Coating Process Parameters and Structural Properties of the Tubular Electrodes of Fuel Cells Based on a Self-Made Coating Device
Abstract
1. Introduction
2. Materials and Equipment
2.1. Main Raw Materials and Equipment
2.2. Coating Device
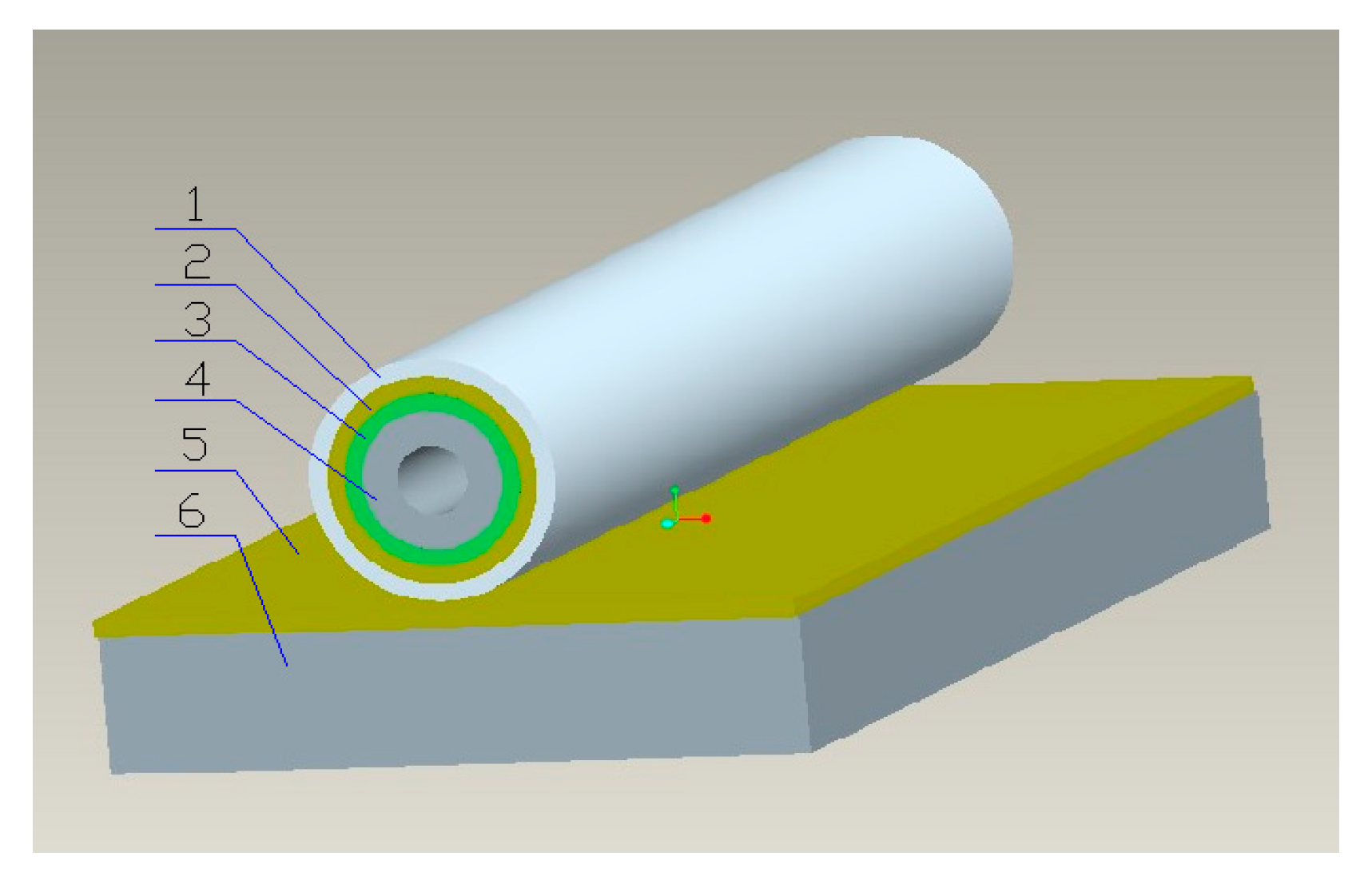
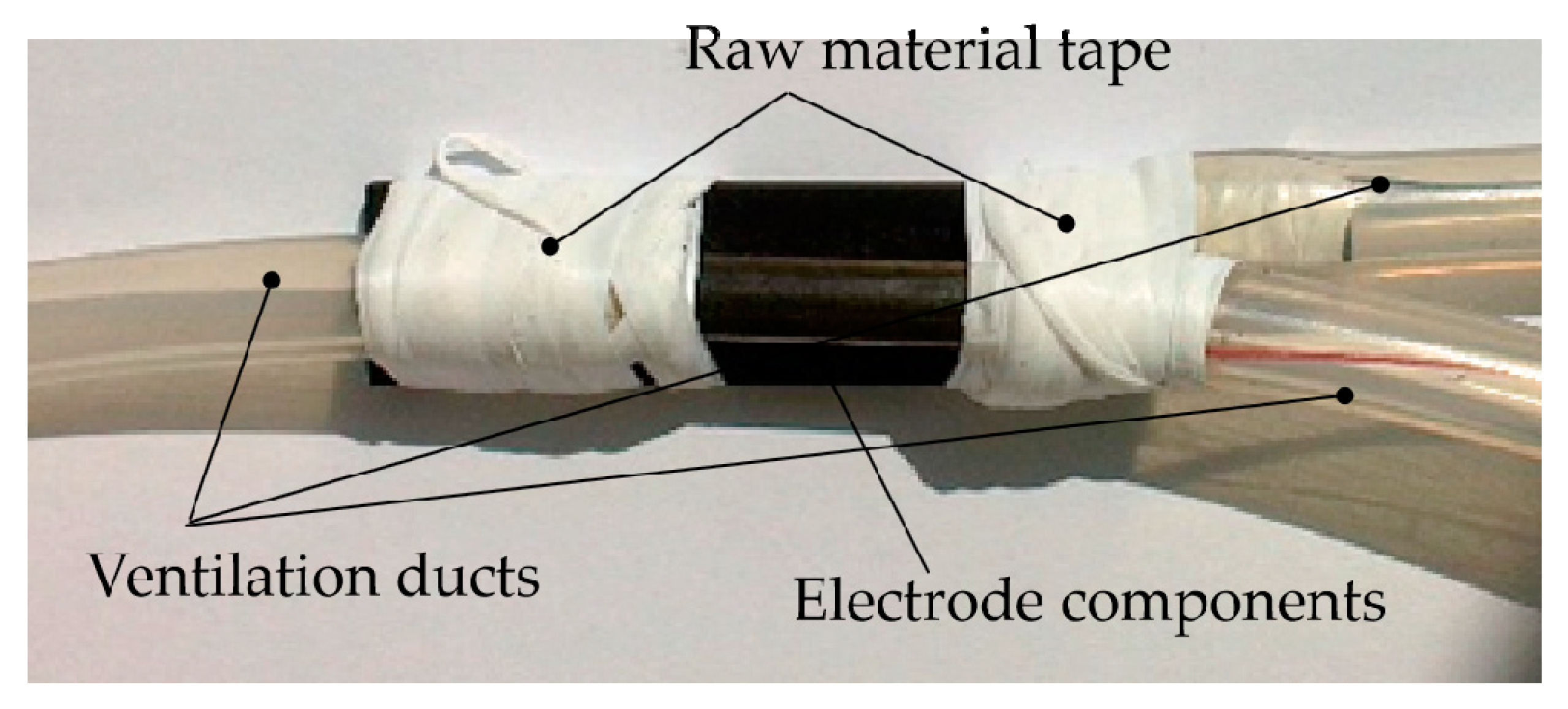
2.2.1. The Structure of the Tubular Electrode
2.2.2. Design of the Coating Device
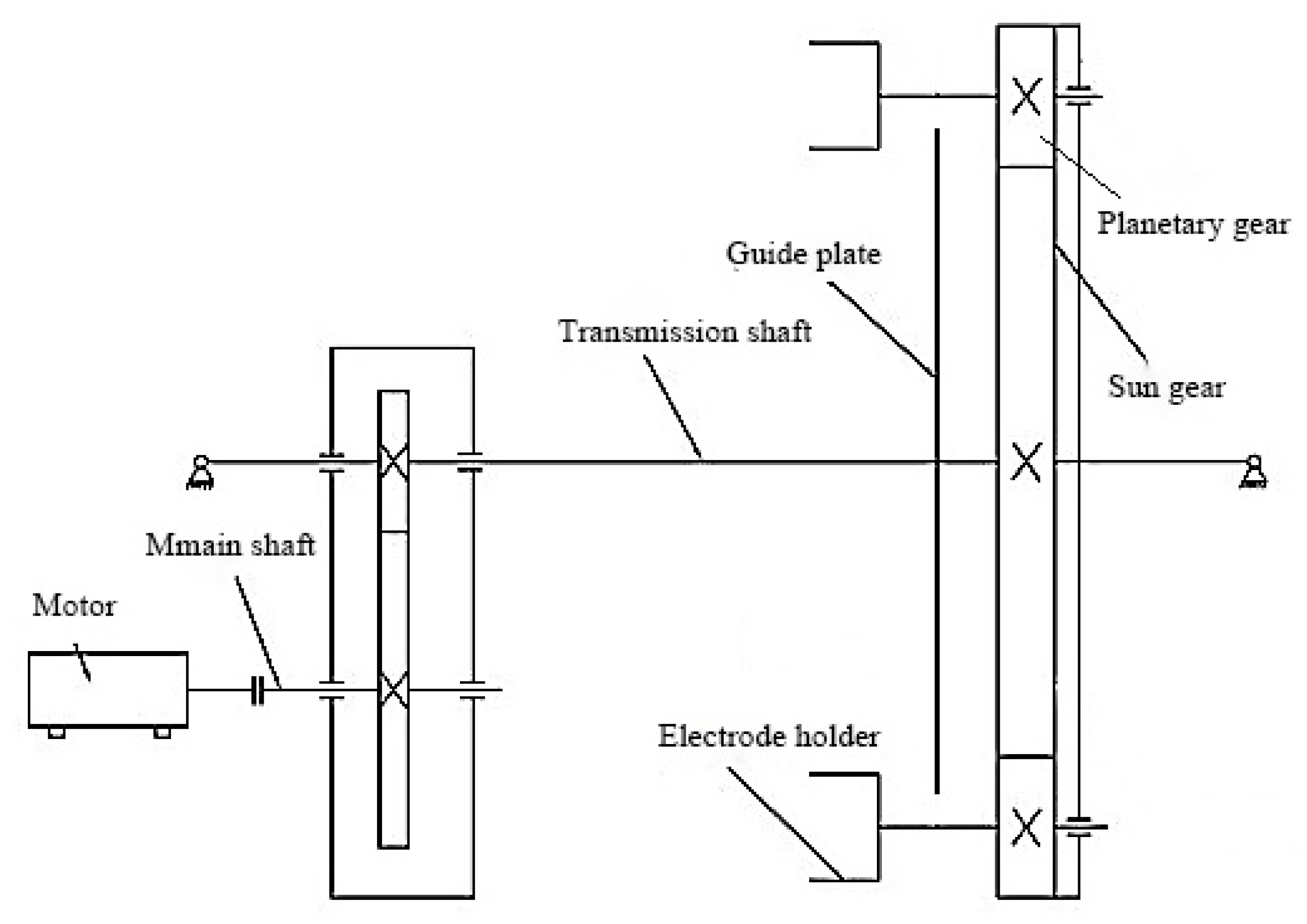
2.2.3. Transmission of the Coating Device
3. Experiments and Results
3.1. Optimization of the Coating Process Parameters
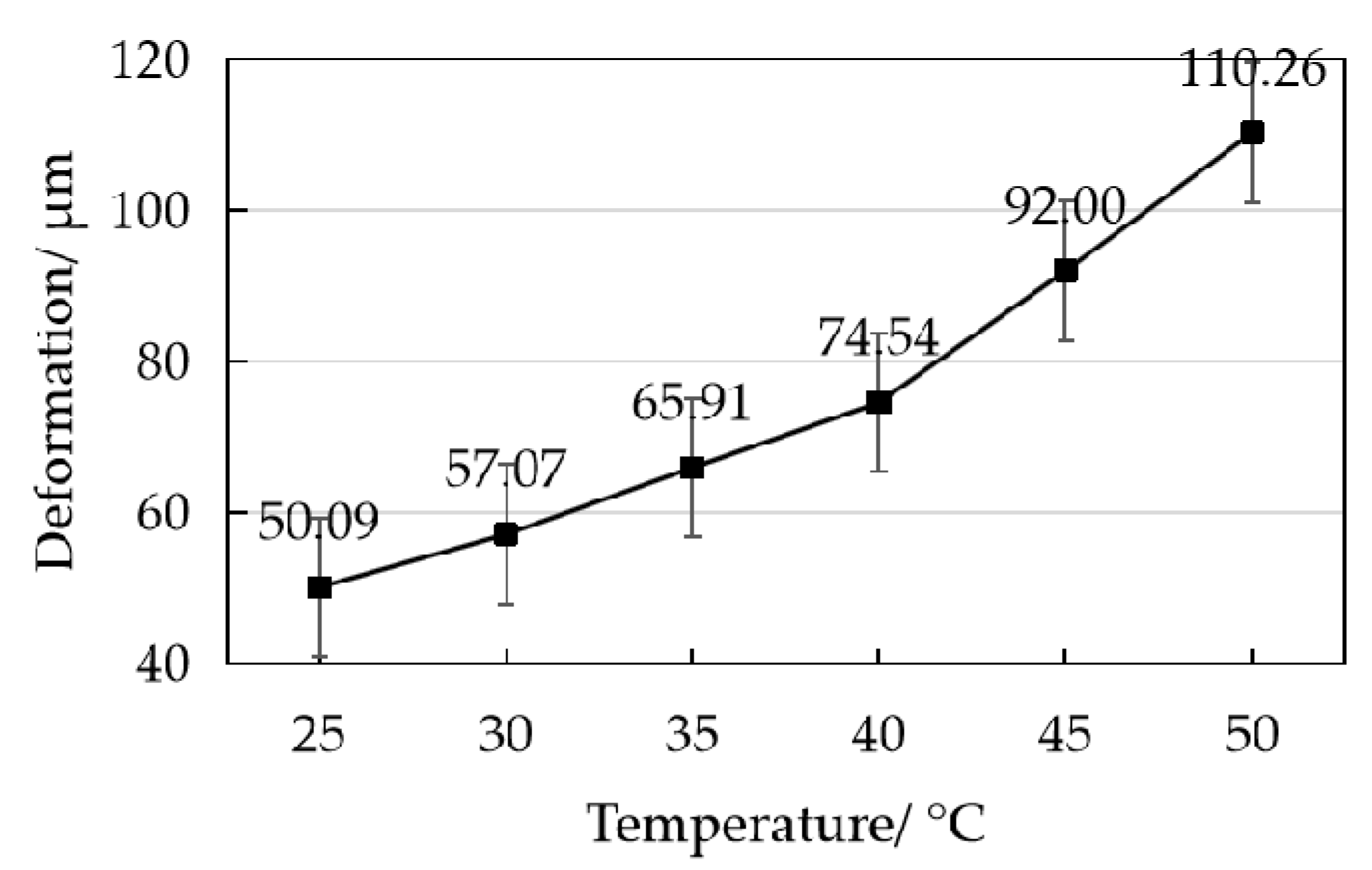
3.1.1. Temperature of Electrode Coating
3.1.2. Speed of Electrode Coating
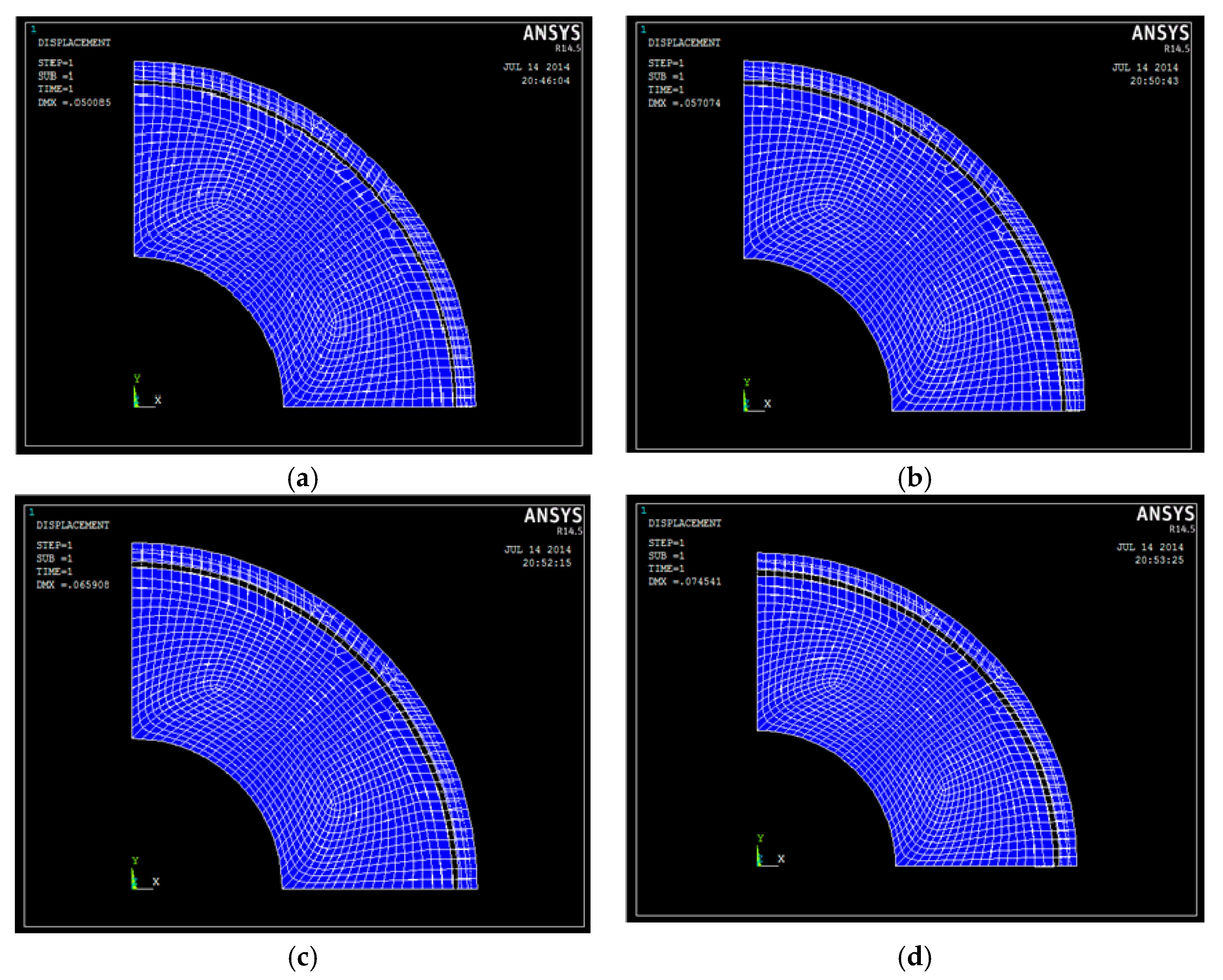
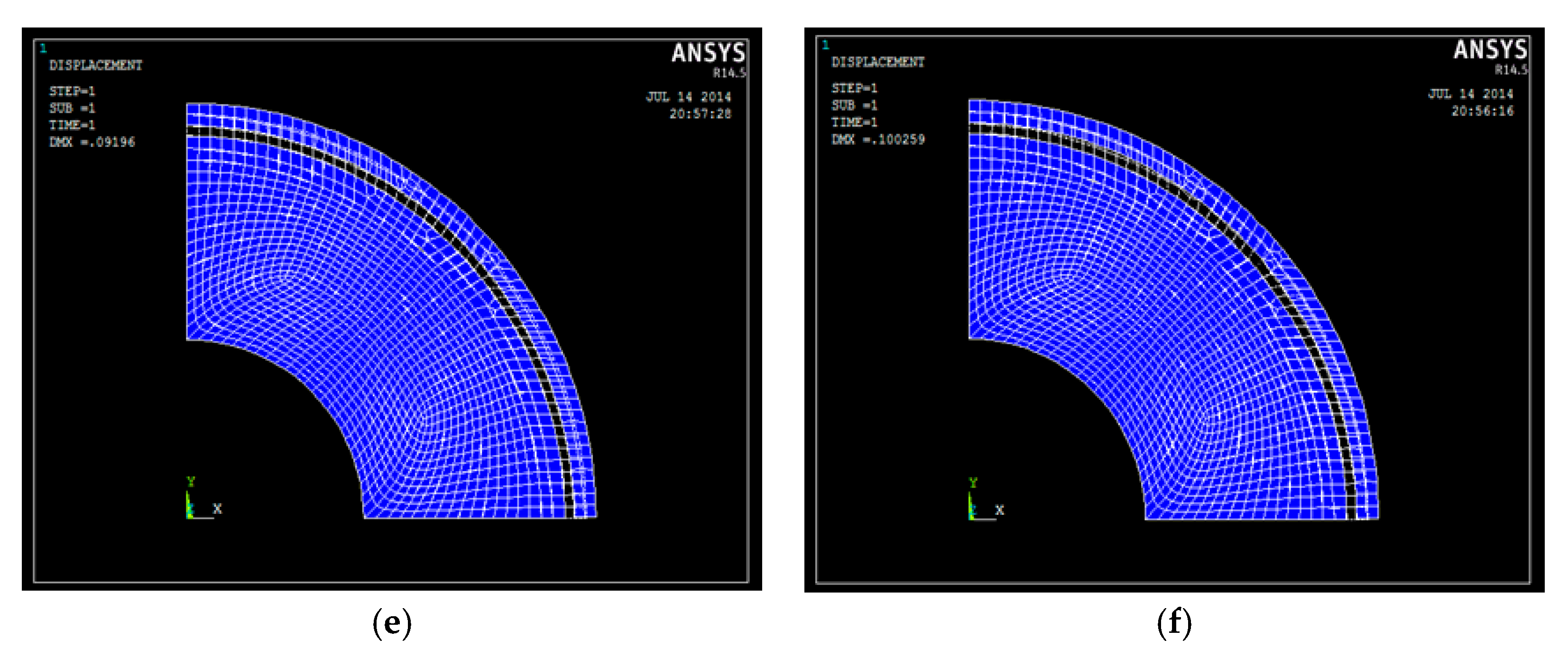
3.2. Research on the Electrode Structure Performance
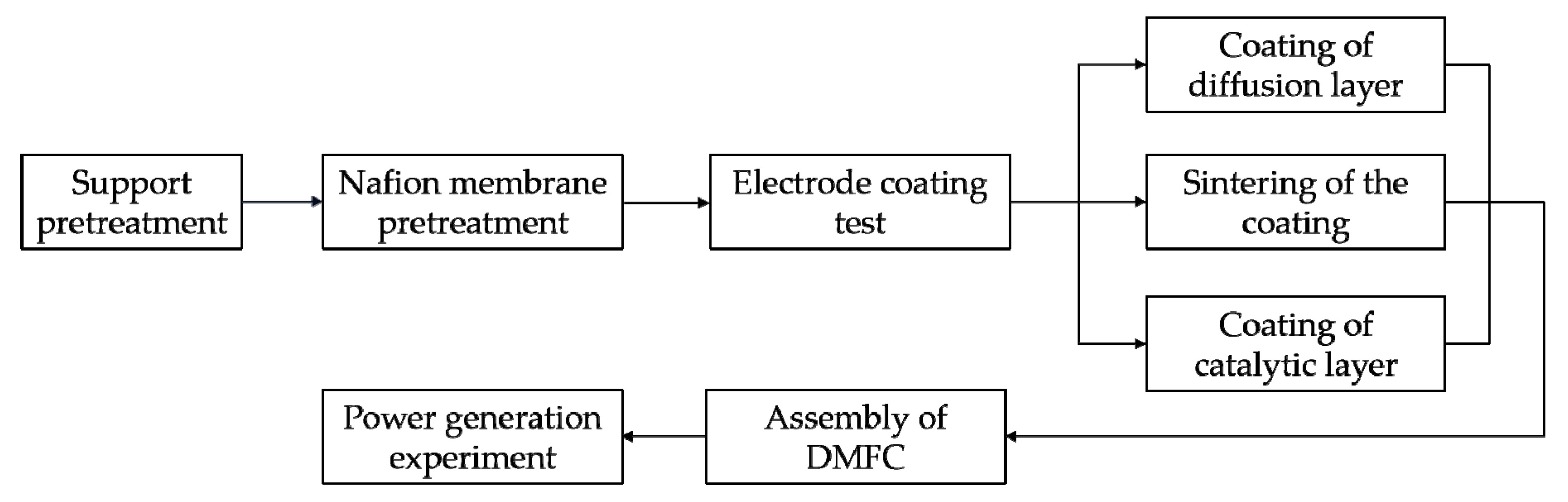
3.2.1. Experimental Process
3.2.2. The Influence of Experimental Parameters on the Coating Performance
- (1)
- Effect of the Electrolyte
- (2)
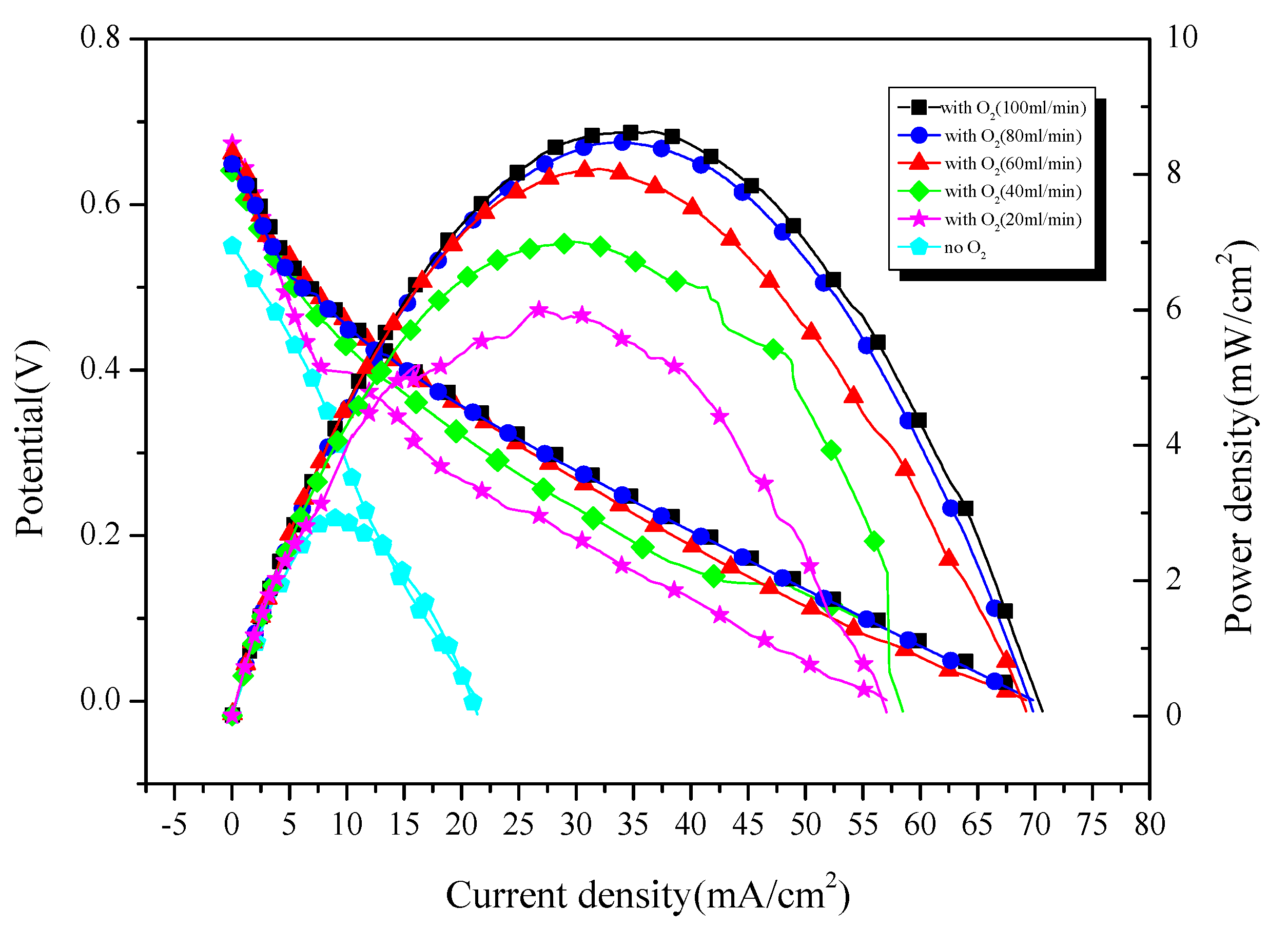
- Effect of Oxidants
- (3)
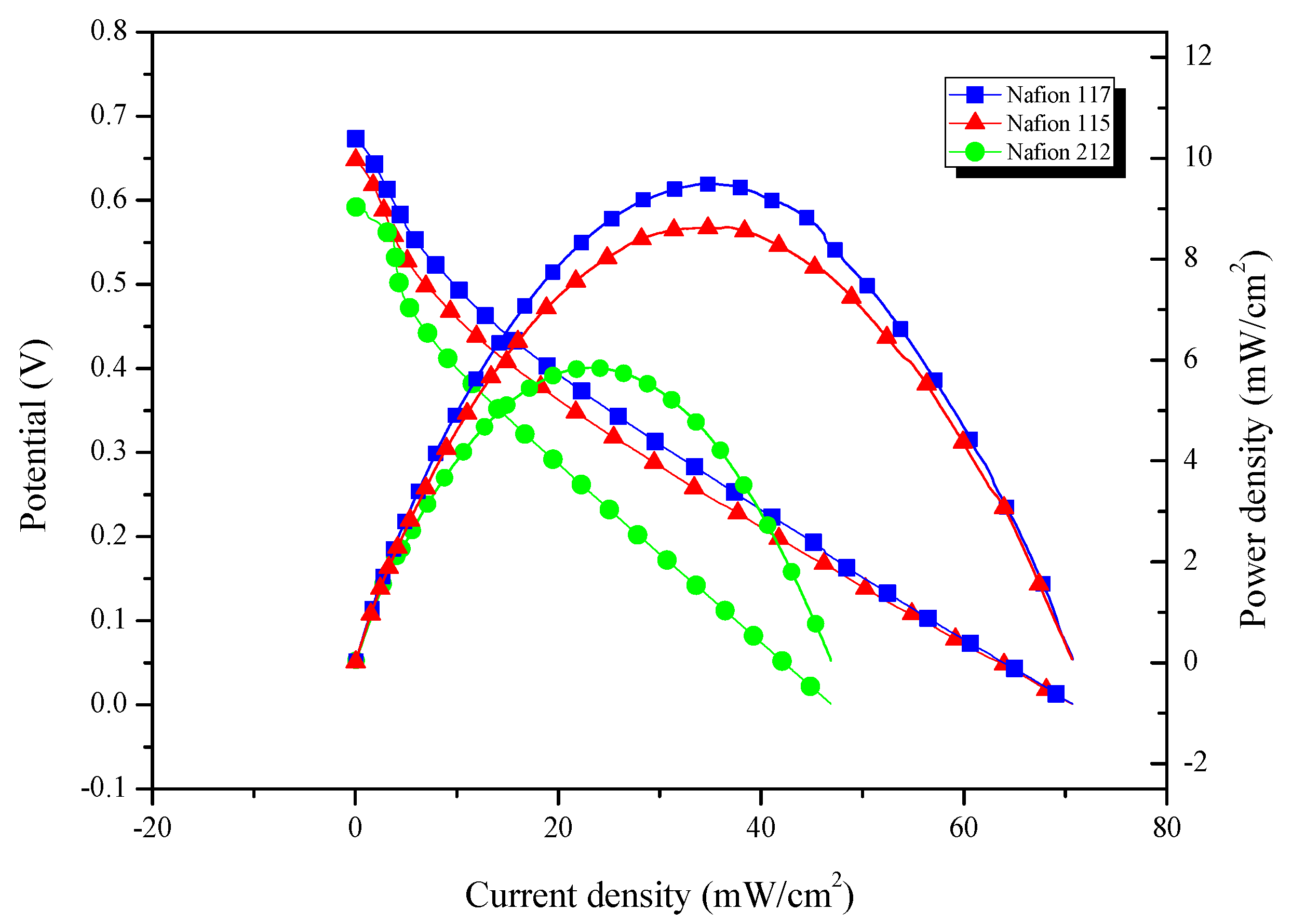
- Effect of PEMs
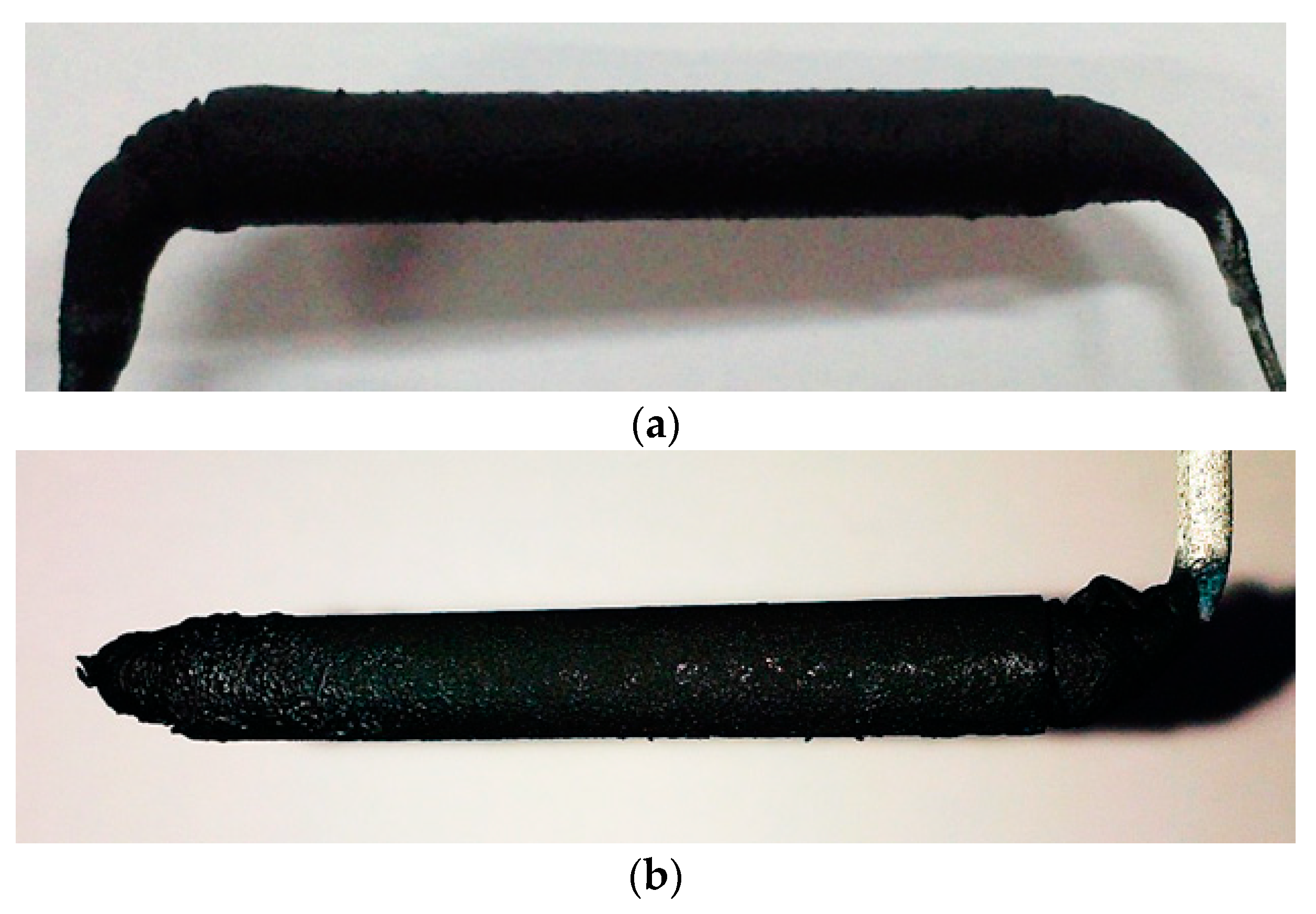
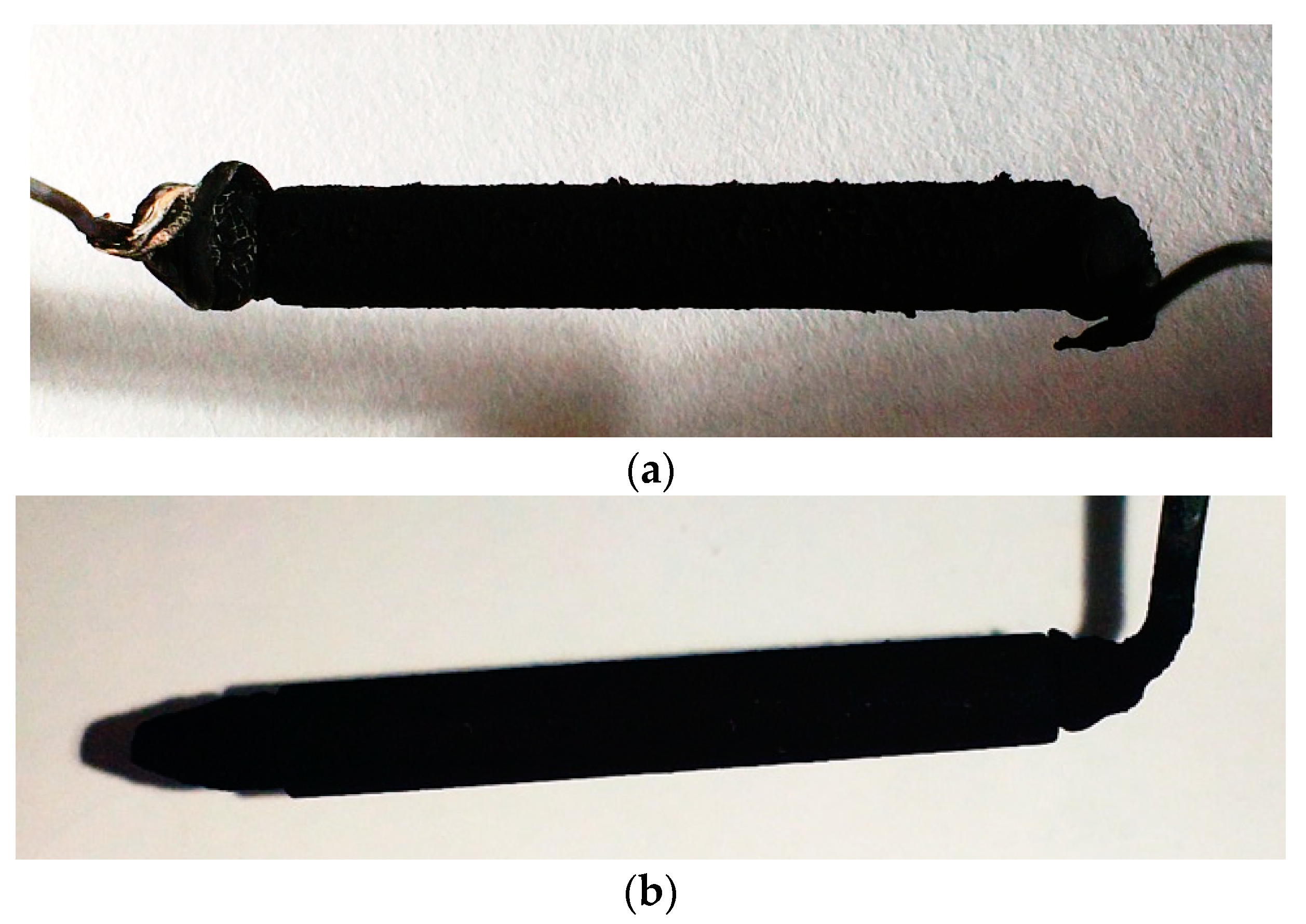
3.2.3. Analysis of Coating Morphology
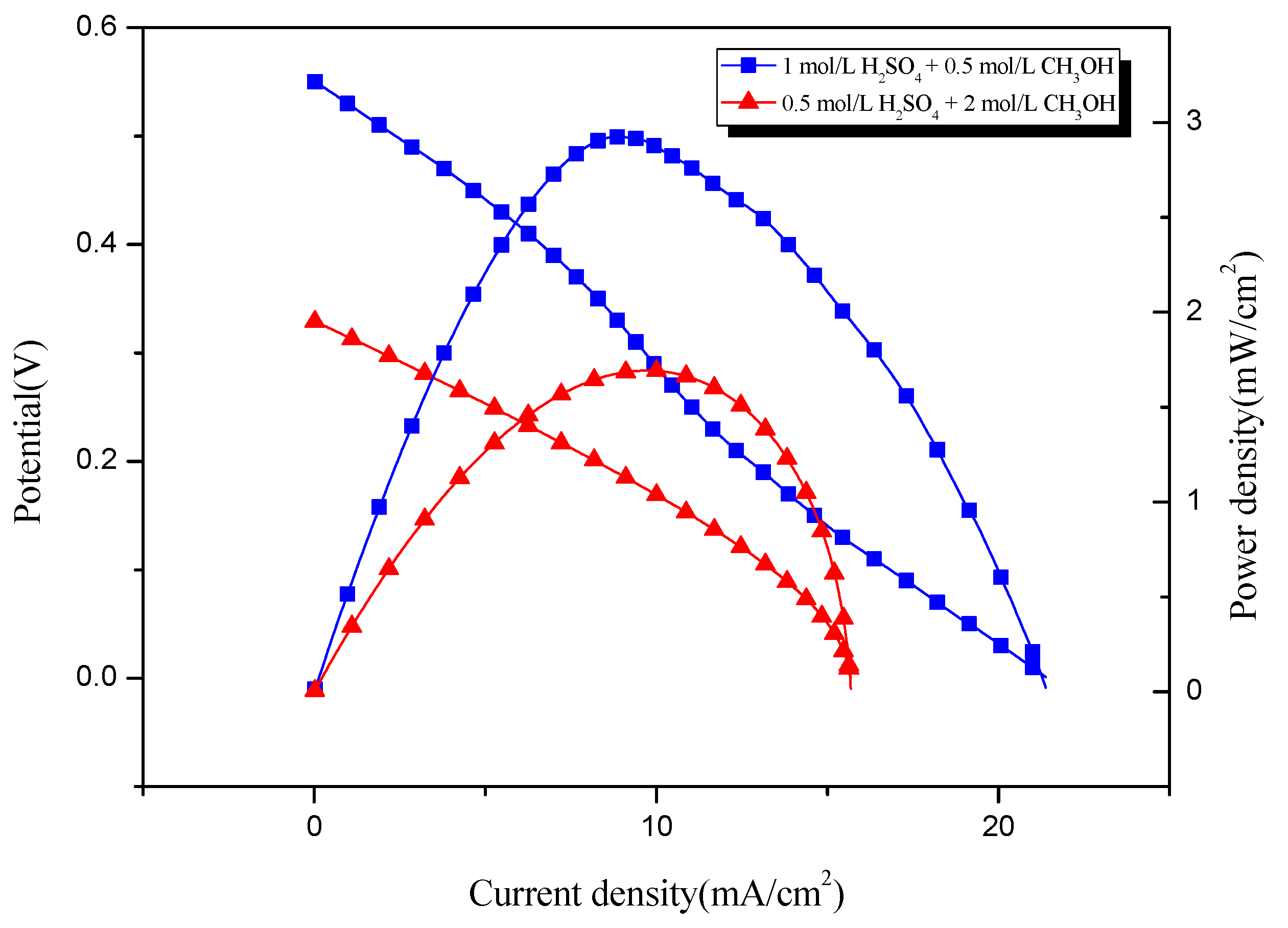
3.2.4. Comparison of Electrochemical Performance
4. Conclusions
- (1)
- A device was designed and fabricated for coating tubular electrodes, and thermal stress coupling experiments were conducted by using ANSYS software, and the optimal coating process of the device was determined by comparing the actual coating data. The optimal coating environment temperature is 30–40 °C, and the better coating speed is 6.67 r/min.
- (2)
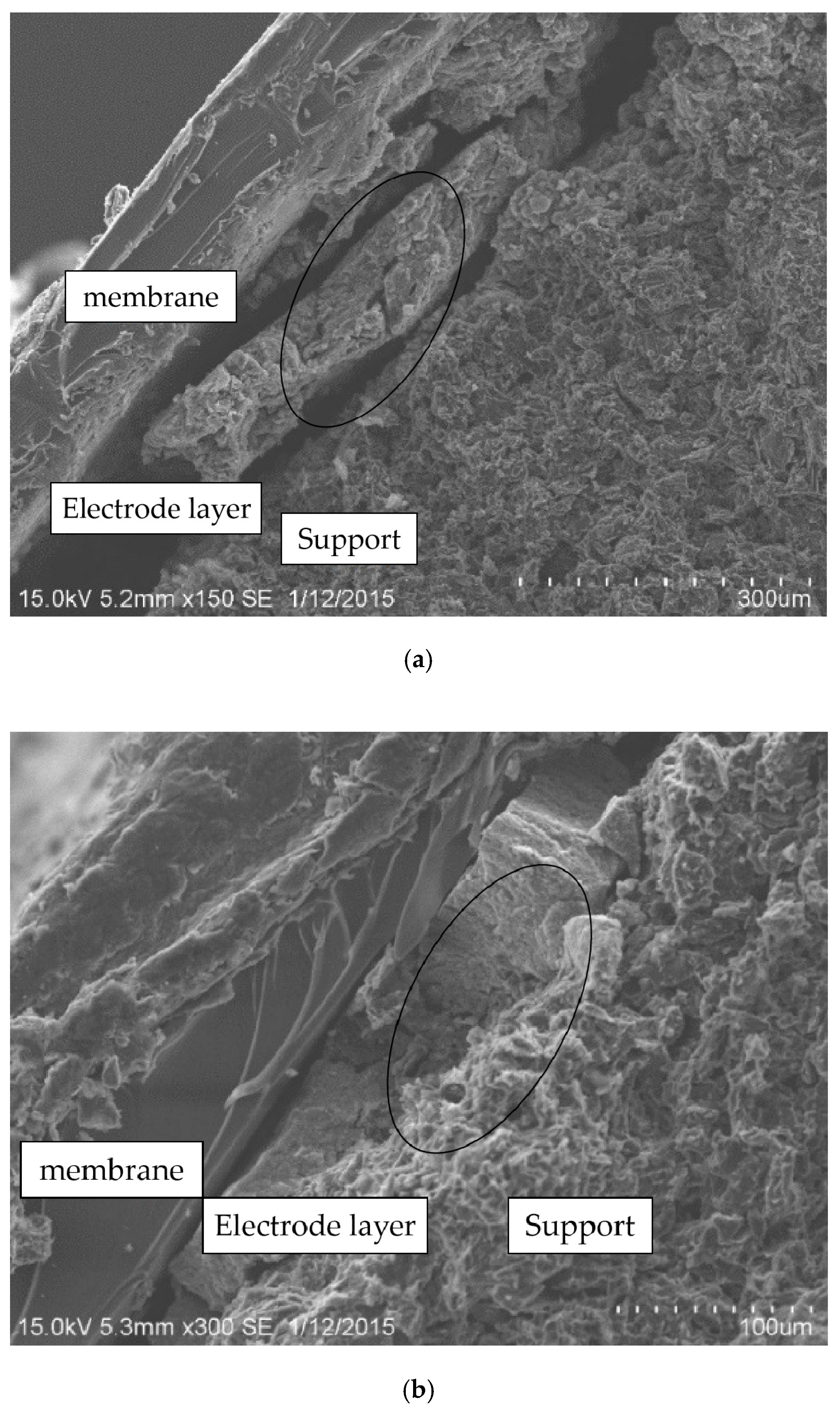
- In terms of macroscopic morphology, the surface uniformity of electrodes coated by self-made devices is better than the electrodes coated manually. The structure of manually coated electrode layers are loose, and are separated from the electrode support and the PEM. Electrode layers coated by the self-made device have a compact structure and a good fit with the electrode support and the PEM.
- (3)
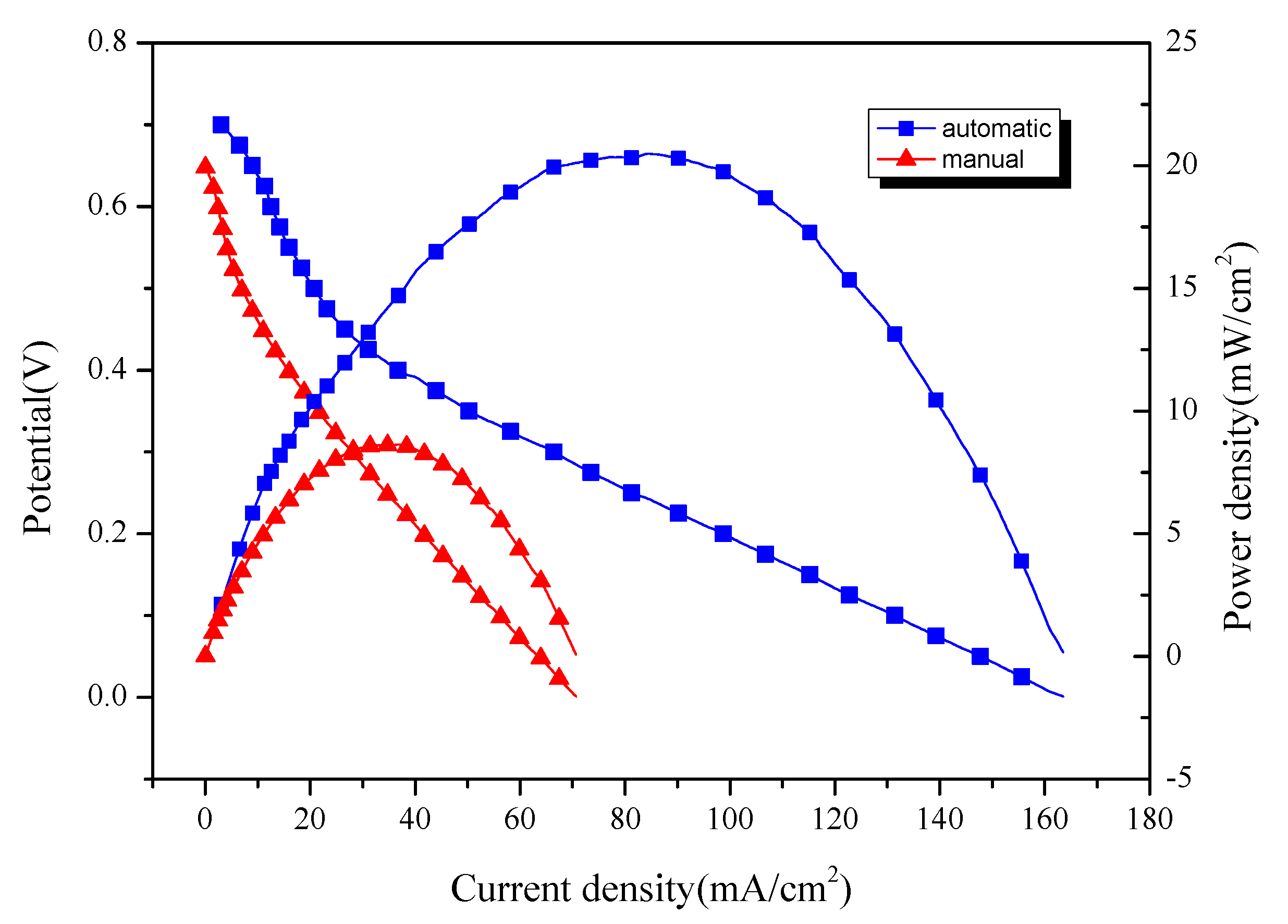
- Through different comparative tests, the optimal power generation experiment conditions are determined as: the PEM is Nafion 117, the electrolyte is 1 mol/L H2SO4 + 0.5 mol/L CH3OH, and the reaction temperature is stable. The power generation experiment shows that the power density of the electrode coated by the self-made device can reach 20.50 mW/cm2, which is about 2.4 times that of the electrode coated manually.
5. Patents
Author Contributions
Funding
Conflicts of Interest
References
- Omer, A.M. Energy, environment and sustainable development. Renew. Sustain. Energy Rev. 2008, 12, 2265–2300. [Google Scholar] [CrossRef]
- Ni, H.; Zhang, J.; Lv, S.; Wang, X.; Zhu, Y.; Gu, T. Preparation and Performance Optimization of Original Aluminum Ash Coating Based on Plasma Spraying. Coatings 2019, 9, 770. [Google Scholar] [CrossRef]
- Shuaishuai, L.; Jiaqiao, Z.; Hongjun, N.; Xingxing, W.; Yu, Z.; Tao, G. Study on Preparation of Aluminum Ash Coating Based on Plasma Spray. Appl. Sci. 2019, 9, 4980. [Google Scholar]
- Lv, S.; Zhang, J.; Ni, H.; Wang, X.; Zhu, Y.; Chen, L. Study on the Coupling Relationship of Low Temperature Fluidity and Oxidation Stability of Biodiesel. Appl. Sci. 2020, 10, 1757. [Google Scholar] [CrossRef]
- Liu, H.; Song, C.; Zhang, L.; Zhang, J.; Wang, H.; Wilkinson, D.P. A review of anode catalysis in the direct methanol fuel cell. J. Power Sources 2006, 155, 95–110. [Google Scholar] [CrossRef]
- Li, L.; Zhang, J.; Wang, Y. Sulfonated poly (ether ether ketone) membranes for direct methanol fuel cell. J. Membr. Sci. 2003, 226, 159–167. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Chan, S.H. Micromachined polymer electrolyte membrane and direct methanol fuel cells—A review. J. Micromechanics Microengineering 2006, 16, R1. [Google Scholar] [CrossRef]
- Martinaiou, I.; Videla, A.H.A.M.; Weidler, N. Activity and degradation study of an Fe-NC catalyst for ORR in Direct Methanol Fuel Cell (DMFC). Appl. Catal. B Environ. 2020, 262, 118217. [Google Scholar] [CrossRef]
- Xing, L.; Shi, W.; Su, H.; Xu, O.; Das, P.K.; Mao, B.; Scott, K. Membrane electrode assemblies for PEM fuel cells: A review of functional graded design and optimization. Energy 2019, 177, 445–464. [Google Scholar] [CrossRef]
- Holzapfel, P.; Bühler, M.; Van, P.C. Directly coated membrane electrode assemblies for proton exchange membrane water electrolysis. Electrochem. Commun. 2020, 110, 106640. [Google Scholar] [CrossRef]
- Ni, H.; Wang, K.; Lv, S.; Wang, X.; Zhuo, L.; Zhang, J. Effects of Concentration Variations on the Performance and Microbial Community in Microbial Fuel Cell Using Swine Wastewater. Energies 2020, 13, 2231. [Google Scholar] [CrossRef]
- Khamseh, S.; Alibakhshi, E.; Ramezanzadeh, B.; Saria, M.G. A tailored pulsed substrate bias voltage deposited (aC: Nb) thin-film coating on GTD-450 stainless steel: Enhancing mechanical and corrosion protection characteristics. Chem. Eng. J. 2020, 404, 126490. [Google Scholar] [CrossRef]
- Xu, P.; Meng, G.; Pershin, L.; Mostaghimi, J.; Coyle, T.W. Control of the hydrophobicity of rare earth oxide coatings deposited by solution precursor plasma spray by hydrocarbon adsorption. J. Mater. Sci. Technol. 2020, 62, 107–118. [Google Scholar] [CrossRef]
- De Crescenzo, C.; Karatza, D.; Musmarra, D.; Chianese, S.; Baxevanis, T.; Dalla, P.T.; Exarchos, D.A.; Dassios, K.G.; Matikas, T.E. Ni-Ti shape memory alloy coatings for structural applications: Optimization of HVOF spraying parameters. Adv. Mater. Sci. Eng. 2018, 7867302. [Google Scholar] [CrossRef]
- Ni, H.; Zhang, J.; Lv, S.; Wang, X.; Zhang, S.; Zhao, N.; Gu, H.; Li, B.; Jiang, J. Design and Study on a Self-Controlled Walking Car. In Proceedings of the 2018 3rd International Conference on Mechanical, Control and Computer Engineering (ICMCCE), Huhhot, China, 14–16 September 2018; pp. 281–285. [Google Scholar] [CrossRef]
- Ni, H.; Pei, Y.; Lv, S.; Ma, J.; Wang, X. Design of Coating Device for Circular Direct Methanol Fuel Cell Cathode. J. Nantong Univ. 2013, 12, 26–28. (In Chinese) [Google Scholar]
- Shin, H.J.; Oytun, F.; Kim, J.W. Cost-effective centrifuge coating method for silver nanowire-based transparent conducting electrode. Electrochim. Acta 2020, 337, 135839. [Google Scholar] [CrossRef]
- Mellbring, O.; Kihlman Øiseth, S.; Krozer, A. Spin coating and characterization of thin high-density polyethylene films. Macromolecules 2001, 34, 7496–7503. [Google Scholar] [CrossRef]
- Fierro, S.; Comninellis, C. Kinetic study of formic acid oxidation on Ti/IrO2 electrodes prepared using the spin coating deposition technique. Electrochim. Acta 2010, 55, 7067–7073. [Google Scholar] [CrossRef]
- Haselrieder, W.; Westphal, B.; Bockholt, H. Measuring the coating adhesion strength of electrodes for lithium-ion batteries. Int. J. Adhes. Adhes. 2015, 60, 1–8. [Google Scholar] [CrossRef]
- Pennestrı, E.; Valentini, P.P. A review of formulas for the mechanical efficiency analysis of two degrees-of-freedom epicyclic gear trains. J. Mech. Des. 2003, 125, 602–608. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Y.; Zhang, J. Effects of tooth-crack-induced mesh stiffness on fault signals of a planetary gear train. Procedia Comput. Sci. 2017, 109, 785–792. [Google Scholar] [CrossRef]
- Behle, S.; Lüettringhaus-Henkel, A.; Eimann, P. Process and Apparatus for the Plasma Coating of Workpieces with Spectral Evaluation of the Process Parameters. U.S. Patent 8,397,667, 19 March 2013. [Google Scholar]
- Janqour, L.M.; Sarabi, A.A. Optimization of coating process parameters and surface characterization for vanadium-based conversion coating on 2024 aluminum alloy. Prog. Org. Coat. 2019, 133, 33–43. [Google Scholar] [CrossRef]
- Röder, F.; Braatz, R.D.; Krewer, U. Direct coupling of continuum and kinetic Monte Carlo models for multiscale simulation of electrochemical systems. Comput. Chem. Eng. 2019, 121, 722–735. [Google Scholar] [CrossRef]
- Wu, D.; Zhong, W. A new strategy for anchoring a functionalized graphene hydrogel in a carbon cloth network to support a lignosulfonate/polyaniline hydrogel as an integrated electrode for flexible high areal-capacitance supercapacitors. J. Mater. Chem. A 2019, 7, 5819–5830. [Google Scholar] [CrossRef]
- Li, F.; Xing, J.; Liu, Y. Thermal Analysis and Stress Analysis of the Heat-Exchange Pipe Based on ANSYS. In Proceedings of the 2011 Fourth International Conference on Information and Computing, Phuket Island, Thailand, 25–27 April 2011; IEEE: New York, NY, USA, 2011; pp. 283–285. [Google Scholar]
- Ni, H.; Zhang, J.; Zhao, N.; Wang, C.; Lv, S.; Ren, F.; Wang, X. Design on the Winter Jujubes Harvesting and Sorting Device. Appl. Sci. 2019, 9, 5546. [Google Scholar] [CrossRef]
- Gao, J.L.; Du, Q.G.; Zhang, X.D. Thermal stress analysis and structure parameter selection for a Bi2Te3-based thermoelectric module. J. Electron. Mater. 2011, 40, 884–888. [Google Scholar] [CrossRef]
- Tyona, M.D. A comprehensive study of spin coating as a thin film deposition technique and spin coating equipment. Adv. Mater. Res. 2013, 2, 181. [Google Scholar] [CrossRef]
- Zhang, J.D.; Hao, N.; Lu, L. High-efficient preparation and screening of electrocatalysts using a closed bipolar electrode array system. J. Electroanal. Chem. 2019, 832, 1–6. [Google Scholar] [CrossRef]
- DeBonis, D.; Mayer, M.; Omosebi, A. Analysis of mechanism of Nafion® conductivity change due to hot pressing treatment. Renew. Energy 2016, 89, 200–206. [Google Scholar] [CrossRef]
- Govindarasu, R.; Somasundaram, S. Studies on Influence of Cell Temperature in Direct Methanol Fuel Cell Operation. Processes 2020, 8, 353. [Google Scholar] [CrossRef]
- Colpan, C.O.; Ouellette, D.; Glüsen, A. Reduction of methanol crossover in a flowing electrolyte-direct methanol fuel cell. Int. J. Hydrog. Energy 2017, 42, 21530–21545. [Google Scholar] [CrossRef]
- Abrego-Martínez, J.C.; Moreno-Zuria, A.; Wang, Y. Fabrication and evaluation of passive alkaline membraneless microfluidic DMFC. Int. J. Hydrog. Energy 2017, 42, 21969–21975. [Google Scholar] [CrossRef]
- Vasile, N.S.; Videla, A.H.A.M.; Simari, C. Influence of membrane-type and flow field design on methanol crossover on a single-cell DMFC: An experimental and multi-physics modeling study. Int. J. Hydrog. Energy 2017, 42, 27995–28010. [Google Scholar] [CrossRef]
- Mushtaq, M.; Wasim, M.; Naeem, M.A.; Khan, M.R.; Yue, S.; Saba, H.; Hussain, T.; Siddiqui, M.Q.; Farooq, A.; Wei, Q. Composite of PLA Nanofiber and Hexadecyl Trimethyl-Ammonium Chloride-Modified Montmorillonite Clay: Fabrication and Morphology. Coatings 2020, 10, 484. [Google Scholar] [CrossRef]


| Raw Material | Type or Parameter | Manufacturer |
|---|---|---|
| Graphite tube | Purity: 99.999% | Beijing Jixing Shengan Industry and Trade Co., Ltd. (Beijing, China) |
| MCMB/G tube | Porosity: 40% | Laboratory self-made |
| Toner | C, Vulcan XC-72R | Cabot corporation (Boston, MA, USA) |
| PTFE emulsion (analytical grade) | 60 wt.% PTFE | DuPont (Wilmington, DE, USA) |
| Pt/C catalyst | Pt/C | Shanghai Hesen Electric Co., Ltd. (Shanghai, China) |
| Nafionemulsion (analytical grade) | 5 wt.% Nafion | DuPont (Wilmington, DE, USA) |
| Graphite plate | Purity: 99.99% | Beijing Jixing Shengan Industry and Trade Co., Ltd. (Beijing, China) |
| PtRu/C catalyst | PtRu/C | Shanghai Hesen Electric Co., Ltd. (Shanghai, China) |
| Parameters | Functional Layers | ||
|---|---|---|---|
| Electrode Support | Electrode Layer | PEM | |
| Elastic moduli/Pa | 10.8 × 106 | 6.13 × 106 | 80 × 106 |
| Thermal expansion coefficient | 5.49 × 10−6 | 22 × 10−6 | 520 × 10−6 |
| Thickness/mm | 1.5 | 0.2 | 0.183 |
| No. | Coating Speed (r/min) | Average Weight Gain (mg) |
|---|---|---|
| 1 | 6.67 | 3.00 ± 1.43 |
| 10 | 2.53 ± 1.34 | |
| 2 | 6.67 | 3.08 ± 1.17 |
| 10 | 2.45 ± 1.64 | |
| 3 | 6.67 | 3.06 ± 1.20 |
| 10 | 1.67 ± 0.96 |
| Name | Parameters |
|---|---|
| Temperature | 80 °C |
| Electrolyte | 1 mol/L H2SO4 + 0.5 mol/L CH3OH solution |
| Oxidant | O2, access rate 100 mL/min |
| PEM | Nafion 117 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, H.; Zhang, J.; Lv, S.; Wang, X.; Pei, Y.; Li, F. Coating Process Parameters and Structural Properties of the Tubular Electrodes of Fuel Cells Based on a Self-Made Coating Device. Coatings 2020, 10, 830. https://doi.org/10.3390/coatings10090830
Ni H, Zhang J, Lv S, Wang X, Pei Y, Li F. Coating Process Parameters and Structural Properties of the Tubular Electrodes of Fuel Cells Based on a Self-Made Coating Device. Coatings. 2020; 10(9):830. https://doi.org/10.3390/coatings10090830
Chicago/Turabian StyleNi, Hongjun, Jiaqiao Zhang, Shuaishuai Lv, Xingxing Wang, Yi Pei, and Fei Li. 2020. "Coating Process Parameters and Structural Properties of the Tubular Electrodes of Fuel Cells Based on a Self-Made Coating Device" Coatings 10, no. 9: 830. https://doi.org/10.3390/coatings10090830
APA StyleNi, H., Zhang, J., Lv, S., Wang, X., Pei, Y., & Li, F. (2020). Coating Process Parameters and Structural Properties of the Tubular Electrodes of Fuel Cells Based on a Self-Made Coating Device. Coatings, 10(9), 830. https://doi.org/10.3390/coatings10090830






