Traditional Herbal Remedies with a Multifunctional Therapeutic Approach as an Implication in COVID-19 Associated Co-Infections
Abstract
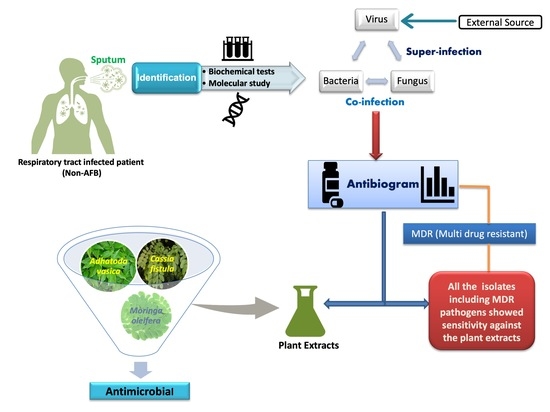
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Processing of Samples
2.3. Identification of the Pathogens
2.4. Antibiotics and Antifungals
2.5. Antibiotic and Antifungal Susceptibility Test
2.6. Preparation of Plant Extracts
2.7. Test for Antimicrobial Activity of Selected Plant Extracts
2.8. Determination of Minimum Inhibitory Concentration (MIC)
2.9. Statistical Analysis
3. Results
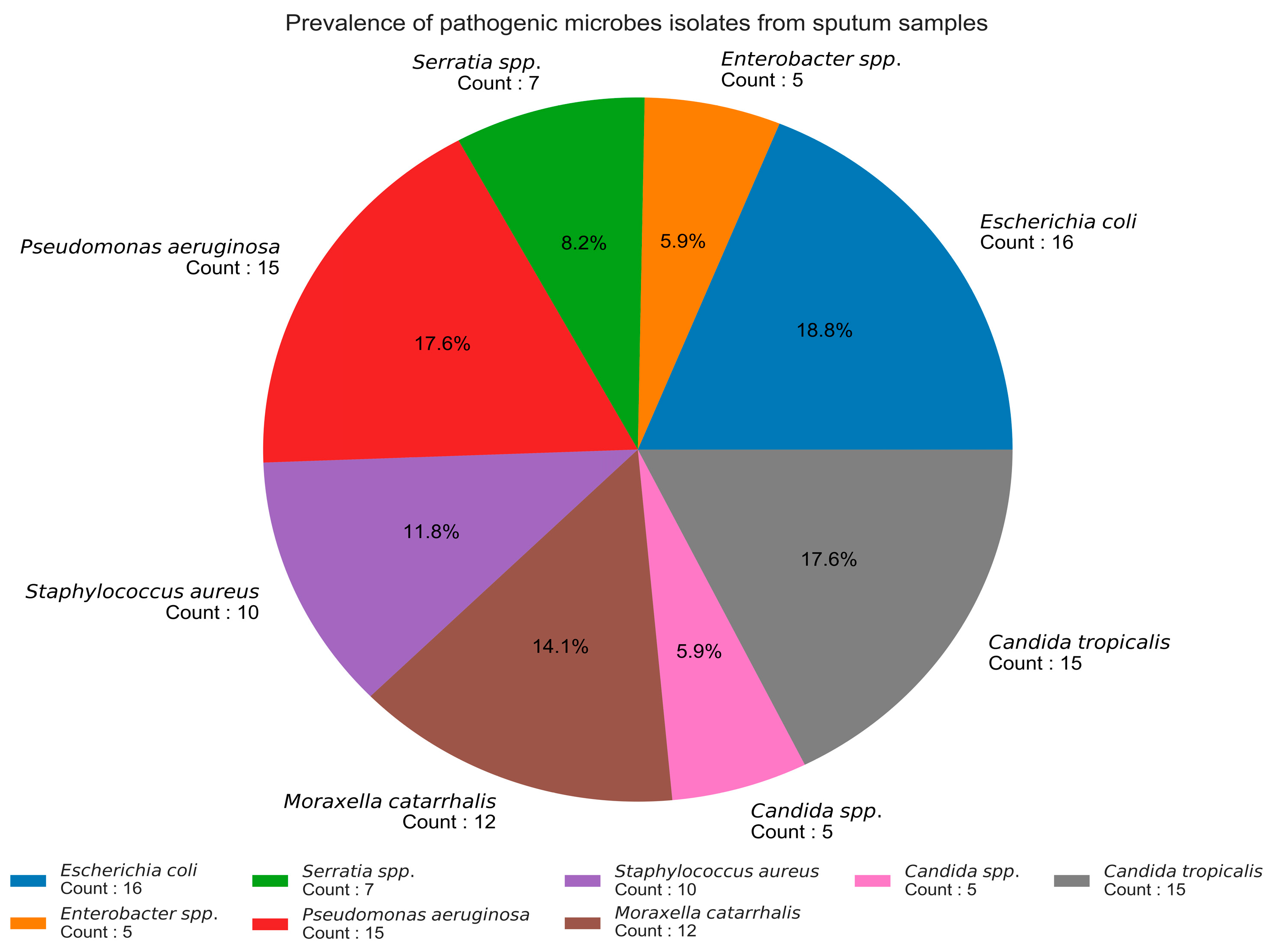
3.1. Prevalence of Pathogens in Sputum Samples
3.2. Antibiotic Susceptibility Pattern of Isolates (Antibiogram)
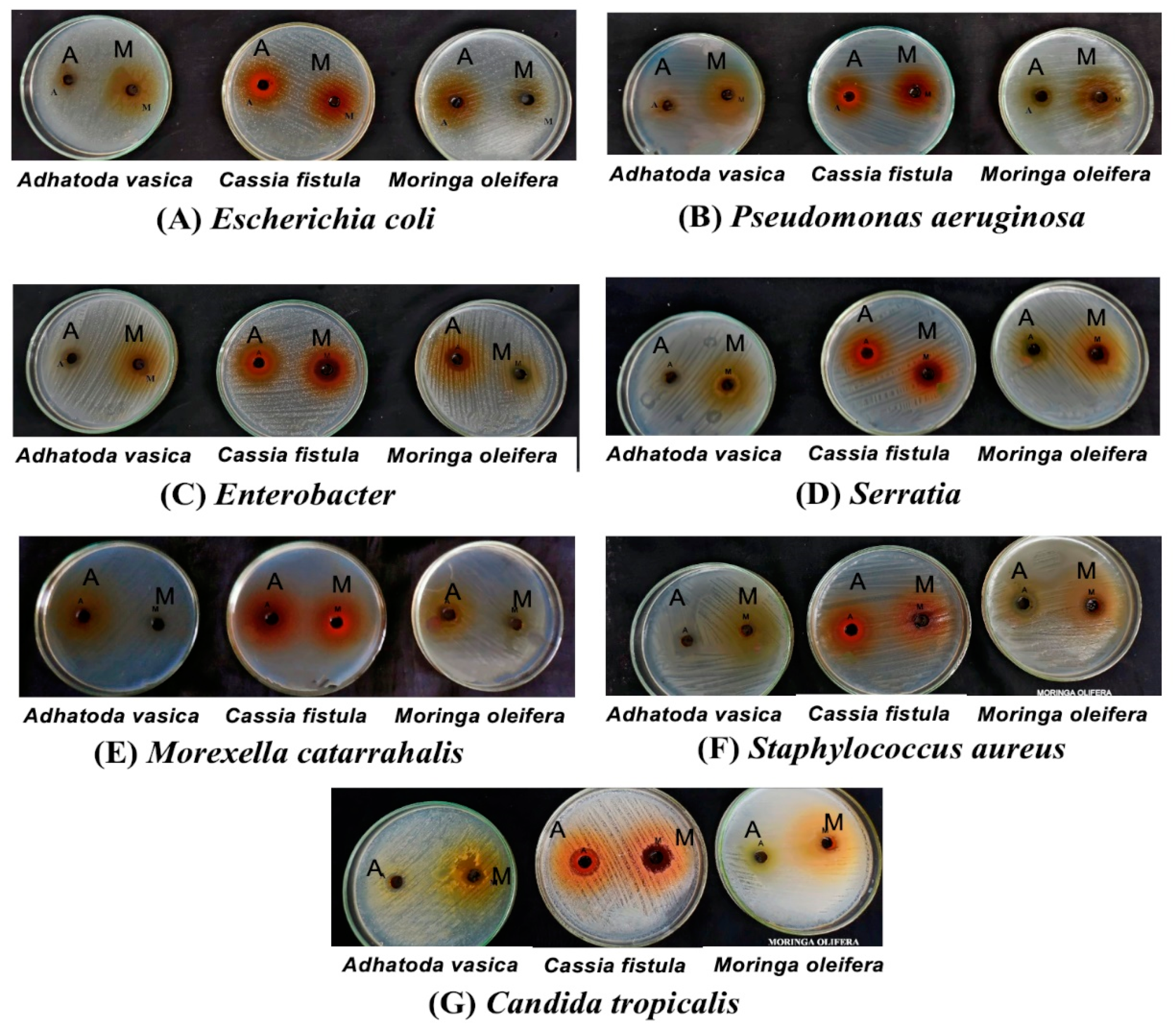
3.3. Antimicrobial Activity of Moringa Oleifera, Cassia Fistula, and Justicia Adhatoda (Adhatoda Vasica)
3.4. MIC Values of the Plant Extract, Viz. Moringa Oleifera, Adhatoda Vasica, and Cassia Fistula, against the Isolated Pathogens
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Data Availability Statement
References
- Sohrabi, C.; Alsafi, Z.; O’Neill, N.; Khan, M.; Kerwan, A.; Jabir, A.A.; Losifidis, C.; Agha, R. World Health Organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19). Int. J. Surg. 2020, 76, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 10229, 1054–1062. [Google Scholar] [CrossRef]
- Cox, M.J.; Loman, N.; Bogaert, D.; O’Grady, J. Co-infections: Potentially lethal and unexplored in COVID-19. Lancet Microbe. 2020, 1, e11. [Google Scholar] [CrossRef]
- Zhou, P.; Liu, Z.; Chen, Y.; Xiao, Y.; Huang, X.; Fan, X.G. Bacterial and fungal infections in COVID-19 patients: A matter of concern. Infect. Control Hosp. Epidemiol. 2020, 1–2. [Google Scholar] [CrossRef]
- MacIntyre, C.R.; Chughtai, A.A.; Barnes, M.; Ridda, I.; Seale, H.; Toms, R.; Heywood, A. The role of pneumonia and secondary bacterial infection in fatal and serious outcomes of pandemic influenza a (H1N1) pdm09. BMC Infect. Dis. 2018, 1, 637. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Chen, R.; Li, Y. The clinical characteristics of secondary infection of lower respiratory in severe acute respiratory syndrome. Chin. J. Respir. Crit. Care Med. 2003, 2, 270–274. [Google Scholar]
- Bengoechea, J.A.; Bamford, C.G.G. SARS-CoV-2, bacterial co-infections, and AMR: The deadly trio in COVID-19? EMBO Mol. Med. 2020. [Google Scholar] [CrossRef]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 5, 475–481. [Google Scholar] [CrossRef]
- Zafar, A.; Hussain, Z.; Lomama, E.; Sibiie, S.; Irfan, S.; Khan, E. Antibiotic susceptibility of pathogens isolated from patients with community-acquired respiratory tract infections in Pakistan- the active study. J. Ayub Med. Coll. 2008, 1, 7–9. [Google Scholar]
- Jacobs, E.; Dalhoff, A.; Korfmann, G. Susceptibility patterns of bacterial isolates from hospitalised patients with respiratory tract infections. Int. J. Antimicrob. Agents 2009, 1, 52–57. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Jyotirmoy, S.; Rekha, S.D. Concept of epidemic diseases in ayurveda. IJHRMLP 2016, 2, 24. [Google Scholar]
- Rastogi, S.; Pandey, D.N.; Singh, R.H. COVID-19 pandemic: A pragmatic plan for ayurveda intervention. J. Ayurveda Integr. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, J.E.; Gay, K.; Barrett, T.J.; Medalla, F.; Chiller, T.M.; Angulo, F.J. Increase in nalidixic acid resistance among non-typhi Salmonella enterica isolates in the United States from 1996 to 2003. Antimicro. Agents Chemother. 2007, 51, 195–197. [Google Scholar] [CrossRef] [PubMed]
- Nostro, A.; Cellini, L.; Di Bartolomeo, S.; Cannatelli, M.A.; Di Campli, E.; Procopio, F. Effects of combining extracts (from propolis or Zingiber officinale) with clarithromycin on Helicobacter pylori. Phytother. Res. 2006, 3, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Bahorun, T.; Neergheen, V.S.; Aruoma, O.I. Phytochemical constituents of Cassia fistula. Afr. J. Biotech. 2005, 4, 1530–1540. [Google Scholar]
- Chaerunisaa, A.Y.; Susilawati, Y.; Muhaimin, M.; Milanda, T.; Hendriani, R.; Subarnas, A. Antibacterial activity and subchronic toxicity of Cassia fistula L. barks in rats. Toxicol. Rep. 2020, 7, 649–657. [Google Scholar] [CrossRef]
- Killedar, G.S.; Nale, B.A.; More, H.N.; Nadaf, J.S.; Pawar, A.A.; Tamboli, S.U. Isolation, characterization, and evaluation of Cassia fistula Linn. seed and pulp polymer for pharmaceutical application. Int. J. Pharm. Investig. 2014, 4, 215. [Google Scholar] [CrossRef]
- Rani, N.Z.A.; Husain, K.; Kumolosasi, E. Moringa genus: A review of phytochemistry and pharmacology. Front. Pharmacol. 2018, 9, 1–26. [Google Scholar]
- Lockett, C.T.; Calvet, C.C.; Grivetti, L.E. Energy and micronutrient composition of dietary and medicinal wild plants consumed during drought. Study of rural Fulani, Northeastern Nigeria. Int. J. Food Sci. Nutr. 2000, 3, 195–208. [Google Scholar]
- Gangwar, K.A.; Ghosh, K.A. Medicinal uses and Pharmacological activity of Adhatoda vasica. Int. J. Herb. Med. 2014, 1, 88–91. [Google Scholar]
- Siyanbola, T.; James, O.O.; Gurunathan, T.; Sasidhar, K.; Ajanaku, K.O.; Ogunniran, K.O.; Adekoya, J.A.; Olasehinde, G.I.; Ajayi, A.A.; Olaofe, O. Synthesis, Characterization and Antimicrobial Evaluation of Polyesteramide Resin from Moringa Oleifera Seed Oil (Moso) for Surface Coating Application. CJPAS Can. J. Pure Appl. Sci. 2015, 1, 3229–3240. [Google Scholar]
- Shukla, S.; Ahirwal, L.; Bajpai, V.K.; Huh, Y.S.; Han, Y.K. Growth inhibitory effects of Adhatoda vasica and its potential at reducing Listeria monocytogenes in chicken meat. Front. Microbiol. 2017, 8, 1–13. [Google Scholar]
- Wijetunge, W.M.A.N.K.; Perera, B.G.K. Preparation of Liquid Medicinal Soap Products Using Adhatoda vasica (Adhatoda) Leaf Extracts. IJMS Int. J. Multidiscip. Stud. 2015, 2, 71. [Google Scholar] [CrossRef]
- Singh, R.M. A green Approach: A corrosion inhibition of mild steel by Adhatoda vasica plant extract in 0.5 M H2SO4. J. Mater. Environ. Sci. 2013, 1, 117–126. [Google Scholar]
- Holt, J.G.; Krieg, N.G.; Sneathm, P.H.A.; Staley, J.T.; Williams, S.T. Bergey’s Manual of Determinative Bacteriology, 9th ed.; William & Wilkins: Philadelphia, PA, USA, 1994. [Google Scholar]
- Zapata, A.; Ramirez, A.S. A Comparative Study of McFarland Turbidity Standards and the Densimat Photometer to Determine Bacterial Cell Density. Curr. Microbiol. 2015, 6, 907–909. [Google Scholar] [CrossRef]
- Hudzicki, J. Kirby-Baurer Disk Diffusion Susceptibility Test Protocol. Am. Soc. Microbiol. 2009. Available online: https://asm.org/getattachment/2594ce26-bd44-47f6-8287-0657aa9185ad/Kirby-Bauer-Disk-Diffusion-Susceptibility-Test-Protocol-pdf.pdf (accessed on 9 July 2020).
- Razmavar, S.; Abdulla, A.M.; Ismail, B.S.; Hassandarvish, P. Antibacterial Activity of Leaf Extracts of Baeckea frutescens against Methicillin-Resistant Staphylococcus aureus. Biomed. Res. Int. 2014, 2014, 5. [Google Scholar] [CrossRef]
- Meignanalakshmi, S.; Kumar, V.S.; Deepika, J.; Begum, F.I. Evaluation of antibacterial activity of methanol extract of leaves of Adhatoda vasica on mastitis pathogens. Hygeia J. Drugs Med. 2013, 1, 1–4. [Google Scholar]
- Obeidat, M.; Shatnawi, M.; Alawi, A.M.; Bi, Z.A.E.; Dmoor, A.H.; Qudah, A.M.; Qudah, E.H.; Otri, I. Antimicrobial activity of crude extracts of some plant leaves. Res. J. Microbiol. 2012, 1, 59–67. [Google Scholar] [CrossRef]
- Das, K.; Tiwari, R.K.S.; Shrivastava, D.K. Techniques for evaluation of medicinal plant products as antimicrobial agent: Current methods and future trends. J. Med. Plants Res. 2010, 2, 104–111. [Google Scholar]
- Atef, N.M.; Shanab, S.M.; Negm, S.I.; Abbas, Y.A. Evaluation of antimicrobial activity of some plant extracts against antibiotic susceptible and resistant bacterial strains causing wound infection. Bull. Nat. Res. Cent. 2019, 43, 144. [Google Scholar] [CrossRef]
- Vishwanath, S.; Chawla, K.; Gopinathan, A. Multidrug resistant Gram-negative bacilli in lower respiratory tract infections. Iran. J. Microbiol. 2013, 4, 323–327. [Google Scholar]
- Amiri, M.R.J.; Siami, R.; Khaledi, A. Tuberculosis Status and Coinfection of Pulmonary Fungal Infections in Patients Referred to Reference Laboratory of Health Centers Ghaemshahr City during 2007–2017. Ethiop. J. Health Sci. 2018, 6, 683–690. [Google Scholar]
- Terraneo, S.; Ferrer, M.; Martín-Loeches, I.; Esperatti, M.; Di Pasquale, M.; Giunta, V.; Rinaudo, M.; Rosa, F.D.; Bassi, L.G.; Centanniet, S. Impact of Candida spp. isolation in the respiratory tract in patients with intensive care unit-acquired pneumonia. Clin. Microbiol. Infect. 2016, 1, 94.e1–94.e8. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.W.; Li, G.; Zheng, M.; Kaur, H.; Magbual, N.; Dalai, S. Superinfections and Coinfections in COVID-19. MedPage Today. 2020. Available online: https://www.medpagetoday.com/infectiousdisease/covid19/86192 (accessed on 9 July 2020).
- Morris, D.E.; Cleary, D.W.; Clarke, S.C. Secondary bacterial infections associated with influenza pandemics. Front. Microbiol. 2017, 8, 1–17. [Google Scholar] [CrossRef]
- Gemmati, D.; Bramanti, B.; Serino, M.L.; Secchiero, P.; Zauli, G.; Tisato, V. COVID-19 and Individual Genetic Susceptibility/Receptivity: Role of ACE1/ACE2 Genes, Immunity, Inflammation and Coagulation. Might the Double X-chromosome in Females Be Protective against SARS-CoV-2 Compared to the Single X-Chromosome in Males? Int. J. Mol. Sci. 2020, 10, 3474. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.M.; Qiao, L.X.; Ai, L.; Zhai, J.J.; Wang, X.X. Isolation of antimicrobial resistant bacteria in upper respiratory tract infections of patients. 3 Biotech 2016, 2, 166. [Google Scholar]
- Budayanti, N.S.; Suryawan, K.; Iswari, I.S.; Sukrama, D.M. The quality of sputum specimens as a predictor of isolated bacteria from patients with lower respiratory tract infections at a Tertiary Referral Hospital, Denpasar, Bali-Indonesia. Front. Med. 2019, 6, 1–6. [Google Scholar] [CrossRef]
- Chartier, L.; Leng, C.; Sire, J.M.; Le Minor, O.; Saman, M.; Bercion, R.; Rahalison, L.; Fontanet, A.; Germany, Y.; L’Her, P.; et al. Factors Associated with Negative Direct Sputum Examination in Asian and African HIV-Infected Patients with Tuberculosis (ANRS 1260). PLoS ONE 2011, 6, 2–7. [Google Scholar] [CrossRef]
- Shelar, A.; Sangshetti, J.; Chakraborti, S.; Singh, A.V.; Patil, R.; Gosavi, S. Helminthicidal and Larvicidal Potentials of Biogenic Silver Nanoparticles Synthesized from Medicinal Plant Momordica charantia. Med. Chem. 2019, 15, 781. [Google Scholar]
- Ansari, M.H.D.; Lavhale, S.; Kalunke, M.R.; Srivastava, L.P.; Pandit, V.; Gade, S.; Yadav, S.; Peter Laux, P.; Luch, A.; Gemmati, D.; et al. Recent Advances in Plant Nanobionics and Nanobiosensors for Toxicology Applications. Curr. Nanosci. 2020, 16, 27. [Google Scholar] [CrossRef]
- Lopes, C.L.; Silva, O.C.M.; Motta, B.C.; Quirós, M.A.; Biavatti, W.M.; Jardel Corrêa de Oliveira, D.C.J.; Guyatt, G. Brazilian medicinal plants to treat upper respiratory tract and bronchial illness: Systematic review and meta analyses—Study protocol. BMJ 2014, 7, e005267. [Google Scholar] [CrossRef] [PubMed]
- Prasannabalaji, N.; Muralitharan, G.; Sivanandan, R.N.; Kumaran, S.; Pugazhvendan, S.R. Antibacterial activities of some Indian traditional plant extracts. Asian Pac. J. Trop. Dis. 2012, 2 (Suppl. S1), S291–S295. [Google Scholar] [CrossRef]
- Eilert, U.; Wolters, B.; Nahrstedt, A. The antibiotic principle of seeds of Moringa oleifera and Moringa stenopetala. Planta Med. 2007, 42, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Thirumal, M.; Srimanthula, S.; Kishore, G. Cassia fistula Linn—Pharmacognostical, Phytochemical and Pharmacological Review. Crit. Rev. Pharm. Sci. 2012, 1, 43–65. [Google Scholar]
- Bag, A.; Bag, A. Treatment of COVID-19 patients: Justicia adhatoda leaves extract is a strong remedy for COVID-19—Case report analysis and docking based study. ChemRxiv 2020. [Google Scholar] [CrossRef]
- Duin, D.V.; Paterson, D. Multidrug-Resistant Bacteria in the Community: Trends and Lessons Learned. Infect. Dis Clin. N. Am. 2010, 2, 377–390. [Google Scholar]
- Singh, A.V.; Ansari, M.H.D.; Rosenkranz, D.; Maharjan, R.S.; Kriegel, F.L.; Gandhi, K.; Kanase, A.; Singh, R.; Laux, P.; Luch, A. Artificial Intelligence and Machine Learning in Computational Nanotoxicology: Unlocking and Empowering Nanomedicine. Adv. Healthc. Mater. 2020, 1–19. [Google Scholar] [CrossRef]
- Singh, A.V.; Ansari, M.H.D.; Laux, P.; Luch, A. Micro-nanorobots: Important considerations when developing novel drug delivery platforms. Expert Opin. Drug Del. 2019, 11, 1259–1275. [Google Scholar] [CrossRef]
- Singh, A.V.; Kishore, V.; Santomauro, G.; Yasa, O.; Bill, J.; Sitti, M. Mechanical Coupling of Puller and Pusher Active Microswimmers Influences Motility. Langmuir 2020, 19, 5435–5443. [Google Scholar] [CrossRef]
- Singh, A.V.; Ansari, M.H.D.; Mahajan, M.; Srivastava, S.; Kashyap, S.; Dwivedi, P.; Pandit, V.; Katha, U. Sperm cell driven microrobots-Emerging opportunities and challenges for biologically inspired robotic design. Micromachines 2020, 11, 448. [Google Scholar] [CrossRef] [PubMed]
| Antibiotics | Pseudomonas Aeruginosa | Enterobacter | Serratia | E. coli | Moraxella Catarrhalis | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| S (%) | R (%) | S (%) | R (%) | S (%) | R (%) | S (%) | R (%) | S (%) | R (%) | |
| ERY (15 µg) | 20 | 80 | 60 | 40 | 57.4 | 42.8 | 12.5 | 87.5 | 0 | 100 |
| CIP (10 µg) | 93.3 | 6.6 | 80 | 20 | 85.7 | 14.2 | 12.5 | 87.5 | 8.3 | 91.6 |
| OFX (10 µg) | 86.6 | 13.3 | 60 | 40 | 71.4 | 28.5 | 43.7 | 56.2 | 83.3 | 16.6 |
| GEM (10 µg) | 86.6 | 13.3 | 80 | 20 | 85.7 | 14.2 | 6.2 | 93.7 | 91.6 | 8.3 |
| PIP (100 µg) | 6.6 | 93.3 | 60 | 40 | 28.5 | 71.4 | 12.5 | 87.5 | 16.6 | 83.3 |
| CXM (25 µg) | 26.6 | 73.3 | 20 | 80 | 57.4 | 42.8 | 18.7 | 81.2 | 8.3 | 91.6 |
| IPM (10 µg) | 80 | 20 | 80 | 20 | 71.4 | 28.5 | 25% | 75 | 83.3 | 16.6 |
| AMP (30 µg) | 33.3 | 66.6 | 60 | 40 | 28.5 | 71.4 | 18.7 | 81.2 | 16.6 | 83.3 |
| CHL (30 µg) | 20 | 80 | 60 | 40 | 85.7 | 14.2 | 12.5 | 87.5 | 16.6 | 83.3 |
| Total | 15 | 5 | 7 | 16 | 12 | |||||
| StaphylococcusAureus | ||
|---|---|---|
| Antibiotics | S (%) | R (%) |
| ERY (15 µg) | 20.0 | 80.0 |
| GEM (10 µg) | 70.0 | 30.0 |
| CXM (25 µg) | 40.0 | 60.0 |
| IPM (10 µg) | 90.0 | 10.0 |
| AMP (30 µg) | 60.0 | 40.0 |
| PEN (10 µg) | 70.0 | 30.0 |
| VAN (30 µg) | 10.0 | 90.0 |
| CO (25 µg) | 20.0 | 80.0 |
| OXA (5 µg) | 60.0 | 40.0 |
| Total | 10 | |
| Antifungals | Candida Tropicalis |
|---|---|
| CLT (10 µg) | R |
| AMB (100 µg) | S |
| KTC (10 µg) | R |
| NYT (100 µg) | I |
| FLC (10 µg) | I |
| ITC (10 µg) | S |
| S. No. | Plant Name | Family Name | Common Name | Parts of the Plant Used |
|---|---|---|---|---|
| 1 | Moringa Oleifera (Lam.) | Moringaceae | Moringa, drumstick tree | Leaves |
| 2 | Cassia Fistula (L.) | Fabaceae | Golden shower, Purging cassia, Indian laburnum pudding-pipe tree | Leaves |
| 3 | Adhatoda Vasica (L.) | Acanthaceae | Malabar Nut (Justicia adhatoda), Adusa | Leaves |
| Pathogenic Isolates | Plant Extracts | |||||
|---|---|---|---|---|---|---|
| Moringa Oleifera | Cassia Fistula | Adhatoda Vasica | ||||
| Acetone | Methanol | Acetone | Methanol | Acetone | Methanol | |
| Pseudomonas aeruginosa | 14.6 ± 0.3 | 16.6 ± 0.6 | 14.3 ± 0.6 | 11.3 ± 0.6 | 17.0 ± 0.0 | 13.3 ± 0.6 |
| Escherichia coli | 12.6 ± 0.3 | 14.3 ± 0.6 | 16.3 ± 0.6 | 13.6 ± 0.3 | 15.6 ± 0.3 | 12.3 ± 0.3 |
| Moraxella catarrhalis | 17.3 ± 0.3 | 17.6 ± 0.6 | 14.6 ± 0.6 | 14.6 ± 0.6 | 13.3 ± 0.3 | 18.3 ± 0.3 |
| Serratia spp. | 16.0 ± 0.5 | 16.3 ± 0.3 | 16.6 ± 0.3 | 13.0 ± 0.5 | 16.3 ± 0.3 | 11.3 ± 0.3 |
| Enterobacter spp. | 12.3 ± 0.3 | 17.6 ± 0.3 | 13.6 ± 0.3 | 15.6 ± 0.3 | 14.6 ± 0.3 | 15.0 ± 0.5 |
| Staphylococcus aureus | 14.6 ± 0.3 | 12.6 ± 0.3 | 18.6 ± 0.3 | 12.3 ± 0.3 | 13.6 ± 0.3 | 11.6 ± 0.3 |
| Candida tropicalis | 15.3 ± 0.3 | 11.3 ± 0.3 | 17.0 ± 0.5 | 17.6 ± 0.3 | 12.3 ± 0.3 | 13.6 ± 0.3 |
| Pathogenic Isolates | MIC Value (mg/mL) | |||||
|---|---|---|---|---|---|---|
| Moringa Oleifera | Adhatoda Vasica | Cassia Fistula | ||||
| Acetone | Methanol | Acetone | Methanol | Acetone | Methanol | |
| Pseudomonas aeruginosa | 125 | 62.5 | 62.5 | 62.5 | 62.5 | 125 |
| Escherichia coli | 125 | 125 | 62.5 | 125 | 15.62 | 62.5 |
| Moraxella catarrhalis | 62.5 | 62.5 | 125 | 31.25 | 62.5 | 62.5 |
| Serratia spp. | 31.25 | 31.25 | 31.25 | 62.5 | 31.25 | 7.81 |
| Enterobacter spp. | 62.5 | 31.25 | 62.5 | 31.25 | 15.62 | 7.81 |
| Staphylococcus aurus | 62.5 | 62.5 | 15.62 | 31.25 | 15.62 | 15.62 |
| Candida tropicalis | 62.5 | 125 | 125 | 125 | 15.62 | 31.25 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiwari Pandey, A.; Pandey, I.; Zamboni, P.; Gemmati, D.; Kanase, A.; Singh, A.V.; Singh, M.P. Traditional Herbal Remedies with a Multifunctional Therapeutic Approach as an Implication in COVID-19 Associated Co-Infections. Coatings 2020, 10, 761. https://doi.org/10.3390/coatings10080761
Tiwari Pandey A, Pandey I, Zamboni P, Gemmati D, Kanase A, Singh AV, Singh MP. Traditional Herbal Remedies with a Multifunctional Therapeutic Approach as an Implication in COVID-19 Associated Co-Infections. Coatings. 2020; 10(8):761. https://doi.org/10.3390/coatings10080761
Chicago/Turabian StyleTiwari Pandey, Aprajita, Ishan Pandey, Paolo Zamboni, Donato Gemmati, Anurag Kanase, Ajay Vikram Singh, and Mohan Prasad Singh. 2020. "Traditional Herbal Remedies with a Multifunctional Therapeutic Approach as an Implication in COVID-19 Associated Co-Infections" Coatings 10, no. 8: 761. https://doi.org/10.3390/coatings10080761
APA StyleTiwari Pandey, A., Pandey, I., Zamboni, P., Gemmati, D., Kanase, A., Singh, A. V., & Singh, M. P. (2020). Traditional Herbal Remedies with a Multifunctional Therapeutic Approach as an Implication in COVID-19 Associated Co-Infections. Coatings, 10(8), 761. https://doi.org/10.3390/coatings10080761