Abstract
The electric double layer (EDL) formed at the water/solid interface is the key to understanding a wide variety of natural phenomena; and nowadays, this knowledge may lead to ideas for technological innovations in the industrial and biomedical fields. The properties of the EDL on a surface directly determine the performance of an EDL-based device. In electrolyte solution, the coions with the same charge sign with the surface are usually ignored. However, they are expected to make a considerable contribution in concentrated conditions when ionic specific effects should be considered. Herein, we investigated the effect of anions in the Hofmeister series, including kosmotropes (Ac−), chaotropes (I−), and intermediate (Cl−), on the properties of the EDL on a negatively charged mica surface by observing intersurface forces using the surface forces apparatus (SFA). The SFA results indicated that at a concentration of 1 M and above, the effect of the monovalent anions in the sodium solutions were mainly correlated with their hydration behaviors. Exclusively in the solutions with strongly hydrated anions, we measured the abnormal long-range repulsions in the diffuse layer region farther away from the surface. By further investigating the solutions with kosmotropic divalent SO42− at relatively low concentrations, we may attribute the observation to the formation of ion pairs and hydrodynamic effects in the presence of concentrated kosmotropic anions. Moreover, these anions can also contribute to the formation of a complete and stable hydration layer near the surface. This work demonstrates the considerable effects of Hofmeister anionic coions on the properties of a simple colloid interface, which has attracted little attention in the past. Therefore, further investigations are desirable in the development of electrolytes and surface materials for the promising EDL-based technologies.
1. Introduction
Upon exposure to an aqueous solution, a surface usually becomes charged due to several mechanisms, such as group dissociation and ion adsorption/exchange [1]. Driven by the Coulomb force, the charged surface can attract the oppositely charged ions in the solution to form a so-called electric double layer (EDL) structure in the vicinity of the surface. The EDL and any interaction arising from it are the reasons for many phenomena observed in colloidal systems, such as clay swelling [2], aggregation and precipitation of particles [3], and adhesion of biomolecules [4]. Owing to many advantages, the EDL have been utilized to build foundations for several revolutionary techniques that are urgently needed for use in the fields of energy, environment and biomedicine [5,6,7]. For instance, the EDL supercapacitor, based on the EDL units on the electrode, can achieve not only a high capacity of energy but also fast and long-term cycling [8]. Clearly, whether the EDL-based devices can achieve the expected performances relies on the characteristics of EDL established on the surface, which represent not only the distribution electrolyte ions in the working area but also their kinetics driven by electric field. Also, note that most applications usually work in conditions containing concentrated electrolytes at concentrations of above 0.1 M [9]. Therefore, it is essential to have a comprehensive understanding of the EDL in concentrated solution in order to efficiently optimize the design and manufacture of electrolytes and surface materials for the devices containing this interfacial structure [10,11,12].
As described by the Gouy–Chapman–Stern (GCS) model [13], the EDL structure can be divided into two regions: Stern layer (Helmholtz layer) and diffuse layer. Most surface charges are screened by one or two layers of bound counterions in the Stern layer, and the rest is balanced within the diffuse layer. For diluted solution, the distribution of ions, as well as the potential in the diffuse layer, has been predicted by the Poisson–Boltzmann (PB) equation; the effective thickness of this layer can be represented by a critical parameter, i.e., the Debye length [1]. When two EDLs from similarly charged surfaces overlap, this can lead to EDL repulsion with entropic nature. By further superimposing this repulsion with the van der Waals (vdW) force, the Derjaguin–Landau–Verwey–Overbeek (DLVO) forces are introduced. With the advancement of measurement technology, measuring the intersurface forces has been demonstrated to be one effective approach for the imaging of the properties of interfacial structure at nanoscale [1,14,15].
Classical PB theory-based models treat ions as point-like charges while ignoring several ionic-specific effects, such as ion size and ion–ion correlation. Normally, the Debye length decreases with increasing the electrolyte concentration. However, these mean field models are broken down under certain conditions, particularly at high concentrations. One typical example is room temperature ionic liquids (ILs) that are usually regarded as having concentrations at several molar levels. By using the surface forces apparatus (SFA), Israelachvili and colleagues [16,17] first reported the long-range repulsions in the imidazolium between mica surfaces, which are similar with that in the corresponding EDL in a diluted solution. The authors further investigated the temperature dependency and proposed that the mechanisms are attributed to ion pairing in the ILs when less than 1% of electrolytes are actually dissociated and participated in the screening of surface charge. Later studies have further demonstrated that this is a universal phenomenon that also takes place in other concentrated aqueous solutions, and the deviation becomes more significant at higher concentrations [9,18]. In attempts to find the origin of the long-range repulsion, both experimental and theoretical studies, considering the effects of strong correlation and finite volume of ions in the system, have proposed that some parameters in the classical models should be modified with effective parameters, including ion concentration [16,18,19], ion charge, and dielectric permittivity [20,21]. In addition to the ion pairing, the underscreening effect observed in the surface force measurements can also be explained by a model of ionic crystal with Schottky defects, which has been verified by Perkin et al. [22,23] in different solutions containing the solute ions with various chemical properties. Although the mechanism beneath this peculiar phenomenon remains to be uncovered, the presence of long-range repulsion appears to be correlated with the concentration, ion size, ion valence, and symmetricity of the electrolytes [23,24,25].
In an aqueous solution, the hydration effect usually arises from the oxide surface, such as mica and silica. The hydration force that dominates the distance in the Stern layer can be sufficiently strong to prevent the surfaces from adhesive contact caused by vdW force [26]. Back to 1983, Israelachvili and Pashley [27] found a repulsive and oscillate region in the force-distance profile of mica surfaces in 10−3 M KNO3; at a distance below 1.5 nm, an oscillation period of 0.25 nm matched the size of water molecule. A later experiment based on the X-ray reflectivity and atomic force microscopy (AFM) identified the density oscillation of the ordered water that is adjacent to the mica surface [28]. However, other investigations prefer to attribute the hydration repulsion to the electrolyte ions with hydration shells. Heuberger et al. [29,30,31], employing the extended SFA, proposed the ordered hydrated cations as the reason for continuous transitions of the solution thickness between two approaching surfaces, and three kinds of hydrated states that could be distinguished from the results. It should be noted that the hydration force possesses not only oscillation behavior, but also the monotonic increase. Two types of hydration forces has been observed to coexist and to be superimposed [32,33]. Despite the discrepancy in the origin of the hydration force, the adsorption state of a certain ion on surface should be determined by its hydration strength, which determines the energy penalty required for the removal of its hydration shell so that it can make a direct contact with the surface [34,35]. Outside the Stern layer, the hydration effect also contributes to the structure in the diffuse layer. Baimpos et al. [33] reported that the absences of the diffuse layer on mica in CsCl solution at concentrations of 0.05–1 M. The authors proposed that with increasing confinement, the Cs+ ions with low hydration energy lose their hydration shells so that they can easily penetrate the lattices of mica, increasing the efficiency of the surface charge screening. Besides, the ion-pairing between cation and anion, which may be responsible for the long-range EDL force, also depends on the difference between their hydration enthalpies, according to the law of matching water affinities (LMWA) [36].
These previous studies on EDL mainly focus on the counterions, i.e., cations for the negatively charged surface; the contribution of anions, except for those that serve as counterions rather than coions, has received little attention [37]. Taking the negatively charged surface as an example, the anions are not usually considered in the analysis of diluted solutions, as their concentrations are much lower than those of cations in the EDL. However, this does not indicate that the role of anions should always be ignored. X-ray photoelectron spectroscopy and X-ray diffraction experiments have provided the evidence on monovalent anions at the water/mica interface showing that the hydration shells are shared with the innermost cations [38,39]. The presence of a specific anion at the interface can influence the coverage of cations on the negatively charged surface [39,40]. Although the effect on water structuring is dependent on the size or charge density of an ion, an anion with a larger size than a cation may even have a higher efficiency in restructuring water molecules [41,42]. Moreover, in a highly concentrated condition, alternative layers of cations and anions can be formed at the interface due to the so-called ‘overscreening effect’ or ‘crowding effect’ [43,44], when the role of anions should become more significant.
In this work, we aim to investigate the effect of anions on the properties of EDL formed on negatively charged mica surfaces in a highly concentrated condition. To reach this aim, we experimentally examined the interactions between mica surfaces in the aqueous sodium solutions containing different anions. The force measurements were performed by the SFA technique, with ultra-fine resolutions of 0.1 nm in distance and 10 nN in force, which provides high sensitivity to any weak interaction and corresponding nanostructure. The anions, including monovalent I−, Cl−, and Ac−, and divalent SO42−, belong to the Hofmeister series. As these anions are known to have different hydration effects [45], we also evaluated their abilities in restructuring the water network. The results and mechanisms behind this are thoroughly discussed in the text. This work presents that although these Hofmeister anions play the roles of coions to mica surface, they indeed have considerable contributions to the interfacial structure, both in the diffuse layer and the Stern layer, mainly depending on their hydration properties. We believe that this topic is worth further investigation on a wide range of electrolytes and surfaces with various properties, which are commonly used in industrial and biomedical fields.
2. Experimental Methods
Chemicals: Sodium iodide (NaI), sodium chloride (NaCl), sodium acetate (NaAc), and sodium sulfate (Na2SO4) (all with purities >99%) were provided by Sigma Aldrich (Shanghai, China). Electrolyte solutions were prepared by dissolving certain amounts of the salts in water, followed by stirring for at least 8 h. Before the test, all solutions were filtered through the syringe filters with 0.22-μm pore size. Glassware and silicon disks were washed with piranha solution to remove organic contaminants and were then stored in ethanol. Milli-Q ultrapure water (Merck Millipore, Molsheim, France; resistivity, 18.25 MΩ cm; total organic carbon < 3 ppb) was used in all solution preparation and glass cleaning.
SFA measurement: A SFA (model 2000, SurForce LLC, Santa Barbara, CA, USA) was employed to measure the intersurface forces, according to the protocol described previously [46]. The setup of an SFA experiment is illustrated in Figure 1. In a typical measurement, two pieces of freshly cleaved mica were used as the surfaces. Mica pieces with thicknesses of 1–3 μm were back-silvered to prepare reflective layers required in the multiple beam interferometry technique, by which the absolute distance (D) between two mica surfaces was determined. After silvering, each mica was glued onto a silicon disk and was then mounted in the SFA chamber with two surfaces facing each other in a cross-cylinder geometry. Initially, the distance D = 0 was calibrated at the position where two surfaces were in contact in dry nitrogen. Droplets of certain aqueous solution (NaI, NaCl, NaAc or Na2SO4) were then injected into the surface gap, and the chamber was sealed. A small amount of ultrapure water may be left at the bottom of the chamber to form a vapor saturated atmosphere. The interactions between the surfaces in the liquids as a function of surface–surface distance was not measured until the equilibrium was reached usually in 2 h. During the measurement, the lower surface was either pushed towards or apart from the fixed upper surface, driven by a motor-driven micrometer. The motor (Faulhaber, Schönaich, Germany) was set to rotate at a constant velocity at ~300 rpm, and the movement was further slowed down using a reducer at a transmission ratio of 900:1, which allowed the change rate of the surface separation to be below 2 nm/s. The interaction energy per unit area (E) was calculated from a normalized force-distance (F/R vs. D) profile by Derjaguin approximation using the following equation: E = F/2π R; where F is the measured normal force, and R is the effective radius of surface. The hardwall distance (DH), which can be used to represent the thickness of medium solution trapped between the surfaces, was obtained from the profile where the distance remained unchanged under high compression (at a load of 20 mM/m). Reproducibility of the results was verified by performing the measurements for two to three times. All experiments were performed at a temperature of 20 ± 0.1 °C.
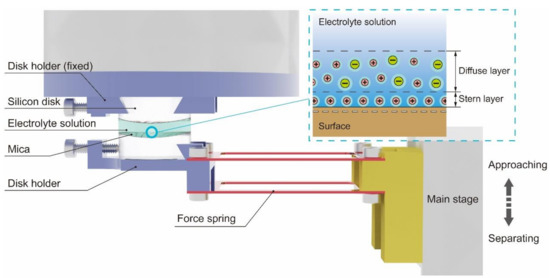
Figure 1.
Schematic diagram of the surface forces apparatus (SFA) system. The electric double layer (EDL) structure formed on a mica surface (both the Stern layer and the diffuse layer) is illustrated in the upper right corner.
Fitting force-distance profile to extended DLVO model: The PB equation can be further linearized to the Debye–Hückel (DH) equation, in which the potential at any distance away from the surface is a function of the Debye length (κ−1) [1]. The Debye length (κ−1) can be expressed as:
where ε and ε0 are the dielectric permittivity of the medium and vacuum, respectively, kB is the Boltzmann constant, T is the temperature, ρi is the number density of ion i in bulk, e is the elementary charge, and zi is the charge of ion i. As can be seen, the Debye length is dependent only on the properties of electrolyte solution; for example, in a 1:1 electrolyte solution at 293 K (20 °C), the Debye length approximately equals nm, where M is the salt concentration in molar/L. Usually, the Debye length can be used to determine the exponentially decaying EDL force in diluted solutions. However, when derivation from the classical theories occurs, effective decay length must be involved.
The conventional DLVO theory regards the overall interactions (F) as a simple summation of van der Waals force (FvdW) and EDL force (FEDL). The normalized force (F/R) can be expressed as follows:
where in the first term, A is the Hamaker constant (2.0 × 10−20 J for micas across the aqueous medium) and D is the separation distance between two mica surfaces. In the second term, the interaction constant Z is defined as [1]; where ψE is the diffuse layer potential at the intermediate plane (D = DE) between the EDL region and the hydration region; λeff (nm) is the effective decay length of the exponential repulsion at the distances farther away from the surface.
The contributions of the hydration effect were also considered by introducing two terms, Fstr/R and Fste/R, to represent the hydration forces with structural (Fstr) and steric (Fste) characteristics, respectively. The hydration forces were isolated by subtracting the DLVO forces from the measured force-distance profile. The additional terms can be expressed in the exponential functions as follows [33]:
where Ai and Wj are the pre-factors of respective hydration forces, Di is the center distance of the structural oscillation, σi is the characteristic width of the oscillation, and λHj is the decay length of the steric hydration force. Because in some measurements more than one oscillation or decay length could be observed in the hydration region, the data was also fitted using m or n as the partitions (when needed). Moreover, the onset of steric hydration force at the distance D = 0 was shifted outwards to DHj, which is where a certain partition transition occurred. For the innermost area, DHj is set to the DH of the hardwall position.
Measurements of pH and Raman spectroscopy: The pHs of the electrolyte solutions were determined using a Mettler Toledo SC S210-B pH meter (Mettler Toledo, Shanghai, China). Each measurement was repeated at least twice. The Raman spectra were recorded on a WITec alpha 300 RA (WITec, Ulm, Germany) using an objective lens with a numerical aperture of 0.55 and at 50× magnification. The Raman scattering photons that were excited using a 532-nm laser with a power of 20 mW were directed into a spectrometer with 600 grooves/mm gratings. The spectra at a band resolution of ~3 cm−1 were collected by a charge-coupled detector. Here, ultrapure water was used as a reference [47].
3. Results and Discussion
3.1. Intersurface Forces in Monovalent Solutions at 0.1 M
The interactions between two bare mica surfaces in three monovalent solutions (NaI, NaCl and NaAc) at a concentration of 0.1 M are shown in Figure 2. The fitting parameters for all the electrolyte solutions examined in this work are listed in Table 1. Due to the screening effect of the concentrated counterion Na+ on the surface, most of the surface charges were balanced within the Stern layer, and the repulsive forces were apparent only at the distances where the contribution of vdW force was considered. In NaI solution, a short-range repulsive force appeared at ~4 nm. After the subtraction of the vdW force from the overall interactions, the rest of the repulsion was found to linearly change with increasing surface separation in a semilogarithmic plot. By fitting the profile with the second term in Equation (2), we could obtain an exponential decay length of 0.96 ± 0.02 nm. This value is consistent with the Debye length of a 1:1 electrolyte solution (at 0.1 M) calculated by Equation (1), clearly indicating that this repulsion was caused by the overlap of the diffuse double layers. When the distance between the two mica surfaces was below ~2 nm, it was not surprising to find the result could no longer be described by the conventional DLVO theory because of the well-known hydration effect, which is usually working at a relatively lower concentration than that is required by the abnormal long-range repulsion mentioned above. As the distance decreased, additional repulsions with two distinct oscillations were superimposed onto the DLVO forces. The oscillatory profile exhibited characteristic widths of 0.29 ± 0.02 and 0.20 ± 0.02 nm, and each oscillation underwent a jump-in. In force-probe techniques, a sudden jump-in is usually caused by mechanical instability that can occur when the gradient of force over distance exceeds the springe constant of system. Similar observations have been observed in several other SFA and AFM studies [27,29,48]. These studies have proposed that the structural oscillation is an indicator of the layering structures at the solid/water interface as it is related to the density variation of ion or water molecules. The mean jump-in distance (∆D) of 0.2 nm corresponds to the diameter of water molecule, and together with the oscillation widths, the results imply that layers of water molecules may be squeezed out from the contact area. Beyond the oscillatory region, an additional repulsion increased with a decay length of 0.13 ± 0.02 nm and the surface separation reached at the DH of 0.6 ± 0.1 nm. According to an earlier report by Hu et al. [37], this innermost repulsion was probably originated from the inner Helmholtz layer. Further changes in structure were not detected in subsequent measurements until the loading was increased up to 20 mN/m, indicating that the remaining hydration layer was stable against the high confinement. The force-distance profile measured in 0.1 M NaI solution showed two distinct regions distributed at the solution/mica interface; the regions were dominated by the EDL force at the distance above ~2 nm and by the hydration force at the distance below ~2 nm. The distance range at which both forces were dominated was found to vary in other solutions. For simplicity, in the following discussion, the region farther away from the surface is referred to as the EDL region, and that near the surface is referred to as the hydration region.
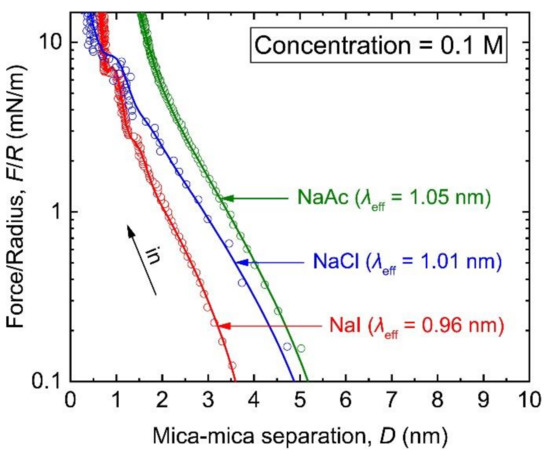
Figure 2.
Semi-log plot of F/R versus D (F/R vs. D profiles) between two mica surfaces in the electrolyte solutions at a concentration of 0.1 M. The solutes include NaI (red), NaCl (blue), and NaAc (green). The decay length (obtained by the fitting of the curves) for each solution is noted in the corresponding bracket. The label “in” denotes the surface approach.

For the NaCl and NaAc solutions, the EDL regions slightly deviated from the DLVO theory; the decay length was 1.01 ± 0.07 nm for NaCl and that was 1.05 ± 0.08 nm for NaAc. The range of the EDL region was even found to extend to above 5 nm in the NaAc solution, which is at least 1 nm longer than that with NaI. In the NaCl solution, two structural oscillations were observed at distances below ~2 nm, each with a distribution width of up to 0.39 ± 0.03 nm and a film thickness changes (∆D) of 0.3 nm. As shown in Table 2, these values are slightly smaller than the diameter of hydrated Na+ ion. Therefore, beside the possible contribution from the water molecules, these jump-in events can also be attributed to the push out of the partially dehydrated Na+ ions, or the dehydration of the cations upon confinement [30]. In comparison, at the same distances in the solution with NaAc, the structural hydration with the jump-in event was not present. Instead, a steric hydration repulsion with an exponential decay length of 0.24 ± 0.03 nm was observed until the end of the measurement.

Table 2.
Specifications of cations and anions discussed in this work.
Interestingly, in the NaCl and NaAc solutions, the diffuse double layer formed at each mica surface had the characteristic lengths of up to ~11 % longer than those predicted by the DH theory. Israelachvili and colleagues have treated the ionic liquids as diluted solution [16,17], from which they found that the EDL force had a decay length that was larger than expected. Since then, the long-range EDL force has been regarded as a common issue in the studies that are caused not only by pure ionic liquids, but also by concentrated electrolyte solutions. The AFM experiments have shown unexpected long-range EDL repulsion in water containing multivalent ions at low concentrations at mM levels [25,49]. For a monovalent electrolyte, the concentration that can trigger such a peculiar phenomenon is relatively high, as has been observed in the literature in NaCl solution at concentrations of above 1 M [9]. Likewise, our results revealed that the critical concentration for monovalent NaCl and NaAc to introduce a minor deviation from the traditional theory could be as low as 0.1 M. In contrast, the results obtained from the NaI solution was in accordance with the common expectation. These findings indicate that the anionic coion may exert a specific effect on the properties of EDL in 1:1 electrolyte solution.
3.2. Intersurface Forces in Monovalent Solutions at 1−3 M
It is well known that the properties of an interfacial structure can vary with ionic concentration. When the concentration was increased to 1 M, the structure can enter into the “solidification” state [29]. As shown in Figure 3a, the interfacial structure at the water/mica interface was further compressed in 1 and 3 M NaI solution. Without any structural oscillation, the intersurface forces were only observable below ~1 nm, and increased steeply with decreasing distance. After being subtracted from the vDW force, these repulsive forces were fitted with decay lengths of 0.31 ± 0.02 nm and 0.27 ± 0.03 nm at concentrations of 1 and 3 M, respectively. It can be noticed that these observable forces are in the range in which strong hydration effect is usually dominated, and the decay length of 0.2–0.4 nm is the characteristic of the hydration force [56]. Moreover, the Debye length in the monovalent solution at 3 M is predicted to be 0.17 nm, which is close to the resolution limit of our apparatus. Therefore, we are inclined to believe that the repulsion attributes to the steric hydration force, at least in the 3 M NaI solution, whereas the diffuse double layer is too restricted to be detected. Based on the SFA results of the three NaI solutions, we may conclude that the DLVO theory can validly describe the interactions in the EDL region via the superposition of the vdW attraction and the EDL repulsion. With respect to the inner region, the hydration effect is the primary factor that overpowers other forces, preventing the achievement of a primary adhesive minimum between surfaces, as predicted by the DLVO theory. When the salt concentration reached 3 M, only the Stern layer composed of hydrated species could be measured, while the structure was shrunk to the distances below ~1 nm.
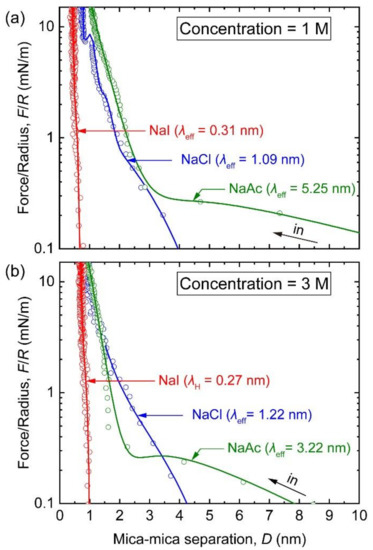
Figure 3.
Semi-log plot of F/R versus D (F/R vs. D profiles) between two mica surfaces in the electrolyte solutions at concentrations of (a) 1 M and (b) 3 M. The solutes include NaI (red), NaCl (blue), and NaAc (green). The decay length for each solution is noted in the corresponding bracket.
In NaCl solutions at 1 M, the long-range repulsion was located at the distances that were much larger than anticipated, which suggests the complete failure of the DLVO theory. After subtracting the vdW force from the profile, this force was apparent at distances of above 1.6 ± 0.3 nm, which is where the onset plane of diffuse EDL was placed. The decay length was fitted to be 1.09 ± 0.11 nm, which is about 3.6 times of the magnitude of the Debye length. Unexpectedly, the deviation was more pronounced in 1 M NaAc solution, when a long tail was found to extend to the distances of above 10 nm with a decay length that was 16 fold (5.25 ± 0.37 nm) larger than the Debye length. Comparing the results from the two solutions, it is apparent that the repulsive force in the EDL region was weaker in NaAc, while the transition plane between the EDL and the hydration regions shifted outwards by ~3 nm. Together with the very long-range EDL region found in NaAc, we may conjecture that the mechanisms behind the intersurface forces measured in these two solutions are different.
In the inner hydration region, the hydration effect strongly dominated other interactions in both solutions. With decreasing distance, the hydration force measured in the NaCl solution increased over two oscillations and with a subtle jump-in in between. The oscillation widths were less than those measured at 0.1 M and were narrower as the distance decreased, indicating that there was possibly dehydration of ions at higher concentrations. Once the external load was increased so that it was strong enough to break the stability of a hydration layer, a jump-in occurred at D = 1.0 ± 0.1 nm, and the ∆D of 0.3 ± 0.1 nm is close to that measured at a concentration of 0.1 M. After the jump-in, the two approaching mica surfaces were in contact at the DH of 0.6 ± 0.1 nm. In the NaAc solution, the steric hydration force showed a decay length of 0.47 ± 0.03 nm starting from 4.6 ± 0.3 nm, and an additional decay length of 0.18 ± 0.02 nm at D < 1.3 ± 0.1 nm. The observation of the hydration force with two decay lengths indicated again that the cations gradually lost their hydration shells at small distances under high compression [57,58]; the large decay length farther away from the interface corresponds to a layer of fully hydrated Na+ ions outside the innermost structure.
When the concentration of NaCl or NaAc was increased to 3 M (Figure 3b), the long-range repulsions observed in the 1 M solution remained present. A slightly increased decay length of 1.22 ± 0.08 nm was observed in 3 M NaCl solution. Like the results at 1 M, the repulsion in NaAc was obviously weaker than that in NaCl. Nevertheless, the characteristic decay length extracted from the profile was found to decrease to 3.22 ± 0.22 nm, rather than being increased; the onset repulsive force was found to move inwards to 2.3 ± 0.3 nm, which is ~2 nm shorter than that at 1 M. At below ~2 nm, the surfaces were driven into the hydration region, where the jump-in was not detected (even in NaCl solution). The decay lengths were measured to be 0.30 ± 0.02 nm for NaCl and 0.28 ± 0.02 nm for NaAc; the difference between them were negligible. Finally, the profile reached the closet contact at the DH of 0.7 ± 0.2 nm in NaCl and at a larger distance of 0.9 ± 0.1 nm in NaAc.
3.3. Ion Pairing in Highly Concentrated Solution
As a widely accepted mechanism for the long-range EDL repulsion in aqueous solution, neutral ion pairing has been proposed in many studies on electrolyte solutions, especially for those using partly dissociated salts [16,25,59]. When the DH theory is valid, the measured decay lengths in 1 M concentration require 76 mM NaCl and 3 mM NaAc, which are correspondent to their dissociation degrees of 8% and 0.3%, respectively. Given that weakly acidic anions of acetate usually undergo hydrolysis in water, we believe that this is evidence of the actual dissociation level of the electrolytes. The hydrolysis of Ac− follows the following reaction (the reaction depends on the base equilibrium constant (Kb) of Ac− which equals 5.6 × 10−10):
where [HAc], [OH−], and [Ac−] are the concentrations of acetic acid, hydroxyl groups, and acetate groups, respectively, after the reaction reaches equilibrium. The pH measurements performed at a concentration of 1 M gave a value of 8.7 for NaAc, while that of NaI and NaCl was 6.4 and 6.8, respectively, from which we can evaluate the OH− concentrations in the solutions. Assuming that the OH− ions in the solution are solely from the hydrolysis process, the concentration of free Ac− ions estimated based on Equation (4) was 45 mM, which infers that only 4.5% or a bit more of NaAc salt has been initially dissociated. The Debye length of the 1:1 electrolyte at this concentration is 1.42 nm, which is too short compared to the measured decay length in 1 M NaAc solution, somehow close to that measured in 1 M NaCl solution.
The anions used here belong to the Hofmeister series, which are typically divided into two groups: kosmotropes (e.g., Ac−), which are usually regarded as the structure makers; and chaotropes (e.g., I−), which are usually regarded as the structure breakers [45]. While the former has higher charge density, thus can provide stronger electrostatic interactions with surrounding water to build its own network, the latter has no such ability. In regard to Cl−, it is located at the middle position in the series to separate the two groups. As the effect of an ion on water structure determines its affinity with another one with the opposite charge to form an ion pair [36,54], we attempted to apply the LMWA to evaluate the potential ion pairing that may occur in the solutions. According to the LMWA, a small difference between the hydration enthalpy of Ac− and that of Na+ (both are kosmotropes; their positive Jones-Dole viscosity B coefficients are shown in Table 2) should allow them to strongly interact with each other; such strong interactions can lead to a higher probability for the ion pairing to occur in the solution, compared with the cases of using the other two anions. In contrast, high activity is expected in NaI solution due to the mismatch in hydration energy between the cation and the anion. That is to say, the affinity of the anions with Na+ cation to form ion pairs are expected to be in the order of Ac− > Cl− > I−. Accordingly, their magnitudes of decay lengths should follow the same order. This would explain why the NaCl solution at 1 M exhibited a decay length that was comparable but somewhat shorter compared to the Debye length predicted from the hydrolysis of NaAc solution, as the former solute has a greater tendency to dissociate.
As the concentration was increased from 1 M to 3 M, the decay length in the NaCl solution was observed to be increased. Although this trend contradicts the prediction by DH theory, it has been reported by earlier studies on highly concentrated solutions [9,22,23]. The theoretical calculation specifically considering the SFA mechanism has shown that the spatial confinement makes the formation of massive ion pairs become favorable even in a simple NaCl solution in highly concentrated condition [19]. During the salt dissociation, the lattice expansion will be suppressed under high confinement, leading to the positive shifting of free energy of dissociation. As the solute concentration increases above a certain value (which is 0.4 M reported in Ref. [19]), the content of total ions in NaCl solution that participate in the screening of surface charge will experience a dramatic decrease, when the decay length is expected to increase. Therefore, the long-range repulsions observed here can be rationalized by the effect of ion pairing to some extent. However, note that results from the NaAc solutions were not the same as that from the NaCl solutions; the decay length was decreased as the concentration was increased to 3 M, rather than being increased. It is obvious that the effect of ion pairing alone is not sufficient to cause such extraordinary intersurface forces as well as structural properties in the NaAc solutions, and other factors should be further explored.
3.4. Hydrodynamic Effect
The specific effect of the anion on water structuring may influence not only the properties of adsorbed layer at the interface, but also the hydrodynamic behavior of bulk water at a relatively high concentration. As the force-probe techniques are usually performed dynamically, the hydrodynamic property of a solution is a common issue in the understanding of the interactions in the confined film [60,61]. According to the semi-empirical Jones–Dole equation, the relative viscosity of the solution is mainly dominated by the term with the B coefficient at a concentration of above 0.05 M, which represents the interaction between solute ion and solvent [62]. Among three monovalent anions, only Ac− has a positive B-coefficient [55]; this shows that its contribution to the viscosity of solution is more significant. Previous studies have demonstrated that the viscosities of the solutions discussed here follows the same order as that of the B-coefficients of the anions at room temperature [63,64]. At an elevated concentration, the viscosity of most aqueous solutions will experience an accelerated increase when another term that scales with the square of the concentration should be added in the Jones-Dole equation [62]. At ~1 M, the viscosity of NaAc solution is only 27% higher than that of NaCl (1.401 vs. 1.104 mPa s); however, it is about double when the concentration is increased to ~3 M (2.948 mPa s vs. 1.388 mPa s) [64]. As the viscous force usually dominates the distances of above 10 nm, the weakly long-range repulsion measured in NaAc is probably caused in part by the hydrodynamic effect of the concentrated electrolytes.
To figure out the hydrodynamic effect on the measured interactions in the NaAc solutions, we introduced an asymmetrical electrolyte Na2SO4 with oxoanion SO42−. The B-coefficient of SO42− is similar to that of Ac−, while its hydration enthalpy is much higher (see Table 2). To maintain the ionic strength, which directly determines the Debye length, we set the concentrations of Na2SO4 to 0.33 and 1 M (the corresponding concentrations in the 1:1 electrolyte solutions are 1 and 3 M, respectively). The viscosities of Na2SO4 solutions are ~1.163 mPa s at ~0.33 M and 1.574 mPa s at ~1 M [64]. As shown in Figure 4, the F/R vs. D profiles measured between mica surfaces in the Na2SO4 solutions were remarkably reminiscent of those in the NaAc solutions. At 0.33 M, the long-range and weak repulsion at above 10 nm had a decay length of 5.16 ± 0.10 nm and an interaction constant Z value of 2.4 ± 0.2 pN, both close to that in 1 M NaAc solution. When the solute concentration was increased to 1 M, the long-range repulsion was suppressed to ~6 nm, and the decay length was also reduced to 3.25 ± 0.14 nm. In the hydration region, only steric hydration forces were observed, of which the decay lengths were measured to be 0.36 ± 0.03 nm at 0.33 M and 0.48 ± 0.03 nm at 1 M. Compared with those obtained from the monovalent solutions, these large decay lengths indicate in the presence of divalent SO42− that the Na+ ions at the interface were in a nearly complete hydrated state. Given that Ac− and SO42− have similar viscosities, the hydrodynamic effect, i.e., the viscosity effect, was further examined by multiplying the approach velocity of the surfaces by 3. As shown by the red curves in Figure 4, the repulsions in the outer region were extended to longer distances at both concentrations. The repulsion strength was also stronger as the Z increased by a factor of 1.5 at the concentration of 0.33 M and by 2.1 at the concentration of 1 M. These results confirm that the hydrodynamic effect of anions contributes to the intersurface forces between two negatively charged surfaces at distances as low as a few nanometers. Further comparing the results from the NaI and NaCl solutions, the absence of the hydrodynamic effect in these solutions highlights the unique and universal contribution from the anionic kosmotropes. Nevertheless, the prediction of the level of the hydrodynamic effect cannot simply rely on the bulky viscosity of a certain solute, since the concentration of Na2SO4 (which was used to replicate the force-distance profile of NaAc solution), as well as its viscosity, was lower than that of NaAc at the same ionic strength. This indicates that, at the interface, the specific properties of kosmotropic anions, such as hydration energy, hydrated size, and charge density, may play the dominant roles.
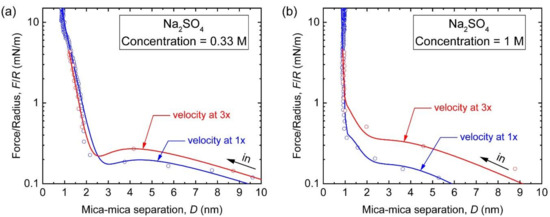
Figure 4.
F/R vs. D profiles (semi-log plots) measured between two mica surfaces during approaching in the Na2SO4 solutions. The concentrations of Na2SO4 are (a) 0.33 M and (b) 1 M. The surfaces were driven at 1× velocity (blue; motor speed = ~300 rpm) to 3× velocity (red; motor speed = ~900 rpm).
3.5. Anion Effect on Water Structuring
To date, several factors have been taken into account to understand the properties of the EDL formed in the examined electrolyte solutions. The main mechanisms are all correlated with the hydration effect of anion. For this reason, we further employed Raman spectra to observe the structuring of the hydrogen bond in the water network in four Na+ solutions. As shown in Figure 5a, the Raman spectrum corresponding to the OH stretching of ultrapure water was observed at 2800–3800 cm−1. Five gaussian sub-bands can be deconvoluted from the spectrum, which are centered at 3014, 3226, 3432, 3572, and 3636 cm−1, respectively. As has been reported previously [47], these bands represent different types of hydrogen bond networks. We found that the double donor-double acceptor (DDAA) centered at 3226 cm−1 and the single donor-single acceptor (DA) centered at 3432 cm−1, which have tetrahedral structure and chains structure of water, respectively [65,66], were consistently the dominant types in the four solutions. The ratio of the intensity at 3226 cm−1 measured in four solutions (NaCl, NaI and NaAc at 1 M, and Na2SO4 at 0.33 M) to that at 3432 cm−1 was plotted in Figure 5b. The results showed that the ratio decreased in the following order: SO42− > Ac− > Cl− > I−, which is in agreement with previous reports [67,68]. The order indicates that among all four anions, I− and Cl− can best destroy the configuration of the tetrahedral water. With that, the percentage of chain structure in the network is expected to increase, and the probability for the hydrogen bonded water to form local clusters is expected to decline. Generally, the water network becomes disordered in the presence of the structure breakers, e.g., I− and Cl−. Thus, the drainage of electrolyte ions and water molecules requires low energy, which is correspondent with the low viscosities of the corresponding Na+ solutions. On the other hand, the increased and stable water structure in the NaAc and Na2SO4 solutions is necessary for the long-range hydrodynamic effect to become effective.
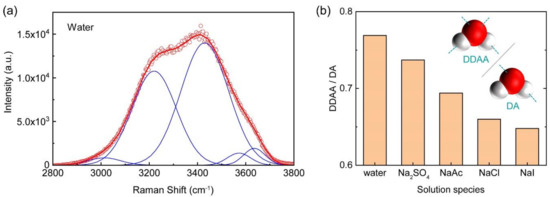
Figure 5.
(a) Raman spectrum showing the OH stretching vibration of ultrapure water (red), which is fitted with five Gaussian sub-bands (blue). (b) Anion effect on water structure of the sodium solutions. The y-axis is the ratio of the intensity of the peak at 3226 cm−1 (highest intensity) to that of the peak at 3432 cm−1. The concentration of the 1:1 electrolyte was 1 M, and that of the 2:1 electrolyte was 0.33 M.
According to the theoretical model for ionic liquids and molten salts [43], ion correlation and finite ion-size lead to “overscreening” and “crowding” in the Stern layer. The occurrence of these two phenomena is also determined by the magnitude of surface potential. As measured by AFM, the absolute surface potential of silica in NaCl solution at a concentration above 1 M is less than 2 mV, indicating that there is a possibility of charge reversion or neutralization [69]. More recently, Gaddam and Ducker [70] have investigated the screening behavior in highly concentrated NaCl and LiCl solutions by monitoring the ion density in a thin film that is confined between two silica surfaces. They observed that the decay length of the EDL structure increases with increasing salt concentration. Meanwhile, the charge inversion of the silica surface was found to occur at a concentration of above 2 M, which was attributed to the overscreening effect. Compared to silica, the mica plane can be more negatively charged at the pH values considered here [71]. It is given that the surface charge density of mica surface in aqueous solution in its fully dissociated state can reach −0.3 C/m2 [72]. Thus, it also provides a substrate that is apt to crowding the electrolyte cations near the surface. For all the three 1:1 electrolyte solutions, higher pH value (in the basic range) in the NaAc solution, due to the hydrolysis of Ac− ions, corresponds to more negative charges on the mica surface. Despite the use of identical cations (Na+) in this study, we did observe a relatively large hardwall distance in NaAc solution at each concentration (see Table 1), which is clear evidence for a strong crowding effect.
Anyway, either the overscreening effect or crowding effect can result in a higher number of both cations and anions while attaching to the surface compared with that observed in a conventional colloid system. The subsequent formation of alternative cation/anion layers can be responsible for the increased decay length of the EDL region in concentrated solutions [33], aside from the contribution of ion-pairing. Moreover, with ions accumulating on the surface to form a wide Stern region, there is also a reduction of ion density in bulk solution that can contribute to the reappearance of the diffuse layer under certain conditions [44]. As the concentration was being further elevated, less anions could stay in the diffuse layer due to the electrolyte dilution along with the underscreening effect. The loss of strongly hydrated species in the region farther away from the surface might cause the diminishing of the hydrodynamic effect and so its working distance. This may provide one probable explanation for why the range of repulsive force in the NaAc or the Na2SO4 solution became shorter when the electrolyte concentration was increased from a moderate level to the highest level, as shown in Figure 3 and Figure 4. Regarding the innermost Stern layer, we think that the impact of anions on the structural properties should be more significant than expected when they are in close contact with the surrounding water molecules and hydrated cations. Experimental data have indicated that bound cations and residual anions share their hydration shells on the mica surface [39]. Here, with identical cations, the anion, which has strong ability to build water structure, may assist the inner cation to maintain a complete hydration field, enhancing the energy required for dehydration. This is supported by the fact that at a concentration lower than 3 M, the order of the decay lengths fitted in the hydration region (λH) follows that of the hydration enthalpies of anions. This correlation between hydration strength of anion and decay length of hydration force is similar to that involving kosmotropic cations [58,73]. In the presence of Ac− and SO42− ions, Na+ ions with strong hydration shells cannot strongly bind to the mica surface, causing them to be easily squeezed out. Consequently, the monotonical and continuous repulsion rather than the structural oscillation dominated the hydration region in the solutions with these two anions. However, it is worth noting that the dependence of the hydration structure on anion hydration may not work at a higher electrolyte concentration. When the concentration was increased to 3 M, the structural oscillation was consistently absent in any solution. The difference in the decay length of steric hydration force of the three 1:1 electrolyte solutions was more subtle, which varied around 0.3 nm that is comparable to the size of the partially dehydrated cation layer. This is possibly due to the formation of numbers of ion pairs between the opposite charged ions in the Stern layer, followed by the release of their individual hydration shells [74]. On the other hand, we also notice that the λH of ~0.3 nm obtained in each solution has been assigned to interfacial water in several works in the literature [33,37,56]. However, we cannot figure out the major component in the hydration layer from the candidate hydrated ion and water molecule at this moment. Considering that using different anions could not lead to a considerable difference in this region, we believe that distinguishing the dominant contribution is beyond the scope of this work, and is worth future investigation.
4. Conclusions
In summary, we measured the interactions between mica surfaces in aqueous sodium solutions containing various Hofmeister anions, which possess specific hydration properties. When the weakly hydrated I− ions were present in solution, no impact on the highly suppressed EDL that was caused by the adsorption of concentrated cations was observed. The measured forces can be described by the extended DLVO model, which is integrated with the hydration terms for the near-surface hydration region. After switching to the ions with higher water affinity, i.e., Cl− or Ac−, a long-range repulsion was present, of which the decay length could reach 5.25 nm in 1 M NaAc solution. Due to a good match in the hydration enthalpies between these anions and sodium cation, this repulsion at high concentrations was probably due to the ion pairing of cation–anion, which might become favorable in confined solutions. Aside from the ion pairing, the extraordinary deviation from the traditional theory in the case with strongly hydrated Ac− was also found to be contributed by the hydrodynamic effect. This effect was confirmed in the divalent solutions with SO42− at relatively low concentrations. As was further indicated by the Raman spectrum, the ability of the examined anions to restructure the water network followed the rank described in the Hofmeister series: the water structuring in the presence of kosmotropic SO42− and Ac− was more stable than that in the presence of Cl− and I−. Accordingly, the ions that accumulated on the surface could possess complete hydration shells with the help of strongly hydrated anions, generating a steric hydration force in the hydration region. In comparison, the presence of weakly hydrated anions caused less ordered water structure and facile ion dehydration, leading to structural oscillation. Therefore, we can conclude that the EDL of mica surface is affected by the anionic coions from the highly concentrated electrolytes, the significance of which mainly depends on their specific hydration and capabilities to restructure water. As mica is a typical metal oxide, the conclusion from this work should be applicable to a wide variety of water/solid interfaces, which needs further confirmation. For practical use, the findings may remind people of the ionic specific effects in the development of new EDL-based nanotechnologies, especially those that are executed in confined spaces, such as the nanochannel-based power generator and DNA sequencing [75,76].
Author Contributions
Conceptualization, Y.K.; methodology, Y.K.; validation, Y.K., Q.Y. and X.Z.; formal analysis, Q.Y. and X.Z.; investigation, Y.K., Q.Y. and X.Z.; resources, Y.K.; data curation, Y.K. and Q.Y.; writing—original draft preparation, Y.K.; writing—review and editing, Q.Y. and Y.Z.; supervision, Y.K.; project administration, Y.K.; funding acquisition, Y.K. and Y.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (51605090), the Fundamental Research Funds for the Central Universities (2242019k1G011), and the Natural Science Foundation of Jiangsu Province (BK20180400, BK20160670).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Israelachvili, J.N. Intermolecular and Surface Forces; Academic Press: Cambridge, MA, USA, 2011; p. 704. [Google Scholar]
- Madsen, F.T.; Müller-Vonmoos, M. The swelling behaviour of clays. Appl. Clay Sci. 1989, 4, 143–156. [Google Scholar] [CrossRef]
- Peukert, W.; Schwarzer, H.-C.; Stenger, F. Control of aggregation in production and handling of nanoparticles. Chem. Eng. Process. Process. Intensif. 2005, 44, 245–252. [Google Scholar] [CrossRef]
- Poortinga, A.T.; Bos, R.; Norde, W.; Busscher, H.J. Electric double layer interactions in bacterial adhesion to surfaces. Surf. Sci. Rep. 2002, 47, 1–32. [Google Scholar] [CrossRef]
- Conway, B.E. Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications; Springer: New York, NY, USA, 1999. [Google Scholar]
- Oren, Y. Capacitive deionization (CDI) for desalination and water treatment—Past, present and future (a review). Desalination 2008, 228, 10–29. [Google Scholar] [CrossRef]
- Yuqing, M.; Jianguo, G.; Jianrong, C. Ion sensitive field effect transducer-based biosensors. Biotechnol. Adv. 2003, 21, 527–534. [Google Scholar] [CrossRef]
- Sharma, P.; Bhatti, T.S. A review on electrochemical double-layer capacitors. Energy Convers. Manag. 2010, 51, 2901–2912. [Google Scholar] [CrossRef]
- Smith, A.M.; Lee, A.A.; Perkin, S. The Electrostatic Screening Length in Concentrated Electrolytes Increases with Concentration. J. Phys. Chem. Lett. 2016, 7, 2157–2163. [Google Scholar] [CrossRef]
- Al-Shara, N.K.; Sher, F.; Yaqoob, A.; Chen, G.Z. Electrochemical investigation of novel reference electrode Ni/Ni(OH)2 in comparison with silver and platinum inert quasi-reference electrodes for electrolysis in eutectic molten hydroxide. Int. J. Hydrog. Energy 2019, 44, 27224–27236. [Google Scholar] [CrossRef]
- Al-Shara, N.K.; Sher, F.; Iqbal, S.Z.; Sajid, Z.; Chen, G.Z. Electrochemical study of different membrane materials for the fabrication of stable, reproducible and reusable reference electrode. J. Energy Chem. 2020, 49, 33–41. [Google Scholar] [CrossRef]
- Al-Shara, N.K.; Sher, F.; Iqbal, S.Z.; Curnick, O.; Chen, G.Z. Design and optimization of electrochemical cell potential for hydrogen gas production. J. Energy Chem. 2020, 52, 421–427. [Google Scholar] [CrossRef]
- Stern, O. The theory of the electrolytic double shift. Z. Elektrochem. Angew. Phys. Chem. 1924, 30, 508–516. [Google Scholar]
- Smith, A.M.; Borkovec, M.; Trefalt, G. Forces between solid surfaces in aqueous electrolyte solutions. Adv. Colloid Interface Sci. 2020, 275, 102078. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Zhang, J.; Gong, L.; Zeng, H. Surface forces and interaction mechanisms of soft thin films under confinement: A short review. Soft Matter 2020, 16, 6697–6719. [Google Scholar] [CrossRef] [PubMed]
- Gebbie, M.A.; Valtiner, M.; Banquy, X.; Fox, E.T.; Henderson, W.A.; Israelachvili, J.N. Ionic liquids behave as dilute electrolyte solutions. Proc. Natl. Acad. Sci. USA 2013, 110, 9674–9679. [Google Scholar] [CrossRef]
- Gebbie, M.A.; Dobbs, H.A.; Valtiner, M.; Israelachvili, J.N. Long-range electrostatic screening in ionic liquids. Proc. Natl. Acad. Sci. USA 2015, 112, 7432–7437. [Google Scholar] [CrossRef]
- Gebbie, M.A.; Smith, A.M.; Dobbs, H.A.; Lee, A.A.; Warr, G.G.; Banquy, X.; Valtiner, M.; Rutland, M.W.; Israelachvili, J.N.; Perkin, S.; et al. Long range electrostatic forces in ionic liquids. Chem. Commun. (Camb.) 2017, 53, 1214–1224. [Google Scholar] [CrossRef]
- Huang, J. Confinement Induced Dilution: Electrostatic Screening Length Anomaly in Concentrated Electrolytes in Confined Space. J. Phys. Chem. C 2018, 122, 3428–3433. [Google Scholar] [CrossRef]
- Kjellander, R. Nonlocal electrostatics in ionic liquids: The key to an understanding of the screening decay length and screened interactions. J. Chem. Phys. 2016, 145, 124503. [Google Scholar] [CrossRef]
- Kjellander, R. Decay behavior of screened electrostatic surface forces in ionic liquids: The vital role of non-local electrostatics. Phys. Chem. Chem. Phys. 2016, 18, 18985–19000. [Google Scholar] [CrossRef]
- Lee, A.A.; Perez-Martinez, C.S.; Smith, A.M.; Perkin, S. Underscreening in concentrated electrolytes. Faraday Discuss. 2017, 199, 239–259. [Google Scholar] [CrossRef]
- Lee, A.A.; Perez-Martinez, C.S.; Smith, A.M.; Perkin, S. Scaling Analysis of the Screening Length in Concentrated Electrolytes. Phys. Rev. Lett. 2017, 119, 026002. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Cabello, F.J.; Trefalt, G.; Maroni, P.; Borkovec, M. Accurate predictions of forces in the presence of multivalent ions by Poisson-Boltzmann theory. Langmuir 2014, 30, 4551–4555. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Maroni, P.; Trefalt, G.; Borkovec, M. Unexpectedly Large Decay Lengths of Double-Layer Forces in Solutions of Symmetric, Multivalent Electrolytes. J. Phys. Chem. B 2019, 123, 1733–1740. [Google Scholar] [CrossRef] [PubMed]
- Pashley, R.M. Hydration forces between mica surfaces in aqueous electrolyte solutions. J. Colloid Interface Sci. 1981, 80, 153–162. [Google Scholar] [CrossRef]
- Israelachvili, J.N.; Pashley, R.M. Molecular Layering of Water at Surfaces and Origin of Repulsive Hydration Forces. Nature 1983, 306, 249–250. [Google Scholar] [CrossRef]
- Cheng, L.; Fenter, P.; Nagy, K.L.; Schlegel, M.L.; Sturchio, N.C. Molecular-Scale Density Oscillations in Water Adjacent to a Mica Surface. Phys. Rev. Lett. 2001, 87, 156103–156104. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Marzal, R.M.; Drobek, T.; Balmer, T.; Heuberger, M.P. Hydrated-ion ordering in electrical double layers. Phys. Chem. Chem. Phys. 2012, 14, 6085–6093. [Google Scholar] [CrossRef]
- Zachariah, Z.; Espinosa-Marzal, R.M.; Spencer, N.D.; Heuberger, M.P. Stepwise collapse of highly overlapping electrical double layers. Phys. Chem. Chem. Phys. 2016, 18, 24417–24427. [Google Scholar] [CrossRef]
- Zachariah, Z.; Espinosa-Marzal, R.M.; Heuberger, M.P. Ion specific hydration in nano-confined electrical double layers. J. Colloid Interface Sci. 2017, 506, 263–270. [Google Scholar] [CrossRef]
- Kilpatrick, J.I.; Loh, S.H.; Jarvis, S.P. Directly probing the effects of ions on hydration forces at interfaces. J. Am. Chem. Soc. 2013, 135, 2628–2634. [Google Scholar] [CrossRef]
- Baimpos, T.; Shrestha, B.R.; Raman, S.; Valtiner, M. Effect of interfacial ion structuring on range and magnitude of electric double layer, hydration, and adhesive interactions between mica surfaces in 0.05-3 M Li(+) and Cs(+) electrolyte solutions. Langmuir 2014, 30, 4322–4332. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Fenter, P.; Nagy, K.L.; Sturchio, N.C. Monovalent ion adsorption at the muscovite (001)-solution interface: Relationships among ion coverage and speciation, interfacial water structure, and substrate relaxation. Langmuir 2012, 28, 8637–8650. [Google Scholar] [CrossRef] [PubMed]
- Ricci, M.; Spijker, P.; Stellacci, F.; Molinari, J.F.; Voitchovsky, K. Direct visualization of single ions in the Stern layer of calcite. Langmuir 2013, 29, 2207–2216. [Google Scholar] [CrossRef] [PubMed]
- Collins, K.D. Charge density-dependent strength of hydration and biological structure. Biophys. J. 1997, 72, 65–76. [Google Scholar] [CrossRef]
- Hu, Q.; Weber, C.; Cheng, H.W.; Renner, F.U.; Valtiner, M. Anion Layering and Steric Hydration Repulsion on Positively Charged Surfaces in Aqueous Electrolytes. Chemphyschem 2017, 18, 3056–3065. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Salmeron, M. An XPS and Scanning Polarization Force Microscopy Study of the Exchange and Mobility of Surface Ions on Mica. Langmuir 1998, 14, 5841–5844. [Google Scholar] [CrossRef]
- Pintea, S.; de Poel, W.; de Jong, A.E.; Vonk, V.; van der Asdonk, P.; Drnec, J.; Balmes, O.; Isern, H.; Dufrane, T.; Felici, R.; et al. Solid-Liquid Interface Structure of Muscovite Mica in CsCl and RbBr Solutions. Langmuir 2016, 32, 12955–12965. [Google Scholar] [CrossRef]
- Odelius, M.; Bernasconi, M.; Parrinello, M. Two Dimensional Ice Adsorbed on Mica Surface. Phys. Rev. Lett. 1997, 78, 2855–2858. [Google Scholar] [CrossRef]
- Gibb, B.C. Hofmeister’s curse. Nat. Chem. 2019, 11, 963–965. [Google Scholar] [CrossRef]
- Hribar, B.; Southall, N.T.; Vlachy, V.; Dill, K.A. How ions affect the structure of water. J. Am. Chem. Soc. 2002, 124, 12302–12311. [Google Scholar] [CrossRef]
- Bazant, M.Z.; Storey, B.D.; Kornyshev, A.A. Double Layer in Ionic Liquids: Overscreening versus Crowding. Phys. Rev. Lett. 2011, 106, 46102–46104. [Google Scholar] [CrossRef]
- Yochelis, A. Transition from non-monotonic to monotonic electrical diffuse layers: Impact of confinement on ionic liquids. Phys. Chem. Chem. Phys. 2014, 16, 2836–2841. [Google Scholar] [CrossRef]
- Kang, B.; Tang, H.; Zhao, Z.; Song, S. Hofmeister Series: Insights of Ion Specificity from Amphiphilic Assembly and Interface Property. ACS Omega 2020, 5, 6229–6239. [Google Scholar] [CrossRef] [PubMed]
- Israelachvili, J.; Min, Y.; Akbulut, M.; Alig, A.; Carver, G.; Greene, W.; Kristiansen, K.; Meyer, E.; Pesika, N.; Rosenberg, K.; et al. Recent advances in the surface forces apparatus (SFA) technique. Rep. Prog. Phys. 2010, 73, 036601. [Google Scholar] [CrossRef]
- Sun, Q. The Raman OH stretching bands of liquid water. Vib. Spectrosc. 2009, 51, 213–217. [Google Scholar] [CrossRef]
- Diao, Y.; Espinosa-Marzal, R.M. Molecular insight into the nanoconfined calcite-solution interface. Proc. Natl. Acad. Sci. USA 2016, 113, 12047–12052. [Google Scholar] [CrossRef]
- Montes Ruiz-Cabello, F.J.; Moazzami-Gudarzi, M.; Elzbieciak-Wodka, M.; Maroni, P.; Labbez, C.; Borkovec, M.; Trefalt, G. Long-ranged and soft interactions between charged colloidal particles induced by multivalent coions. Soft Matter 2015, 11, 1562–1571. [Google Scholar] [CrossRef] [PubMed]
- Marcus, Y. Thermodynamics of solvation of ions. Part 5—Gibbs free energy of hydration at 298.15 K. J. Chem. Soc. Faraday Trans. 1991, 87, 2995–2999. [Google Scholar] [CrossRef]
- Marcus, Y. Effect of ions on the structure of water: Structure making and breaking. Chem. Rev. 2009, 109, 1346–1370. [Google Scholar] [CrossRef]
- Caminiti, R.; Paschina, G.; Pinna, G.; Magini, M. Experimental evidence of interactions SO2−4 −H2O in an aqueous solution. Chem. Phys. Lett. 1979, 64, 391–395. [Google Scholar] [CrossRef]
- Smith, D.W. Ionic hydration enthalpies. J. Chem. Educ. 1977, 54. [Google Scholar] [CrossRef]
- Salis, A.; Ninham, B.W. Models and mechanisms of Hofmeister effects in electrolyte solutions, and colloid and protein systems revisited. Chem. Soc. Rev. 2014, 43, 7358–7377. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.B., Jr.; Strottmann, J.M.; Stellwagen, E. Prediction of neutral salt elution profiles for affinity chromatography. Proc. Natl. Acad. Sci. USA 1981, 78, 2287–2291. [Google Scholar] [CrossRef] [PubMed]
- Parsegian, V.A.; Zemb, T. Hydration forces: Observations, explanations, expectations, questions. Curr. Opin. Colloid Interface Sci. 2011, 16, 618–624. [Google Scholar] [CrossRef]
- Pashley, R.M. Hydration forces between mica surfaces in electrolyte solutions. Adv. Colloid Interface Sci. 1982, 16, 57–62. [Google Scholar] [CrossRef]
- Chapel, J.P. Electrolyte Species Dependent Hydration Forces between Silica Surfaces. Langmuir 1994, 10, 4237–4243. [Google Scholar] [CrossRef]
- Tulpar, A.; Subramanian, V.; Ducker, W. Decay Lengths of Double-Layer Forces in Solutions of Partly Associated Ions. Langmuir 2001, 17, 8451–8454. [Google Scholar] [CrossRef]
- Chan, D.Y.C.; Horn, R.G. The drainage of thin liquid films between solid surfaces. J. Chem. Phys. 1985, 83, 5311–5324. [Google Scholar] [CrossRef]
- Israelachvili, J.N. Measurement of the viscosity of liquids in very thin films. J. Colloid Interface Sci. 1986, 110, 263–271. [Google Scholar] [CrossRef]
- Jenkins, H.D.B.; Marcus, Y. Viscosity B-Coefficients of Ions in Solution. Chem. Rev. 1995, 95, 2695–2724. [Google Scholar] [CrossRef]
- Abdulagatov, I.M.; Zeinalova, A.B.; Azizov, N.D. Viscosity of Aqueous Electrolyte Solutions at High Temperatures and High Pressures. ViscosityB-coefficient. Sodium Iodide. J. Chem. Eng. Data 2006, 51, 1645–1659. [Google Scholar] [CrossRef]
- Rumble, J.R.; Lide, D.R.; Bruno, T.J. CRC Handbook of Chemistry and Physics: A Ready-Reference Book of Chemical and Physical Data, 99th ed.; John, R.R., David, R.L., Thomas, J.B., Eds.; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Head-Gordon, T.; Johnson, M.E. Tetrahedral structure or chains for liquid water. Proc. Natl. Acad. Sci. USA 2006, 103, 7973–7977. [Google Scholar] [CrossRef] [PubMed]
- Walrafen, G.E. Raman Spectral Studies of Water Structure. J. Chem. Phys. 1964, 40, 3249–3256. [Google Scholar] [CrossRef]
- Gong, Y.; Zhou, Y.; Wu, H.; Wu, D.; Huang, Y.; Sun, C.Q. Raman spectroscopy of alkali halide hydration: Hydrogen bond relaxation and polarization. J. Raman Spectrosc. 2016, 47, 1351–1359. [Google Scholar] [CrossRef]
- Nishi, N.; Nakabayashi, T.; Kosugi, K. Raman Spectroscopic Study on Acetic Acid Clusters in Aqueous Solutions: Dominance of Acid−Acid Association Producing Microphases. J. Phys. Chem. A 1999, 103, 10851–10858. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Hampton, M.A.; Nguyen, A.V. Atomic Force Microscopy Study of Forces between a Silica Sphere and an Oxidized Silicon Wafer in Aqueous Solutions of NaCl, KCl, and CsCl at Concentrations up to Saturation. J. Phys. Chem. C 2013, 117, 2113–2120. [Google Scholar] [CrossRef]
- Gaddam, P.; Ducker, W. Electrostatic Screening Length in Concentrated Salt Solutions. Langmuir 2019, 35, 5719–5727. [Google Scholar] [CrossRef]
- Nishimura, S.; Tateyama, H.; Tsunematsu, K.; Jinnai, K. Zeta potential measurement of muscovite mica basal plane-aqueous solution interface by means of plane interface technique. J. Colloid Interface Sci. 1992, 152, 359–367. [Google Scholar] [CrossRef]
- Dziadkowiec, J.; Javadi, S.; Bratvold, J.E.; Nilsen, O.; Royne, A. Surface forces apparatus measurements of interactions between rough and reactive calcite surfaces. Langmuir 2018, 34, 7248–7263. [Google Scholar] [CrossRef]
- Van Lin, S.R.; Grotz, K.K.; Siretanu, I.; Schwierz, N.; Mugele, F. Ion-Specific and pH-Dependent Hydration of Mica-Electrolyte Interfaces. Langmuir 2019, 35, 5737–5745. [Google Scholar] [CrossRef]
- Marcus, Y.; Hefter, G. Ion pairing. Chem. Rev. 2006, 106, 4585–4621. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Graf, M.; Liu, K.; Ovchinnikov, D.; Dumcenco, D.; Heiranian, M.; Nandigana, V.; Aluru, N.R.; Kis, A.; Radenovic, A. Single-layer MoS2 nanopores as nanopower generators. Nature 2016, 536, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Ai, Y.; Qian, S. Electrokinetic particle translocation through a nanopore. Phys. Chem. Chem. Phys. 2011, 13, 4060–4071. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).