. Orthod. 2020, 42, 635–642. [Google Scholar]
- KoLe, H. Surgical operations on the alveolar ridge to correct occlusal abnormalities. Oral Surg. Oral Med. Oral Pathol. 1959, 12, 515–529. [Google Scholar]
- Serge, K.; Duke, R. Mouth: Experimental animal research into segmental alveolar movement after corticotomy. Afr. Zool. 1976, 40, 127–142. [Google Scholar]
- Wilcko, W.M.; Wilcko, T.; Bouquot, J.E.; Ferguson, D.J. Rapid orthodontics with alveolar reshaping: Two case reports of decrowding. Int. J. Periodontics Restor. Dent. 2001, 21, 9–19. [Google Scholar]
- Wang, L.; Lee, W.; Lei, D.L.; Liu, Y.P.; Yamashita, D.D.; Yen, S.L.K. Tissue responses in corticotomy- and osteotomy-assisted tooth movements in rats: Histology and immunostaining. Am. J. Orthod. Dentofac. Orthop. 2009, 136, 1–11. [Google Scholar]
- Yue, F.; Cheng, Y.; Breschi, A.; Vierstra, J.; Wu, W.; Ryba, T.; Sandstrom, R.; Ma, Z.; Davis, C.; Pope, B.D.; et al. A comparative encyclopedia of DNA elements in the mouse genome. Nature 2014, 515, 355–364. [Google Scholar]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Müller, R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar]
- Baloul, S.S.; Gerstenfeld, L.C.; Morgan, E.F.; Carvalho, R.S.; Van Dyke, T.E.; Kantarci, A. Mechanism of action and morphologic changes in the alveolar bone in response to selective alveolar decortication-facilitated tooth movement. Am. J. Orthod. Dentofac. Orthop. 2011, 139, S83–S101. [Google Scholar]
- Sebaoun, J.D.; Kantarci, A.; Turner, J.W.; Carvalho, R.S.; Van Dyke, T.E.; Ferguson, D.J. Modeling of trabecular bone and lamina dura following selective alveolar decortication in rats. J. Periodontol. 2008, 79, 1679–1688. [Google Scholar]
- Minkin, C. Bone acid phosphatase: Tartrate-resistant acid phosphatase as a marker of osteoclast function. Calcif. Tissue Int. 1982, 34, 285–290. [Google Scholar]
- Aubin, J.E.; Bonnelye, E. Osteoprotegerin and its Ligand: A New Paradigm for Regulation of Osteoclastogenesis and Bone Resorption. Osteoporos. Int. 2000, 11, 905–913. [Google Scholar]
- Kostenuik, P.; Shalhoub, V. Osteoprotegerin: A physiological and pharmacological inhibitor of bone resorption. Curr. Pharm. Des. 2001, 7, 613–635. [Google Scholar]
- Zhou, Y.; He, X.; Zhang, D. Study of bone remodeling in corticotomy-assisted orthodontic tooth movement in rats. J. Cell. Biochem. 2019, 120, 15952–15962. [Google Scholar] [CrossRef] [PubMed]
- Karner, C.M.; Long, F. Wnt signaling and cellular metabolism in osteoblasts. Cell. Mol. Life Sci. 2017, 74, 1649–1657. [Google Scholar] [CrossRef] [PubMed]
- Maupin, K.A.; Droscha, C.J.; Williams, B.O. A comprehensive overview of skeletal phenotypes associated with alterations in Wnt/β-catenin signaling in humans and mice. Bone Res. 2013, 1, 27–71. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Li, Y.; Wang, X.; Zou, S.; Hu, J.; Luo, E. The Effect of Uniaxial Mechanical Stretch on Wnt/β-Catenin Pathway in Bone Mesenchymal Stem Cells. J. Craniofac. Surg. 2016, 28, 113. [Google Scholar] [CrossRef]
- Han, Y.; Bai, T.; Liu, W. Controlled Heterogeneous Stem Cell Differentiation on a Shape Memory Hydrogel Surface. Sci. Rep. 2014, 4, 5815. [Google Scholar] [CrossRef]
- Lee, M.H.; Javed, A.; Kim, H.J.; Shin, H.I.; Gutierrez, S.; Choi, J.Y.; Rosen, V.; Stein, J.L.; van Wijnen, A.J.; Stein, G.S.; et al. Transient upregulation of CBFA1 in response to bone morphogenetic protein-2 and transforming growth factor beta1 in C2C12 myogenic cells coincides with suppression of the myogenic phenotype but is not sufficient for osteoblast differentiation. J. Cell. Biochem. 1999, 73, 114–125. [Google Scholar] [CrossRef]
- Nakashima, K.; Zhou, X.; Kunkel, G.; Zhang, Z.; Deng, J.M.; Behringer, R.R.; de Crombrugghe, B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 2002, 108, 17–29. [Google Scholar] [CrossRef]
- Wong, S.K.; Mohamad, N.; Ibrahim, N.; Chin, K.; Shuid, A.N.; Ima-Nirwana, S. The Molecular Mechanism of Vitamin E as a Bone-Protecting Agent: A Review on Current Evidence. Int. J. Mol. Sci. 2019, 20, 1453. [Google Scholar] [CrossRef]
- Komori, T. Regulation of osteoblast differentiation by Runx2. Adv. Exp. Med. Biol. 2010, 658, 43–49. [Google Scholar]
- Zhang, C. Molecular mechanisms of osteoblast-specific transcription factor Osterix effect on bone formation. J. Peking Univ. 2012, 44, 659–665. [Google Scholar]
- Lee, W.; Karapetyan, G.; Moats, R.; Yamashita, D.D.; Moon, H.B.; Ferguson, D.J.; Yen, S. Corticotomy-/osteotomy-assisted tooth movement microCTs differ. J. Dent. Res. 2008, 87, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.; Li, C.; Zheng, Z. Remote Corticotomy Accelerates Orthodontic Tooth Movement in a Rat Model. BioMed Res. Int. 2019, 2019, 4934128. [Google Scholar] [CrossRef] [PubMed]


| Gene | Forward Primer (5′→3′) | Reverse Primer (3′→5′) |
|---|---|---|
| Axin2 | CCTGGCTCCAGAAGATCAC | TAGGTGACAACCAGCTCAC |
| Cyclin D1 | GCGTACCCTGACACCAATCTC | ACTTGAAGTAAGATACGGAGGGC |
| Runx2 | GCCGGGAATGATGAGAAC | TGGGGAGGATTTGTGAAGA |
| Osterix | GCTGGAGAGGGAAAGGG | GCCGAACAACCCAAAACT |
| Cathepsin K | ACTTGGGAGACATGACCAG | CATTACTGTAGGATCGAGAGGG |
| IL-6 | TACCACTTCACAAGTCGGA | AATTGCCATTGCACAACTC |
| ALP | TGGGGAGGATTTGTGAAGA | TGGAGACATTTTCCCGTTC |
| BSP | GGTGAAAGTGACTGATTCTGG | GAGGACACAGCATTCTGTG |
| RANKL | ACTCTGGAGAGTGAAGACAC | GTTGCAGTTCCTTCTGCAC |
| OPG | GAAAGGAAATGCAACACATGAC | CACAGGGTGACATCTATTCCA |
| GAPDH | AGGTCGGTGTGAACGGATTTG | TGTAGACCATGTAGTTGAGGTCA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, R.; Huang, L.; Xiao, X.; Guan, Y.; Jiang, Y.; Yin, X.; Zou, S.; Ye, Q. Biomechanical Interfaces of Corticotomies on Periodontal Tissue Remodeling during Orthodontic Tooth Movement. Coatings 2021, 11, 1. https://doi.org/10.3390/coatings11010001
Liu R, Huang L, Xiao X, Guan Y, Jiang Y, Yin X, Zou S, Ye Q. Biomechanical Interfaces of Corticotomies on Periodontal Tissue Remodeling during Orthodontic Tooth Movement. Coatings. 2021; 11(1):1. https://doi.org/10.3390/coatings11010001
Chicago/Turabian StyleLiu, Ruojing, Li Huang, Xiaoyue Xiao, Yuzhe Guan, Yukun Jiang, Xing Yin, Shujuan Zou, and Qingsong Ye. 2021. "Biomechanical Interfaces of Corticotomies on Periodontal Tissue Remodeling during Orthodontic Tooth Movement" Coatings 11, no. 1: 1. https://doi.org/10.3390/coatings11010001
APA StyleLiu, R., Huang, L., Xiao, X., Guan, Y., Jiang, Y., Yin, X., Zou, S., & Ye, Q. (2021). Biomechanical Interfaces of Corticotomies on Periodontal Tissue Remodeling during Orthodontic Tooth Movement. Coatings, 11(1), 1. https://doi.org/10.3390/coatings11010001





