Adsorption of Titanium Halides on Nitride and Oxide Surfaces during Atomic Layer Deposition: A DFT Study
Abstract
1. Introduction
2. Computational Methods
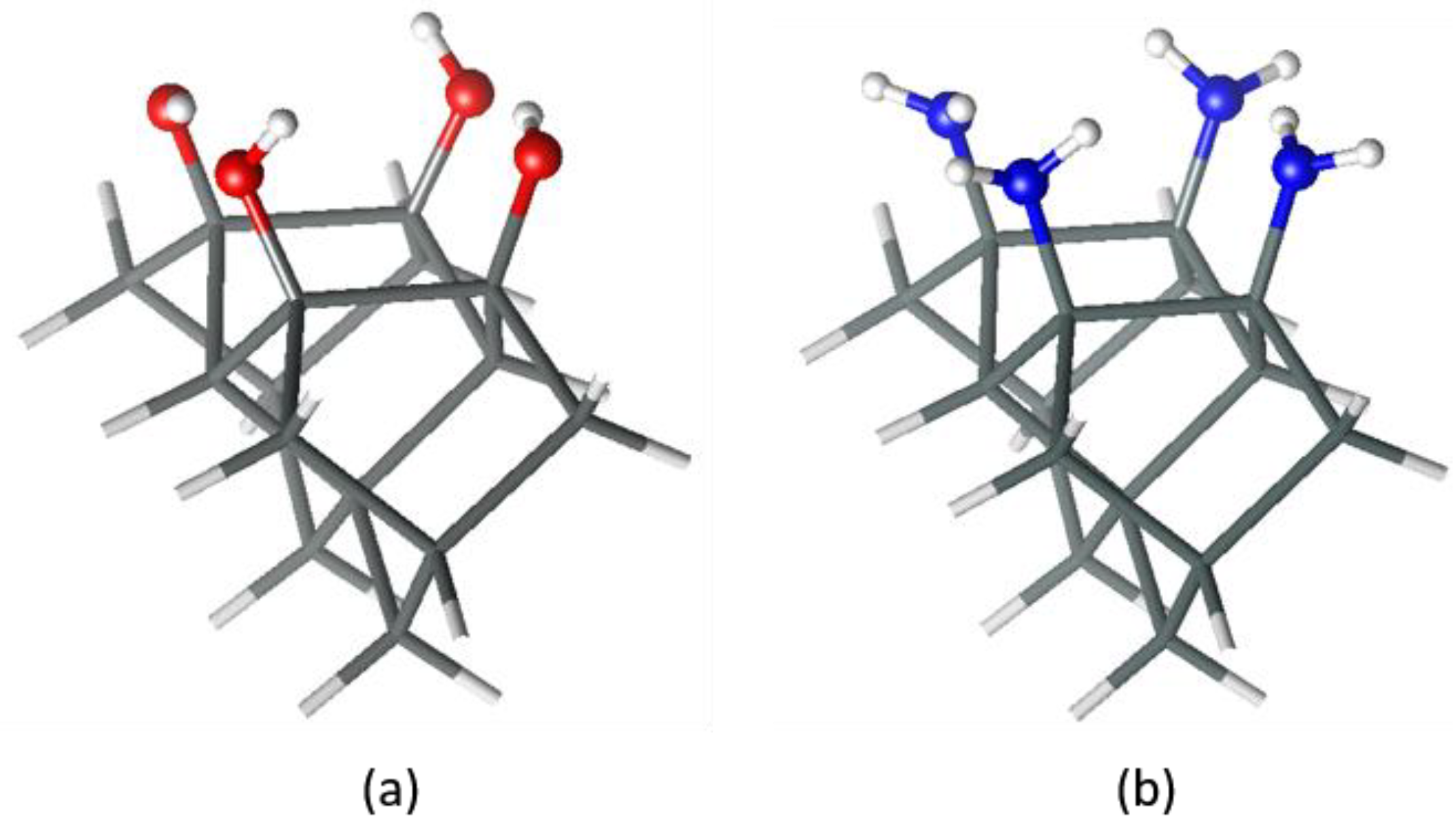
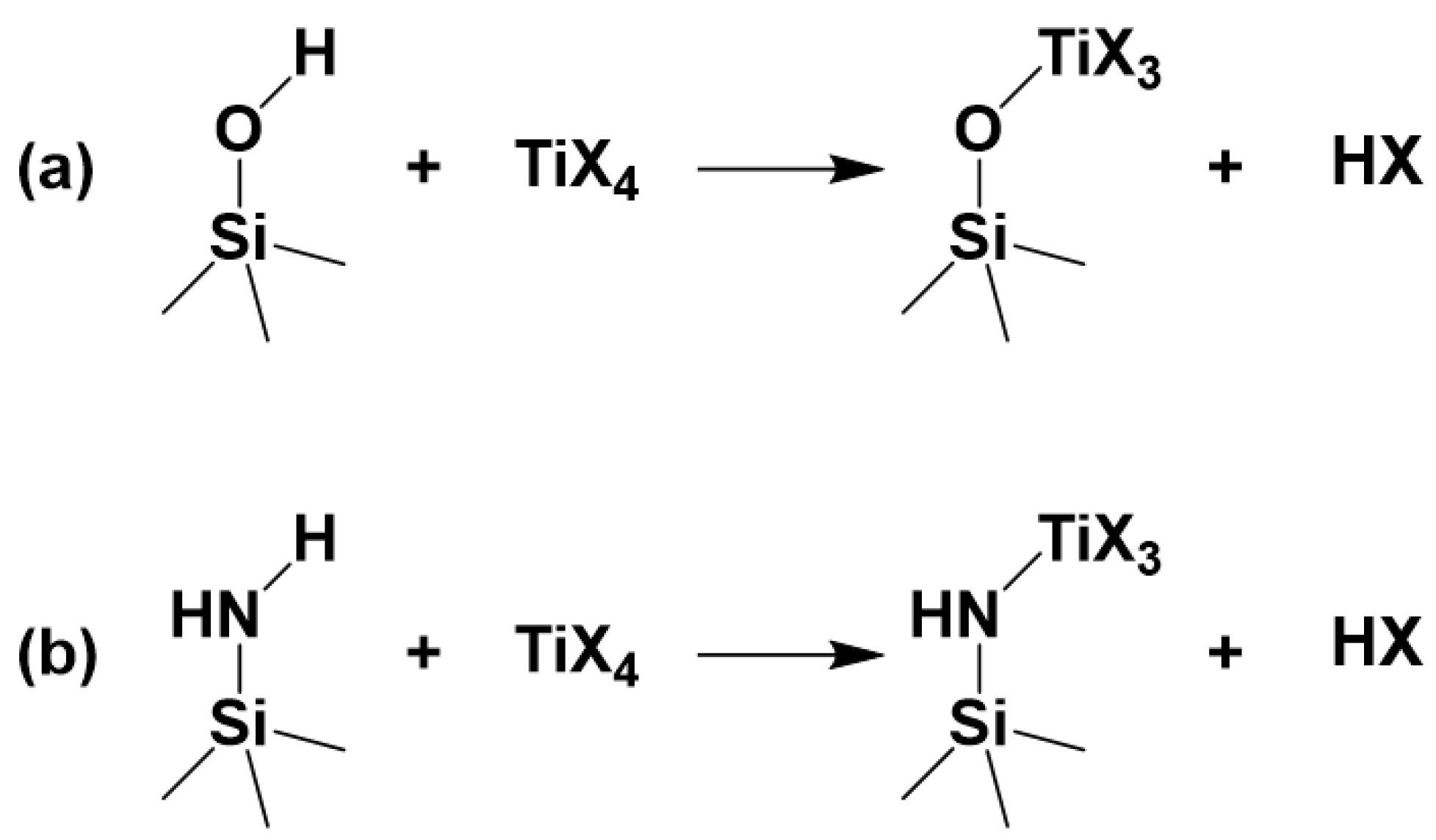
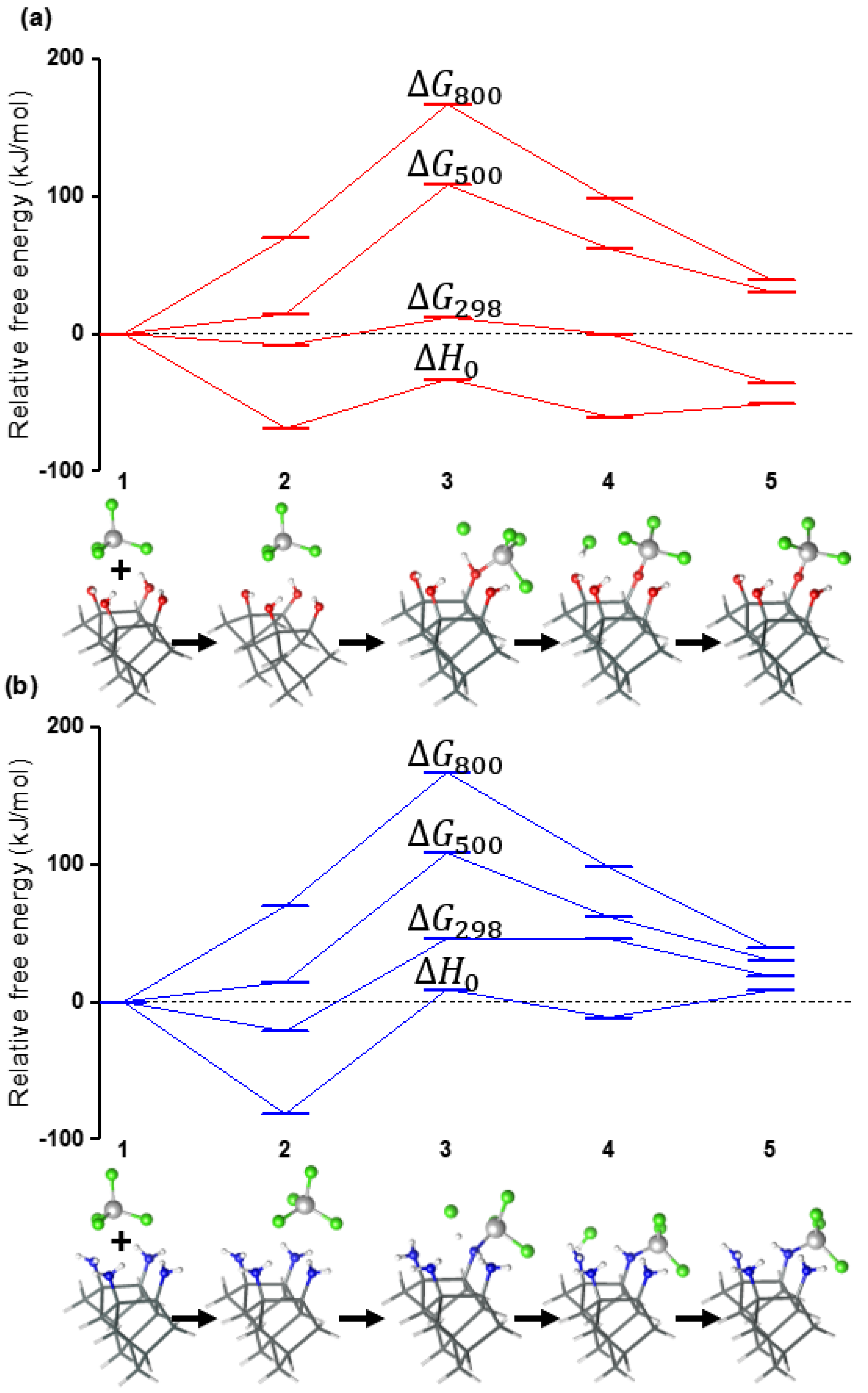
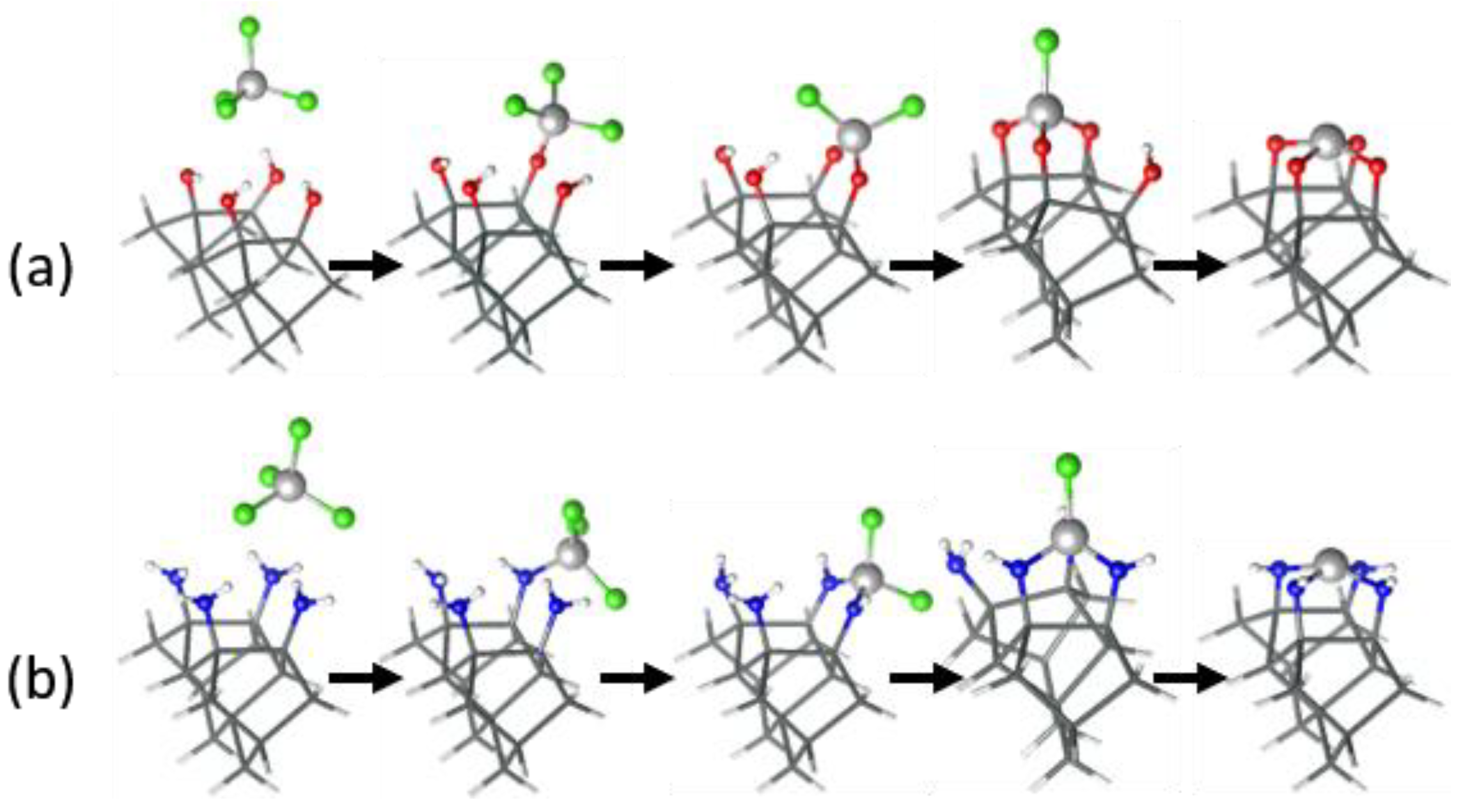
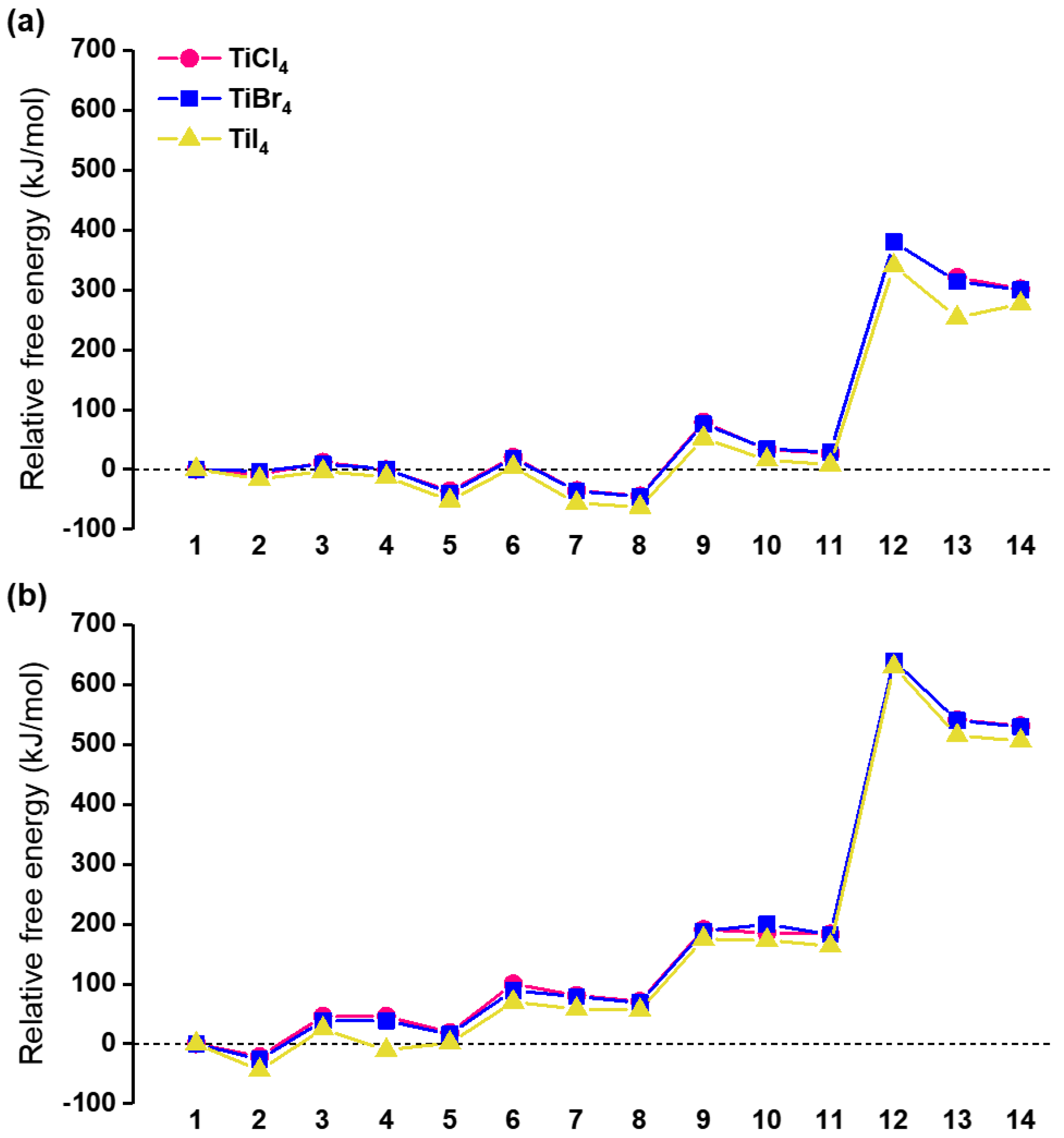
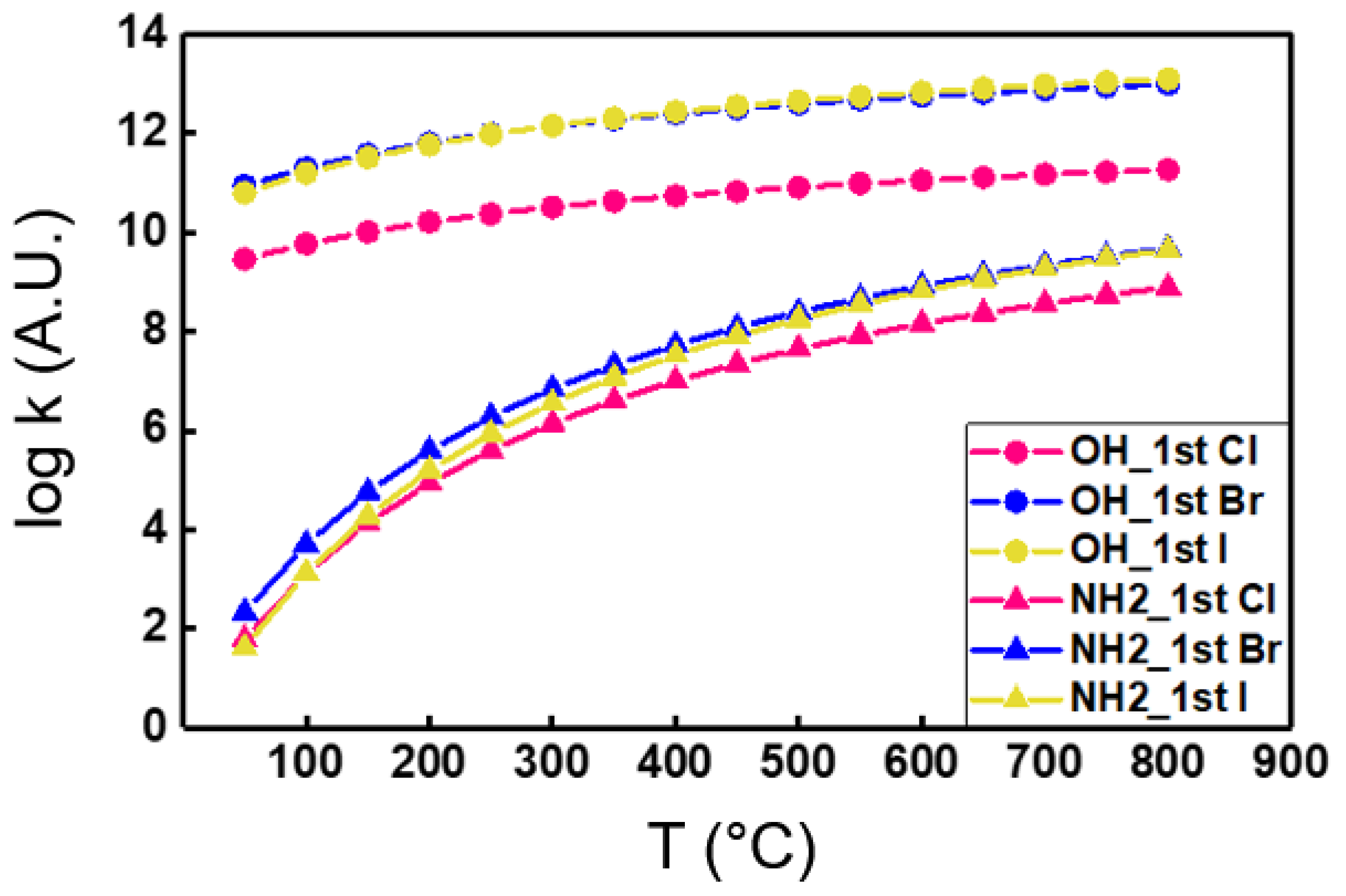
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wang, Y.; Han, R.; Liu, X.; Kang, J. The challenges for physical limitations in Si microelectronics. In Proceedings of the 1998 5th International Conference on Solid-State and Integrated Circuit Technology. Proceedings (Cat. No.98EX105), Beijing, China, 23 October 1998; pp. 25–30. [Google Scholar] [CrossRef]
- Westlinder, J.; Schram, T.; Pantisano, L.; Cartier, E.; Kerber, A.; Lujan, G.S.; Olsson, J.; Groeseneken, G. On the thermal stability of atomic layer deposited TiN as gate electrode in MOS devices. IEEE Electron Device Lett. 2003, 24, 550–552. [Google Scholar] [CrossRef]
- Sinke, W.; Frijlink, G.P.A.; Saris, F.W. Oxygen in titanium nitride diffusion barriers. Appl. Phys. Lett. 1985, 47, 471–473. [Google Scholar] [CrossRef]
- Grigorov, G.I.; Grigorov, K.G.; Stoyanova, M.; Vignes, J.L.; Langeron, J.P.; Denjean, P. Aluminium diffusion in titanium nitride films. Efficiency of TiN barrier layers. Appl. Phys. A 1993, 57, 195–197. [Google Scholar] [CrossRef]
- Reyes-Coronado, D.; Rodríguez-Gattorno, G.; Espinosa-Pesqueira, M.E.; Cab, C.; de Coss, R.; Oskam, G. Phase-pure TiO2 nanoparticles: Anatase, brookite and rutile. Nanotechnology 2008, 19, 145605. [Google Scholar] [CrossRef] [PubMed]
- Seok, T.J.; Liu, Y.; Jung, H.J.; Kim, S.B.; Kim, D.H.; Kim, S.M.; Jang, J.H.; Cho, D.-Y.; Lee, S.W.; Park, T.J. Field-effect device using quasi-two-dimensional electron gas in mass-producible atomic-layer-deposited Al2O3/TiO2 ultrathin (<10 nm) film heterostructures. ACS Nano 2018, 12, 10403–10409. [Google Scholar] [CrossRef]
- Kim, I.; Kumta, P.N. Hydrazide sol–gel synthesis of nanostructured titanium nitride: Precursor chemistry and phase evolution. J. Mater. Chem. 2003, 13, 2028–2035. [Google Scholar] [CrossRef]
- Liang, Y.; Sun, S.; Deng, T.; Ding, H.; Chen, W.; Chen, Y. The Preparation of TiO2 Film by the sol-gel method and evaluation of its self-cleaning property. Materials 2018, 11, 450. [Google Scholar] [CrossRef]
- Wang, X.-P.; Yu, Y.; Hu, X.-F.; Gao, L. Hydrophilicity of TiO2 films prepared by liquid phase deposition. Thin Solid Films 2000, 371, 148–152. [Google Scholar] [CrossRef]
- Su, J.; Boichot, R.; Blanquet, E.; Mercier, F.; Pons, M. Chemical vapor deposition of titanium nitride thin films: Kinetics and experiments. CrystEngComm 2019, 21, 3974–3981. [Google Scholar] [CrossRef]
- Jagadeesan, S.; Doh, Y.H.; Choi, K.-H. Low-temperature fabrication of TiO2 film on flexible substrate by atmospheric roll-to-roll CVD. J. Coat. Technol. Res. 2017, 14, 701–708. [Google Scholar] [CrossRef]
- Singh, J.; Khan, S.A.; Shah, J.; Kotnala, R.K.; Mohapatra, S. Nanostructured TiO2 thin films prepared by RF magnetron sputtering for photocatalytic applications. Appl. Surf. Sci. 2017, 422, 953–961. [Google Scholar] [CrossRef]
- Knoops, H.C.M.; Potts, S.E.; Bol, A.A.; Kessels, W.M.M. Atomic layer deposition. In Handbook of Crystal Growth; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1101–1134. [Google Scholar] [CrossRef]
- Chaukulkar, R.P.; Agarwal, S. Atomic layer deposition of titanium dioxide using titanium tetrachloride and titanium tetraisopropoxide as precursors. J. Vac. Sci. Technol. A 2013, 31, 031509. [Google Scholar] [CrossRef]
- Xie, S.; Cai, J.; Wang, Q.; Wang, L.; Liu, Z. Properties and morphology of TiN films deposited by atomic layer deposition. Tsinghua Sci. Technol. 2014, 19, 144–149. [Google Scholar] [CrossRef]
- Bronneberg, A.C.; Höhn, C.; van de Krol, R. Probing the interfacial chemistry of ultrathin ALD-grown TiO2 films: An in-line XPS study. J. Phys. Chem. C 2017, 121, 5531–5538. [Google Scholar] [CrossRef]
- Pore, V.; Rahtu, A.; Leskelä, M.; Ritala, M.; Sajavaara, T.; Keinonen, J. Atomic layer deposition of photocatalytic TiO2 thin films from titanium tetramethoxide and water. Chem. Vap. Depos. 2004, 10, 143–148. [Google Scholar] [CrossRef]
- Zhuiykov, S.; Akbari, M.K.; Hai, Z.; Xue, C.; Xu, H.; Hyde, L. Data set for fabrication of conformal two-dimensional TiO2 by atomic layer deposition using tetrakis (dimethylamino) titanium (TDMAT) and H2O precursors. Data Brief 2017, 13, 401–407. [Google Scholar] [CrossRef]
- Lee, C.-S.; Kim, J.; Son, J.Y.; Choi, W.; Kim, H. Photocatalytic functional coatings of TiO2 thin films on polymer substrate by plasma enhanced atomic layer deposition. Appl. Catal. B 2009, 91, 628–633. [Google Scholar] [CrossRef]
- Sowińska, M.; Brizzi, S.; Das, C.; Kärkkänen, I.; Schneidewind, J.; Naumann, F.; Gargouri, H.; Henkel, K.; Schmeißer, D. Analysis of nitrogen species in titanium oxynitride ALD films. Appl. Surf. Sci. 2016, 381, 42–47. [Google Scholar] [CrossRef]
- Xie, Q.; Musschoot, J.; Deduytsche, D.; Meirhaeghe, R.L.V.; Detavernier, C.; Berghe, S.V.d.; Jiang, Y.-L.; Ru, G.-P.; Li, B.-Z.; Qu, X.-P. Growth kinetics and crystallization behavior of tio2 films prepared by plasma enhanced atomic layer deposition. J. Electrochem. Soc. 2008, 155, H688–H692. [Google Scholar] [CrossRef]
- Maxwell, J.L.; Black, M.R.; Chavez, C.A.; Maskaly, K.R.; Espinoza, M.; Boman, M.; Landstrom, L. Growth of normally-immiscible materials (NIMs), binary alloys, and metallic fibers by hyperbaric laser chemical vapor deposition. Appl. Phys. A 2008, 91, 507–514. [Google Scholar] [CrossRef]
- Kim, H. Atomic layer deposition of metal and nitride thin films: Current research efforts and applications for semiconductor device processing. J. Vac. Sci. Technol. B 2003, 21, 2231. [Google Scholar] [CrossRef]
- Wolf, S.; Breeden, M.; Kwak, I.; Park, J.H.; Kavrik, M.; Naik, M.; Alvarez, D.; Spiegelman, J.; Kummel, A.C. Low temperature thermal ALD TaNX and TiNX films from anhydrous N2H4. Appl. Surf. Sci. 2018, 462, 1029–1035. [Google Scholar] [CrossRef]
- Yu, I.-S.; Cheng, H.-E.; Chang, C.-C.; Lin, Y.-W.; Chen, H.-T.; Wang, Y.-C.; Yang, Z.-P. Substrate-insensitive atomic layer deposition of plasmonic titanium nitride films. Opt. Mater. Express 2017, 7, 777. [Google Scholar] [CrossRef]
- Miikkulainen, V.; Leskelä, M.; Ritala, M.; Puurunen, R.L. Crystallinity of inorganic films grown by atomic layer deposition: Overview and general trends. J. Appl. Phys. 2013, 113, 021301. [Google Scholar] [CrossRef]
- Moriwaki, M.; Yamada, T. Influences of Residual Chlorine in CVD-TiN Gate Electrode on the gate oxide reliability in multiple-thickness oxide technology. Jpn. J. Appl. Phys. 2001, 40, 2679–2684. [Google Scholar] [CrossRef]
- Krylov, I.; Zoubenko, E.; Weinfeld, K.; Kauffmann, Y.; Xu, X.; Ritter, D.; Eizenberg, M. Obtaining low resistivity (∼100 μΩ cm) TiN films by plasma enhanced atomic layer deposition using a metalorganic precursor. J. Vac. Sci. Technol. A 2018, 36, 051505. [Google Scholar] [CrossRef]
- Mäntymäki, M.; Hämäläinen, J.; Puukilainen, E.; Munnik, F.; Ritala, M.; Leskelä, M. Atomic layer deposition of LiF thin films from Lithd and TiF4 precursors. Chem. Vap. Depos. 2013, 19, 111–116. [Google Scholar] [CrossRef]
- Mäntymäki, M.; Hämäläinen, J.; Puukilainen, E.; Sajavaara, T.; Ritala, M.; Leskelä, M. Atomic layer deposition of LiF thin films from Lithd, Mg(thd)2, and TiF4 precursors. Chem. Mater. 2013, 25, 1656–1663. [Google Scholar] [CrossRef]
- Raghavachari, K.; Halls, M.D. Quantum chemical studies of semiconductor surface chemistry using cluster models. Mol. Phys. 2004, 102, 381–393. [Google Scholar] [CrossRef]
- Frisch, M.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Petersson, G.; Nakatsuji, H.; et al. Gaussian 16. Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297. [Google Scholar] [CrossRef] [PubMed]
- Ochterski, J.W. Thermochemistry in Gaussian. Available online: https://gaussian.com/thermo/ (accessed on 15 July 2020).
- Lewars, E. Computational Chemistry; Springer: Dordrecht, The Netherlands, 2003. [Google Scholar]
- Kim, T.H.; Nandi, D.K.; Ramesh, R.; Han, S.-M.; Shong, B.; Kim, S.-H. Some insights into atomic layer deposition of MoNx using Mo(CO)6 and NH3 and its diffusion barrier application. Chem. Mater. 2019, 31, 8338–8350. [Google Scholar] [CrossRef]
- Ansari, M.Z.; Nandi, D.K.; Janicek, P.; Ansari, S.A.; Ramesh, R.; Cheon, T.; Shong, B.; Kim, S.-H. Low-temperature atomic layer deposition of highly conformal tin nitride thin films for energy storage devices. ACS Appl. Mater. Interfaces 2019, 11, 43608–43621. [Google Scholar] [CrossRef] [PubMed]
- Rietze, C.; Titov, E.; Lindner, S.; Saalfrank, P. Thermal isomerization of azobenzenes: On the performance of Eyring transition state theory. J. Phys. Condens. Matter 2017, 29, 314002. [Google Scholar] [CrossRef]
- Lu, H.-L.; Chen, W.; Ding, S.-J.; Xu, M.; Zhang, D.W.; Wang, L.-K. Quantum chemical study of the initial surface reactions in atomic layer deposition of TiN on the SiO2 surface. J. Phys. Condens. Matter 2006, 18, 5937–5944. [Google Scholar] [CrossRef]
- Haran, M.; Engstrom, J.R.; Clancy, P. Ab initio calculations of the reaction mechanisms for metal−nitride deposition from organo-metallic precursors onto functionalized self-assembled monolayers. J. Am. Chem. Soc. 2006, 128, 836–847. [Google Scholar] [CrossRef]
- Killampalli, A.S.; Ma, P.F.; Engstrom, J.R. The reaction of tetrakis(dimethylamido)titanium with self-assembled alkyltrichlorosilane monolayers possessing −OH, −NH2, and −CH3 terminal groups. J. Am. Chem. Soc. 2005, 127, 6300–6310. [Google Scholar] [CrossRef]
- Heil, S.B.S.; Langereis, E.; Roozeboom, F.; van de Sanden, M.C.M.; Kessels, W.M.M. Low-temperature deposition of TiN by plasma-assisted atomic layer deposition. J. Electrochem. Soc. 2006, 153, G956. [Google Scholar] [CrossRef]
- Strobel, A.; Schnabel, H.-D.; Reinhold, U.; Rauer, S.; Neidhardt, A. Room temperature plasma enhanced atomic layer deposition for TiO2 and WO3 films. J. Vac. Sci. Technol. A 2016, 34, 01A118. [Google Scholar] [CrossRef]
- Afshar, A.; Cadien, K.C. Growth mechanism of atomic layer deposition of zinc oxide: A density functional theory approach. Appl. Phys. Lett. 2013, 103, 251906. [Google Scholar] [CrossRef]
- Silva, T.C.; dos S. Pires, M.; de Castro, A.A.; Lacerda, L.C.T.; Rocha, M.V.J.; Ramalho, T.C. Structure and bonding in NbX5 X = (F, Cl, Br and I) complexes: A molecular orbital perspective in the C–H bond activation. Theor. Chem. Acc. 2018, 137, 146. [Google Scholar] [CrossRef]
- Boeyens, J.C.A. The periodic electronegativity table. Z. Naturforsch. B 2008, 63, 199–209. [Google Scholar] [CrossRef]
- Chen, C.-H.; Su, M.-D. The Mechanism of C–X (X=F, Cl, Br, and I) bond activation in CX4 by a stabilized dialkylsilylene. Chem. -Eur. J. 2007, 13, 6932–6941. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.K.; Choi, C.H. Initial adsorption mechanisms of TiCl4 on OH/Si(100)-2×1. Chem. Phys. Lett. 2008, 457, 69–73. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Yu, N.K.; Jang, D.; Jung, E.; Noh, H.; Moon, J.; Kil, D.; Shong, B. Adsorption of Titanium Halides on Nitride and Oxide Surfaces during Atomic Layer Deposition: A DFT Study. Coatings 2020, 10, 712. https://doi.org/10.3390/coatings10080712
Park J, Yu NK, Jang D, Jung E, Noh H, Moon J, Kil D, Shong B. Adsorption of Titanium Halides on Nitride and Oxide Surfaces during Atomic Layer Deposition: A DFT Study. Coatings. 2020; 10(8):712. https://doi.org/10.3390/coatings10080712
Chicago/Turabian StylePark, Jeongwoo, Neung Kyung Yu, Donghak Jang, Eunae Jung, Hyunsik Noh, Jiwon Moon, Deoksin Kil, and Bonggeun Shong. 2020. "Adsorption of Titanium Halides on Nitride and Oxide Surfaces during Atomic Layer Deposition: A DFT Study" Coatings 10, no. 8: 712. https://doi.org/10.3390/coatings10080712
APA StylePark, J., Yu, N. K., Jang, D., Jung, E., Noh, H., Moon, J., Kil, D., & Shong, B. (2020). Adsorption of Titanium Halides on Nitride and Oxide Surfaces during Atomic Layer Deposition: A DFT Study. Coatings, 10(8), 712. https://doi.org/10.3390/coatings10080712




