Assessment of the Chemical Composition in Different Dental Implant Types: An Analysis through EDX System
Abstract
1. Introduction
2. Material and Methods
2.1. Surface Morphological Analysis of Dental Implants
2.2. Chemical Analysis of Dental Implants
3. Results
3.1. Surface Morphological Analysis of Dental Implants
3.2. Chemical Analysis of Dental Implants
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Elani, H.W.; Starr, J.R.; Da Silva, J.D.; Gallucci, G.O. Trends in dental implant use in the U.S., 1999–2016, and projections to 2026. J. Dent. Res. 2018, 97, 1424–1430. [Google Scholar] [CrossRef]
- Block, M.S. Dental implants: The last 100 Years. J. Oral Maxillofac. Surg. 2018, 76, 11–26. [Google Scholar] [CrossRef]
- Mishra, S.K.; Chowdhary, R. Patient’s oral health-related quality of life and satisfaction with implant supported overdentures—A systematic review. J. Oral Biol. Craniofac. Res. 2019, 9, 340–346. [Google Scholar] [CrossRef]
- Muller, F.; Salem, K.; Barbezat, C.; Herrmann, F.R.; Schimmel, M. Knowledge and attitude of elderly persons towards dental implants. Gerodontology 2012, 29, e914–e923. [Google Scholar] [CrossRef]
- Javed, F.; Ahmed, H.B.; Crespi, R.; Romanos, G.E. Role of primary stability for successful osseointegration of dental implants: Factors of influence and evaluation. Interv. Med. Appl. Sci. 2013, 5, 162–167. [Google Scholar] [CrossRef]
- Giudice, A.; Bennardo, F.; Antonelli, A.; Barone, S.; Wagner, F.; Fortunato, L.; Traxler, H. Influence of clinician’s skill on primary implant stability with conventional and piezoelectric preparation techniques: An ex-vivo study. J. Biol. Regul. Homeost. Agents 2020, 34, 739–745. [Google Scholar]
- Delgado-Ruiz, R.; Romanos, G. Potential causes of titanium particle and ion release in implant dentistry: A systematic review. Int. J. Mol. Sci. 2018, 19, 3585. [Google Scholar] [CrossRef]
- Olefjord, I.; Hansson, S. Surface analysis of four dental implant systems. Int. J. Oral Maxillofac. Implant. 1993, 8, 32–40. [Google Scholar]
- Dohan Ehrenfest, D.M.; Vazquez, L.; Park, Y.J.; Sammartino, G.; Bernard, J.P. Identification card and codification of the chemical and morphological characteristics of 14 dental implant surfaces. J. Oral. Implantol. 2011, 37, 525–542. [Google Scholar] [CrossRef]
- Guler, B.; Uraz, A.; Cetiner, D. The chemical surface evaluation of black and white porous titanium granules and different commercial dental implants with energy-dispersive x-ray spectroscopy analysis. Clin. Implant. Dent. Relat. Res. 2019, 21, 352–359. [Google Scholar] [CrossRef]
- Klauber, C.; Lenz, L.J.; Henry, P.J. Oxide thickness and surface contamination of six endosseous dental implants determined by electron spectroscopy for chemical analysis: A preliminary report. Int. J. Oral. Maxillofac. Implant. 1990, 5, 264–271. [Google Scholar]
- Massaro, C.; Rotolo, P.; De Riccardis, F.; Milella, E.; Napoli, A.; Wieland, M.; Textor, M.; Spencer, N.D.; Brunette, D.M. Comparative investigation of the surface properties of commercial titanium dental implants. Part I: Chemical composition. J. Mater. Sci. Mater. Med. 2002, 13, 535–548. [Google Scholar] [CrossRef]
- Kang, B.S.; Sul, Y.T.; Oh, S.J.; Lee, H.J.; Albrektsson, T. XPS, AES and SEM analysis of recent dental implants. Acta Biomater. 2009, 5, 2222–2229. [Google Scholar] [CrossRef]
- Dohan Ehrenfest, M.D.; Del Corso, M.; Kang, B.S.; Leclercq, P.; Mazor, Z.; Horowitz, R.A.; Shibli, H.L.W. Identification card and codification of the chemical and morphological characteristics of 62 dental implant surfaces. Part 3: Sand-blasted/acid-etched (SLA Type) and related surfaces (Group 2A, main subtractive process). POSEIDO 2014, 2, 37–55. [Google Scholar]
- Duddeck, D.U.; Albrektsson, T.; Wennerberg, A.; Larsson, C.; Beuer, F. On the cleanliness of different oral implant systems: A pilot study. J. Clin. Med. 2019, 8, 1280. [Google Scholar] [CrossRef]
- Schupbach, P.; Glauser, R.; Bauer, S. Al2O3 particles on titanium dental implant systems following sandblasting and acid-etching process. Int. J. Biomater. 2019, 2019, 6318429. [Google Scholar] [CrossRef]
- Jacobs, J.J.; Skipor, A.K.; Black, J.; Urban, R.; Galante, J.O. Release and excretion of metal in patients who have a total hip-replacement component made of titanium-base alloy. J. Bone Joint Surg. Am. Vol. 1991, 73, 1475–1486. [Google Scholar] [CrossRef]
- Martin-Camean, A.; Jos, A.; Puerto, M.; Calleja, A.; Iglesias-Linares, A.; Solano, E.; Camean, A.M. In vivo determination of aluminum, cobalt, chromium, copper, nickel, titanium and vanadium in oral mucosa cells from orthodontic patients with mini-implants by Inductively coupled plasma-mass spectrometry (ICP-MS). J. Trace Elem. Med. Biol. 2015, 32, 13–20. [Google Scholar] [CrossRef]
- Lutzner, J.; Gunther, K.P.; Postler, A.; Morlock, M. Metal ion release after hip and knee arthroplasty—Causes, biological effects and diagnostics. Z. Orthop. Unf. 2019. [Google Scholar] [CrossRef]
- Costa, B.C.; Tokuhara, C.K.; Rocha, L.A.; Oliveira, R.C.; Lisboa-Filho, P.N.; Costa Pessoa, J. Vanadium ionic species from degradation of Ti-6Al-4V metallic implants: In vitro cytotoxicity and speciation evaluation. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 96, 730–739. [Google Scholar] [CrossRef]
- Grenon, M.S.; Robledo, J.; Ibanez, J.C.; Sanchez, H.J. Titanium diffusion in shinbone of rats with osseointegrated implants. J. Microsc. 2016, 264, 182–188. [Google Scholar] [CrossRef]
- Li, H.; Huang, T.; Wang, Y.; Pan, B.; Zhang, L.; Zhang, Q.; Niu, Q. Toxicity of alumina nanoparticles in the immune system of mice. Nanomedicine 2020, 15, 927–946. [Google Scholar] [CrossRef]
- Chappard, D.; Bizot, P.; Mabilleau, G.; Hubert, L. Aluminum and bone: Review of new clinical circumstances associated with Al(3+) deposition in the calcified matrix of bone. Morphologie 2016, 100, 95–105. [Google Scholar] [CrossRef]
- Goc, A. Biological activity of vanadium compounds. Cent. Eur. J. Biol. 2006, 1, 314–332. [Google Scholar] [CrossRef]
- Park, Y.J.; Song, Y.H.; An, J.H.; Song, H.J.; Anusavice, K.J. Cytocompatibility of pure metals and experimental binary titanium alloys for implant materials. J. Dent. 2013, 41, 1251–1258. [Google Scholar] [CrossRef]
- Niu, Q.; Yang, Y.; Zhang, Q.; Niu, P.; He, S.; Di Gioacchino, M.; Conti, P.; Boscolo, P. The relationship between Bcl-gene expression and learning and memory impairment in chronic aluminum-exposed rats. Neurotox. Res. 2007, 12, 163–169. [Google Scholar] [CrossRef]
- Zhang, Q.L.; Boscolo, P.; Niu, P.Y.; Wang, F.; Shi, Y.T.; Zhang, L.; Wang, L.P.; Wang, J.; Di Gioacchino, M.; Conti, P.; et al. How do rat cortical cells cultured with aluminum die: Necrosis or apoptosis? Int. J. Immunopathol. Pharmacol. 2008, 21, 107–115. [Google Scholar] [CrossRef]
- Zhang, Q.L.; Li, M.Q.; Ji, J.W.; Gao, F.P.; Bai, R.; Chen, C.Y.; Wang, Z.W.; Zhang, C.; Niu, Q. In vivo toxicity of nano-alumina on mice neurobehavioral profiles and the potential mechanisms. Int. J. Immunopathol. Pharmacol. 2011, 24, 23S–29S. [Google Scholar]
- Li, X.; Han, Y.; Guan, Y.; Zhang, L.; Bai, C.; Li, Y. Aluminum induces osteoblast apoptosis through the oxidative stress-mediated JNK signaling pathway. Biol. Trace Elem. Res. 2012, 150, 502–508. [Google Scholar] [CrossRef]
- Diaz-Corte, C.; Fernandez-Martin, J.L.; Barreto, S.; Gomez, C.; Fernandez-Coto, T.; Braga, S.; Cannata, J.B. Effect of aluminium load on parathyroid hormone synthesis. Nephrol. Dial. Transplant. 2001, 16, 742–745. [Google Scholar] [CrossRef]
- Goldstein, J.I.; Newbury, D.E.; Michael, J.R.; Ritchie, N.W.; Scott, J.H.J.; Joy, D.C. Scanning Electron Microscopy and X-Ray Microanalysis, 3rd ed.; Springer: New York, NY, USA, 2017; pp. 209–234. [Google Scholar]
- Souza, F.A.; Queiroz, T.P.; Sonoda, C.K.; Okamoto, R.; Margonar, R.; Guastaldi, A.C.; Nishioka, R.S.; Garcia Junior, I.R. Histometric analysis and topographic characterization of cp Ti implants with surfaces modified by laser with and without silica deposition. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 1677–1688. [Google Scholar] [CrossRef]
- He, X.; Reichl, F.X.; Wang, Y.; Michalke, B.; Milz, S.; Yang, Y.; Stolper, P.; Lindemaier, G.; Graw, M.; Hickel, R.; et al. Analysis of titanium and other metals in human jawbones with dental implants—A case series study. Dent. Mater. 2016, 32, 1042–1051. [Google Scholar] [CrossRef]
- Beger, B.; Goetz, H.; Morlock, M.; Schiegnitz, E.; Al-Nawas, B. In vitro surface characteristics and impurity analysis of five different commercially available dental zirconia implants. Int. J. Implant. Dent. 2018, 4, 13. [Google Scholar] [CrossRef]
- Souza, F.A.; Furtado, T.S.M.; Dayube, U.R.C.; Melo, W.M.; Nishioka, R.S.; Poli, P.P.; Maiorana, C.; de Carvalho, P.S.P. Comparative in vivo study of alloy titanium implants with two different surfaces: Biomechanical and SEM analysis. Clin. Oral Investig. 2019, 23, 4383–4397. [Google Scholar] [CrossRef]
- Gehrke, S.A.; Perez-Diaz, L.; Mazon, P.; De Aza, P.N. Biomechanical effects of a new macrogeometry design of dental implants: An In Vitro Experimental Analysis. J. Funct. Biomater. 2019, 10, 47. [Google Scholar] [CrossRef]
- Chang, J.Z.; Tsai, P.I.; Kuo, M.Y.; Sun, J.S.; Chen, S.Y.; Shen, H.H. Augmentation of DMLS biomimetic dental implants with weight-bearing strut to balance of biologic and mechanical demands: From bench to animal. Materials 2019, 12, 164. [Google Scholar] [CrossRef]
- Juodzbalys, G.; Sapragoniene, M.; Wennerberg, A.; Baltrukonis, T. Titanium dental implant surface micromorphology optimization. J. Oral Implantol. 2007, 33, 177–185. [Google Scholar] [CrossRef]
- Dohan Ehrenfest, M.D.; Del Corso, M.; Kang, B.S.; Leclercq, P.; Mazor, Z.; Horowitz, R.A.; Russe, P.; Oh, H.K.; Zou, D.R.; Shibli, J.A.; et al. Identification card and codification of the chemical and morphological characteristics of 62 dental implant surfaces. Part 5: Chemically coated surfaces (Group 3, coating) and implant collar surfaces (Group 4, collar). POSEIDO 2014, 2, 81–104. [Google Scholar]
- Smeets, R.; Stadlinger, B.; Schwarz, F.; Beck-Broichsitter, B.; Jung, O.; Precht, C.; Kloss, F.; Grobe, A.; Heiland, M.; Ebker, T. Impact of dental implant surface modifications on osseointegration. BioMed. Res. Int. 2016, 2016, 6285620. [Google Scholar] [CrossRef]
- Wennerberg, A.; Albrektsson, T. Effects of titanium surface topography on bone integration: A systematic review. Clin. Oral Implant. Res. 2009, 20 (Suppl. 4), 172–184. [Google Scholar] [CrossRef]
- Leader Italia Implant Catalogue. Available online: http://partotasvir.com/catalogue/LEADER%20Italia%20Implantology%20Catalogue%20%20HD%20version%201.2.pdf (accessed on 11 July 2020).
- Senna, P.; Antoninha Del Bel Cury, A.; Kates, S.; Meirelles, L. Surface damage on dental implants with release of loose particles after insertion into bone. Clin. Implant. Dent. Relat. Res. 2015, 17, 681–692. [Google Scholar] [CrossRef]
- Deppe, H.; Wolff, C.; Bauer, F.; Ruthenberg, R.; Sculean, A.; Mucke, T. Dental implant surfaces after insertion in bone: An in vitro study in four commercial implant systems. Clin. Oral Investig. 2018, 22, 1593–1600. [Google Scholar] [CrossRef]
- Gu, Y.X.; Du, J.; Si, M.S.; Mo, J.J.; Qiao, S.C.; Lai, H.C. The roles of PI3K/Akt signaling pathway in regulating MC3T3-E1 preosteoblast proliferation and differentiation on SLA and SLActive titanium surfaces. J. Biomed. Mater. Res. A 2013, 101, 748–754. [Google Scholar] [CrossRef]
- Cicciu, M.; Fiorillo, L.; Herford, A.S.; Crimi, S.; Bianchi, A.; D’Amico, C.; Laino, L.; Cervino, G. Bioactive titanium surfaces: Interactions of eukaryotic and prokaryotic cells of nano devices applied to dental practice. Biomedicines 2019, 7, 12. [Google Scholar] [CrossRef]
- Nicholson, J.W. Titanium alloys for dental implants: A review. Prosthesis 2020, 2, 100–116. [Google Scholar] [CrossRef]
- Straumann® Roxolid®. Available online: https://www.straumann.com/es/es/profesionales-de-laodontologia/ciencia/bibliografia/roxolid.html (accessed on 10 May 2020).
- He, X.; Reichl, F.X.; Milz, S.; Michalke, B.; Wu, X.; Sprecher, C.M.; Yang, Y.; Gahlert, M.; Rohling, S.; Kniha, H.; et al. Titanium and zirconium release from titanium- and zirconia implants in mini pig maxillae and their toxicity in vitro. Dent. Mater. 2020, 36, 402–412. [Google Scholar] [CrossRef]
- Tardelli, J.D.; da Costa Valente, M.L.; de Oliveira, T.T.; dos Reis, A.C. Influence of chemical composition on cell viability on titanium surfaces: A systematic review. J. Prosthet. Dent. 2020, in press. [Google Scholar] [CrossRef]
- Zinelis, S.; Silikas, N.; Thomas, A.; Syres, K.; Eliades, G. Surface characterization of SLActive dental implants. Eur. J. Esthet. Dent. 2012, 7, 72–92. [Google Scholar]
- Nie, J. Exposure to aluminum in daily life and alzheimer’s disease. In Neurotoxicity of Aluminum, Advances in Experimental Medicine and Biology, 1st ed.; Niu, Q., Ed.; Springer Nature: Singapore, 2018; pp. 99–111. [Google Scholar]
- Gupta, V.B.; Anitha, S.; Hegde, M.L.; Zecca, L.; Garruto, R.M.; Ravid, R.; Shankar, S.K.; Stein, R.; Shanmugavelu, P.; Jagannatha Rao, K.S. Aluminium in Alzheimer’s disease: Are we still at a crossroad? Cell. Mol. Life Sci. 2005, 62, 143–158. [Google Scholar] [CrossRef]
- Okazaki, Y.; Gotoh, E. Comparison of metal release from various metallic biomaterials in vitro. Biomaterials 2005, 26, 11–21. [Google Scholar] [CrossRef]
- Katic, J.; Šaric, A.; Despotovic, I.; Matijakovic, N.; Petkovic, M.; Petrovic, Z. Bioactive coating on titanium dental implants for improved anticorrosion protection: A combined experimental and theoretical study. Coatings 2019, 6, 612. [Google Scholar] [CrossRef]
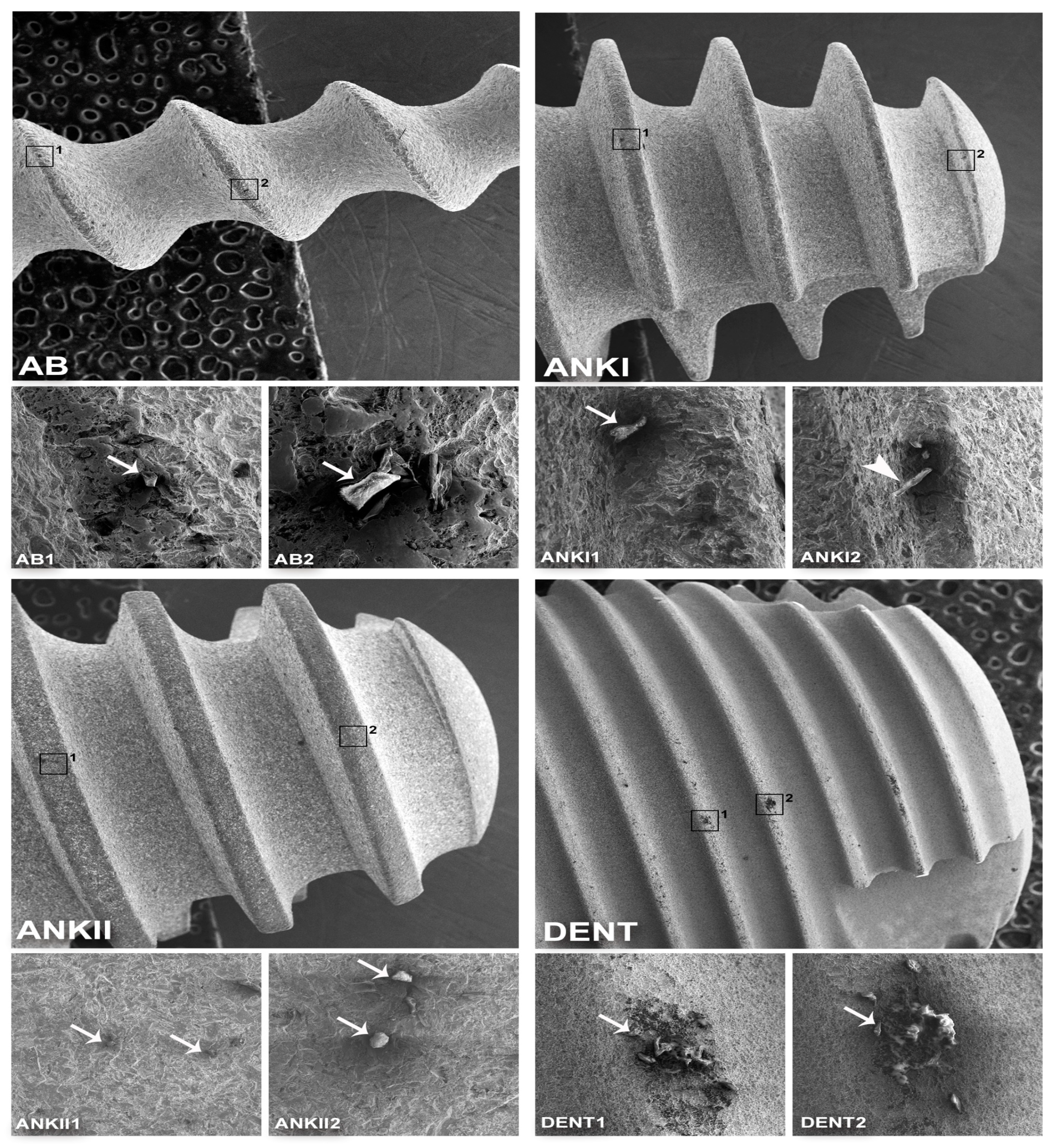
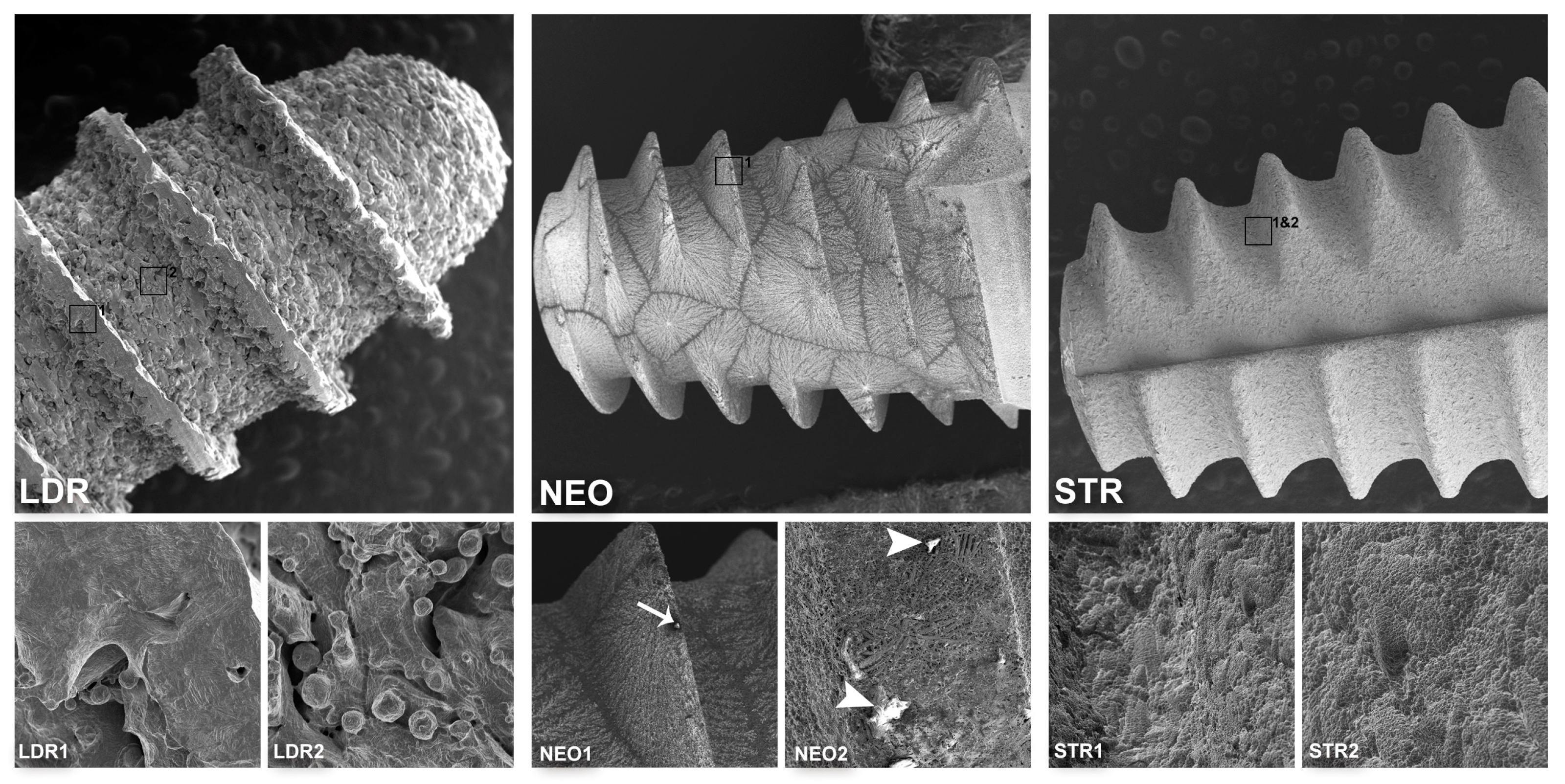
| Implant | Manufacturer/Country | Size | Material | Surface Treatment | Ref. | Lot Number | Cost (USD) |
|---|---|---|---|---|---|---|---|
| AB (ARRP) | Alpha-Bio Tec, Tel Aviv, Israel | 3.0 × 13 mm | Ti grade 5 | Al2O3 sand-blasted/ etched | 2423 | 1104109 | <100 |
| ANKI (B17) | Ankylos Friadent, Mannheim, Germany | 4.5 × 17 mm | Ti grade 2 | Al2O3 sand-blasted/ etched | 31010430 | B160003263 | >100 |
| ANKII (B11) | Ankylos C/X, Dentsply Friadent, Mannheim, Germany | 4.5 × 11 mm | Ti grade 2 | Al2O3 sand-blasted/ etched | 31010050 | 013567 | >100 |
| DENT (Superline) | Dentium, Seoul, Korea | 7.0 × 7 mm | Ti grade 4 | Sand-blasted/ etched | FX7007SW | F10D02410 | <100 |
| LDRI (Tixos MC) | Leader, Milano, Italy | 3.75 × 10 mm | Ti grade 5 | ND (DLMF) | 09ITX3710 | E1014381 | >100 |
| LDRII (Tixos MC) | Leader, Milano, Italy | 3.3 × 10 mm | Ti grade 5 | ND (DLMF) | 09ITX3310 | E1114652 | >100 |
| NEO (Helix GM Acqua) | Neodent, Curitiba, Brazil | 3.75 × 8 mm | Ti grade 4 | Al2O3 sand-blasted/ etched/ immersed in NaCl solution | 140.976 | 800341931 | <100 |
| STR (Bone level, Roxolid SLA) | Straumann, Basel, Switzerland | 4.1 × 14 mm | Ti–Zr | Al2O3 sand-blasted/ etched | 021.5514 | NN454 | >100 |
| Sample | Ti | O | C | Al | Zr | Cl | Na |
|---|---|---|---|---|---|---|---|
| AB | 70.6 ± 12.4 | – | 24.5 ± 12.8 | 4.6 ± 0.9 | – | – | – |
| ANKI | 73.8 ± 20.6 | 24.7 ± 0.4 | 9.2 ± 5.5 | 6.5 ± 0.5 | – | – | – |
| ANKII | 67.1 ± 0.1 | 19.2 ± 0.9 | 4.2 ± 3.8 | 11.6 ± 2.1 | – | – | – |
| DENT | 100 | – | – | – | – | – | – |
| LDRI | 91.5 ± 4.7 | – | 4.6 ± 0.6 | 5 ± 1.6 | – | – | – |
| LDRII | 93.1 ± 1.3 | – | 2.5 ± 0.3 | 6.1 ± 0.2 | – | – | – |
| NEO | 92.9 ± 4.1 | – | 4.7 ± 2.3 | – | – | 1.2 ± 0.04 | 1.2 ± 0.2 |
| STR | 81.4 ± 3.2 | 5.6 ± 0.7 | 2.4 ± 0.4 | – | 13 ± 0.4 | – | – |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dias, F.J.; Fuentes, R.; Navarro, P.; Weber, B.; Borie, E. Assessment of the Chemical Composition in Different Dental Implant Types: An Analysis through EDX System. Coatings 2020, 10, 882. https://doi.org/10.3390/coatings10090882
Dias FJ, Fuentes R, Navarro P, Weber B, Borie E. Assessment of the Chemical Composition in Different Dental Implant Types: An Analysis through EDX System. Coatings. 2020; 10(9):882. https://doi.org/10.3390/coatings10090882
Chicago/Turabian StyleDias, Fernando José, Ramón Fuentes, Pablo Navarro, Benjamin Weber, and Eduardo Borie. 2020. "Assessment of the Chemical Composition in Different Dental Implant Types: An Analysis through EDX System" Coatings 10, no. 9: 882. https://doi.org/10.3390/coatings10090882
APA StyleDias, F. J., Fuentes, R., Navarro, P., Weber, B., & Borie, E. (2020). Assessment of the Chemical Composition in Different Dental Implant Types: An Analysis through EDX System. Coatings, 10(9), 882. https://doi.org/10.3390/coatings10090882







