Comparison of Carbon Thin Films with Low Secondary Electron Yield Deposited in Neon and Argon
Abstract
1. Introduction
2. Apparatus and Methods
2.1. Sample Preparation
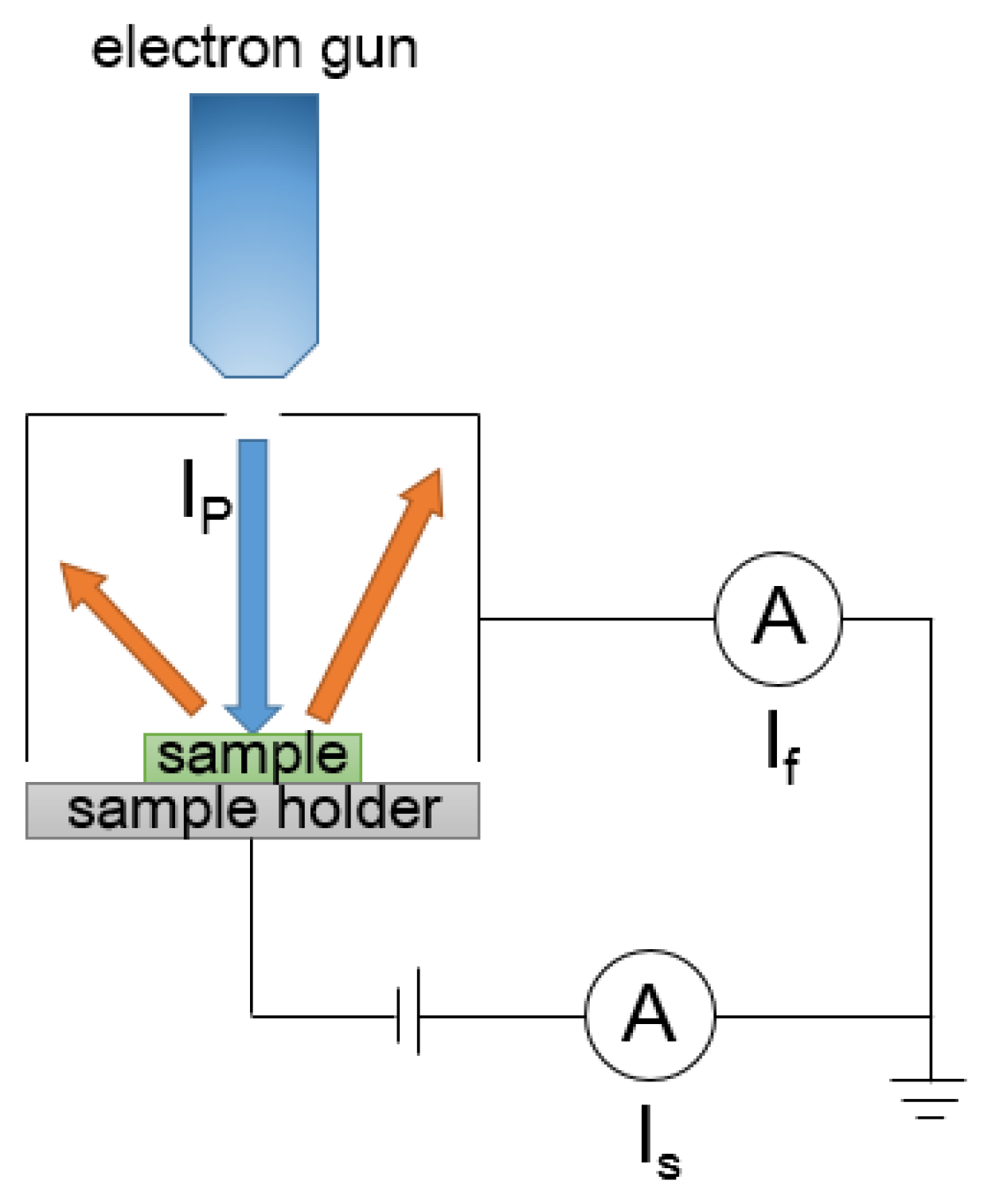
2.2. SEY Measurement
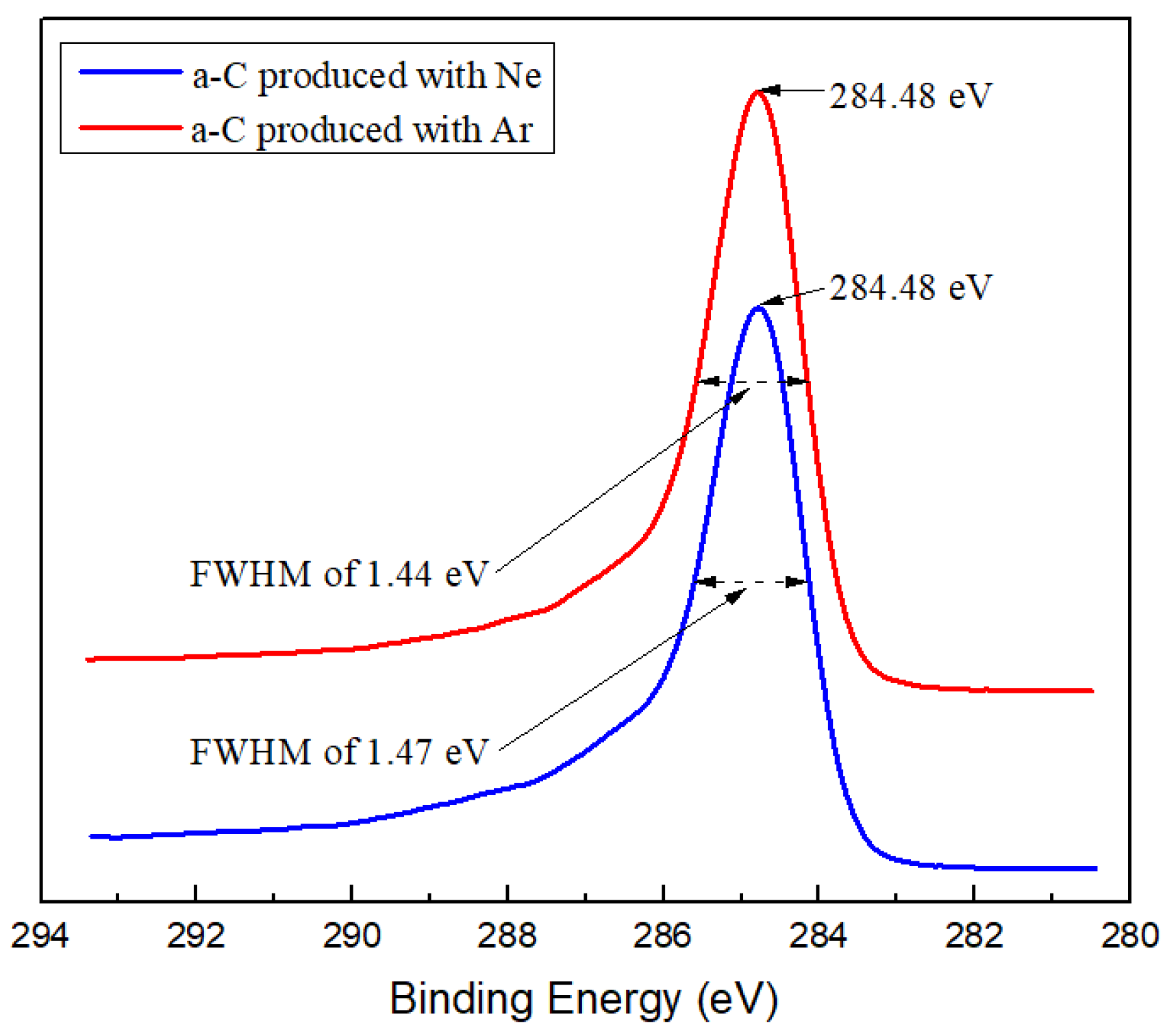
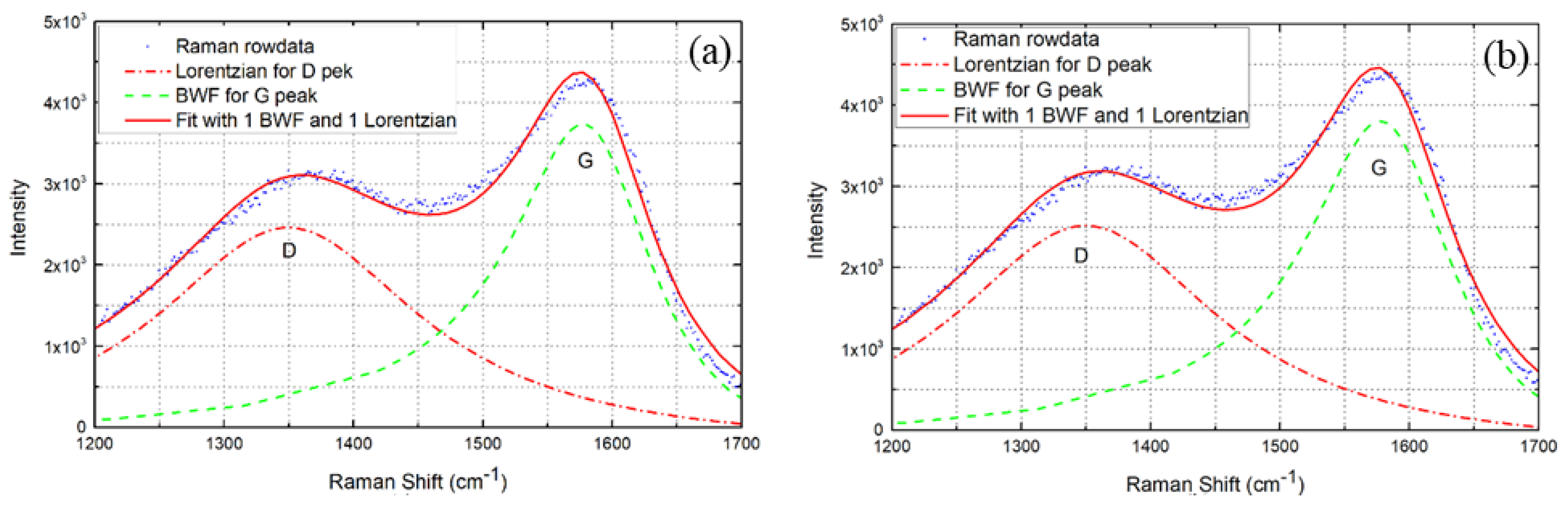
2.3. Characterization Method
3. Results
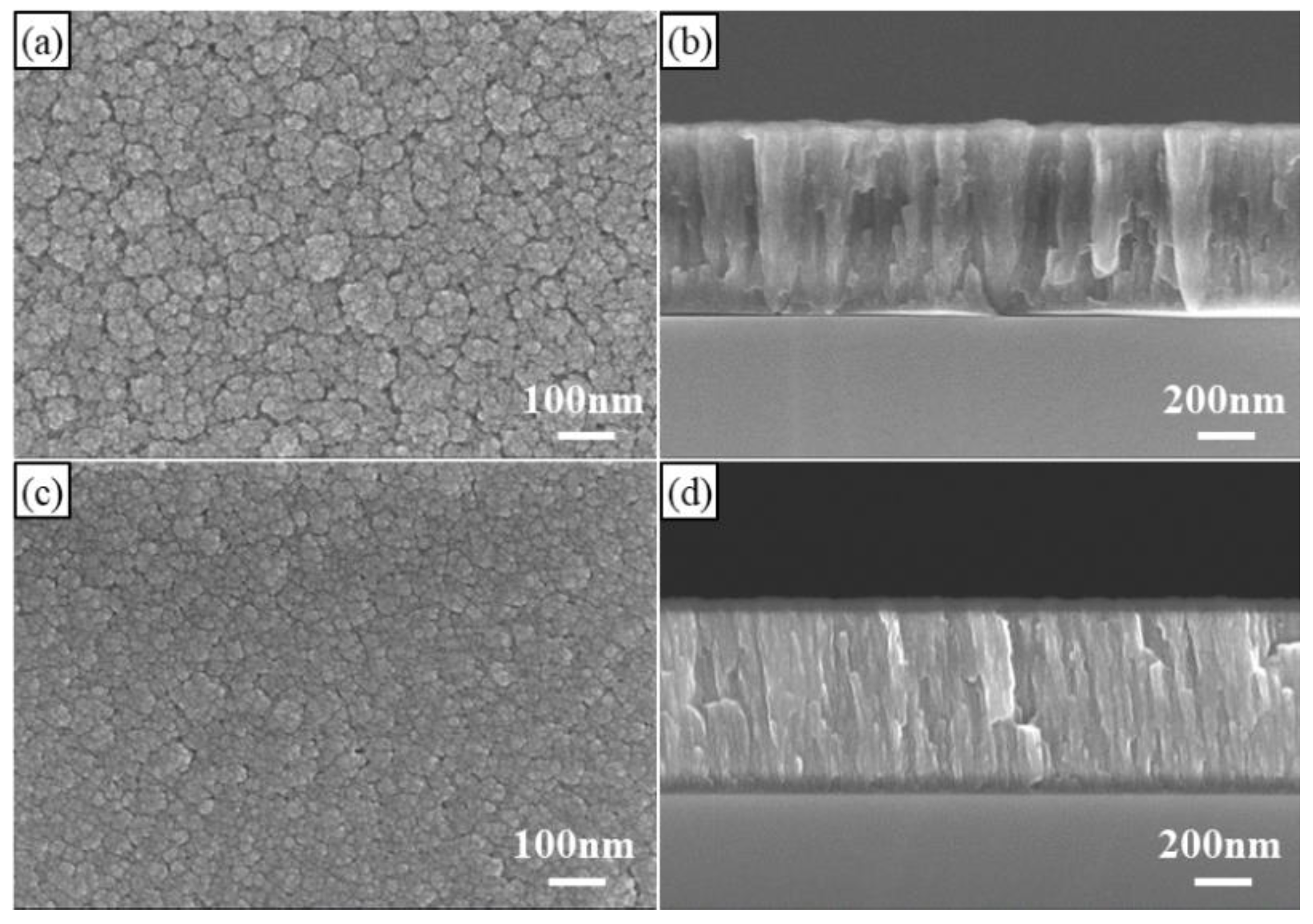
3.1. Microstructure Characterization
3.2. SEY of Amorphous Carbon Films
4. Discussion
5. Summary and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cimino, R.; Demma, T. Electron cloud in accelerators. Int. J. Mod. Phys. A 2014, 29, 1430023. [Google Scholar] [CrossRef]
- Fukuma, H. Electron Cloud Observations and Predictions at KEKB, PEP-II and SuperB Factories. arXiv 2013, arXiv:1307.8330. [Google Scholar]
- Tobiyama, M.; Flanagan, J.W.; Fukuma, H.; Kurokawa, S.; Ohmi, K.; Win, S.S. Coupled bunch instability caused by an electron cloud. Phys. Rev. Spec. Top. Accel. Beams 2006, 9. [Google Scholar] [CrossRef]
- Iadarola, G.; Rumolo, G. Electron cloud in the CERN accelerators (PS, SPS, LHC). arXiv 2013, arXiv:1309.6795. [Google Scholar]
- Larciprete, R.; Cimino, R.; Commisso, M.; Grosso, D.R.; Flammini, R. The chemical origin of SEY at technical surfaces. arXiv 2013, arXiv:1308.1290. [Google Scholar]
- Romanov, G. Simulation of Multipacting with Space Charge Effect. Am. J. Phys. Appl. 2017, 5, 99–105. [Google Scholar] [CrossRef]
- Suetsugu, Y.; Fukuma, H.; Pivi, M.; Wang, L. Continuing study on electron-cloud clearing techniques in high-intensity positron ring: Mitigation by using groove surface in vertical magnetic field. Nucl. Instrum. Meth. Phys. Res. Sect. A 2009, 604, 449–456. [Google Scholar] [CrossRef]
- Suetsugu, Y.; Fukuma, H.; Shibata, K.; Kek, T.; Pivi, M.T.F.; Wang, L. Experimental Studies on Grooved Surfaces to Suppress Secondary Electron Emission. In Proceedings of the IPAC’10, Kyoto, Japan, 23–28 May 2010; pp. 2021–2023. [Google Scholar]
- Fischer, W.; Blaskiewicz, M.; Brennan, J.M.; Huang, H.; Hseuh, H.C.; Ptitsyn, V.; Roser, T.; Thieberger, P.; Trbojevic, D.; Wei, J.; et al. Electron cloud observations and cures in the Relativistic Heavy Ion Collider. Rev. Spec. Top. Accel. Beams 2008, 11, 041002. [Google Scholar] [CrossRef]
- Fukuma, H.; Flanagan, J.; Hosoyama, K.; Ieiri, T.; Kawamoto, T.; Kubo, T.; Suetake, A.; Uno, S.; Win, S.S.; Yoshioka, M. Status of solenoid system to suppress the electron cloud effects at the KEKB. In AIP Conference Proceedings; Chou, W., Mori, Y., Neuffer, D., Ostiguy, J.F., Eds.; American Institute of Physics: College Park, MD, USA, 2002; Volume 642, pp. 357–359. [Google Scholar]
- Vallgren, C.Y.; Arduini, G.; Bauche, J.; Calatroni, S.; Chiggiato, P.; Cornelis, K.; Pinto, P.C.; Henrist, B.; Metral, E.; Neupert, H.; et al. Amorphous carbon coatings for the mitigation of electron cloud in the CERN Super Proton Synchrotron. Phys Rev Spec Top. Accel. Beams 2011, 14, 071001. [Google Scholar] [CrossRef]
- Gompel, M.V.; Pinto, P.C.; Vollenberg, W.; Chiggiato, P.; Cruikshank, P.; Pasquino, C.C.; Sapountzis, A.; Taborelli, M.; Espinos, J.P. Amorphous Carbon Thin Film Coating of the SPS Beamline: Evaluation of the First Coating Implementation. In Proceedings of the IPAC’17, Geneva, Switzerland, 14–19 May 2017; pp. 44–47. [Google Scholar]
- Salemme, R.; Baglin, V.; Bregliozzi, G. Amorphous carbon coatings at cryogenic temperatures with LHC type beams: First results with the COLDEX experiment. In Proceedings of the IPAC’15, Richmond, VA, USA, 3–8 May 2015; pp. 3112–3114. [Google Scholar]
- Salemme, R.; Baglin, V.; Bregliozzi, G.; Chiggiato, P. Vacuum Performance of Amorphous Carbon Coating at Cryogenic Temperature with Presence of Proton Beams. In Proceedings of the IPAC’16, Busan, Korea, 8–13 May 2016; pp. 3663–3666. [Google Scholar]
- Nishiwaki, M.; Kato, S. Study of the secondary electron yield of carbon materials. J. Vac. Soc. Jpn. 2005, 118, 48. [Google Scholar]
- Woods, M.E.; Hopkins, B.J.; Matthews, G.F.; McCracken, G.M.; Sewell, P.; Fahrang, H. An investigation of the secondary-electron emission of carbon samples exposed to a hydrogen plasma. J. Phys. D Appl. Phys. 1987, 20, 1136–1142. [Google Scholar] [CrossRef]
- Ripalda, J.M.; Montero, I.; Vazquez, L.; Raboso, D.; Galan, L. Secondary electron emission and photoemission studies on surface films of carbon nitride. J. Appl. Phys. 2006, 99, 043513. [Google Scholar] [CrossRef]
- Alfonso, E.; Olaya, J.; Cubillos, G. Thin film growth through sputtering technique and its applications. In Crystallization-Science and Technology; Andreeta, M.R.B., Ed.; IntechOpen: Rijeka, Croatia, 2012. [Google Scholar]
- Gou, L.; Qi, C.S.; Ran, J.G.; Zheng, C.Q. SiC film deposition by DC magnetron sputtering. Thin. Solid Films 1999, 345, 42–44. [Google Scholar] [CrossRef]
- Jung, H.S.; Park, H.H.; Mendieta, I.R.; Smith, D.A. Enhancement of sp3 hybridized C in amorphous carbon films by Ar ion bombardment and Si incorporation. J. Appl. Phys. 2003, 94, 4828–4834. [Google Scholar] [CrossRef]
- Bruining, H. Physics and Applications of Secondary Electron Emission; Pergamon Press: Oxford, UK, 1954; pp. 70–96. [Google Scholar]
- Comelli, G.; Stoehr, J.; Robinson, C.J.; Jark, W. Structural studies of argon-sputtered amorphous carbon films by means of extended x-ray-absorption fine structure. Phys. Rev. B 1988, 38, 7511–7519. [Google Scholar] [CrossRef]
- Diaz, J.; Paolicelli, G.; Ferrer, S.; Comin, F. Separation of the sp3 and sp2 components in the C1s photoemission spectra of amorphous carbon films. Phys. Rev. B 1996, 54, 8064–8069. [Google Scholar] [CrossRef]
- Merel, P.; Tabbal, M.; Chaker, M.; Moisa, S.; Margot, J. Direct evaluation of the sp3 content in diamond-like-carbon films by XPS. Appl. Surf. Sci. 1998, 136, 105–110. [Google Scholar] [CrossRef]
- Jackson, S.T.; Nuzzo, R.G. Determining hybridization differences for amorphous carbon from the XPS C1s envelope. Appl. Surf. Sci. 1995, 90, 195–203. [Google Scholar] [CrossRef]
- Robertson, J.F.R. Diamond-like amorphous carbon. Mater. Sci. Eng. R Rep. 2002, 37, 129–281. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Resonant Raman spectroscopy of disordered, amorphous, and diamondlike carbon. Phys. Rev. B 2001, 64, 075414. [Google Scholar] [CrossRef]
- Kunz, C. Synchrotron radiation: Overview. In Photoemission in Solids II; Ley, L., Cardona, M., Eds.; Springer: Berlin/Heidelberg, Germany, 1979; Volume 27, pp. 299–348. [Google Scholar]
- Nishimura, K.; Itotani, T.; Ohya, K. Influence of Surface Roughness on Secondary Electron Emission and Electron Backscattering from Metal Surface. Jpn. J. Appl. Phys. 1994, 33, 4727–4734. [Google Scholar] [CrossRef]
- Yin Vallgren, C.; Chiggiato, P.; Costa Pinto, P.; Neupert, H.; Rumolo, G.; Shaposhnikova, E.; Taborelli, M.; Kato, S. Performance of carbon coatings for mitigation of electron cloud in the SPS. In Proceedings of the IPAC’11, San Sebastián, Spain, 4–9 September 2011; pp. 1590–1592. [Google Scholar]
- Ellmer, K.; Hinze, J.; Klaer, J. Copper indium disulfide solar cell absorbers prepared in a one-step process by reactive magnetron sputtering from copper and indium targets. Thin. Solid Films 2002, 413, 92–97. [Google Scholar] [CrossRef]
- Wasa, K.; Adachi, H.; Kitabatake, M. Thin Film Materials Technology: Sputtering of Compound Mater; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Willis, R.F.; Fitton, B.; Painter, G.S. Secondary-electron emission spectroscopy and the observation of high-energy excited states in graphite: Theory and experiment. Phys. Rev. B 1974, 9, 1926–1937. [Google Scholar] [CrossRef]
- Ascarelli, P.; Cappelli, E.; Pinzari, F.; Rossi, M.C.; Salvatori, S.; Merli, P.G.; Migliori, A. Secondary electron emission from diamond: Physical modeling and application to scanning electron microscopy. J. Appl. Phys. 2001, 89, 689–696. [Google Scholar] [CrossRef]
- Pan, L.S.; Kania, D.R. Diamond: Electronic Properties and Applications; Springer: New York, NY, USA, 1995; pp. 50–60. [Google Scholar]
- Nishiwaki, M.; Kato, S. Residual gas analysis and electron cloud measurement of DLC and TiN coated chambers at KEKB LER. In Proceedings of the IPAC’10, Kyoto, Japan, 23–28 May 2010; pp. 3852–3854. [Google Scholar]
- Lifshitz, Y.; Kasi, S.R.; Rabalais, J.W.; Eckstein, W. Subplantation model for film growth from hyperthermal species. Phys. Rev. B 1990, 41, 10468–10480. [Google Scholar] [CrossRef]
- Tang, J.; Berg, J.S.; Zheng, S.; Han, T.; Liu, B.; Gupta, R.; Ruan, M.; Chou, W.; Yuan, Y.; Sun, B.; et al. Concept for a future Super Proton-Proton Collider. arXiv 2015, arXiv:1507.03224. [Google Scholar]
- Hartung, W.H.; Conway, J.; Dennett, C.A.; Greenwald, S.; Kim, J.S.; Li, Y.; Moore, T.P.; Omanovic, V. Measurements of secondary electron yield of metal surfaces and films with exposure to a realistic accelerator environment. In Proceedings of the IPAC’13, Shanghai, China, 12–17 May 2013; pp. 3493–3495. [Google Scholar]
- Hartung, W.H.; Asner, D.M.; Conway, J.V.; Dennett, C.A.; Greenwald, S.; Kim, J.S.; Li, Y.; Moore, T.P.; Omanovic, V.; Palmer, M.A.; et al. In-situ measurements of the secondary electron yield in an accelerator environment: Instrumentation and methods. Nucl. Instrum. Meth. A 2015, 783, 95–109. [Google Scholar] [CrossRef]
- Kirby, R.E.; King, F.K. Secondary electron emission yields from PEP-II accelerator materials. Nucl. Instrum. Meth. A 2001, 469, 1–12. [Google Scholar] [CrossRef]



| Parameter | Unit | Value |
|---|---|---|
| Sputtering Gas | - | Ne, Ar |
| Power | W | 250 |
| Pressure | mbar | 3.8 × 10−2 |
| Magnetic Field | Gauss | 150 |
| Gas Flow | sccm | 5 |
| Sputtering Conditions | D Peak (cm−1) | G Peak (cm−1) | ID/IG |
|---|---|---|---|
| Ne Gas | 1350 | 1580 | 1.036 |
| Ar Gas | 1349 | 1586 | 1.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wang, Y.; Wang, S.; Wei, W.; Ge, X.; Zhu, B.; Shao, J.; Wang, Y. Comparison of Carbon Thin Films with Low Secondary Electron Yield Deposited in Neon and Argon. Coatings 2020, 10, 884. https://doi.org/10.3390/coatings10090884
Zhang Y, Wang Y, Wang S, Wei W, Ge X, Zhu B, Shao J, Wang Y. Comparison of Carbon Thin Films with Low Secondary Electron Yield Deposited in Neon and Argon. Coatings. 2020; 10(9):884. https://doi.org/10.3390/coatings10090884
Chicago/Turabian StyleZhang, Yuxin, Yigang Wang, Sihui Wang, Wei Wei, Xiaoqin Ge, Bangle Zhu, Jieqiong Shao, and Yong Wang. 2020. "Comparison of Carbon Thin Films with Low Secondary Electron Yield Deposited in Neon and Argon" Coatings 10, no. 9: 884. https://doi.org/10.3390/coatings10090884
APA StyleZhang, Y., Wang, Y., Wang, S., Wei, W., Ge, X., Zhu, B., Shao, J., & Wang, Y. (2020). Comparison of Carbon Thin Films with Low Secondary Electron Yield Deposited in Neon and Argon. Coatings, 10(9), 884. https://doi.org/10.3390/coatings10090884




