Anti-Corrosive Coating of Carbon-Steel Assisted by Polymer-Camphorsulfonic Acid Embedded within Graphene
Abstract
:1. Introduction
2. Experimental
2.1. Materials
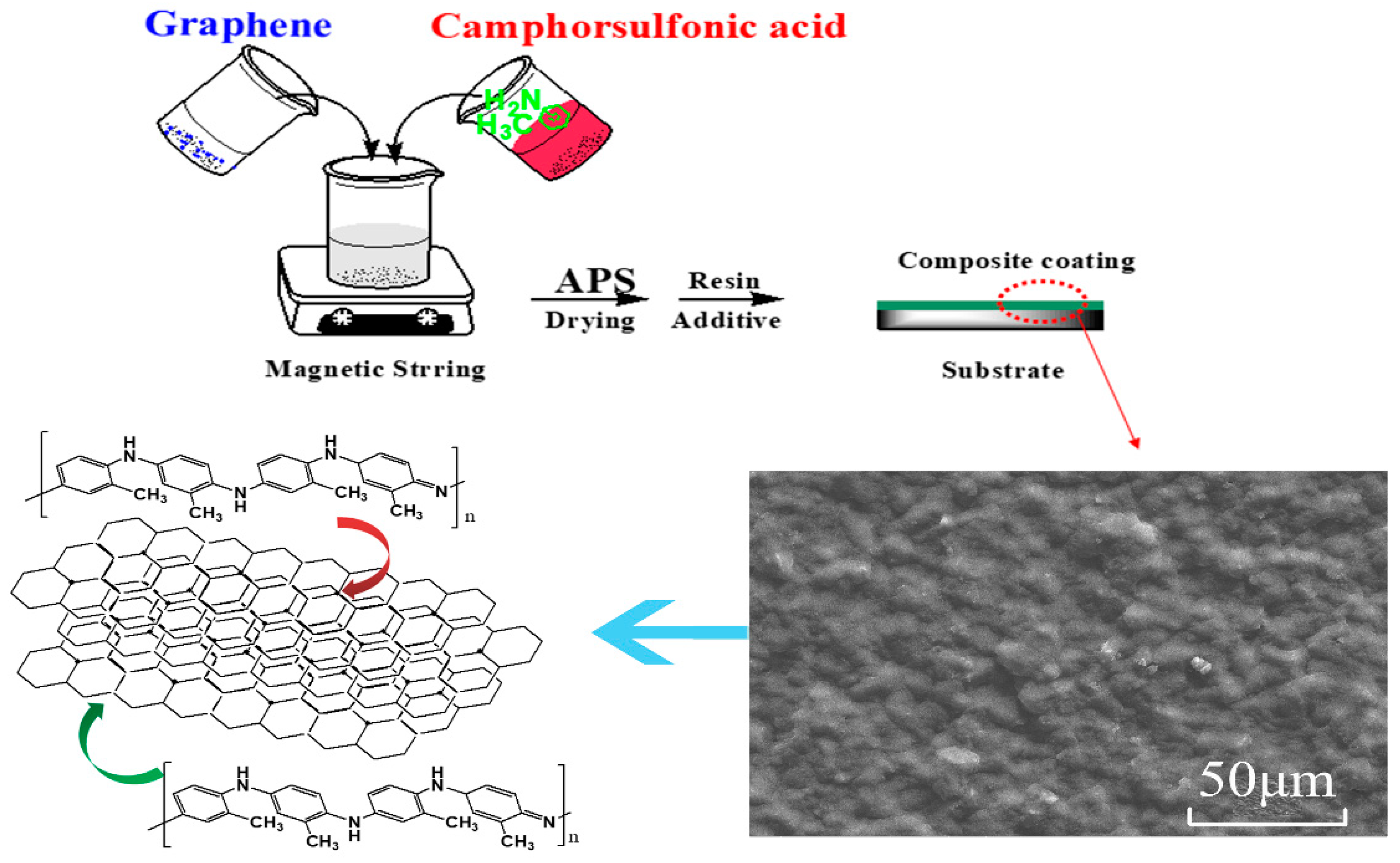
2.2. Preparation
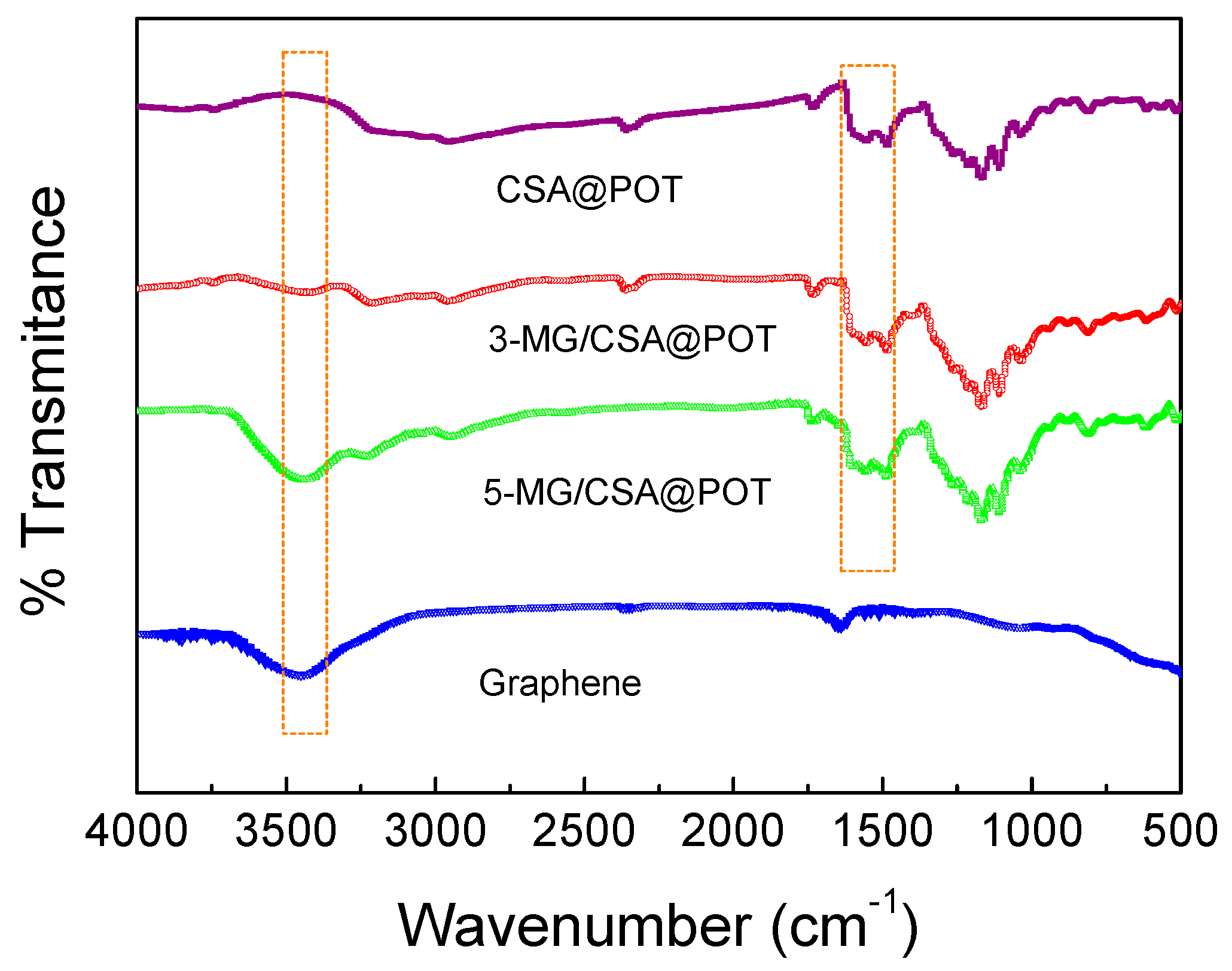
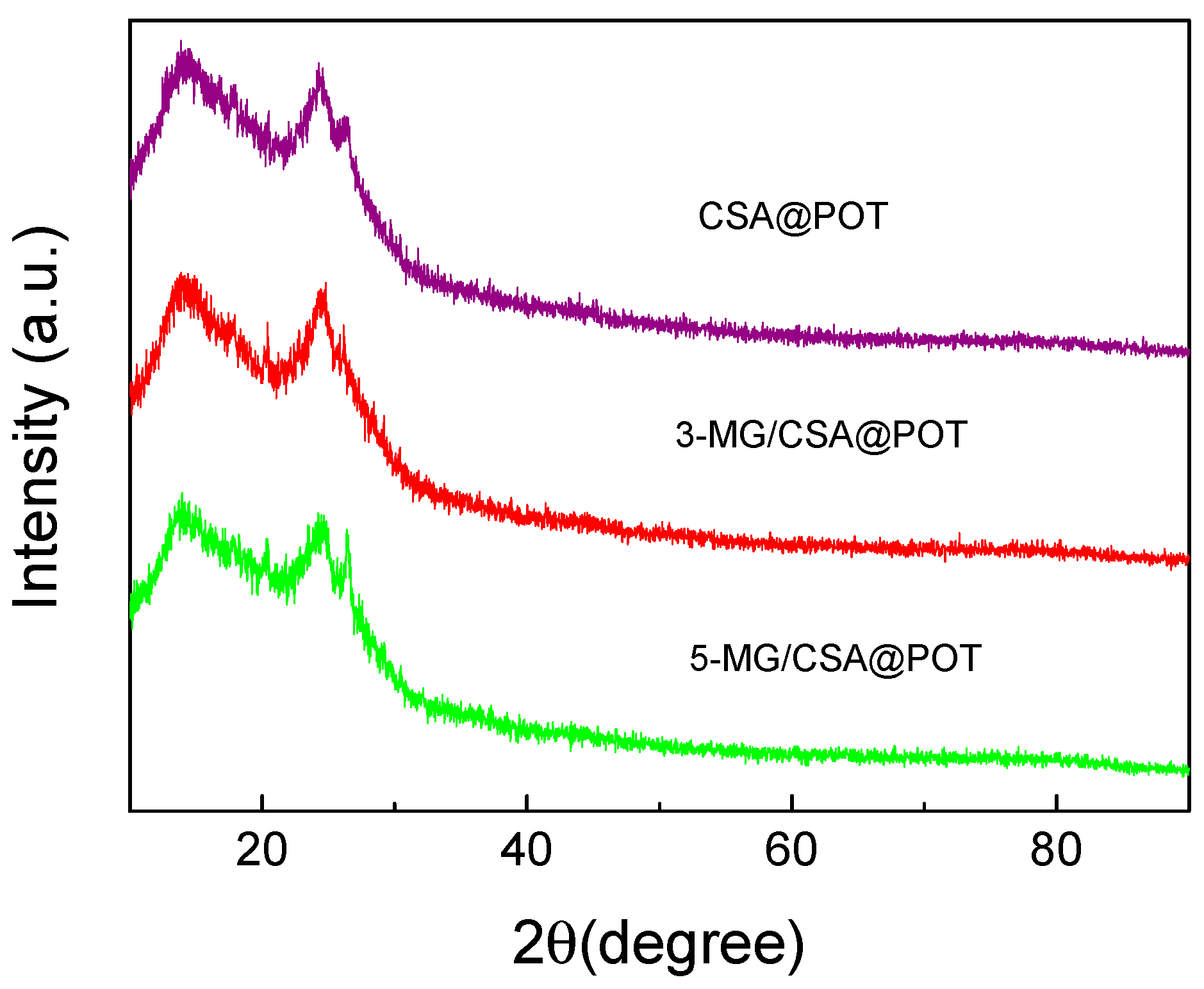
2.3. Characterization
3. Results and Discussion
3.1. Structure and Morphology
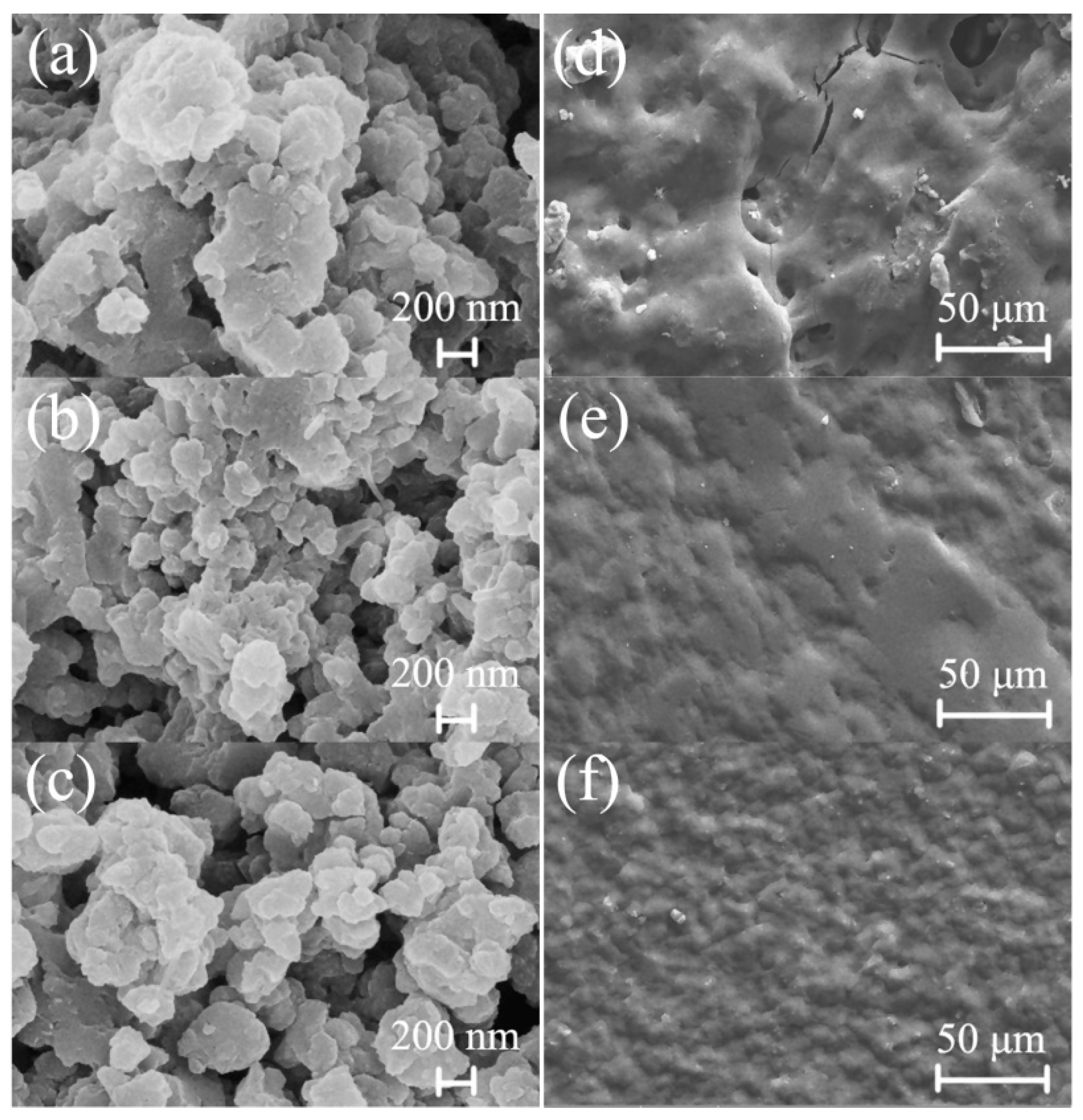
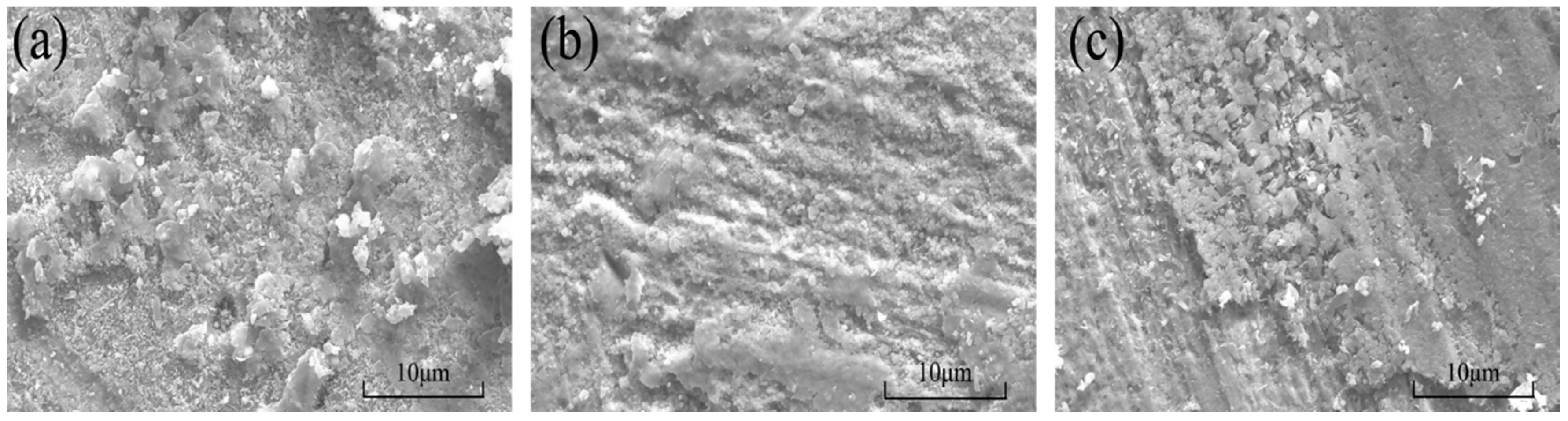
3.2. Morphological Characterization of the Composite Powders and Coatings
3.3. Analysis of the Corrosion Resistances of the Coatings
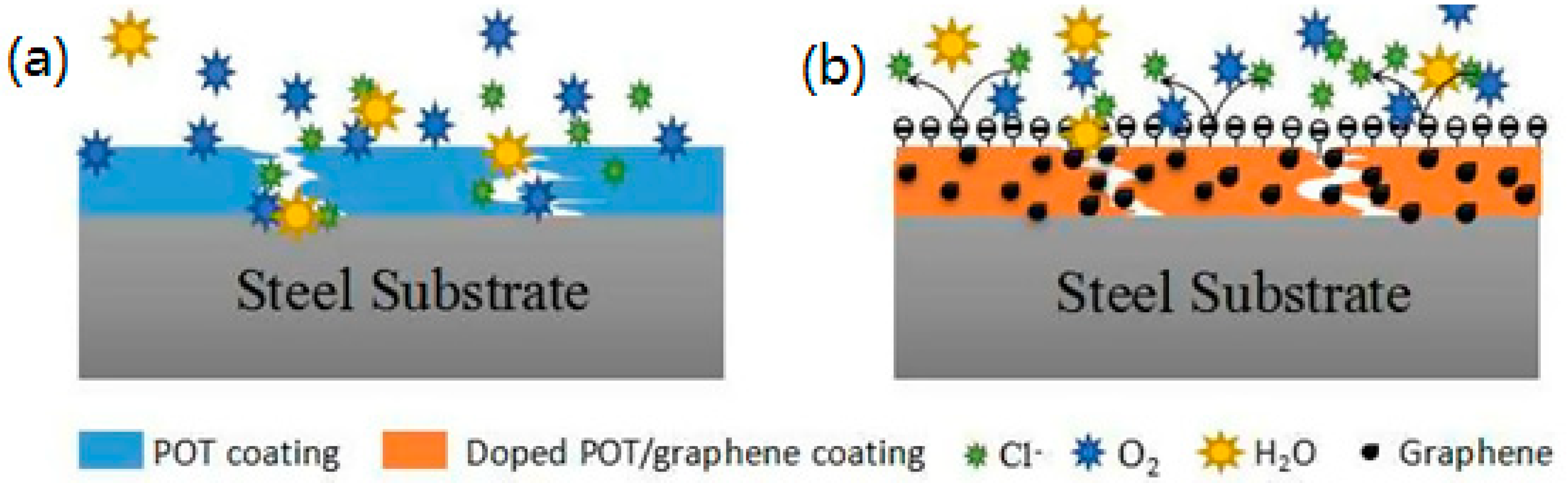
3.4. Mechanism
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kumar, S.A.; Bhandari, H.; Sharma, C.; Khatoon, F.; Dhawan, S.K. A new smart coating of polyaniline-SiO2 composite for protection of mild steel against corrosion in strong acidic medium. Polym. Int. 2013, 62, 1192–1201. [Google Scholar] [CrossRef]
- Wessling, B.; Posdorfer, J. Corrosion prevention with an organic metal (polyaniline): Corrosion test results. Electrochim. Acta 1999, 44, 2139–2147. [Google Scholar] [CrossRef]
- Liu, X.; Hou, P.; Zhao, X.; Ma, X.; Hou, B. The polyaniline-modified TiO2 composites in water-based epoxy coating for corrosion protection of Q235 steel. J. Coat. Technol. Res. 2019, 16, 71–80. [Google Scholar] [CrossRef]
- Hájková, T.; Kalendová, A.; Kohl, M. Anticorrosion and physical properties of organic coatings containing perovskites surface modified by polyaniline or polypyrrole phosphates. Chem. Pap. 2017, 71, 439–448. [Google Scholar] [CrossRef]
- González, M.B.; Saidman, S.B. Electrodeposition of polypyrrole on 316L stainless steel for corrosion prevention. Corros. Sci. 2011, 53, 276–282. [Google Scholar] [CrossRef]
- Nautiyal, A.; Qiao, M.; Cook, J.E.; Zhang, X.; Huang, T. High performance polypyrrole coating for corrosion protection and biocidal applications. Appl. Surf. Sci. 2018, 427, 922–930. [Google Scholar] [CrossRef]
- Deshpande, P.P.; Jadhav, N.G.; Gelling, V.J.; Sazou, D. Conducting polymers for corrosion protection: A review. J. Coat. Technol. Res. 2014, 11, 473–494. [Google Scholar] [CrossRef]
- De Leon, A.C.C.; Pernites, R.B.; Advincula, R.C. Superhydrophobic Colloidally Textured Polythiophene Film as Superior Anticorrosion Coating. ACS Appl. Mater. Inter. 2012, 4, 3169–3176. [Google Scholar] [CrossRef]
- Sivakkumar, S.R.; Saraswathi, R. Application of poly(o-phenylenediamine) in rechargeable cells. J. Appl. Electrochem. 2004, 34, 1147–1152. [Google Scholar] [CrossRef]
- Jamal, R.; Abdiryim, T.; Nurulla, I. Comparative studies of solid-state synthesized poly(o-methoxyaniline) and poly(o-toluidine). Polym. Advan. Technol. 2008, 19, 1461–1466. [Google Scholar] [CrossRef]
- Yin, P.; Kilmartin, P.A. Formation of poly-2,5-dimethoxyaniline on steels. Curr. Appl. Phys. 2004, 4, 141–143. [Google Scholar] [CrossRef]
- Carrillo, I.; Sánchez De La Blanca, E.; González-Tejera, M.J. Influence of the electropolymerisation time on the nucleation mechanism, structure and morphology of polyfurane/perchlorate doped films. Polymer 2001, 42, 9447–9453. [Google Scholar] [CrossRef]
- Abdiryim, T.; Xiao-Gang, Z.; Jamal, R. Synthesis and characterization of poly(o-toluidine) doped with organic sulfonic acid by solid-state polymerization. J. Appl. Polym. Sci. 2005, 96, 1630–1634. [Google Scholar] [CrossRef]
- Kulkarni, M.V.; Viswanath, A.K.; Khanna, P.K. Synthesis and humidity sensing properties of conducting poly(N-methyl aniline) doped with different acids. Sens. Actuators B Chem. 2006, 115, 140–149. [Google Scholar] [CrossRef]
- Hu, C.; Li, J.; Kong, Y.; Ding, Y.; Li, Y. Preparation and characterization of poly(o-toluidine)/nano TiO composite and Study of their anticorrosion properties blended with epoxy resin. J. Polym. Mater. 2017, 4, 797–808. [Google Scholar]
- Aussawasathien, D.; Sahasithiwat, S.; Menbangpung, L. Electrospun camphorsulfonic acid doped poly(o-toluidine)—Polystyrene composite fibers: Chemical vapor sensing. Synth. Met. 2008, 158, 259–263. [Google Scholar] [CrossRef]
- Shinde, V.; Sainkar, S.R.; Patil, P.P. Corrosion protective poly(o-toluidine) coatings on copper. Corros. Sci. 2005, 47, 1352–1369. [Google Scholar] [CrossRef]
- Uzoma, P.C.; Liu, F.; Xu, L.; Zhang, Z.; Han, E.; Ke, W.; Arukalam, I.O. Superhydrophobicity, conductivity and anticorrosion of robust siloxane-acrylic coatings modified with graphene nanosheets. Prog. Org. Coat. 2019, 127, 239–251. [Google Scholar] [CrossRef]
- Ke-feng, C.; Yun-hui, F.; Kai-he, Z.; Peng, L.; Jin-sheng, G. Preparation and Performance Evaluation of Graphene Based Conductive Anti-corrosive Coatings. Surf. Technol. 2018, 12, 246–254. [Google Scholar]
- Liu, Q.; Ma, R.; Du, A.; Zhang, X.; Yang, H.; Fan, Y.; Zhao, X.; Cao, X. Investigation of the anticorrosion properties of graphene oxide doped thin organic anticorrosion films for hot-dip galvanized steel. Appl. Surf. Sci. 2019, 2, 646–654. [Google Scholar] [CrossRef]
- Jiang, F.; Zhao, W.; Wu, Y.; Wu, Y.; Liu, G.; Dong, J.; Zhou, K. A polyethyleneimine-grafted graphene oxide hybrid nanomaterial: Synthesis and anti-corrosion applications. Appl. Surf. Sci. 2019, 15, 963–973. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Zhang, S. Preparation, Characterization and Corrosion Evaluation of Poly(o-toluidine), Poly(m-toluidine), and Poly(p-toluidine) Blended with Waterborne Polyurethane. JOM-US 2018, 70, 2660–2666. [Google Scholar] [CrossRef]
- Hu, C.; Li, Y.; Kong, Y.; Ding, Y. Preparation of poly(o-toluidine)/nano ZnO/epoxy composite coating and evaluation of its corrosion resistance properties. Synth. Met. 2016, 214, 62–70. [Google Scholar] [CrossRef]
- Ismail, Y.A.; Ahmad, A.; Mohammad, F. Synthesis, Electrical, Electronic and Charge Transport Properties of Poly(aniline-co-p-toluidine). J. Macromol. Sci. Part A 2008, 45, 650–657. [Google Scholar] [CrossRef]
- Jiang, L.; Syed, J.A.; Lu, H.; Meng, X. In-situ electrodeposition of conductive polypyrrole-graphene oxide composite coating for corrosion protection of 304SS bipolar plates. J. Alloys Compd. 2019, 770, 35–47. [Google Scholar] [CrossRef]
- Yeh, T.; Huang, T.; Huang, H.; Huang, Y.; Cai, Y.; Lin, S.; Wei, Y.; Yeh, J. Electrochemical investigations on anticorrosive and electrochromic properties of electroactive polyurea. Polym. Chem. 2012, 3, 2209–2216. [Google Scholar] [CrossRef]
- Cui, M.; Ren, S.; Pu, J.; Wang, Y.; Zhao, H.; Wang, L. Poly(o-phenylenediamine) modified graphene toward the reinforcement in corrosion protection of epoxy coatings. Corros. Sci. 2019, 159, 108131. [Google Scholar] [CrossRef]
- Musa, A.Y.; Kadhum, A.A.H.; Mohamad, A.B.; Takriff, M.S.; Chee, E.P. Inhibition of aluminum corrosion by phthalazinone and synergistic effect of halide ion in 1.0M HCl. Curr. Appl. Phys. 2012, 12, 325–330. [Google Scholar] [CrossRef]
- Chaudhari, S.; Patil, P.P.; Mandale, A.B.; Patil, K.R.; Sainkar, S.R. Use of poly(o-toluidine)/ZrO2 nanocomposite coatings for the corrosion protection of mild steel. J. Appl. Polym. Sci. 2007, 106, 220–229. [Google Scholar] [CrossRef]
- Dominis, A.J.; Spinks, G.M.; Wallace, G.G. Comparison of polyaniline primers prepared with different dopants for corrosion protection of steel. Prog. Org. Coat. 2003, 48, 43–49. [Google Scholar] [CrossRef]
- Grgur, B.N.; Elkais, A.R.; Gvozdenović, M.M.; Drmanić, S.Ž.; Trišović, T.L.; Jugović, B.Z. Corrosion of mild steel with composite polyaniline coatings using different formulations. Prog. Org. Coat. 2015, 79, 17–24. [Google Scholar] [CrossRef]
- Le, D.P.; Yoo, Y.H.; Kim, J.G.; Cho, S.M.; Son, Y.K. Corrosion characteristics of polyaniline-coated 316L stainless steel in sulphuric acid containing fluoride. Corros. Sci. 2009, 51, 330–338. [Google Scholar] [CrossRef]
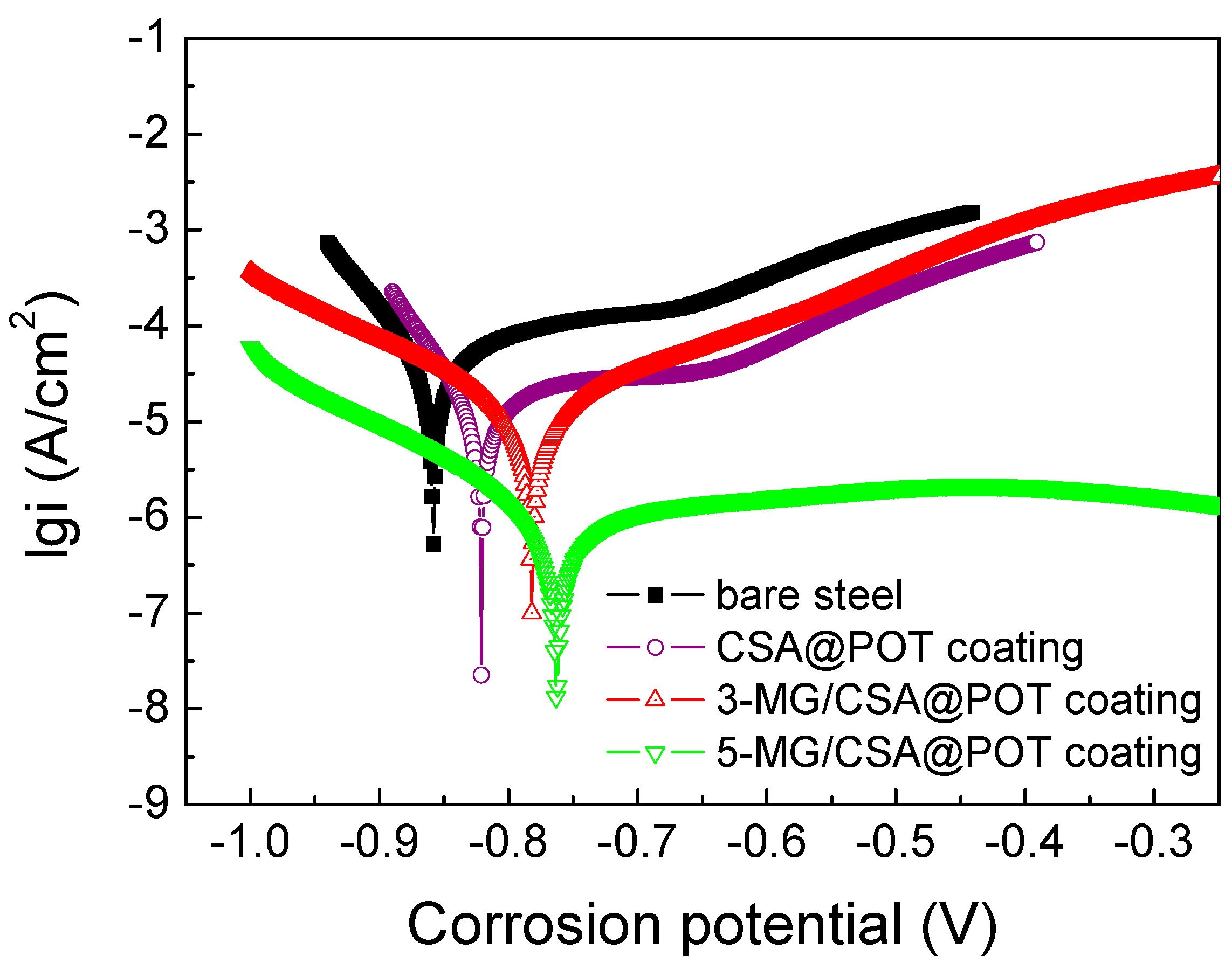
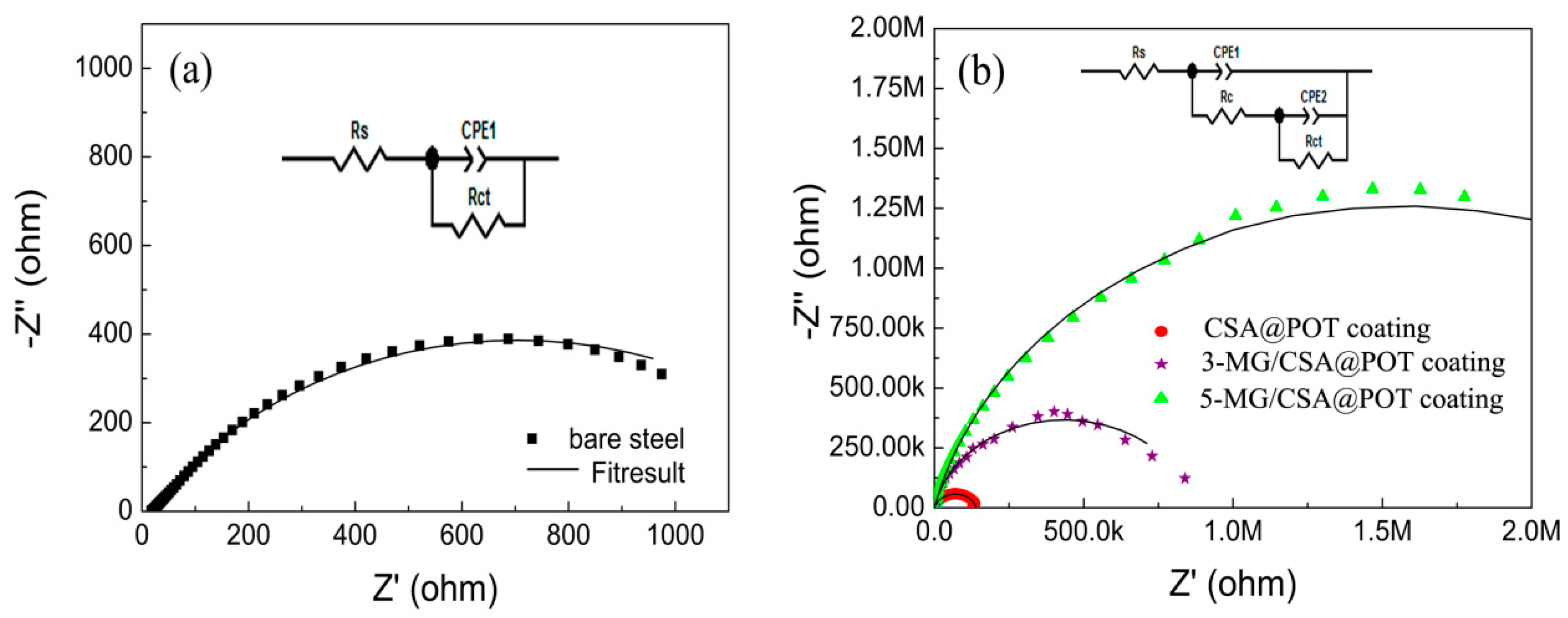
| Coatings | Potentio-Dynamic Polarization | Electrochemical Impedance Spectroscopy | |||||
|---|---|---|---|---|---|---|---|
| Ecorr (V) | icorr (A/cm2) | CR (mm/year) | Rs (Ω·cm2) | Rc (Ω·cm2) | Rct (Ω·cm2) | IE (%) | |
| Bare steel | −0.86 | −4.50 | 3.16 × 10−5 | 18.15 | - | 1227 | - |
| CSA@POT-WPU | −0.82 | −5.01 | 9.78 × 10−6 | 26.20 | 156.9 | 139,630 | 99.12 |
| 3-MG/CSA@POT-WPU | −0.78 | −5.21 | 6.17 × 10−6 | 28.15 | 247.3 | 869,260 | 99.85 |
| 5-MG/CSA@POT-WPU | −0.67 | −5.99 | 1.02 × 10−6 | 27.57 | 243.4 | 3.16 × 106 | 99.96 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhai, Y.; Pan, K.; Zhang, E. Anti-Corrosive Coating of Carbon-Steel Assisted by Polymer-Camphorsulfonic Acid Embedded within Graphene. Coatings 2020, 10, 879. https://doi.org/10.3390/coatings10090879
Zhai Y, Pan K, Zhang E. Anti-Corrosive Coating of Carbon-Steel Assisted by Polymer-Camphorsulfonic Acid Embedded within Graphene. Coatings. 2020; 10(9):879. https://doi.org/10.3390/coatings10090879
Chicago/Turabian StyleZhai, Yingying, Kefeng Pan, and Ende Zhang. 2020. "Anti-Corrosive Coating of Carbon-Steel Assisted by Polymer-Camphorsulfonic Acid Embedded within Graphene" Coatings 10, no. 9: 879. https://doi.org/10.3390/coatings10090879
APA StyleZhai, Y., Pan, K., & Zhang, E. (2020). Anti-Corrosive Coating of Carbon-Steel Assisted by Polymer-Camphorsulfonic Acid Embedded within Graphene. Coatings, 10(9), 879. https://doi.org/10.3390/coatings10090879




