Directly-Patternable Bi2O3 Nanoparticle for Polymer Nanocomposite Capacitor
Abstract
1. Introduction
2. Materials and Methods
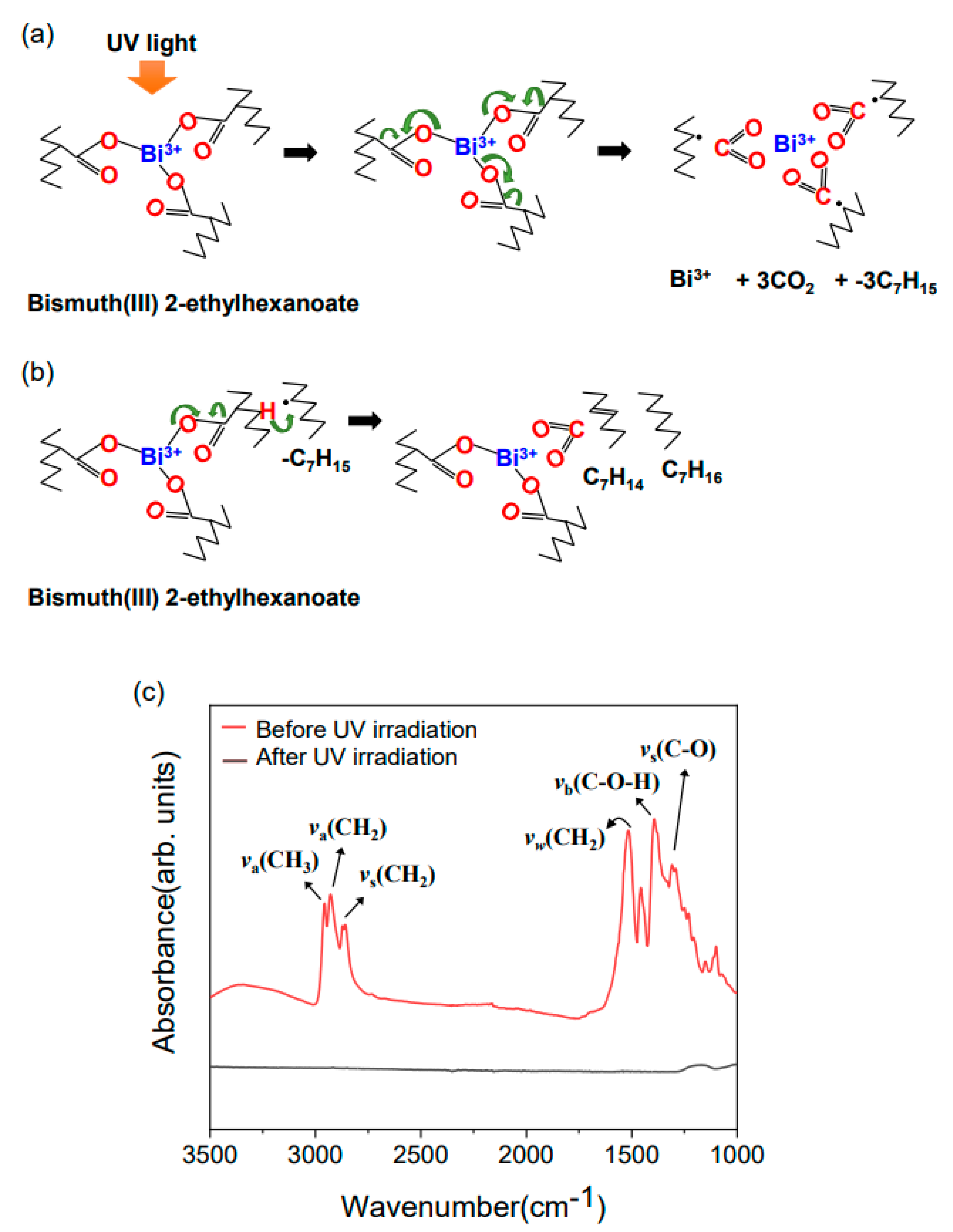
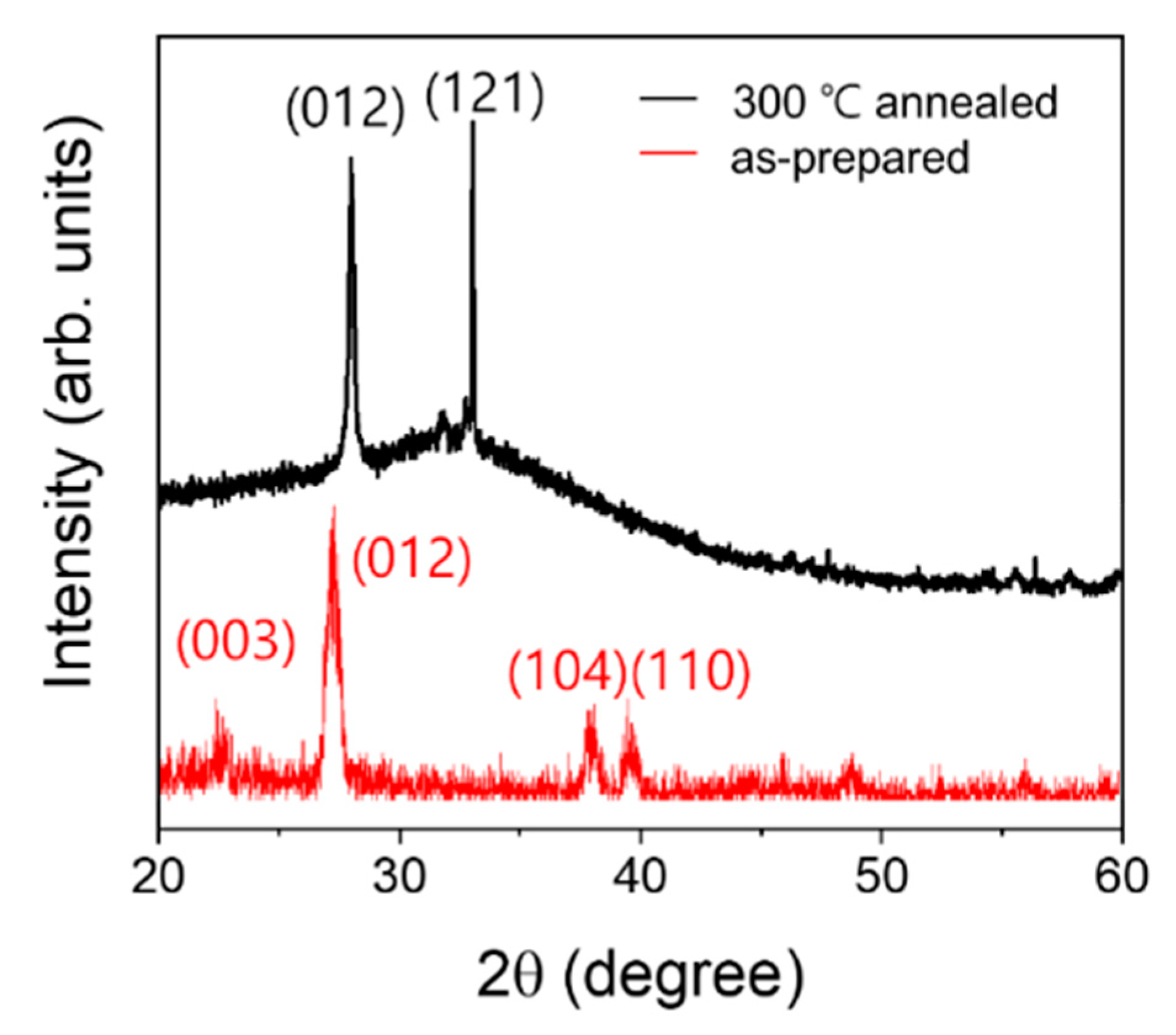
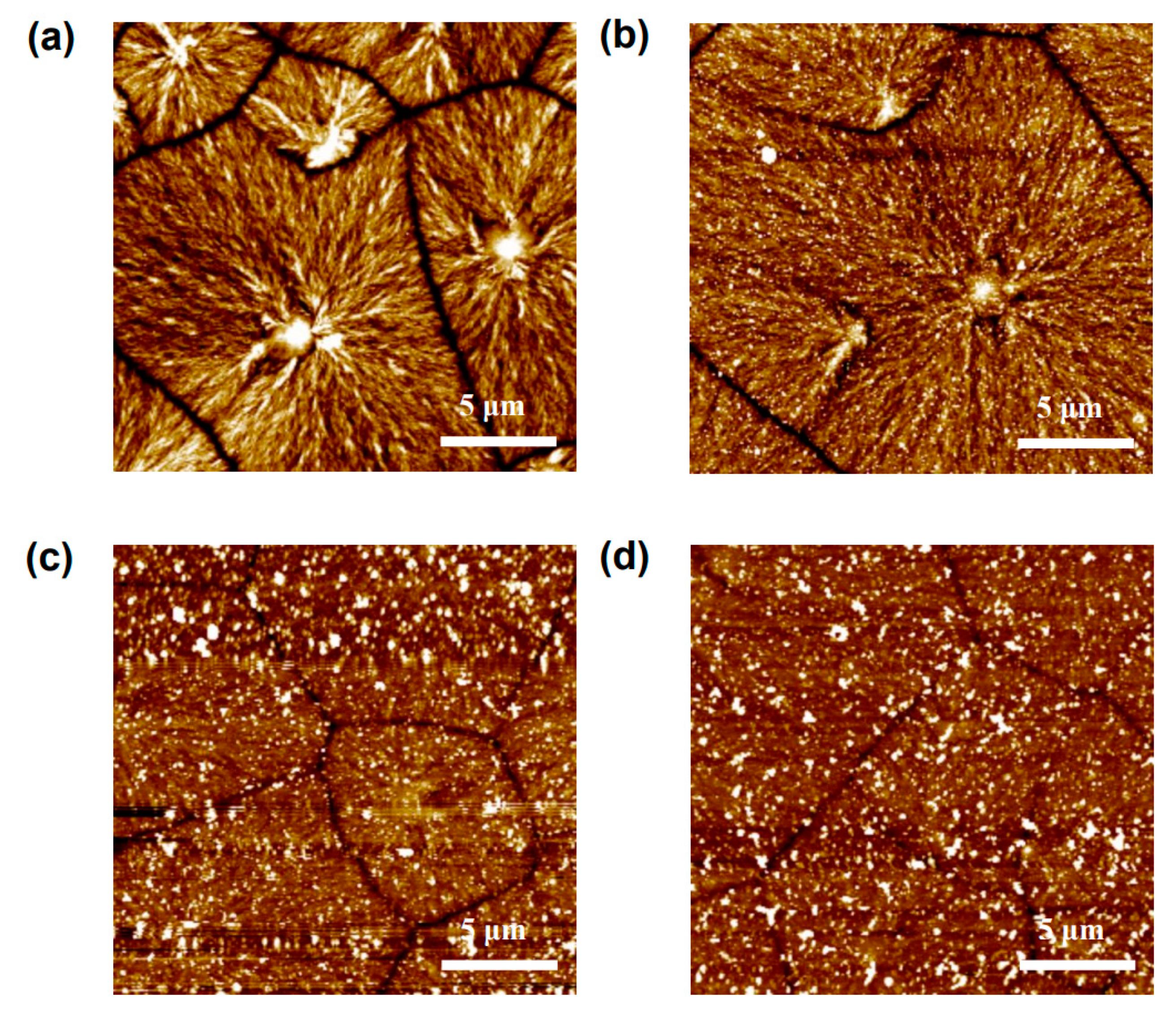
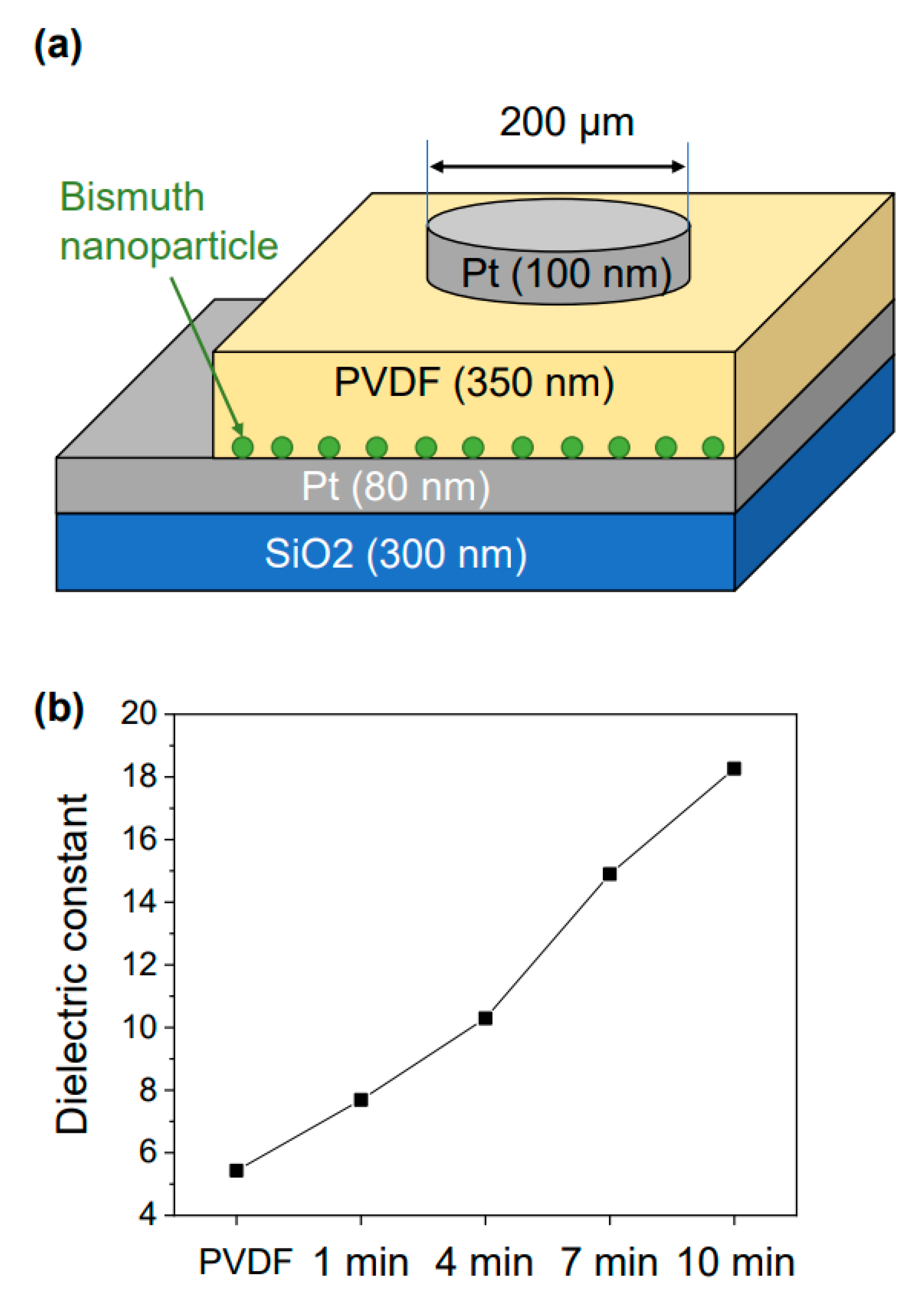
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, P.; Xue, S.; Wang, J. New challenges of miniaturization of electronic devices: Electromigration and thermomigration in lead-free solder joints. Mater. Des. 2020, 192, 108726. [Google Scholar] [CrossRef]
- Annuar, S.; Mahmoodian, R.; Hamdi, M.; Tu, K.N. Intermetallic compounds in 3D integrated circuits technology: A brief review. Sci. Technol. Adv. Mater 2017, 18, 693–703. [Google Scholar] [CrossRef]
- Hwang, J.S.; Kim, W.S.; Park, H.H.; Kim, T.S. The effect of intermediate anneal on the ferroelectric properties of direct-patternable PZT films. Sens. Actuators A Phys. 2005, 117, 137–142. [Google Scholar] [CrossRef]
- Grove, T.T.; Masters, M.F.; Miers, R.E. Determining dielectric constants using a parallel plate capacitor. Am. J. Phys. 2005, 73, 52–56. [Google Scholar] [CrossRef]
- Baojin, C.; Xin, Z.; Kailiang, R.; Bret, N.; Minren, L.; Qing, W.; Bauer, F.; Zhang, Q.M. A dielectric polymer with high electric energy density and fast discharge speed. Science 2006, 313, 334–336. [Google Scholar]
- Xie, L.Y.; Huang, X.Y.; Jiang, P.K.; Huang, Y.H.; Yang, K. Core-shell structured hyperbranched aromatic polyamide/BaTiO3 hybrid filler for poly (vinylidene fluoride-trifluoroethylene -chlorofluoroethylene) nanocomposites with the dielectric constant comparable to that of percolative composites. Appl. Mater. Interface 2013, 5, 1747–1756. [Google Scholar] [CrossRef]
- Joyce, D.M.; Ouchen, F.; Grote, J.G. Re-engineering the polymer capacitor, layer by layer. Adv. Energy Mater. 2016, 6, 1600676. [Google Scholar] [CrossRef]
- Tan, D.; Zhang, L.; Chen, Q.; Irwin, P. High-temperature capacitor polymer films. J. Electron. Mater. 2014, 43, 4569–4575. [Google Scholar] [CrossRef]
- Han, W.J.; Lee, H.S.; Bangi, U.-K.H.; Yoo, B.W.; Park, H.H. Dielectric properties of poly(4-vinylphenol) with embedded PbO nanoparticles. Polym. Adv. Technol. 2015, 27, 245–249. [Google Scholar] [CrossRef]
- Mukhin, N.; Sokolova, I.; Chigirev, D.; Rudaja, L.; Lebedeva, G.; Kastro, R.; Bol’shakov, M.; Schmidt, M.-P.; Hirsch, S. Composite ferroelectric coatings based on a heat-resistant polybenzoxazole polymer matrix. Coatings 2020, 10, 286. [Google Scholar] [CrossRef]
- Xie, J.; Zhu, Z.; Tao, H.; Zhou, S.; Liang, Z.; Li, Z.; Yao, R.; Wang, Y.; Ning, H.; Peng, J. Research progress of high dielectric constant zirconia-based materials for gate dielectric application. Coatings 2020, 10, 698. [Google Scholar] [CrossRef]
- Fröhlich, K.; Ťapajna, M.; Rosová, A.; Dobročka, E.; Hušeková, K.; Aarik, J.; Aidla, A. Growth of high-dielectric-constant TiO films in capacitors with RuO electrodes. Electrochem. Solid-State Lett. 2008, 11, G19. [Google Scholar]
- Halford, J.H.; Hacker, H. Dielectric properties of bismuth trioxide thin films. Thin Solid Film. 1969, 4, 265–279. [Google Scholar] [CrossRef]
- Komatsu, S.; Abe, K.; Fukushima, N. Effects of ambient gas on dielectric constant of sputtered SrTiO3 epitaxial thin films. Jpn. J. Appl. Phys. 1998, 37, 5651–5654. [Google Scholar] [CrossRef]
- Valipour, P.; Ghasemi, S.E.; Khosravani, M.R.; Ganji, D.D. Theoretical analysis on nonlinear vibration of fluid flow in single-walled carbon nanotube. J. Theor. Appl. Phys. 2016, 10, 211–218. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, L.; Zhang, Y. Vibration of single-walled carbon nanotubes with elastic boundary conditions. Int. J. Mech. Sci. 2017, 122, 156–166. [Google Scholar] [CrossRef]
- Yang, K.; Huang, X.; Huang, Y.; Xie, L.; Jiang, P. Fluoro-polymer@BaTiO3 hybrid nanoparticles prepared via RAFT polymerization: Toward ferroelectric polymer nanocomposites with high dielectric constant and low dielectric loss for energy storage application. Chem. Mater. 2013, 25, 2327–2338. [Google Scholar] [CrossRef]
- Singh, D.; Singh, N.; Garg, A.; Gupta, R.K. Engineered thiol anchored Au-BaTiO3/PVDF polymer nanocomposite as efficient dielectric for electronic applications. Compos. Sci. Technol. 2019, 174, 158–168. [Google Scholar]
- Wang, L.; Dang, Z.M. Carbon nanotube composites with high dielectric constant at low percolation threshold. Appl. Phys. Lett. 2005, 87, 042903. [Google Scholar] [CrossRef]
- Hong, R.Y.; Chen, Q. Dispersion of inorganic nanoparticles in polymer matrices: Challenges and solutions. Adv. Polym. Sci. 2014, 267, 1–38. [Google Scholar]
- Park, H.H.; Park, H.H.; Hill, R.H. Direct-patterning of SnO2 thin film by photochemical metal-organic deposition. Sens. Actuators A 2006, 132, 429–433. [Google Scholar] [CrossRef]
- Lee, H.S.; Choi, H.J.; Chung, S.W.; Park, H.H. Effect of SrTiO3 buffer layer on the phase formation and properties of direct-patternable BiFeO3 thin films fabricated using photochemical metal-organic deposition. Ceram. Soc. Jpn. 2010, 118, 1024–1027. [Google Scholar] [CrossRef][Green Version]
- BuonoCore, G.E.; Cabello, G.; Klahn, A.H.; Lucero, A.; Nuñez, M.V.; Torrejón, B.; Castillo, C. Growth and characterization of molybdenum oxide thin films prepared by photochemical metal–organic deposition (PMOD). Polyhedron 2010, 29, 1551–1554. [Google Scholar] [CrossRef]
- Zhu, H.J.; Hill, R.H. The photochemical metal organic deposition of manganese oxide films from films of manganese(II) 2-ethylhexanoate: A mechanistic study. J. Non-Cryst. Solids 2002, 311, 174–184. [Google Scholar] [CrossRef]
- Wu, B.E.; Chiang, C.Y. Photochemical metal organic deposition of FeOx catalyst on BiVO4 for improving solar-driven water oxidation efficiency. J.Taiwan Inst. Chem. Eng. 2017, 80, 1014–1021. [Google Scholar] [CrossRef]
- Andronic, L.S.; Hill, R.H. The mechanism of the photochemical metal organic deposition of lead oxide films from thin films of lead (II) 2-ethylhexanoate. J. Photochem. Photobiol. A Chem. 2002, 152, 259–265. [Google Scholar] [CrossRef]
- Tradel, S.; Li, G.; Zhang, X.; Hill, R.H. Positive and Negative Lithography by Photochemical Metalorganic Deposition from Metal 2-ethylhexanoates. J. Photopolym. Sci. Technol. 2006, 19, 467–475. [Google Scholar] [CrossRef]
- Yang, J.; Xie, T.; Liu, C.; Xu, L. Facile fabrication of dumbbell-Like β-Bi2O3/graphene nanocomposites and their highly efficient photocatalytic activity. Materials 2018, 11, 1359. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, L.; Wang, K.; Zheng, Z. Low temperature synthesis of δ-Bi2O3 solid spheres and their conversion to hierarchical BiOI nests via the Kirkendall effect. CrystEngComm 2011, 13, 5460. [Google Scholar] [CrossRef]
- Epifani, M.; Tang, P.Y.; Genç, A.; Morante, J.R.; Arbiol, J.; Díaz, R.; Wicker, S. The ethylhexanoate route to metal oxide nanocrystals: Synthesis of CoO nanooctahedra from Co(II) 2-Ethylhexanoate. Eur. J. Inorg. Chem. 2016, 24, 3963–3968. [Google Scholar] [CrossRef]
- Shi, Y.; Li, G.; Hill, R.H. Preparation of nanocomposite thin films by sol-gel and photochemical metal–organic deposition of Ba0.5Sr0.5TiO3 and PbZr0.48Ti0.52O3. Mater. Sci. Semicond. Process. 1999, 2, 297–301. [Google Scholar] [CrossRef]
- Trudel, S.; Jones, C.H.W.; Hill, R.H. Magnetic properties of nanocrystalline iron oxide/amorphous manganese oxide nanocomposite thin films prepared via photochemical metal-organic deposition. J. Mater. Chem. 2007, 17, 2206. [Google Scholar] [CrossRef]
- Chen, C.W.; Chiang, C.Y. Molybdenum-containing amorphous metal oxide catalysts for oxygen evolution reaction. Int. J. Hydrog. Energy 2017, 42, 29773–29780. [Google Scholar] [CrossRef]
- Pan, L.; Wang, Q.; Li, Y.; Zhang, C. Amorphous cobalt-cerium binary metal oxides as high performance electrocatalyst for oxygen evolution reaction. J. Catal 2020, 384, 14–21. [Google Scholar] [CrossRef]
- Ligang, L.; Shao, Q.; Huang, X. Amorphous Oxide Nanostructures for Advanced Electrocatalysis. Chem. Eur. J. 2019, 25, 1–19. [Google Scholar]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, K.-H.; Lee, H.-S. Directly-Patternable Bi2O3 Nanoparticle for Polymer Nanocomposite Capacitor. Coatings 2020, 10, 752. https://doi.org/10.3390/coatings10080752
Son K-H, Lee H-S. Directly-Patternable Bi2O3 Nanoparticle for Polymer Nanocomposite Capacitor. Coatings. 2020; 10(8):752. https://doi.org/10.3390/coatings10080752
Chicago/Turabian StyleSon, Ki-Hoon, and Hong-Sub Lee. 2020. "Directly-Patternable Bi2O3 Nanoparticle for Polymer Nanocomposite Capacitor" Coatings 10, no. 8: 752. https://doi.org/10.3390/coatings10080752
APA StyleSon, K.-H., & Lee, H.-S. (2020). Directly-Patternable Bi2O3 Nanoparticle for Polymer Nanocomposite Capacitor. Coatings, 10(8), 752. https://doi.org/10.3390/coatings10080752





