Research Progress of High Dielectric Constant Zirconia-Based Materials for Gate Dielectric Application
Abstract
1. Introduction
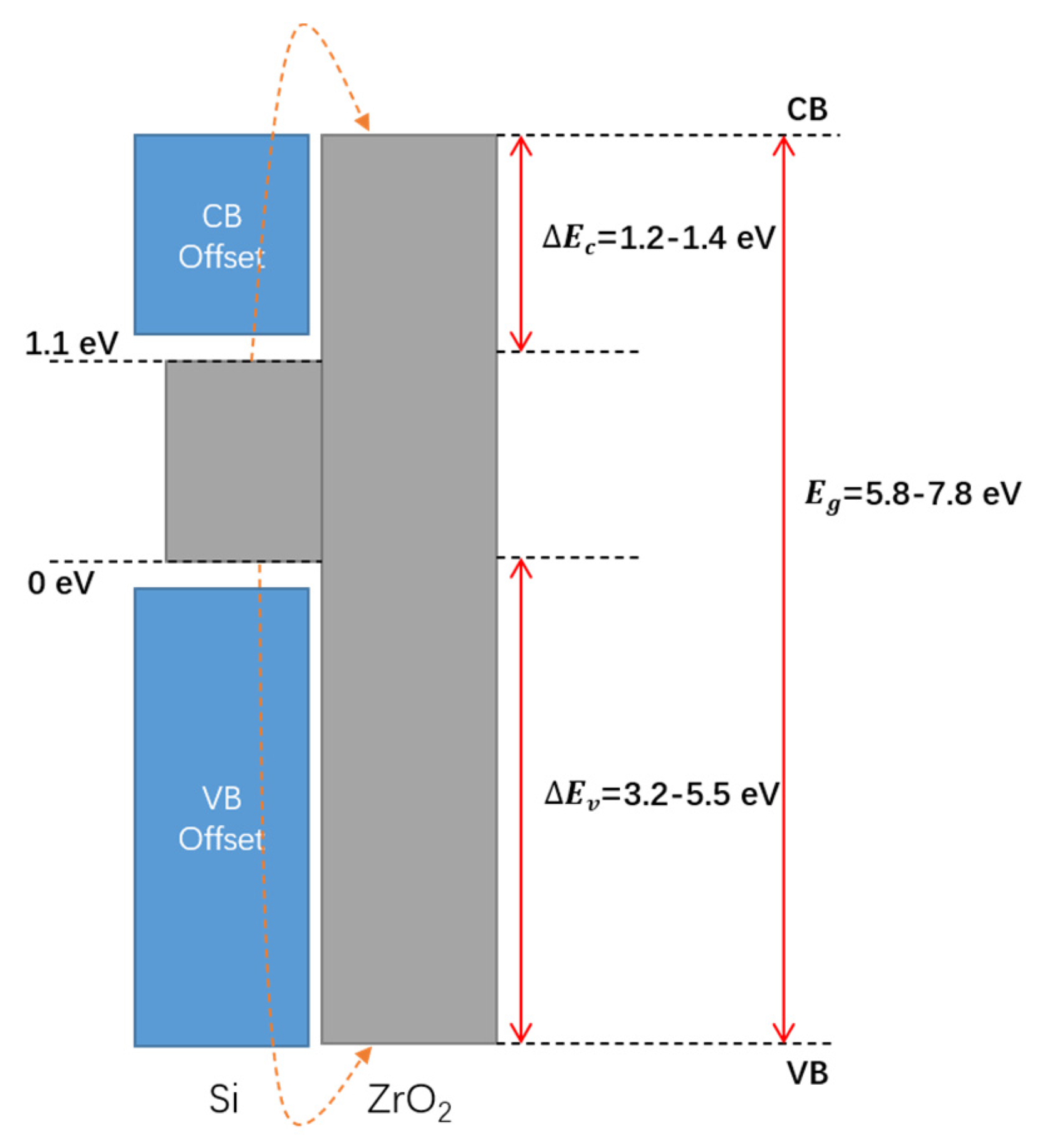
2. Zr-Based High-k Dielectrics
3. ZrO2 Thin Films Deposition
3.1. Atomic Layer Deposition
3.2. Physical Vapor Deposition
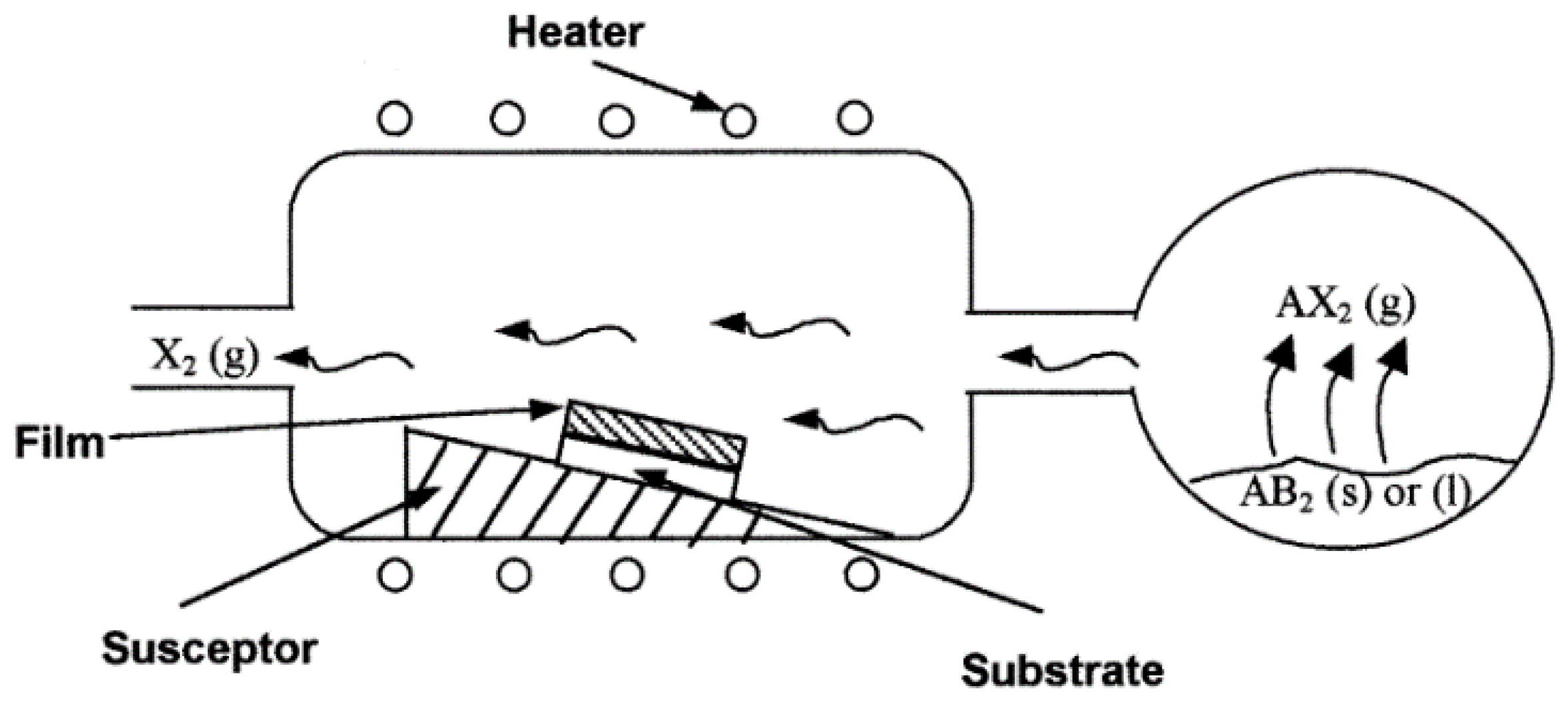
3.3. Chemical Vapor Deposition
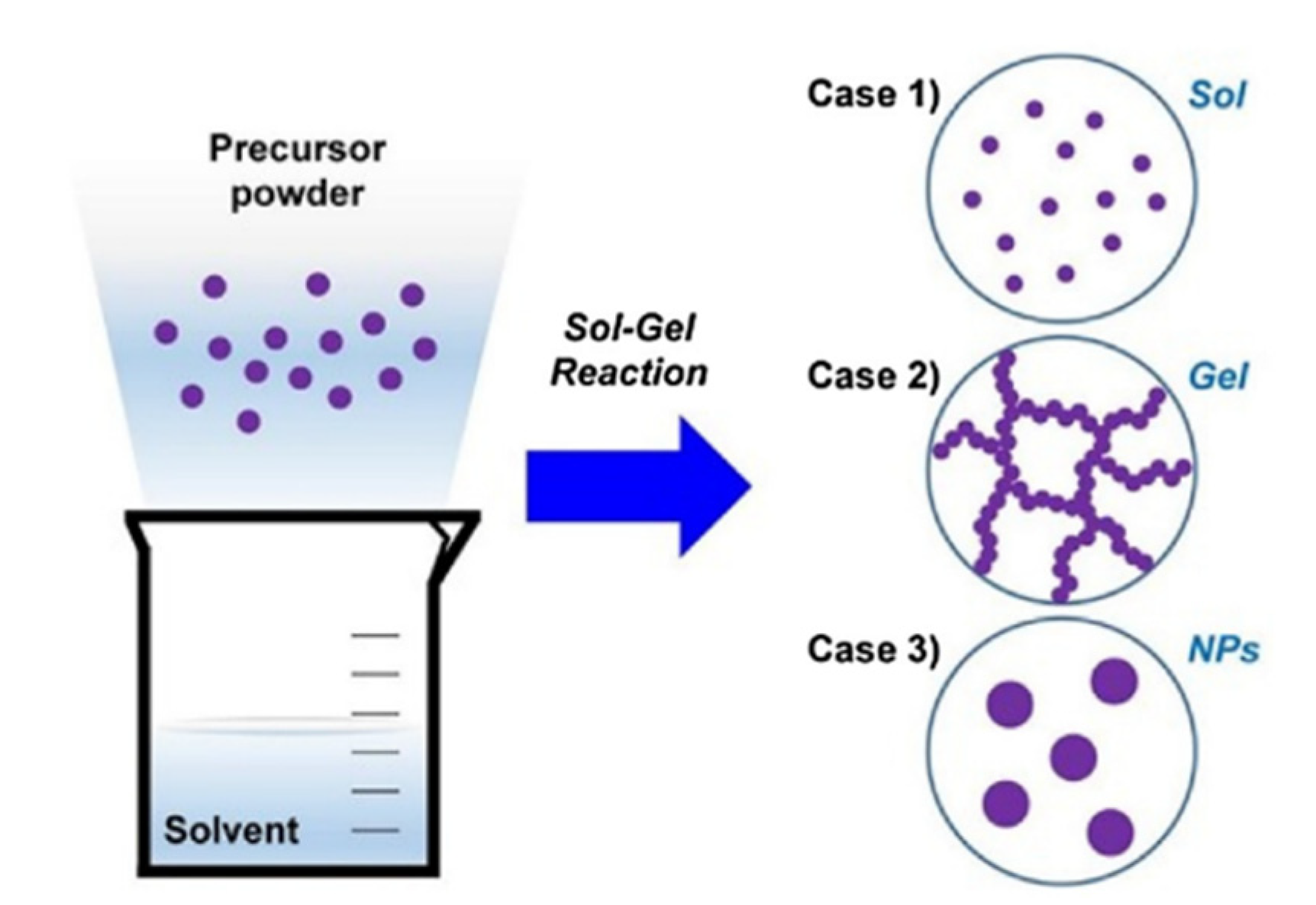
3.4. Sol–Gel Method
4. Methods to Improve the Properties of ZrO2 Films
4.1. Doping of Non-Metallic Elements
4.2. Metal Elements Doping
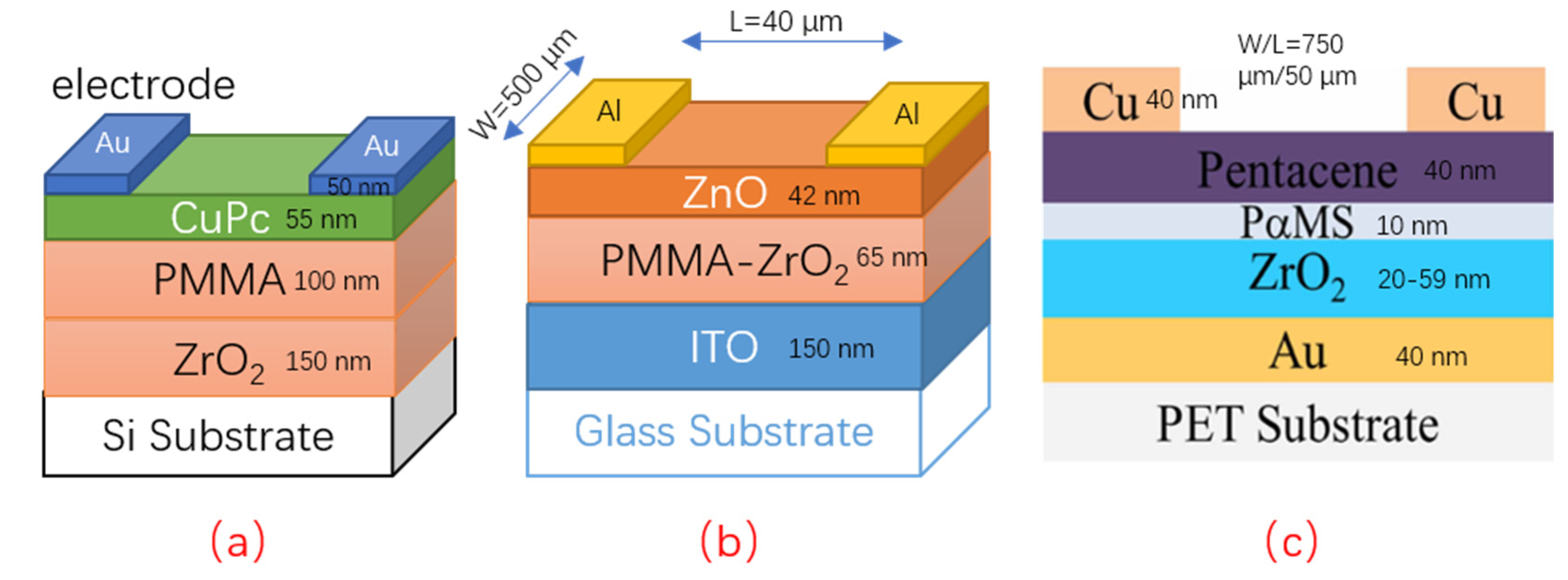
4.3. Coupling with Organic Materials
4.4. Crystalline ZrO2 Dielectric
4.5. Low-Temperature Solution Process
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Panda, D.; Tseng, T.-Y. Growth, dielectric properties, and memory device applications of ZrO2 thin films. Thin Solid Films 2013, 531, 1–20. [Google Scholar] [CrossRef]
- Wilk, G.D.; Wallace, R.M.; Anthony, J.M. High-κ gate dielectrics: Current status and materials properties considerations. J. Appl. Phys. 2001, 89, 5243–5275. [Google Scholar] [CrossRef]
- Chang, J.P.; Lin, Y.S. Dielectric property and conduction mechanism of ultrathin zirconium oxide films. Appl. Phys. Lett. 2001, 79, 3666–3668. [Google Scholar] [CrossRef]
- Jae Sang, L.; Seongpil, C.; Sang-Mo, K.; Sang Yeol, L. High-Performance a-IGZO TFT With ZrO2 Gate Dielectric Fabricated at Room Temperature. IEEE Electron Device Lett. 2010, 31, 225–227. [Google Scholar] [CrossRef]
- Lee, S.Y.; Chang, S.; Lee, J.-S. Role of high-k gate insulators for oxide thin film transistors. Thin Solid Films 2010, 518, 3030–3032. [Google Scholar] [CrossRef]
- Kol, S.; Oral, A. Hf-Based High-κ Dielectrics: A Review. Acta Phys. Pol. A 2019, 136, 873–881. [Google Scholar] [CrossRef]
- Robertson, J.; Wallace, R.M. High-K materials and metal gates for CMOS applications. Mater. Sci. Eng. R Rep. 2015, 88, 1–41. [Google Scholar] [CrossRef]
- Yu, S.; Guan, X.; Wong, H.S.P. Conduction mechanism of TiN/HfOx/Pt resistive switching memory: A trap-assisted-tunneling model. Appl. Phys. Lett. 2011, 99, 063507. [Google Scholar] [CrossRef]
- Ha, Y.G.; Everaerts, K.; Hersam, M.C.; Marks, T.J. Hybrid gate dielectric materials for unconventional electronic circuitry. Acc Chem. Res. 2014, 47, 1019–1028. [Google Scholar] [CrossRef]
- Copel, M.; Gribelyuk, M.; Gusev, E. Structure and stability of ultrathin zirconium oxide layers on Si(001). Appl. Phys. Lett. 2000, 76, 436–438. [Google Scholar] [CrossRef]
- Wong, Y.H.; Cheong, K.Y. ZrO2 thin films on Si substrate. J. Mater. Sci. Mater. Electron. 2010, 21, 980–993. [Google Scholar] [CrossRef]
- Jõgi, I.; Kukli, K.; Ritala, M.; Leskelä, M.; Aarik, J.; Aidla, A.; Lu, J. Atomic layer deposition of high capacitance density Ta2O5–ZrO2 based dielectrics for metal–insulator–metal structures. Microelectron. Eng. 2010, 87, 144–149. [Google Scholar] [CrossRef]
- Vlček, J.; Rezek, J.; Houška, J.; Čerstvý, R.; Bugyi, R. Process stabilization and a significant enhancement of the deposition rate in reactive high-power impulse magnetron sputtering of ZrO2 and Ta2O5 films. Surf. Coat. Technol. 2013, 236, 550–556. [Google Scholar] [CrossRef]
- Hwang, S.M.; Lee, S.M.; Park, K.; Lee, M.S.; Joo, J.; Lim, J.H.; Kim, H.; Yoon, J.J.; Kim, Y.D. Effect of annealing temperature on microstructural evolution and electrical properties of sol-gel processed ZrO2/Si films. Appl. Phys. Lett. 2011, 98, 022903. [Google Scholar] [CrossRef]
- Zhao, X.; Vanderbilt, D. Phonons and lattice dielectric properties of zirconia. Phys. Rev. B 2002, 65, 075105. [Google Scholar] [CrossRef]
- Vanderbilt, D.; Zhao, X.; Ceresoli, D. Structural and dielectric properties of crystalline and amorphous ZrO2. Thin Solid Films 2005, 486, 125–128. [Google Scholar] [CrossRef]
- Lee, B.; Choi, K.J.; Hande, A.; Kim, M.J.; Wallace, R.M.; Kim, J.; Senzaki, Y.; Shenai, D.; Li, H.; Rousseau, M.; et al. A novel thermally-stable zirconium amidinate ALD precursor for ZrO2 thin films. Microelectron. Eng. 2009, 86, 272–276. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, J.Y.; Boyd, I. Formation of stable zirconium oxide on silicon by photo-assisted sol–gel processing. Appl. Surf. Sci. 2002, 186, 190–194. [Google Scholar] [CrossRef]
- Mih, T.A.; Paul, S.; Milanov, A.P.; Bhakta, R.; Devi, A. Capacitance-Voltage Analysis of ZrO2 Thin Films Deposited by Thermal MOCVD Technique. ECS Trans. 2009, 25, 901–907. [Google Scholar] [CrossRef]
- Hojabri, A. Structural and optical characterization of ZrO2 thin films grown on silicon and quartz substrates. J. Theor. Appl. Phys. 2016, 10, 219–224. [Google Scholar] [CrossRef]
- Kim, M.-S.; Ko, Y.-D.; Hong, J.-H.; Jeong, M.-C.; Myoung, J.-M.; Yun, I. Characteristics and processing effects of ZrO2 thin films grown by metal-organic molecular beam epitaxy. Appl. Surf. Sci. 2004, 227, 387–398. [Google Scholar] [CrossRef]
- Jena, S.; Tokas, R.B.; Thakur, S.; Sahoo, N.K. Optical properties of electron beam evaporated ZrO2:10% SiO2 thin films: Dependence on structure. Indian J. Phys. 2016, 90, 951–957. [Google Scholar] [CrossRef]
- Tang, W.T.; Ying, Z.F.; Hu, Z.G.; Li, W.W.; Sun, J.; Xu, N.; Wu, J.D. Synthesis and characterization of HfO2 and ZrO2 thin films deposited by plasma assisted reactive pulsed laser deposition at low temperature. Thin Solid Films 2010, 518, 5442–5446. [Google Scholar] [CrossRef]
- Leskelä, M.; Niinistö, J.; Ritala, M. Atomic Layer Deposition. In Comprehensive Materials Processing; Elsevier: Amsterdam, The Netherlands, 2014; pp. 101–123. [Google Scholar] [CrossRef]
- Niinistö, L.; Nieminen, M.; Päiväsaari, J.; Niinistö, J.; Putkonen, M.; Nieminen, M. Advanced electronic and optoelectronic materials by Atomic Layer Deposition: An overview with special emphasis on recent progress in processing of high-k dielectrics and other oxide materials. Phys. Status Solid 2004, 201, 1443–1452. [Google Scholar] [CrossRef]
- Kukli, K.; Kemell, M.; Köykkä, J.; Mizohata, K.; Vehkamäki, M.; Ritala, M.; Leskelä, M. Atomic layer deposition of zirconium dioxide from zirconium tetrachloride and ozone. Thin Solid Films 2015, 589, 597–604. [Google Scholar] [CrossRef]
- Hausmann, D.M.; Kim, E.; Becker, J.; Gordon, R.G. Atomic Layer Deposition of Hafnium and Zirconium Oxides Using Metal Amide Precursors. Am. Chem. Soc. 2002, 14, 4350–4358. [Google Scholar] [CrossRef]
- Rossnagel, S.M. Thin film deposition with physical vapor deposition and related technologies. J. Vac. Sci. Technol. A Vac. Surf. Films 2003, 21, S74–S87. [Google Scholar] [CrossRef]
- Baptista, A.; Silva, F.; Porteiro, J.; Míguez, J.; Pinto, G. Sputtering Physical Vapour Deposition (PVD) Coatings: A Critical Review on Process Improvement and Market Trend Demands. Coatings 2018, 8, 402. [Google Scholar] [CrossRef]
- Khojier, K.; Savaloni, H.; Jafari, F. Structural, electrical, and decorative properties of sputtered zirconium thin films during post-annealing process. J. Theor. Appl. Phys. 2013, 7, 55. [Google Scholar] [CrossRef]
- Patel, U.S.; Patel, K.H.; Chauhan, K.V.; Chawla, A.K.; Rawal, S.K. Investigation of Various Properties for Zirconium Oxide Films Synthesized by Sputtering. Procedia Technol. 2016, 23, 336–343. [Google Scholar] [CrossRef]
- Prabakar, K.; Park, A.; Cho, N.; Lee, W.I.; Hwangbo, C.K.; Lee, J.G.; Lee, C. rf-Magnetron sputter deposited ZrO2 dielectrics for metal–insulator–semiconductor capacitors. Vacuum 2008, 82, 1367–1370. [Google Scholar] [CrossRef]
- Jones, A.C.; Hitchman, M.L. Chapter 1. Overview of Chemical Vapour Deposition. In Chemical Vapour Deposition; The Royal Society of Chemistry: Cambridge, UK, 2008; pp. 1–36. [Google Scholar]
- Cabello, G.; Lillo, L.; Buono-Core, G.E. Zr(IV) and Hf(IV) β-diketonate complexes as precursors for the photochemical deposition of ZrO2 and HfO2 thin films. J. Non-Cryst. Solids 2008, 354, 982–988. [Google Scholar] [CrossRef]
- Wu, C.H.; Huang, B.W.; Chang, K.M.; Wang, S.J.; Lin, J.H.; Hsu, J.M. The Performance Improvement of N2 Plasma Treatment on ZrO2 Gate Dielectric Thin-Film Transistors with Atmospheric Pressure Plasma-Enhanced Chemical Vapor Deposition IGZO Channel. J. Nanosci. Nanotechnol. 2016, 16, 6044–6048. [Google Scholar] [CrossRef] [PubMed]
- Cárcamo-León, P.; Torres-Huerta, A.M.; Domínguez-Crespo, M.; Ramírez-Meneses, E. Synthesis of Transparent ZrO2 Thin Films by MOCVD. ECS Trans. 2009, 25, 475–482. [Google Scholar] [CrossRef]
- Karlsson, P.G.; Göthelid, E.; Richter, J.H.; Sandell, A. Initial stages of ZrO2 chemical vapor deposition on Si(100)-(2×1) from zirconium tetra-tert-butoxide. Surf. Sci. 2008, 602, 1803–1809. [Google Scholar] [CrossRef]
- Choy, K.L. Chemical vapour deposition of coatings. Prog. Mater. Sci. 2003, 48, 57–170. [Google Scholar] [CrossRef]
- Xu, W.; Li, H.; Xu, J.B.; Wang, L. Recent Advances of Solution-Processed Metal Oxide Thin-Film Transistors. ACS Appl. Mater. Interfaces 2018, 10, 25878–25901. [Google Scholar] [CrossRef]
- Park, S.; Kim, C.-H.; Lee, W.-J.; Sung, S.; Yoon, M.-H. Sol-gel metal oxide dielectrics for all-solution-processed electronics. Mater. Sci. Eng. R Rep. 2017, 114, 1–22. [Google Scholar] [CrossRef]
- Van Elshocht, S.; Hardy, A.; Adelmann, C.; Caymax, M.; Conard, T.; Franquet, A.; Richard, O.; Van Bael, M.K.; Mullens, J.; De Gendt, S. Impact of Process Optimizations on the Electrical Performance of High-k Layers Deposited by Aqueous Chemical Solution Deposition. J. Electrochem. Soc. 2008, 155, G91. [Google Scholar] [CrossRef]
- Dutta, S.; Pandey, A.; Yadav, I.; Thakur, O.P.; Kumar, A.; Pal, R.; Chatterjee, R. Growth and electrical properties of spin coated ultrathin ZrO2 films on silicon. J. Appl. Phys. 2013, 114, 014105. [Google Scholar] [CrossRef]
- Aoki, Y.; Kunitake, T. Solution-based Fabrication of High-κ Gate Dielectrics for Next-Generation Metal-Oxide Semiconductor Transistors. Adv. Mater. 2004, 16, 118–123. [Google Scholar] [CrossRef]
- Boratto, M.H.; Congiu, M.; dos Santos, S.B.O.; Scalvi, L.V.A. Annealing temperature influence on sol-gel processed zirconium oxide thin films for electronic applications. Ceram. Int. 2018, 44, 10790–10796. [Google Scholar] [CrossRef]
- Ishii, H.; Kidera, T.; Nakajima, A.; Yokoyama, S. Atomic-layer deposition of ZrO2 with a Si nitride barrier layer. Appl. Phys. Lett. 2002, 81, 2824–2826. [Google Scholar]
- Lü, S.; Yin, J.; Xia, Y.; Gao, L.; Liu, Z. Study on the thermal stability and electrical properties of the high-k dielectrics (ZrO2)x(SiO2)1−x. Sci. China Ser. E Technol. Sci. 2009, 52, 2222–2226. [Google Scholar] [CrossRef]
- Wilk, G.D.; Wallace, R.M. Stable zirconium silicate gate dielectrics deposited directly on silicon. Appl. Phys. Lett. 2000, 76, 112–114. [Google Scholar] [CrossRef]
- Ouyang, L.; Ching, W.Y. Electronic structure and dielectric properties of dielectric gate material (ZrO2)x(SiO2)1−x. J. Appl. Phys. 2004, 95, 7918–7924. [Google Scholar] [CrossRef]
- Puthenkovilakam, R.; Carter, E.A.; Chang, J.P. First-principles exploration of alternative gate dielectrics:Electronic structure of ZrO2/Si and ZrSiO4/Si interfaces. Phys. Rev. B 2004, 69, 155329. [Google Scholar] [CrossRef]
- Wang, W.-C.; Tsai, M.-C.; Lin, Y.-P.; Tsai, Y.-J.; Lin, H.-C.; Chen, M.-J. Suppression of interfacial layer in high-K gate stack with crystalline high-K dielectric and AlN buffer layer structure. Mater. Chem. Phys. 2016, 184, 291–297. [Google Scholar] [CrossRef]
- Jeon, S.; Choi, C.-J.; Seong, T.-Y.; Hwang, H. Electrical characteristics of ZrOxNy prepared by NH3 annealing of ZrO2. Appl. Phys. Lett. 2001, 79, 245–247. [Google Scholar] [CrossRef]
- Nieh, R.; Choi, R.; Gopalan, S.; Onishi, K.; Kang, C.S.; Cho, H.-J.; Krishnan, S.; Lee, J.C. Evaluation of silicon surface nitridation effects on ultra-thin ZrO2 gate dielectrics. Appl. Phys. Lett. 2002, 81, 1663–1665. [Google Scholar] [CrossRef]
- Ishii, H.; Nakajima, A.; Yokoyama, S. Growth and electrical properties of atomic-layer deposited ZrO2/Si-nitride stack gate dielectrics. J. Appl. Phys. 2004, 95, 536–542. [Google Scholar] [CrossRef]
- Yana, G.; Xifeng, L.; Longlong, C.; Jifeng, S.; Xiao Wei, S.; Jianhua, Z. High Mobility Solution-Processed Hafnium Indium Zinc Oxide TFT With an Al-Doped ZrO2 Gate Dielectric. IEEE Electron Device Lett. 2014, 35, 554–556. [Google Scholar] [CrossRef]
- Liang, Z.; Zhou, S.; Cai, W.; Fu, X.; Ning, H.; Chen, J.; Yuan, W.; Zhu, Z.; Yao, R.; Peng, J. Zirconium-Aluminum-Oxide Dielectric Layer with High Dielectric and Relatively Low Leakage Prepared by Spin-Coating and the Application in Thin-Film Transistor. Coatings 2020, 10, 282. [Google Scholar] [CrossRef]
- Peng, J.; Wei, J.; Zhu, Z.; Ning, H.; Cai, W.; Lu, K.; Yao, R.; Tao, H.; Zheng, Y.; Lu, X. Properties-Adjustable Alumina-Zirconia Nanolaminate Dielectric Fabricated by Spin-Coating. Nanomaterials 2017, 7, 419. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Xu, J.; Liu, L.; Deng, Y.; Lai, P.T.; Tang, W.M. Optimizing Al-doped ZrO2 as the gate dielectric for MoS2 field-effect transistors. Nanotechnology 2020, 31, 135206. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liang, Z.; Yao, R.; Zuo, W.; Zhou, S.; Zhu, Z.; Wang, Y.; Qiu, T.; Ning, H.; Peng, J. Effect of Zirconium Doping on Electrical Properties of Aluminum Oxide Dielectric Layer by Spin Coating Method with Low Temperature Preparation. Coatings 2020, 10, 620. [Google Scholar] [CrossRef]
- Zhang, J.; Ding, X.; Li, J.; Zhang, H.; Jiang, X.; Zhang, Z. Performance enhancement in InZnO thin-film transistors with compounded ZrO2–Al2O3 nanolaminate as gate insulators. Ceram. Int. 2016, 42, 8115–8119. [Google Scholar] [CrossRef]
- Gholipur, R.; Bahari, A.; Ebrahimzadeh, M. Deposition of Nanostructurated ZrxLa1−xOy Thin Films on P-type Si(100) Substrate by the Sol-Gel Route. Silicon 2015, 9, 173–181. [Google Scholar] [CrossRef]
- Xiao, D.Q.; He, G.; Lv, J.G.; Wang, P.H.; Liu, M.; Gao, J.; Jin, P.; Jiang, S.S.; Li, W.D.; Sun, Z.Q. Interfacial modulation and electrical properties improvement of solution-processed ZrO2 gate dielectrics upon Gd incorporation. J. Alloys Compd. 2017, 699, 415–420. [Google Scholar] [CrossRef]
- Bang, S.; Lee, S.; Jeon, S.; Kwon, S.; Jeong, W.; Kim, S.; Jeon, H. Physical and Electrical Properties of Hafnium–Zirconium–Oxide Films Grown by Atomic Layer Deposition. J. Electrochem. Soc. 2008, 155, H633–H637. [Google Scholar] [CrossRef]
- Lee, T.I.; Ahn, H.J.; Kim, M.J.; Shin, E.J.; Lee, S.H.; Shin, S.W.; Hwang, W.S.; Yu, H.-Y.; Cho, B.J. Ultrathin EOT (0.67 nm) High-k Dielectric on Ge MOSFET Using Y Doped ZrO2 With Record-Low Leakage Current. IEEE Electron Device Lett. 2019, 40, 502–505. [Google Scholar] [CrossRef]
- Jeong, J.W.; Hwang, H.S.; Choi, D.; Ma, B.C.; Jung, J.; Chang, M. Hybrid Polymer/Metal Oxide Thin Films for High Performance, Flexible Transistors. Micromachines 2020, 11, 264. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.H.; Yoon, S.-M.; Kang, S.Y.; Kim, C.A.; Ahn, S.D.; Suh, K.S. Organic field-effect transistors with thermal-cured polyacrylate gate dielectric films. Thin Solid Films 2008, 516, 1574–1577. [Google Scholar] [CrossRef]
- Syamala Rao, M.G.; Pacheco-Zuñiga, M.A.; Garcia-Cerda, L.A.; Gutiérrez-Heredia, G.; Torres Ochoa, J.A.; Quevedo López, M.A.; Ramírez-Bon, R. Low-temperature sol-gel ZrHfO2-PMMA hybrid dielectric thin-films for metal oxide TFTs. J. Non-Cryst. Solids 2018, 502, 152–158. [Google Scholar] [CrossRef]
- Ha, Y.-g.; Jeong, S.; Wu, J.; Kim, M.-G.; Dravid, V.P.; Facchetti, A.; Marks, T.J. Flexible Low-Voltage Organic Thin-Film Transistors Enabled by Low-Temperature, Ambient Solution-Processable Inorganic/Organic Hybrid Gate Dielectrics. J. Am. Chem. Soci. 2010, 132, 17426–17434. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Liu, M.; Tu, D.; Liu, G.; Liu, X.; Ji, Z. Low-Voltage Organic Field-Effect Transistor With PMMA/ZrO2 Bilayer Dielectric. IEEE Trans. Electron Devices 2009, 56, 370–376. [Google Scholar] [CrossRef]
- Alvarado-Beltran, C.G.; Almaral-Sanchez, J.L.; Mejia, I.; Quevedo-Lopez, M.A.; Ramirez-Bon, R. Sol-Gel PMMA-ZrO2 Hybrid Layers as Gate Dielectric for Low-Temperature ZnO-Based Thin-Film Transistors. ACS Omega 2017, 2, 6968–6974. [Google Scholar] [CrossRef]
- Son, B.-G.; Je, S.Y.; Kim, H.J.; Jeong, J.K. Modification of a polymer gate insulator by zirconium oxide doping for low temperature, high performance indium zinc oxide transistors. RSC Adv. 2014, 4, 45742–45748. [Google Scholar] [CrossRef]
- Hwang, J.; Lee, J.; Kim, Y.; Lee, E.; Wang, Y.; Kim, H. Hybrid gate insulator for OTFT using dip-coating method. Curr. Appl. Phys. 2011, 11, S154–S157. [Google Scholar] [CrossRef]
- Gong, Y.; Zhao, K.; He, H.; Cai, W.; Tang, N.; Ning, H.; Wu, S.; Gao, J.; Zhou, G.; Lu, X.; et al. Solution processable high quality ZrO2 dielectric films for low operation voltage and flexible organic thin film transistor applications. J. Phys. D App. Phys. 2018, 51, 115105. [Google Scholar] [CrossRef]
- Kim, M.J.; Pak, K.; Choi, J.; Lee, T.I.; Hwang, W.S.; Im, S.G.; Cho, B.J. Ultrathin ZrOx-Organic Hybrid Dielectric (EOT 3.2 nm) via Initiated Chemical Vapor Deposition for High-Performance Flexible Electronics. ACS Appl. Mater. Interfaces 2019, 11, 44513–44520. [Google Scholar] [CrossRef] [PubMed]
- Fischer, D.; Kersch, A. The effect of dopants on the dielectric constant of HfO2 and ZrO2 from first principles. Appl. Phys. Lett. 2008, 92, 012908. [Google Scholar] [CrossRef]
- Wu, Y.-H.; Wu, M.-L.; Lyu, R.-J.; Wu, J.-R.; Chen, L.-L.; Lin, C.-C. Crystalline ZrO2-gated Ge metal-oxide-semiconductor capacitors fabricated on Si substrate with Y2O3 as passivation layer. Appl. Phys. Lett. 2011, 98, 203502. [Google Scholar] [CrossRef]
- Huang, J.-J.; Huang, L.-T.; Tsai, M.-C.; Lee, M.-H.; Chen, M.-J. Enhancement of electrical characteristics and reliability in crystallized ZrO2 gate dielectrics treated with in-situ atomic layer doping of nitrogen. Appl. Surf. Sci. 2014, 305, 214–220. [Google Scholar] [CrossRef]
- Tsipas, P.; Volkos, S.N.; Sotiropoulos, A.; Galata, S.F.; Mavrou, G.; Tsoutsou, D.; Panayiotatos, Y.; Dimoulas, A.; Marchiori, C.; Fompeyrine, J. Germanium-induced stabilization of a very high-k zirconia phase in ZrO2/GeO2 gate stacks. Appl. Phys. Lett. 2008, 93, 082904. [Google Scholar] [CrossRef]
- Lin, C.-M.; Chang, H.-C.; Wong, I.H.; Luo, S.-J.; Liu, C.W.; Hu, C. Interfacial layer reduction and high permittivity tetragonal ZrO2 on germanium reaching ultrathin 0.39 nm equivalent oxide thickness. Appl. Phys. Lett. 2013, 102, 232906. [Google Scholar] [CrossRef]
- Liu, H.; Han, G.; Xu, Y.; Liu, Y.; Liu, T.-J.K.; Hao, Y. High-Mobility Ge pMOSFETs With Crystalline ZrO2 Dielectric. IEEE Electron Device Lett. 2019, 40, 371–374. [Google Scholar] [CrossRef]
- Wu, Y.-H.; Chen, L.-L.; Lyu, R.-J.; Li, M.-Y.; Wu, H.-C. Tetragonal ZrO2/Al2O3 Stack as High-κ Gate Dielectric for Si-Based MOS Devices. IEEE Electron Device Lett. 2010, 31, 1014–1016. [Google Scholar] [CrossRef]
- Huang, J.-J.; Tsai, Y.-J.; Tsai, M.-C.; Lee, M.-H.; Chen, M.-J. Double nitridation of crystalline ZrO2/Al2O3 buffer gate stack with high capacitance, low leakage and improved thermal stability. Appl. Surf. Sci. 2015, 330, 221–227. [Google Scholar] [CrossRef]
- Wang, S.; Xia, G. A facile low-cost preparation of high-k ZrO2 dielectric films for superior thin-film transistors. Ceram. Int. 2019, 45, 23666–23672. [Google Scholar] [CrossRef]
- Banger, K.K.; Yamashita, Y.; Mori, K.; Peterson, R.L.; Leedham, T.; Rickard, J.; Sirringhaus, H. Low-temperature, high-performance solution-processed metal oxide thin-film transistors formed by a ‘sol-gel on chip’ process. Nat. Mater. 2011, 10, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Yoon, D.H.; Rim, Y.S.; Kim, H.J. Low-Temperature Solution-Processed ZrO2 Gate Insulators for Thin-Film Transistors Using High-Pressure Annealing. Electrochem. Solid-State Lett. 2011, 14, E35. [Google Scholar] [CrossRef]
- Hwan Hwang, Y.; Seo, J.-S.; Moon Yun, J.; Park, H.; Yang, S.; Ko Park, S.-H.; Bae, B.-S. An ‘aqueous route’ for the fabrication of low-temperature-processable oxide flexible transparent thin-film transistors on plastic substrates. NPG Asia Mater. 2013, 5, e45. [Google Scholar] [CrossRef]
- Kim, M.G.; Kanatzidis, M.G.; Facchetti, A.; Marks, T.J. Low-temperature fabrication of high-performance metal oxide thin-film electronics via combustion processing. Nat. Mater. 2011, 10, 382–388. [Google Scholar] [CrossRef]
- Wang, B.; Yu, X.; Guo, P.; Huang, W.; Zeng, L.; Zhou, N.; Chi, L.; Bedzyk, M.J.; Chang, R.P.H.; Marks, T.J.; et al. Solution-Processed All-Oxide Transparent High-Performance Transistors Fabricated by Spray-Combustion Synthesis. Adv. Electron. Mater. 2016, 2, 1500427. [Google Scholar] [CrossRef]
- Xia, G.; Wang, S. Rapid and facile low-temperature solution production of ZrO2 films as high-k dielectrics for flexible low-voltage thin-film transistors. Ceram. Int. 2019, 45, 16482–16488. [Google Scholar] [CrossRef]
- Oluwabi, A.T.; Gaspar, D.; Katerski, A.; Mere, A.; Krunks, M.; Pereira, L.; Oja Acik, I. Influence of Post-UV/Ozone Treatment of Ultrasonic-Sprayed Zirconium Oxide Dielectric Films for a Low-Temperature Oxide Thin Film Transistor. Materials 2019, 13, 6. [Google Scholar] [CrossRef]
- Gong, Y.; Zhao, K.; Yan, L.; Wei, W.; Yang, C.; Ning, H.; Wu, S.; Gao, J.; Zhou, G.; Lu, X.; et al. Room Temperature Fabrication of High Quality ZrO2 Dielectric Films for High Performance Flexible Organic Transistor Applications. IEEE Electron Device Lett. 2018, 39, 280–283. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, J.; Zhou, Z.; Ning, H.; Cai, W.; Wei, J.; Zhou, S.; Yao, R.; Lu, X.; Peng, J. A Simple, Low Cost Ink System for Drop-on-Demand Printing High Performance Metal Oxide Dielectric Film at Low Temperature. ACS Appl. Mater. Interfaces 2019, 11, 5193–5199. [Google Scholar] [CrossRef]
- Dong, X.; Xia, G.; Zhang, Q.; Li, L.; Gong, H.; Bi, J.; Wang, S. Room-temperature UV-ozone assisted solution process for zirconium oxide films with high dielectric properties. Ceram. Int. 2017, 43, 15205–15213. [Google Scholar] [CrossRef]



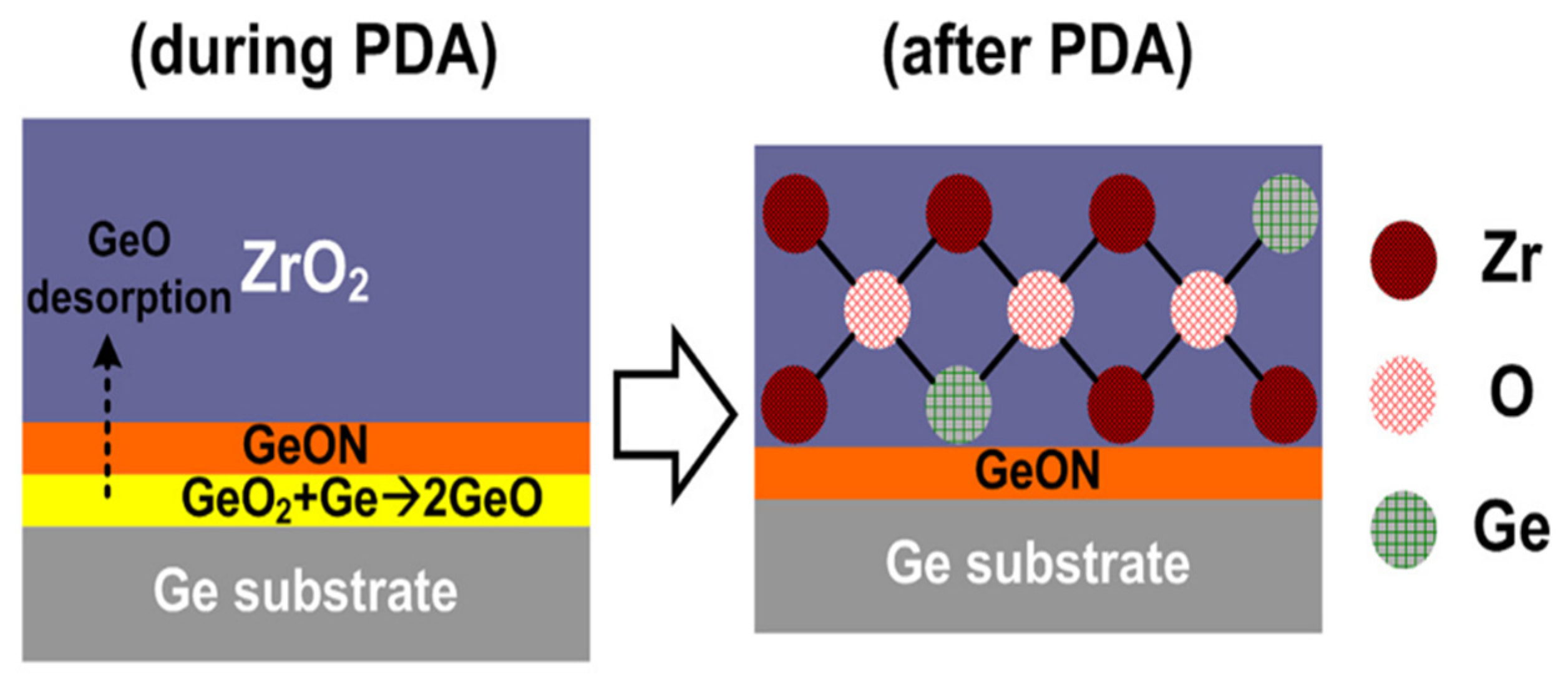
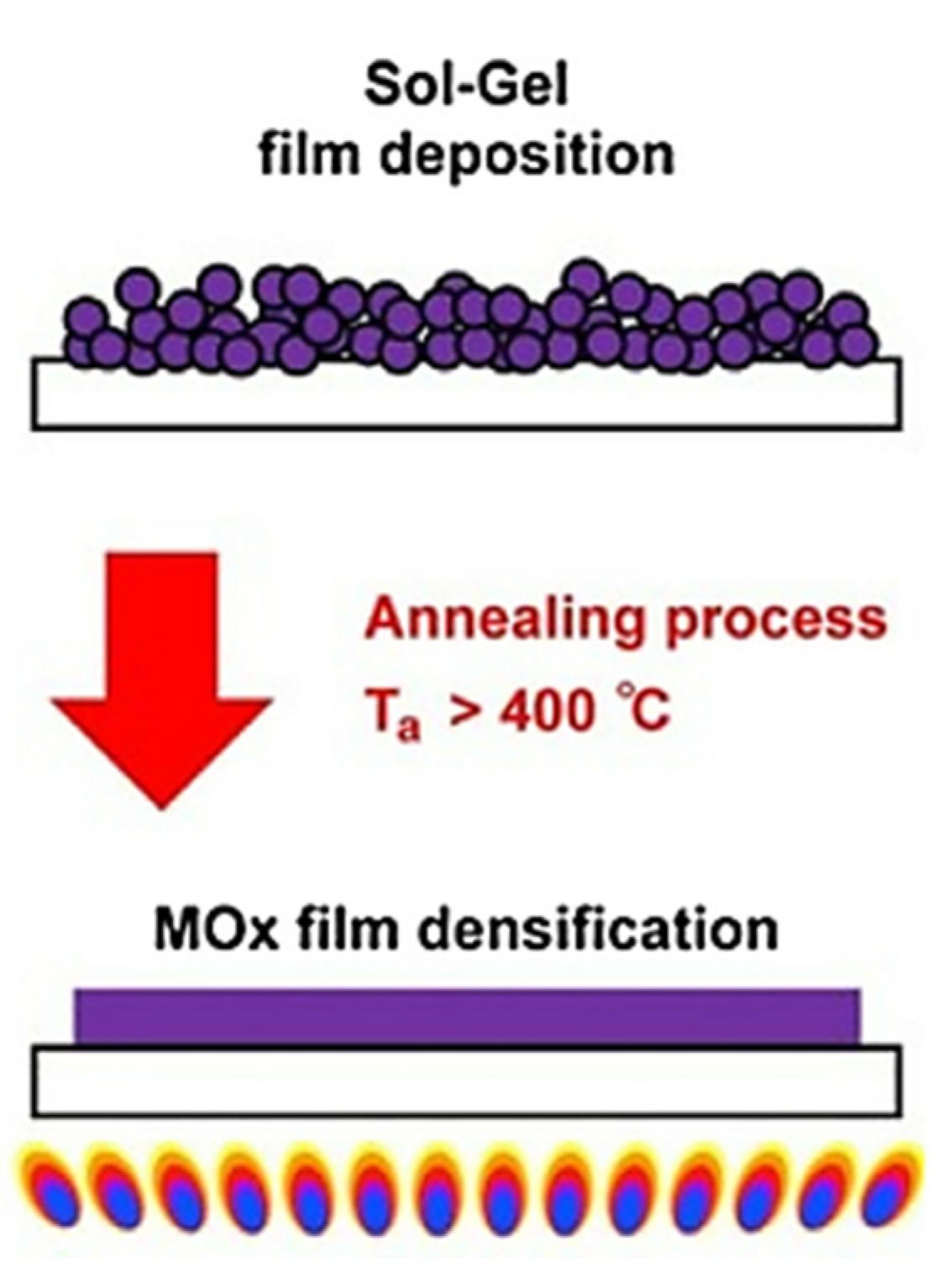
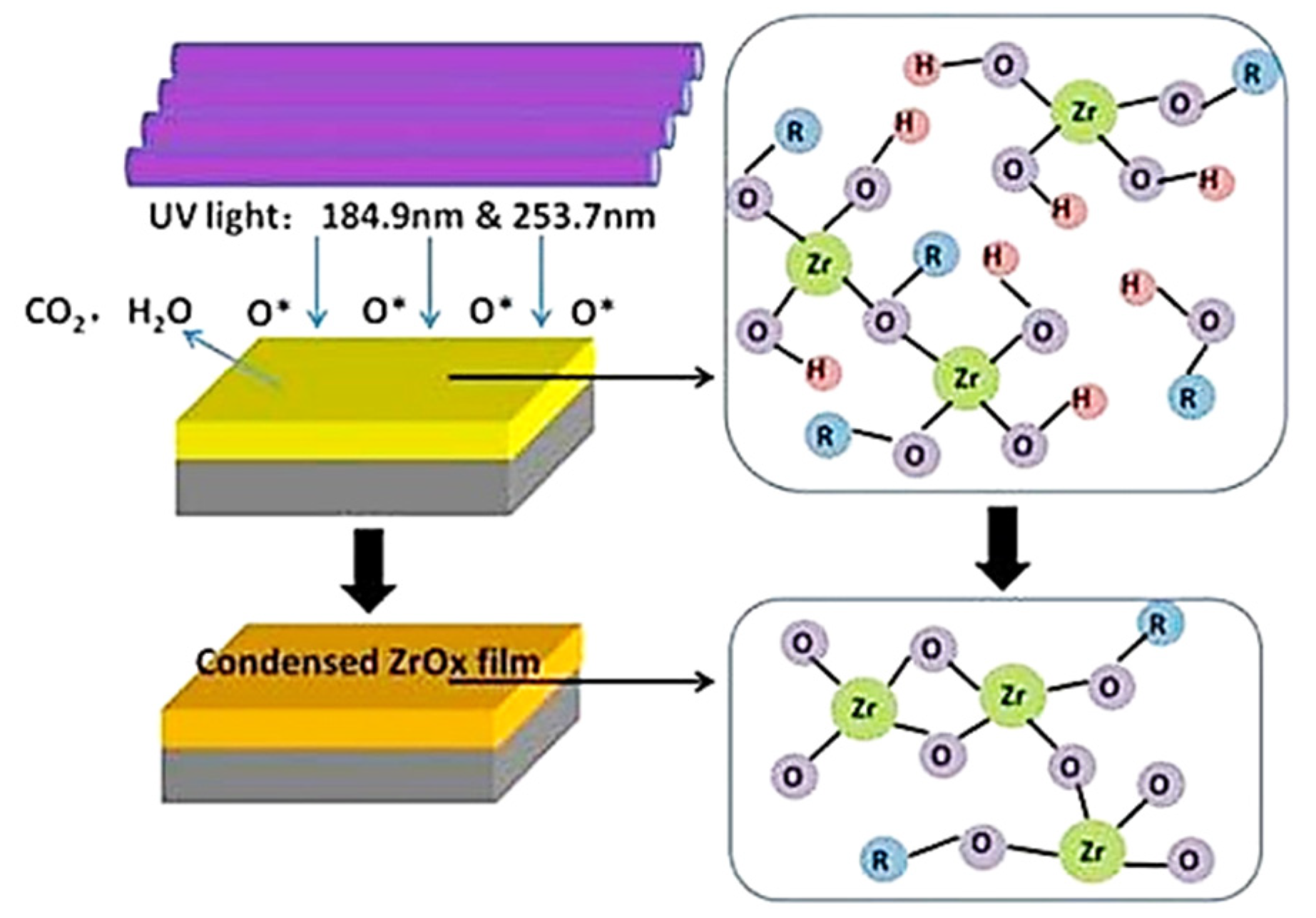
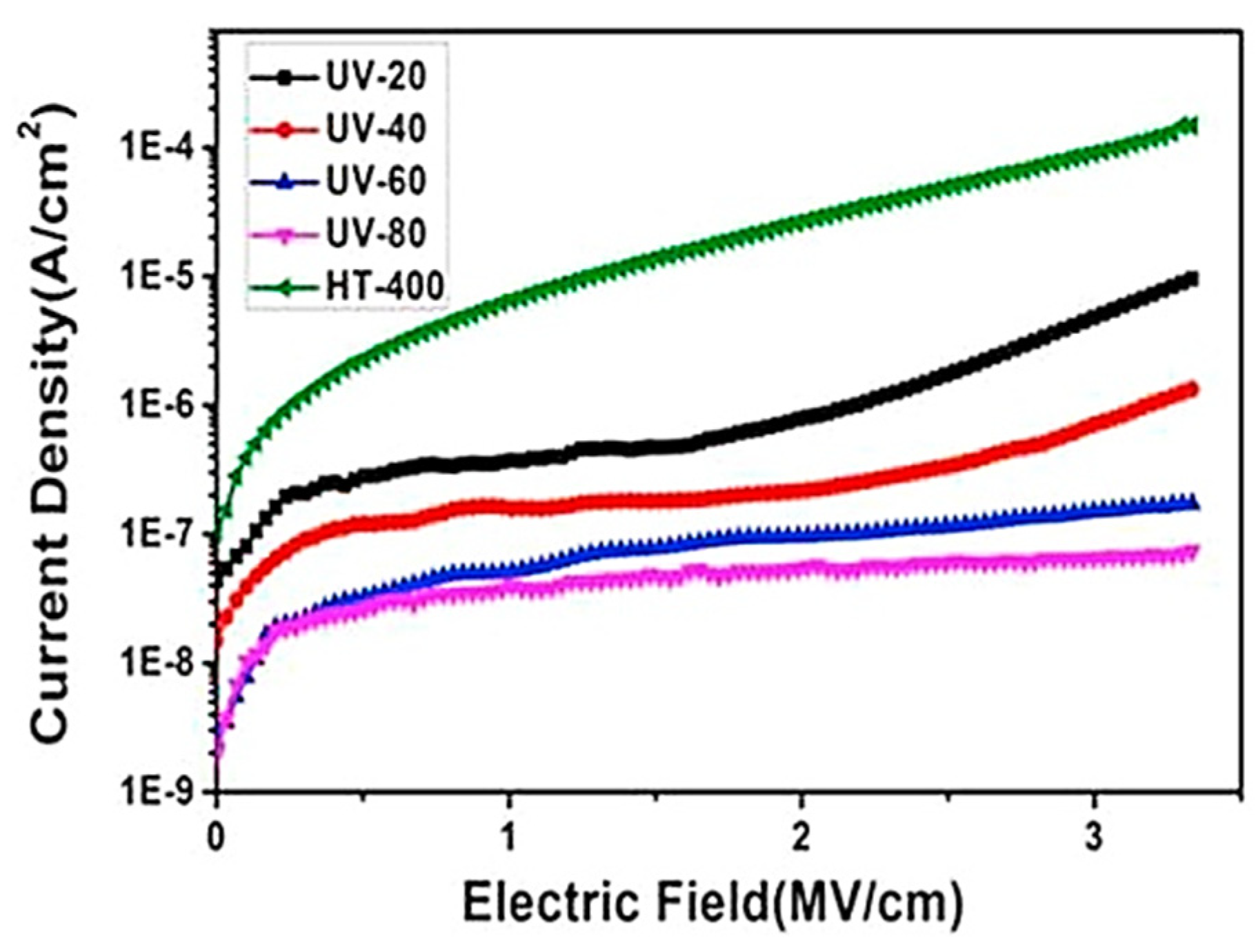
| Material Structure | Leakage Current Density (A/cm2) | EOT (nm) | Carrier Mobility (cm2/V·s) | Density of States (eV−1cm−2) | k Value | Threshold Voltage (V) | Deposition Method | Application | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| ZrO2 | – | – | 28 | – | 25 | 3.2 | RF magnetron sputtering | TFT | [4] |
| N2 + ZrO2 | 10−9~10−8 | – | 22.5 | 7.06 × 1012 | 0.1 | EBE and PECVD | TFT | [35] | |
| HfZrO2 | 3.6 × 10−5 | 1.6~1.8 | – | – | 29~37 | – | ALD | MIM | [62] |
| ZrO2/Si-N | - | 1.6 | – | 5 × 1012 | 11.5 | – | ALD | MIM | [53] |
| ZrAlO | 6.2 × 10−7 | 19.3 | 40.6 | 3.30 × 1012 | 19.67 | 0.71 | ALD | FET | [57] |
| t-ZrO2/Al2O3 | 3.43 × 10−5 | 1.09 | – | 3.35 × 1011 | 21 | – | Remote plasma ALD | MOS | [81] |
| PMMA-ZrO2 | 10−6~10−5 | – | 0.48 | - | 4~12 | 3.3 | Sol−Gel | TFT | [69] |
| Gd-ZrO2 | 1.8 × 10−6 | 4.9 | – | 1.34 × 1011 | 10.3 | – | Sol−Gel | MOS | [61] |
| ZrHfO2-PMMA | 7.7 × 10−6 | – | 2.45 | – | 7.2~9.4 | 1.2 | Sol−Gel | TFT | [66] |
| PMMA-ZrO2 | 3 × 10−9 | – | 5.7 × 10−2 | 7.5 × 1010 | – | 0.8 | EBE and Sol−Gel | OFET | [68] |
| Y-ZrO2 | 1.14 × 10−7 | 0.67 | 68 | 1.2 × 1012 | 30~33 | – | co-sputtering | FET | [63] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, J.; Zhu, Z.; Tao, H.; Zhou, S.; Liang, Z.; Li, Z.; Yao, R.; Wang, Y.; Ning, H.; Peng, J. Research Progress of High Dielectric Constant Zirconia-Based Materials for Gate Dielectric Application. Coatings 2020, 10, 698. https://doi.org/10.3390/coatings10070698
Xie J, Zhu Z, Tao H, Zhou S, Liang Z, Li Z, Yao R, Wang Y, Ning H, Peng J. Research Progress of High Dielectric Constant Zirconia-Based Materials for Gate Dielectric Application. Coatings. 2020; 10(7):698. https://doi.org/10.3390/coatings10070698
Chicago/Turabian StyleXie, Junan, Zhennan Zhu, Hong Tao, Shangxiong Zhou, Zhihao Liang, Zhihang Li, Rihui Yao, Yiping Wang, Honglong Ning, and Junbiao Peng. 2020. "Research Progress of High Dielectric Constant Zirconia-Based Materials for Gate Dielectric Application" Coatings 10, no. 7: 698. https://doi.org/10.3390/coatings10070698
APA StyleXie, J., Zhu, Z., Tao, H., Zhou, S., Liang, Z., Li, Z., Yao, R., Wang, Y., Ning, H., & Peng, J. (2020). Research Progress of High Dielectric Constant Zirconia-Based Materials for Gate Dielectric Application. Coatings, 10(7), 698. https://doi.org/10.3390/coatings10070698







