1. Introduction
Recently, a novel coronavirus named severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) was identified as the causative agent of a respiratory disease referred to as COVID-19 (coronavirus disease 2019) by the World Health Organization (WHO). Being a member of the severe acute respiratory syndrome (SARS) and the Middle East respiratory syndrome (MERS), COVID-19 is caused by betacoronavirus, which affects the lower respiratory tract and manifests as pneumonia in humans [
1]. Bacterial and fungal infections as prognostic implications are most often associated with viral pneumonia in critically hospitalized patients [
2]. Respiratory virus infections predispose patients to co-infection, which contributes to increased disease mortality and morbidity. Co-infection can be termed as the presence of the organism itself in the patient, prior to the viral infection, or it may be associated as a persistent chronic infection; otherwise, it may be due to hospital-acquired infections [
3]. The presence of bacterial and/or fungal co-infections can likely be an important factor affecting mortality, and as of late this has received inadequate attention in COVID-19 patients [
4]. An earlier report of the pandemic in 2009 stated a significant association of either bacterial, fungal, or both infections in H1N1 patients [
5], which accorded for poor outcomes. Zhou and colleagues [
2] showed that in the current coronavirus disease 2019 (COVID-19) pandemic, almost 50% of patients who died had secondary bacterial infections; the authors in [
6] also reported both bacterial and fungal co-infections. Data suggest that almost 71% of the admitted patients with COVID-19 received antibiotic prophylactics without any information on their sensitivities towards the identified organisms, including no record of the duration and type of antimicrobial treatment, thus implicating the urgency for detailed information on co-infections to assess their role in the prognosis of COVID-19 patients. Co-infections are complex to diagnose, which becomes even more complicated with the excessive use of antibiotics in COVID-19 patients, causing antimicrobial resistance and multiple drug resistance [
3], which has been well supported by the findings of Bengoechea et al. [
7], who reported that the trio of SARS-CoV-2, bacterial co-infections, and antimicrobial resistance (AMR) contributed towards the more severe aspects of COVID-19. The co-infections are trivial in clinical examination; hence, microbiological examination can add great value to diagnosis, especially sputum culture, which is not possible presently due to the safety issues of clinicians dealing with it, but our previous knowledge of the history and details about patients’ microbial analysis of sputum can provide the armamentarium in co-infection diagnosis and treatment [
4]. Moreover, the recent infection control strategies of SARS-CoV-2 focus on interpersonal transmission and cross infection, in turn bypassing the eradication of bacterial or fungal secondary infection [
8]. The infections of the respiratory tract are amongst the most widespread and most serious infections that compel an individual to seek medical attention and prescription of antibiotics [
9]. In the past few decades, antibiotics have lost their effectiveness against many diseases by inducing in vivo toxic reactions as well as the global emergence of resistance within bacterial communities against them [
10].
Drugs obtained from natural sources play an important part in combating human diseases [
11]. Clinical interventions worldwide are being done without any evidence-based treatment for COVID-19 to date. The Indian system of medicine, or Ayurveda, needs the same strategic implementation, and it has become even more relevant by its factual and elaborate descriptions on causation and management of epidemics, referred to as Janapadodhwamsa [
12,
13]. Advance research in understanding the genomics of resistance and synthetic formulation development using scaling strategies could provide promising alternatives [
14]. Natural products such as antimicrobials in the management of infectious diseases provide strong alternatives in traditional medicine. Several plant compounds have proven their record as antibacterial agents against resistant strains [
15]. Amongst them,
Cassia fistula Linn. (Leguminosae) has the potential to be an alternative medicine for the treatment of infectious diseases using entire parts of the plant [
16]. The plant leaves have laxative, antiperiodic, depurative, anti-inflammatory properties, and are useful in various diseases [
17]. Furthermore, they are used as standardized formulations in hand sanitizers and soaps, and they need further optimization for them to be used as disinfectant and antiseptics. Apart from that,
Cassia fistula seed gum is used as a film coating material in different dosage forms for a variety of purposes [
18]. Traditionally, the medicinal plant of the genus
Moringa has been used to cure wounds and diseases such as common cold and diabetes. Thirteen varieties of
Moringa have been extensively cultivated in Asia and Africa for multiple uses, amongst which
Moringa oleifera is the most revered species [
19].
Moringa oleifera (Lam) has high nutritional value and is considered a complete food due to its effective range of medicinal uses. The extracts of the leaves are considered to have biological properties and are normally found to differ with the type of solvent used to isolate the active compounds [
20,
21].
Moringa oleifera seed oil (MOSO) is considered for the production of organic surface coating resins, which showed excellent antimicrobial inhibitive properties with a thermal stability profile that is best use in the surface coating industry also [
22].
Adhatoda vasica (Malabar nut) is a perennial plant that is well known in Ayurveda for its medicinal properties.
Adhatoda vasica has been well known for its action against serious respiratory infections, with its leaves used for centuries as an expectorant with demonstrated antispasmodic activity against severe respiratory infections such as asthma and chronic bronchitis [
23]. A previous study documented the efficient use of the methanolic extract of
Adhatoda vasica in the production of antibacterial
Adhatoda liquid soap [
24]. One excellent use of
Adhatoda vasica extract was shown by its inhibition efficiency of corrosion of steel surface [
25].
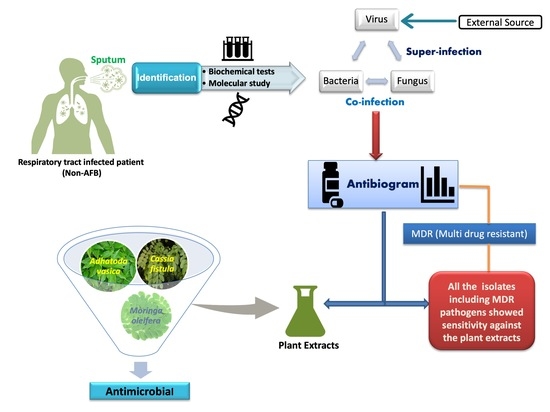
This study shows the antibacterial and antifungal activity of the aforementioned well-known medicinal plant extracts on clinically relevant antibacterial and antifungal strains isolated from sputum samples. The results from this study implicate that medics can diagnose bacterial or fungal infections in COVID-19 patients with their drug sensitivity antibiogram, in turn providing them evidence-based detailed insight into specific prognosis and therapy, leading to marked precision in the prevention and control of infection-associated complications, which in turn contributes to lessening the number of deaths amongst COVID-19 patients [
2]. Thus, the Indian system of medicine traditionally known as Ayurveda comes to the rescue at the forefront by providing all the necessary armamentarium, including a holistic approach in combating this global viral pandemic.
2. Materials and Methods
2.1. Sample Collection
Non-AFB (Non-acid fast bacilli) sputum samples were collected from 120 OPD (outdoor patients) suffering from respiratory tract infection with informed consent and ethical clearance from India. Samples were collected aseptically into well-labeled sterile wide-mouthed glass bottles. A patient’s registering proforma with brief medical histories such as name, age, sex, hospitalization records within previous years, antibiotics prescribed, personal hygiene status, and other medication data, etc. were maintained.
2.2. Processing of Samples
Sputum samples were inoculated on different commercial media (HiMedia) such as nutrient agar, MacConkey agar, mannitol salt agar, Sabouraud dextrose agar, while chocolate agar and blood agar were prepared in the lab using sheep blood. Inoculated agar plates were incubated at 37 °C for bacterial isolates and 26 °C for fungal isolates for 24 to 48 h.
2.3. Identification of the Pathogens
The bacterial isolates were maintained in a nutrient broth (NB) at 37 °C while fungal isolates of
candida spp. were maintained on Sabouraud dextrose agar (SDA) at 26 °C. The isolates were identified by cultural, morphological, gram-staining, and biochemical characteristics (according to Bergey’s Manual of Determinative Bacteriology) [
26], and the selected multidrug resistant (MDR) bacteria were further subjected to molecular identification by Sanger sequencing using the 3500XL genetic analyzer (Applied Biosystems, Waltham, MA, USA) for bacterial 16S rRNA and fungal 18S rRNA. The sequences obtained were used to create a phylogenetic tree using MEGA software. The sequences obtained were also submitted to the NCBI database with available accession numbers.
2.4. Antibiotics and Antifungals
Commercial antibiotics were procured from HiMedia Laboratories Pvt. Ltd., Mumbai, India, including ampicillin (AMP) 30 µg, ciprofloxacin (CIP) 10 µg, ofloxacin (OFX) 10 µg, erythromycin (ERY) 10 µg, gentamicin (GEM) 10 µg, cefuroxime (CXM) 30 µg, imipenem (IPM) 10 µg, chloramphenicol (CHL) 30 µg, piperacillin (PIP) 100 µg, oxacillin (OXA) 5 µg, vancomycin (VAN) 30 µg, penicillin G (PEN) 10 µg, co-trimoxazole (CO) 25 µg, clotrimazole (CLT) 10 µg, amphotericin B (AMB) 100 µg, ketoconazole (KTC) 10 µg, nystatin (NYT) 100 IU, fluconazole (FLC) 10 µg, and itraconazole (ITC) 10 µg.
2.5. Antibiotic and Antifungal Susceptibility Test
Antibacterial and antifungal susceptibility testing was performed by Kirby Bauer’s disc diffusion method according to the National Committee for Clinical Laboratory Standards guidelines of the Clinical Laboratory Standards Institute, USA. Antibiotic discs were placed on Mueller–Hinton agar plates, previously swabbed with the target bacterial isolate at a concentration of 10
5 CFU/mL (McFarland standard [
27]), and were incubated for 24 h at 37 °C (bacteria) and 25 °C for
Candida spp. Antimicrobial activity is defined as the diameter (mm) of the clear inhibitory zone formed around the discs [
28].
2.6. Preparation of Plant Extracts
Fresh leaves of
Moringa oleifera, Justicia adhatoda (Syn. Adhatoda vasica), and
Cassia fistula were taken, identified by a taxonomist at the University of Allahabad. Fresh leaves from selected species of plants were washed preliminarily 2–3 times with tap water followed by distilled water and dried at 50 °C before being ground to a fine powder in a blender machine. Following this, 10 g of powdered material was soaked in a 100 mL solvent such as acetone and methanol and kept at 30 °C for 6–7 days in a rotary shaker. After that, it was filtered through Whatman filter paper No. 1. The filtrate was then evaporated using a rotatory evaporator to obtain concentrated, powdered extracts. All the extracts were concentrated as per need and stored in sterile vials at 4 °C for further use [
29,
30].
2.7. Test for Antimicrobial Activity of Selected Plant Extracts
The antimicrobial potential of the selected plant extracts was screened by the agar well diffusion method [
31]. An inoculum suspension concentration of 10
5 CFU/mL (McFarland standard) was swabbed uniformly to solidified Muller–Hinton Agar (MHA) media. Holes of 5 mm in diameter were made in seeded agar using a sterile cork borer. An aliquot of 100 µL from each plant extract (100 mg/mL DMSO) was added into each well and was allowed to stand for 1h for proper diffusion, and thereafter incubated at 37 °C for 24 h (bacteria) and 26 °C for Candida isolates. The antimicrobial activity was evaluated by measuring the zone of inhibition (mm).
2.8. Determination of Minimum Inhibitory Concentration (MIC)
The minimum inhibitory concentration was assessed by the broth dilution method. The plant extracts were diluted serially to obtain concentrations of 500, 250, 125, 62.5, 31.25, 15.62, 7.81, 3.90, and 1.95 mg/mL, respectively in separate tubes. Here, 100 μL of 10
5 CFU/mL (McFarland standard) of the tested strains of equal volume was transferred into each tube of nutrient broth and plant extracts, and incubated at 37 °C for 24 h respectively. Control tubes with one having nutrient broth was inoculated with bacteria as a positive control, and the other one containing nutrient broth with plant extract was used as a negative control. The test compounds that inhibit the visible microbial growth (no turbidity) at the lowest concentration after incubation of 24 h were taken as the MIC value [
32,
33].
2.9. Statistical Analysis
Data obtained from the study were analyzed using the Graph Pad Prism Version 5.0 for a Windows (Graph Pad Software) statistical package program. All experiments were performed in triplicates, and values were expressed as mean ± SEM (standard error of mean). Results obtained were statistically analyzed by using one-way Analysis of Variance (ANOVA).
4. Discussion
According to a cohort study, 20 out of 90 people with severe acute respiratory syndrome reported additional lower respiratory tract infections in 2003, and these infections were responsible for the need of a major invasive operative procedure in 70.6% of critical SARS patients. The pathogens causing secondary infections in SARS patients were diverse. The negative bacilli, including
Candida, were the most common of them [
6]. Isolates along with selected MDR characteristics have been identified based on culture, morphological, biochemical, and molecular characteristics. If any pathogen shows resistance against three or more antibiotics, it is called a multidrug-resistant pathogen. Bacterial isolates such as
Pseudomonas aeruginosa,
Escherichia coli, and
Moraxella catarrhalis (gram negative) showed MDR characteristics. Transmission of MDR bacteria to the community is a crucial process of triggering infectious disease outbreak and is correlated with elevated morbidity, death, healthcare expenses, and antibiotic usage [
34]. Isolates have been tested for antibiotic resistance using different antibiotics that displayed differing degrees of susceptibility and resistance to various antibiotics and antifungals. In many cases, clinicians have misinterpreted fungal pulmonary infection due to a lack of specific clinical symptoms, which causes a high rate of morbidity and mortality. Different studies on pulmonary tuberculosis reported the prevalence of around 15–32%
Candida spp. co-infection [
35]. Immunocompromised patients with invasive pneumonia reported significant
Candida spp. infection [
36]. Bacterial and fungal co-infections with marked drug resistance were confirmed after ruling out for tuberculosis (TB) infection in the respiratory tract infected (RTI) patients. This made them the ultimate model for superinfection. According to the Centre for Disease Control (CDC), a superinfection is an infection following a previous infection that is specifically caused by microorganisms that are or have become antibiotic-resistant even before the superinfection, while a co-infection is an infection occurring in tandem with an initial infection [
37]. Thus, the difference is clear that co-infections occur simultaneously, whereas superinfections develop following the initial infection [
38]. A rapid diagnosis of co-infection is required; this emphasizes the need for methods that are capable of detecting a wide array of potential pathogens with drug-resistant strains as well as subsequent monitoring for infection development. Such a study would provide valuable surveillance data, thereby helping in the antibiotic prescribing policy. Nevertheless, very little importance has been given to secondary bacterial and fungal infections with a scarcity of standardized diagnostic procedures. Likewise, few challenges prevail in diagnosing secondary infection in COVID-19 patients [
39]. A rapid characterization of co-infection in the most severe COVID-19 patients is essential in management and treatment, which in turn will also improve antimicrobial stewardship throughout the pandemic, saving more lives than causing deaths [
3]. Various pathogenic bacteria present in respiratory tract infections have shown marked resistance against a wide range of commonly used antibiotics. The increase in resistance of gram-negative bacteria (faster than in gram-positive bacteria) is higher than active drug candidates or antimicrobials with a few drug development programs, making an insufficient therapeutic cover for almost the next two decades [
40].
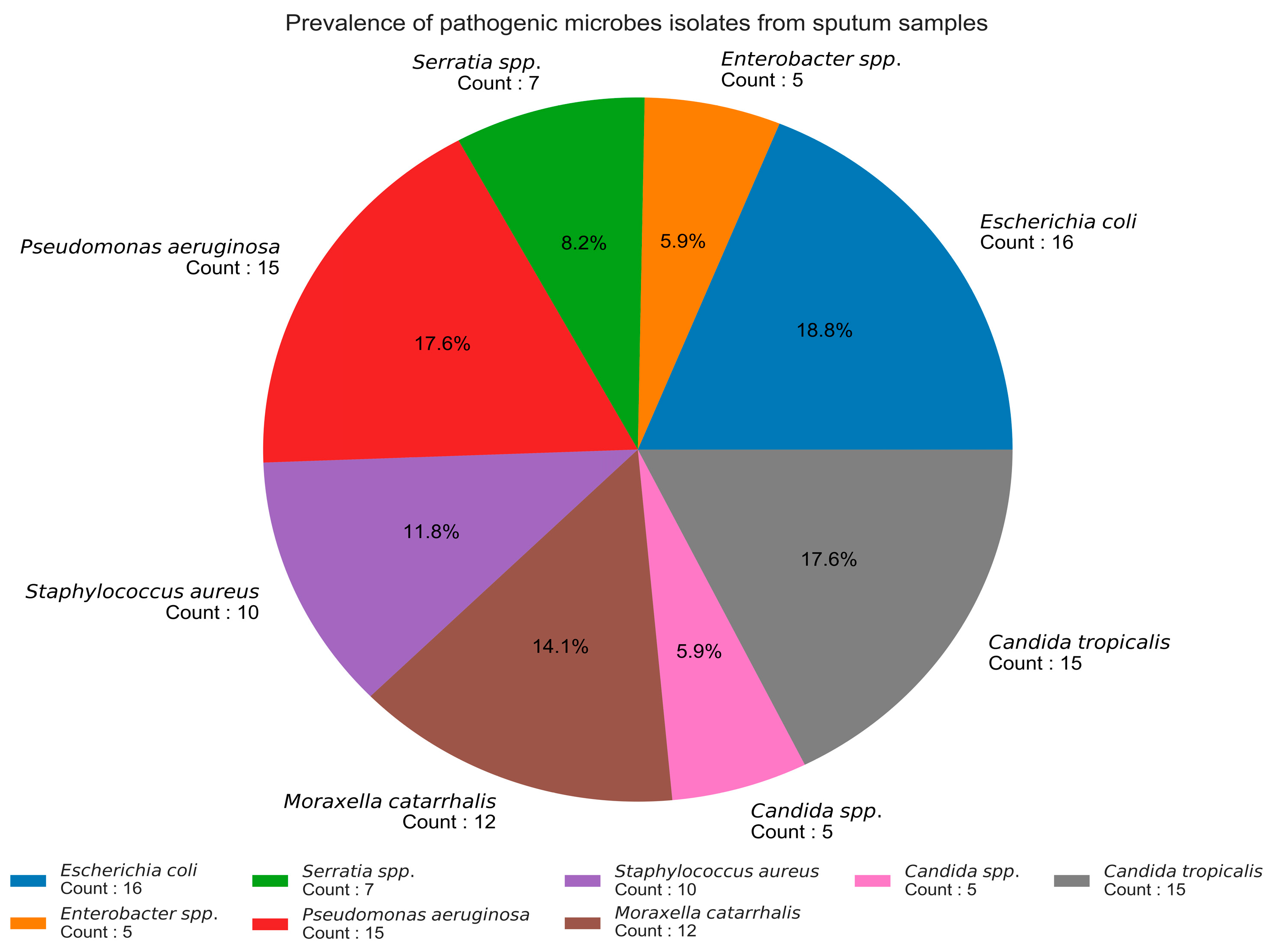
In this study, 120 sputum samples were collected, out of which 85 (70.83%) patients showed a positive incidence for different bacterial and fungal pathogens, which included
S. aureus,
Pseudomonas aeruginosa, and
Candida spp., and were also reported by [
41]. All samples were non-AFB sputum samples but also showed the presence of other pathogens, which has been already confirmed in a Cambodian study by [
42]. Hence, it was concluded from the study that the sputum samples that were negative for AFB also showed the incidence of pathogenic bacterial isolates such as
Escherichia coli,
Pseudomonas aeruginosa,
Moraxella catarrhalis,
Staphylococcus aureus,
Serratia spp.,
Enterobacter spp., and
Candida spp. as fungal isolates. Data regarding the bacterial or fungal infection associated with viral pneumonia caused by coronavirus are limited. All the isolated pathogens show sensitivity against the selected plant extracts used. Plant-based products have recently demonstrated massive potential in designing therapeutics and diagnostics [
43,
44]. Herbal intervention for respiratory illness associated with cough provides estimates of the effectiveness of treatments and their associated side effects [
45]. Increasing demand for the bioprospection of plants for drugs is steadily escalating, which entails screening medicinal plants with promising biological activity. Medicinal plants are the gift of nature to cure a number of diseases amongst human beings [
46]. Three medicinal plants are used in our study, viz.
Moringa oleifera, Cassia fistula, and
Adhatoda vasica.
Moringa oleifera seed oil (MOSO) can be considered for the production of organic surface coating resins. The polyester-amide (MOPEA) of MOSO was successfully synthesized and characterized, and it showed excellent antimicrobial inhibitive properties. The remarkable solubility property of MOPEA in most tested solvents reveals its thermal stability and indicates how promising the resin will become when applied in the surface coating industry [
22]. The use of
Cassia fistula as standardized formulations in the form of hand sanitizers and soaps has been widely used recently, and it needs further optimization for it to be used as a disinfectant and antiseptic. Apart from that,
Cassia fistula seed gum has been used as a film coating material for drugs as well as for a variety of other pharmaceutical and bioengineering coating purposes [
18]. The applicability of the
Adhatoda vasica methanol extract in the preparation of medicinal liquid-based soaps, which demonstrated substantial antibacterial activity in liquid form compared to the dry extract type, has been well documented in previous studies [
24]. The extract of the
Adhatoda vasica effectively prevented the corrosion of a steel surface in a 0.5 M H
2SO
4 solution. The anticorrosion activity of the
Adhatoda vasica extract increased significantly on increasing the concentration of extract and decreased with a rise in temperature while the potentiodynamic polarization measurement study confirmed its mixed-type inhibition activity [
25].
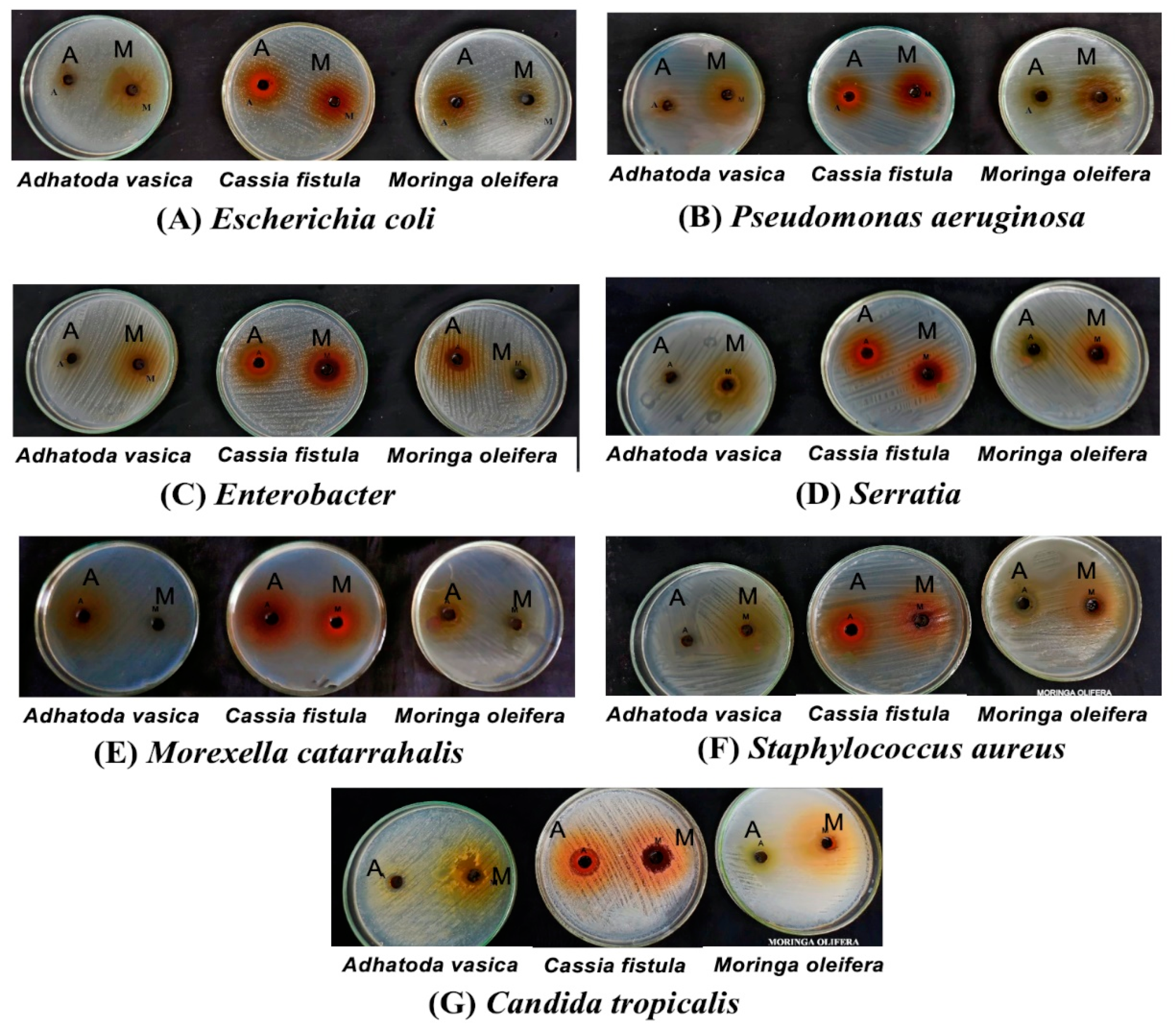
Pathogenic isolates are tested against acetone and methanol extracts from selected medicinal plants, which showed a broad range of antimicrobial activity, marking a 100% sensitivity against the isolated pathogens including MDR isolates. Acetone and the methanol extract of
Moringa oleifera showed the highest zone of inhibition (17.3 ± 0.3, 17.6 ± 0.6) against MDR isolates.
M. oleifera extracts from seeds, roots, leaves, and a mixture in ethyl acetate, acetone, and ethanol as solvents were respectively assessed for antibacterial and antifungal potential on infected dental patients [
47]. However, Rani et al. exhibited the
Moringa extract to be a stronger inhibitor against gram-positive species than gram-negative species. The compound 4-[(-
l-rhamnosyloxy)-benzyl] isothiocyanate is responsible for the antimicrobial activity of
M. oleifera against
Staphylococcus aureus,
Staphylococcus epidermis,
Bacillus subtilis,
Epidermophyton floccosum, and
Trichophyton rubrum, as shown by [
19]. Acetone extract of
Cassia fistula gives the highest zone of inhibition (18.6 ± 0.3) against
S. aureus. Different parts of
C. fistula are used as an important source of naturally occurring bioactive compounds and show activity against a wide range of pathogenic microorganisms [
48]. However, the acetone extract of
Adhatoda vasica gives the highest zone of inhibition (17.0 ± 0.0) against
Pseudomonas aeruginosa.
A. vasica has been used indigenously by natives as folkloric medicine for ages, especially in the treatment of respiratory disorders such as coughs, colds, asthma, etc. [
23]. Bag et al. showed that different parts of
Adhatoda vasica are used against a wide range of chronic infections, amongst which the leaves show potential activity in COVID-19 patients as shown by [
49], consequently manifesting strong evidence about plant extracts as more effective than the available antibiotics by showing their full sensitivity against respiratory tract infected microbial pathogens. Herbal drugs present more effective and safer options for upper respiratory tract infection (URTI) patients and are recognized as key materials for future research [
45]. A recent increase in antibiotic resistance amongst major pathogenic groups including respiratory tract infections (RTI) has made alarming concerns in the diagnosis and treatment with the existing therapeutics. Hence, alternative treatment strategies and effective prognosis of RTI is the urgent need of the hour. Significant immunomodulatory and anti-inflammatory activities by plants used in our study also have been reported, which increase in manifolds their efficacy in treating patients with multifactorial disorders, thereby proving their multifunctional potential [
50]. The medicinal plants used in the study has tremendous potential to be used by industry experts in various forms, such as toothpaste, mouth wash, water purifying agents, tablet coatings, tissue-engineered wound dressing materials, as well as in the form of surface coatings of steel and other biomedical engineered devices and nanotechnological based bioproducts [
19].
As per the Indian system of medicine, the present protocol by the Ministry of Ayurveda, Yoga and Naturopathy, Unani, Siddha and Homoeopathy (AYUSH) in India has recommended the use of plants such as
Moringa oleifera and
Adhatoda vasica for administration to patients with mild infection of COVID-19 who are in quarantine [
13]. As per our study, we also suggest and recommend the use of a magical trio of
Moringa oleifera (
antiviral) and
Adhatoda vasica (
antibacterial) along with
Cassia fistula (
antifungal) in asymptomatic as well as symptomatic patients along with patients having any secondary or respiratory co-infections. Accordingly, our study will provide a scientific evidence-based proactive strategy in treating COVID-19 cases in the future, thereby supplementing the present AYUSH protocol with cheaper and effective dosages easily available within a given treatment regimen. In the context with the recent Covid-19 pandemic, more multidisciplinary approaches such as artificial intelligence [
51,
52] and nanotechnology-enabled approaches could be front-runners to fight against the transmission as well as advocate the prevention and therapeutics of such cases. Further, we have to understand the recently emerging complex cellular physiology of the bacteria [
53,
54] and infectious viruses to implement better preventive foresight.