High Temperature Corrosion Behaviors of 20G Steel, Hastelloy C22 Alloy and C22 Laser Coating under Reducing Atmosphere with H2S
Abstract
:1. Introduction
2. Materials and Methods
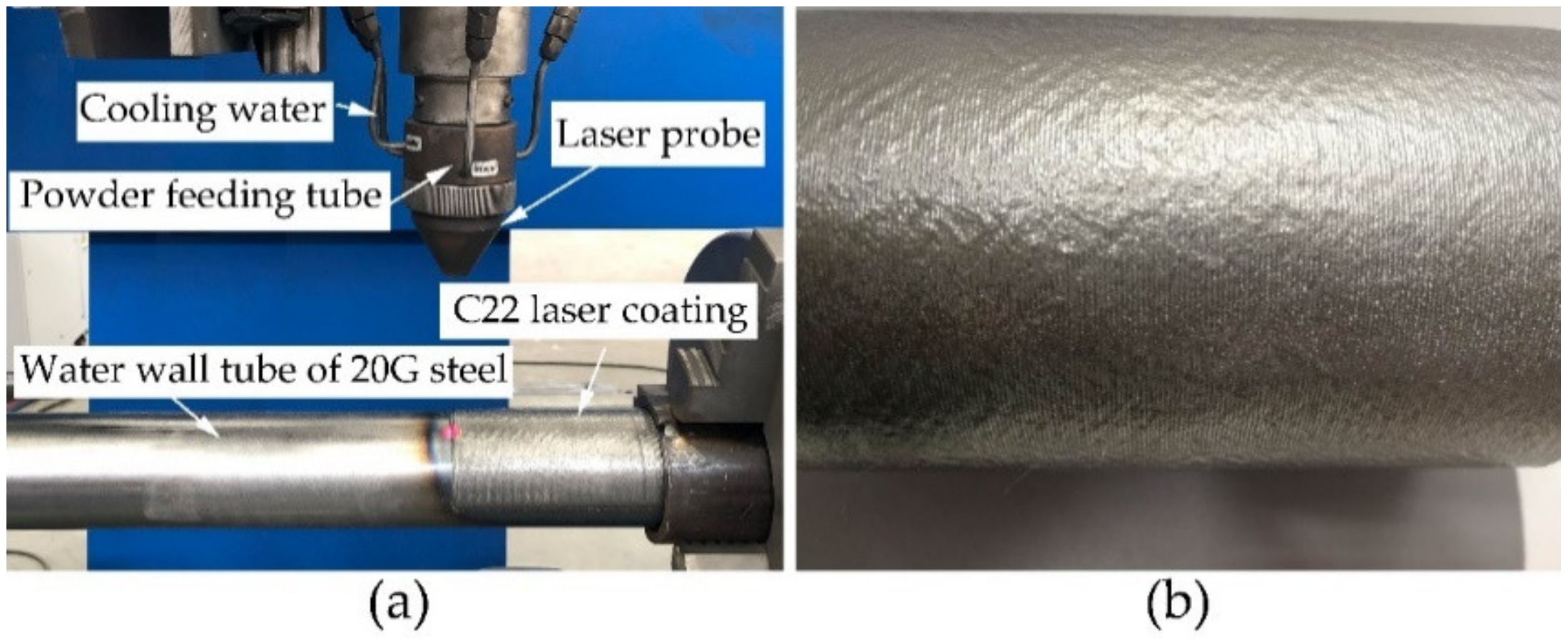
2.1. Materials and Laser-Cladding Process
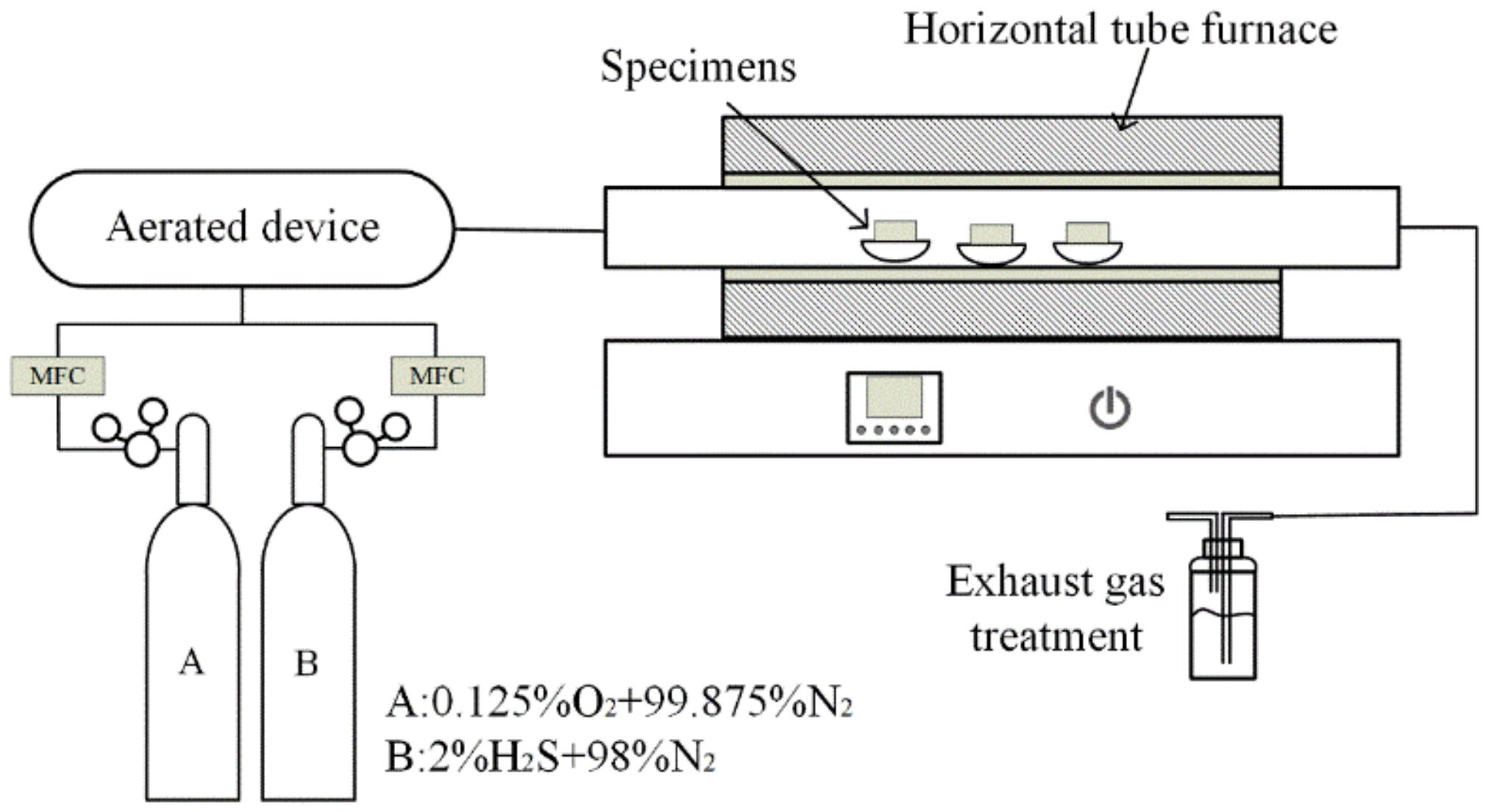
2.2. Hot Corrosion Studies
3. Results
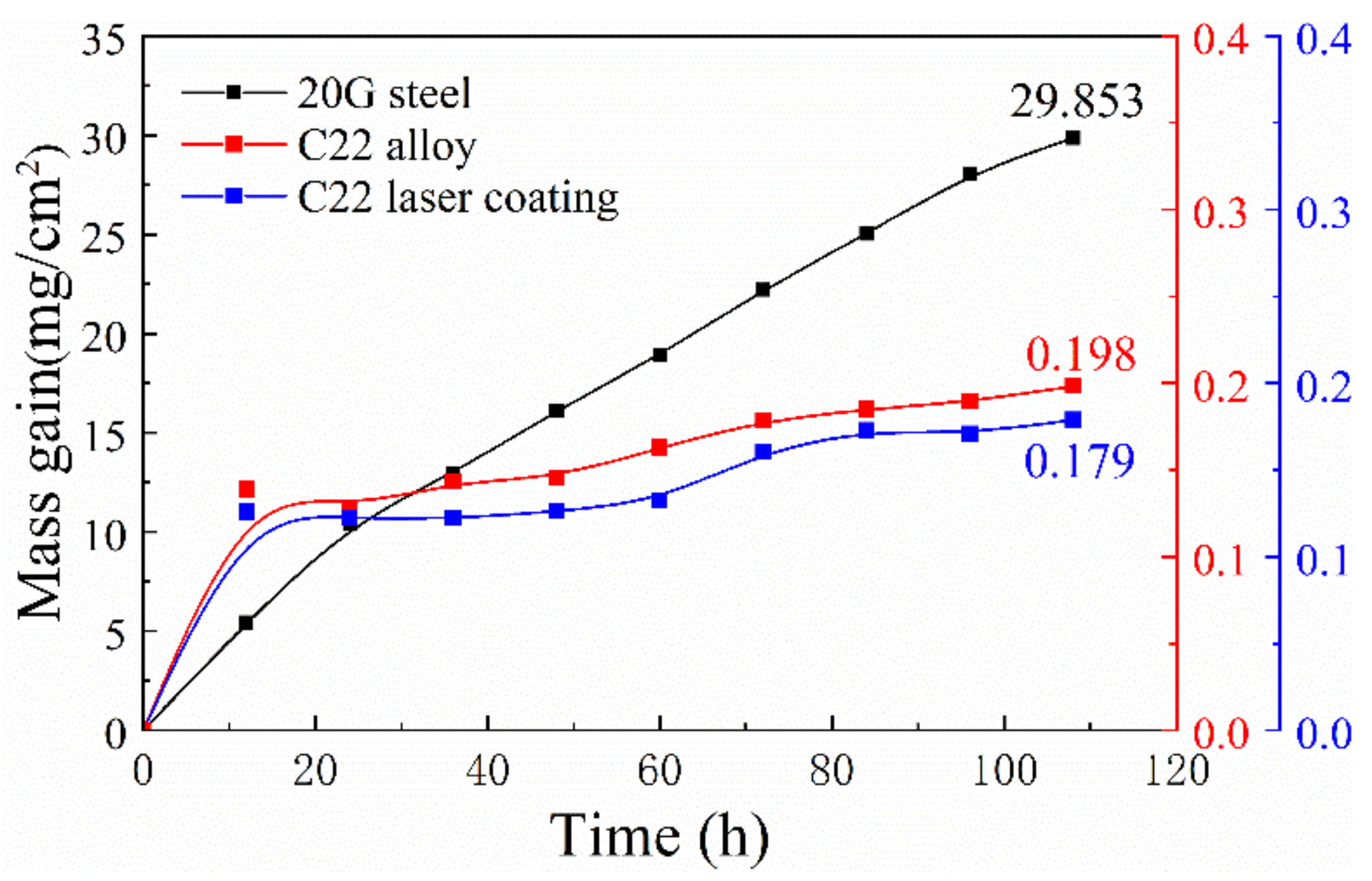
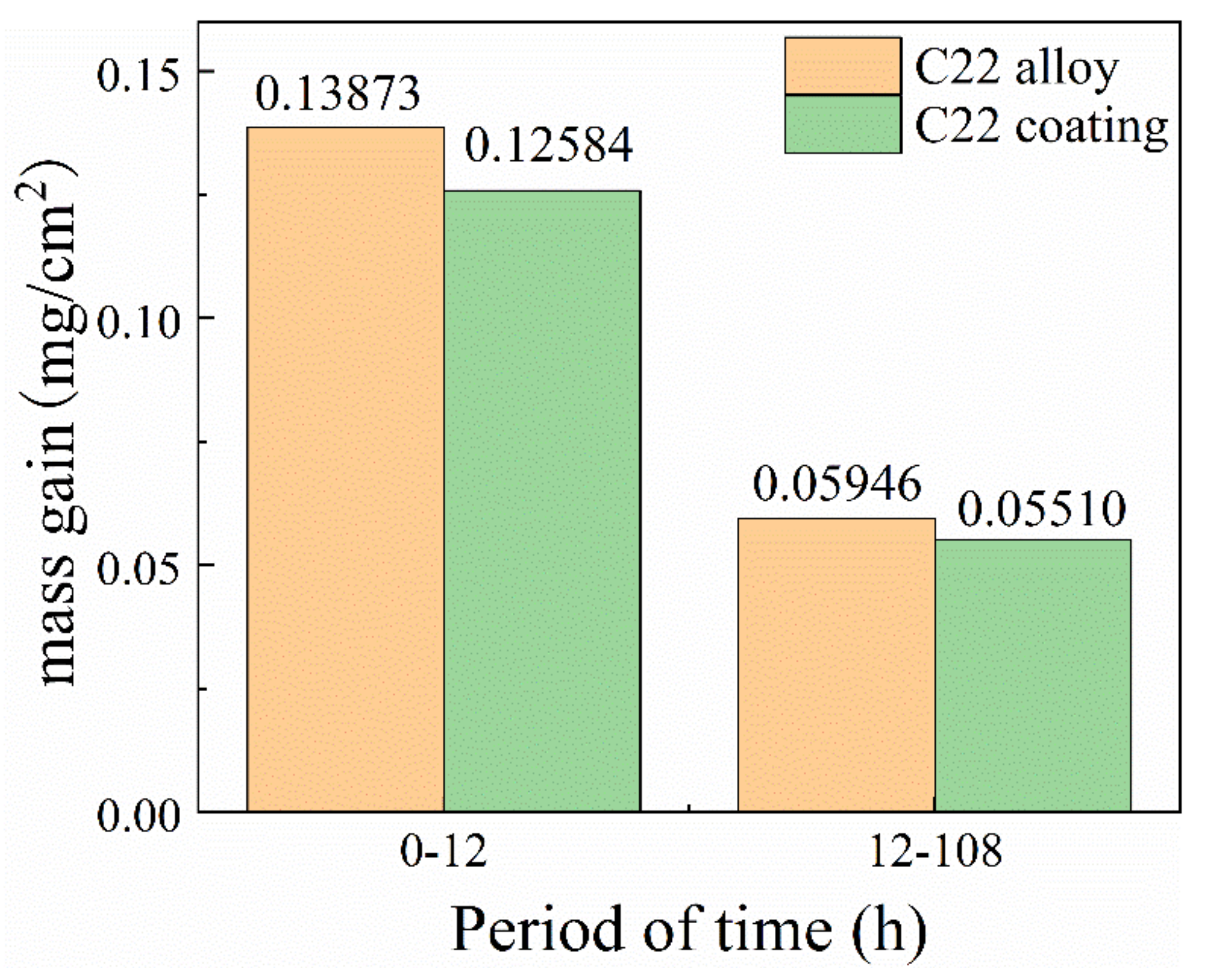
3.1. Corrosion Dynamics Curve
3.2. Corrosion of 20G Steel, C22 Alloy and C22 Laser Coating
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fan, W.; Lin, Z.; Kuang, J.; Li, Y. Impact of air staging along furnace height on NOx emissions from pulverized coal combustion. Fuel Process. Technol. 2010, 91, 625–634. [Google Scholar] [CrossRef]
- Flamma, M. Low NOx combustion technologies for high temperature applications. Energy Convers. Manag. 2001, 42, 1919–1935. [Google Scholar] [CrossRef]
- Javed, M.T.; Irfan, N.; Gibbs, B.M. Control of combustion-generated nitrogen oxides by selective non-catalytic reduction. J. Environ. Manag. 2007, 83, 251–289. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; You, R.; Wang, Z.; Zhang, H.; Zhou, Z.; Zhou, J.; Guan, J.; Qiu, L. Effects of near-wall air application in a pulverized-coal 300 MWe utility boiler on combustion and corrosive gases. Energy Fuels 2017, 31, 10075–10081. [Google Scholar] [CrossRef]
- Chou, S.F.; Daniel, P.L.; Blazewicz, A.J.; Dudek, R.F. Hydrogen sulfide corrosion in low-NOx combustion systerms. J. Mater. Energy Syst. 1986, 7, 361–369. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, Y.; Dong, K.; Liu, H.; Shen, Y.; Cen, K. Influence of the gas particle flow characteristics of a low-NOx swirl burner on the formation of high temperature corrosion. Fuel 2014, 134, 595–602. [Google Scholar] [CrossRef]
- Harb, J.N.; Smith, E.E. Fireside corrosion in pc-fired boilers. Prog. Energy Combust. Sci. 1990, 16, 155–190. [Google Scholar] [CrossRef]
- Xu, L.-G.; Huang, Y.-J.; Wang, J.; Zou, L.; Yue, J.-F. High-temperature corrosion properties of water wall material 15CrMoG under reducing atmosphere. J. Zhejiang Univ. (Eng. Sci.) 2018, 52. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, Z.; Cheng, D.; Wang, Y.; Deng, X. High temperature corrosion of water wall materials T23 and T24 in simulated furnace atmospheres. Chin. J. Chem. Eng. 2012, 20, 814–822. [Google Scholar] [CrossRef]
- Xu, W.; Tan, H.; Liu, Y.; Wei, B.; Hui, S.e. Research on determination of high temperature corrosion tendency of water walls and limiting concentration range of H2S near walls. Electr. Power 2018, 51. [Google Scholar] [CrossRef]
- Jones, R.H.; Baer, D.R.; Windisch, J.C.F. Corrosion enhanced enrichment of sulfur and implications for alloy 22. In Proceedings of the NACE International Corrosion Conference, San Diego, CA, USA, 12–16 March 2006. [Google Scholar]
- Petrov, A.I.; Razuvaeva, M.V. Stress corrosion cracking of metals and alloys in aggressive H2S–CO2–Cl– environments. Tech. Phys. 2020, 64, 1814–1820. [Google Scholar] [CrossRef]
- Rebak, R.B.; Szklarska-Amialowska, Z. The mechanism of stress corrosion cracking of alloy 600 in high temperature water. Corros. Sci. 1996, 38, 971–988. [Google Scholar] [CrossRef]
- Davis, J.R. Nickel, Cobalt, and Their Alloys, 1st ed.; ASM International Materials Park: Cleveland, OH, USA, 2000; pp. 125–186. [Google Scholar]
- Pardo, A.; Merino, M.C.; Coy, A.E.; Viejo, F.; Arrabal, R.; Matykina, E. Pitting corrosion behaviour of austenitic stainless steels—Combining effects of Mn and Mo additions. Corros. Sci. 2008, 50, 1796–1806. [Google Scholar] [CrossRef]
- Ebrahimi, N.; Jakupi, P.; Korinek, A.; Barker, I.; Moser, D.E.; Shoesmith, D.W. Sigma and random grain boundaries and their effect on the corrosion of the Ni-Cr-Mo alloy 22. J. Electrochem. Soc. 2016, 163, C232–C239. [Google Scholar] [CrossRef]
- Jakupi, P.; Noël, J.J.; Shoesmith, D.W. Intergranular corrosion resistance of ∑3 grain boundaries in alloy 22. Electrochem. Solid-State Lett. 2010, 13. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Z.; Wang, Y.; Tang, J. A comparative study on the high temperature corrosion of TP347H stainless steel, C22 alloy and laser-cladding C22 coating in molten chloride salts. Corros. Sci. 2014, 83, 396–408. [Google Scholar] [CrossRef]
- He, X.; Dunn, D.S. Crevice corrosion penetration rates of alloy 22 in chloride-containing waters. Corros. Sci. 2007, 63, 145–158. [Google Scholar] [CrossRef]
- Jakupi, P.; Wang, F.; Noël, J.J.; Shoesmith, D.W. Corrosion product analysis on crevice corroded Alloy-22 specimens. Corros. Sci. 2011, 53, 1670–1679. [Google Scholar] [CrossRef]
- Chiang, K.T.; Dunn, D.S.; Cragnolino, G.A. Effect of simulated ground water chemistry on stress corrosion cracking of alloy 22. Corros. Sci. 2007, 63, 940–950. [Google Scholar] [CrossRef]
- Newman, R.C. The dissolution and passivation kinetics of stainless alloys containing molybdenum-1. Coulometric studies of Fe-Cr and Fe-Cr-Mo alloys. Corros. Sci. 1985, 25, 331–339. [Google Scholar] [CrossRef]
- Hayes, J.R.; Gray, J.J.; Szmodis, A.W.; Orme, C.A. Influence of chromium and molybdenum on the corrosion of nickel-based alloys. Corros. Sci. 2006, 62, 491–500. [Google Scholar] [CrossRef]
- Lloyd, A.C.; Noël, J.J.; McIntyre, S.; Shoesmith, D.W. Cr, Mo and W alloying additions in Ni and their effect on passivity. Electrochim. Acta 2004, 49, 3015–3027. [Google Scholar] [CrossRef]
- Jakupi, P.; Noël, J.J.; Shoesmith, D.W. The evolution of crevice corrosion damage on the Ni–Cr–Mo–W alloy-22 determined by confocal laser scanning microscopy. Corros. Sci. 2012, 54, 260–269. [Google Scholar] [CrossRef]
- Jakupi, P.; Zagidulin, D.; Noël, J.J.; Shoesmith, D.W. The impedance properties of the oxide film on the Ni–Cr–Mo Alloy-22 in neutral concentrated sodium chloride solution. Electrochim. Acta 2011, 56, 6251–6259. [Google Scholar] [CrossRef]
- Wang, Q.-Y.; Wang, X.-Z.; Luo, H.; Luo, J.-L. A study on corrosion behaviors of Ni–Cr–Mo laser coating, 316 stainless steel and X70 steel in simulated solutions with H2S and CO2. Surf. Coat. Technol. 2016, 291, 250–257. [Google Scholar] [CrossRef]
- Haemers, T.A.M.; Rickerby, D.G.; Lanza, F.; Geiger, F.; Mittemeijer, E.J. Laser cladding of stainless steel with hastelloy. Adv. Eng. Mater. 2001, 3, 242–245. [Google Scholar] [CrossRef]
- Barnes, S.; Timms, N.; Bryden, B.; Pashby, I. High power diode laser cladding. J. Mater. Process. Technol. 2003, 138, 411–416. [Google Scholar] [CrossRef]
- Cui, C.; Guo, Z.; Liu, Y.; Xie, Q.; Wang, Z.; Hu, J.; Yao, Y. Characteristics of cobalt-based alloy coating on tool steel prepared by powder feeding laser cladding. Optics Laser Technol. 2007, 39, 1544–1550. [Google Scholar] [CrossRef]
- Huang, Y. Characterization of dilution action in laser-induction hybrid cladding. Optics Laser Technol. 2011, 43, 965–973. [Google Scholar] [CrossRef]
- Chen, L.; Bai, S.-L. The anti-corrosion behavior under multi-factor impingement of Hastelloy C22 coating prepared by multilayer laser cladding. Appl. Surf. Sci. 2018, 437, 1–12. [Google Scholar] [CrossRef]
- Fujimagari, H.; Hagiwara, M.; Kojima, T. Laser cladding technology to small diameter pipes. Nucl. Eng. Des. 2000, 195, 289–298. [Google Scholar] [CrossRef]
- Wang, Q.-Y.; Zhang, Y.-F.; Bai, S.-L.; Liu, Z.-D. Microstructures, mechanical properties and corrosion resistance of Hastelloy C22 coating produced by laser cladding. J. Alloys Compd. 2013, 553, 253–258. [Google Scholar] [CrossRef]
- Li, X.-Z.; Liu, Z.-D.; Li, H.-C.; Wang, Y.-T.; Li, B. Investigations on the behavior of laser cladding Ni–Cr–Mo alloy coating on TP347H stainless steel tube in HCl rich environment. Surf. Coat. Technol. 2013, 232, 627–639. [Google Scholar] [CrossRef]
- Standardization Administration of China. GB/T 5310–2017: Seamless Steel Tubes and Pipes for High Pressure Boiler; Standards Press of China: Beijing, China, 2017; p. 7. [Google Scholar]
- Zhao, H.; Wei, Y. Discussion on the mechanisms and factors of the gas side high temperature corrosion in water wall tubes for coal fired boilers. Power Eng. 2002, 22, 1070–1074. [Google Scholar] [CrossRef]
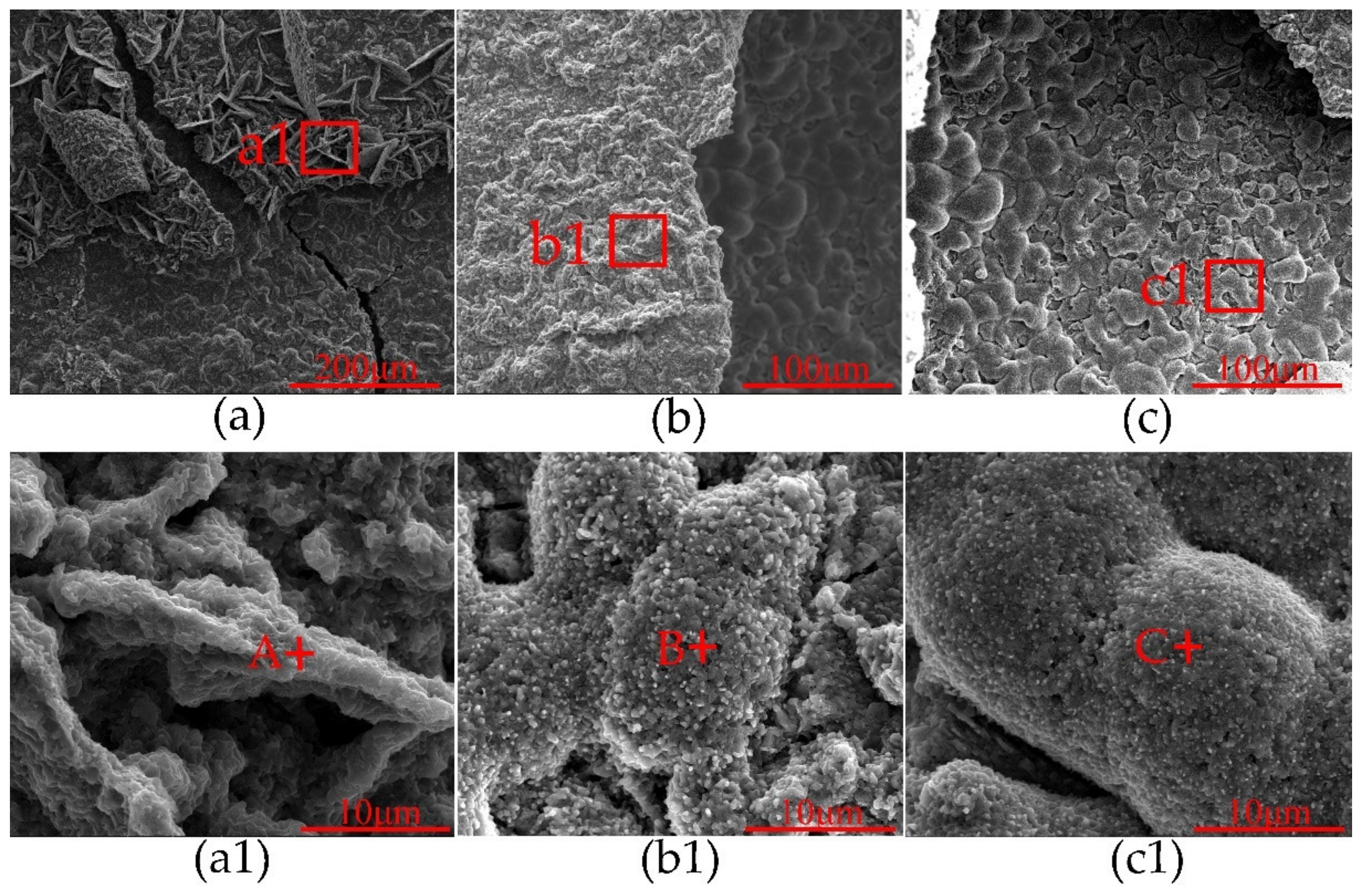
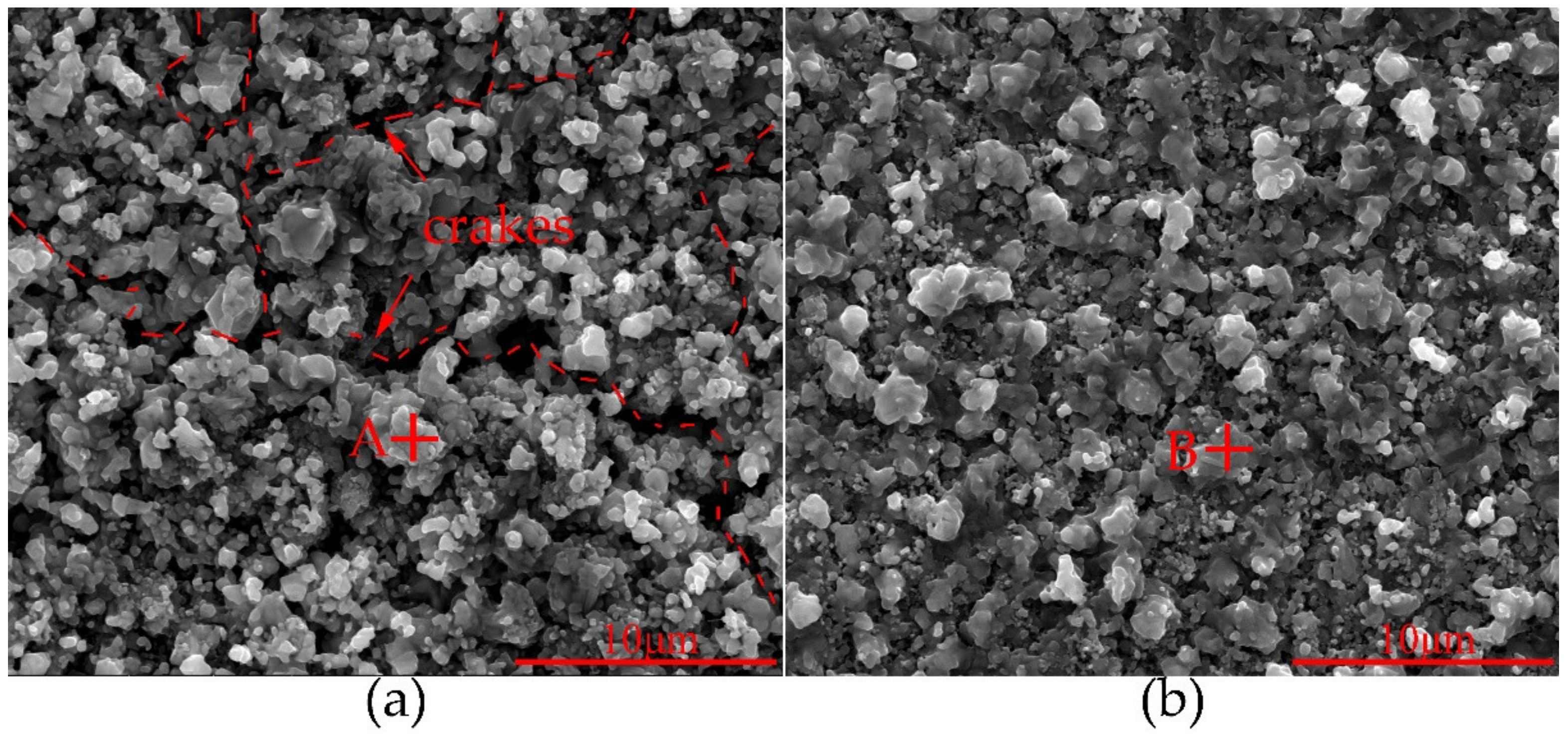
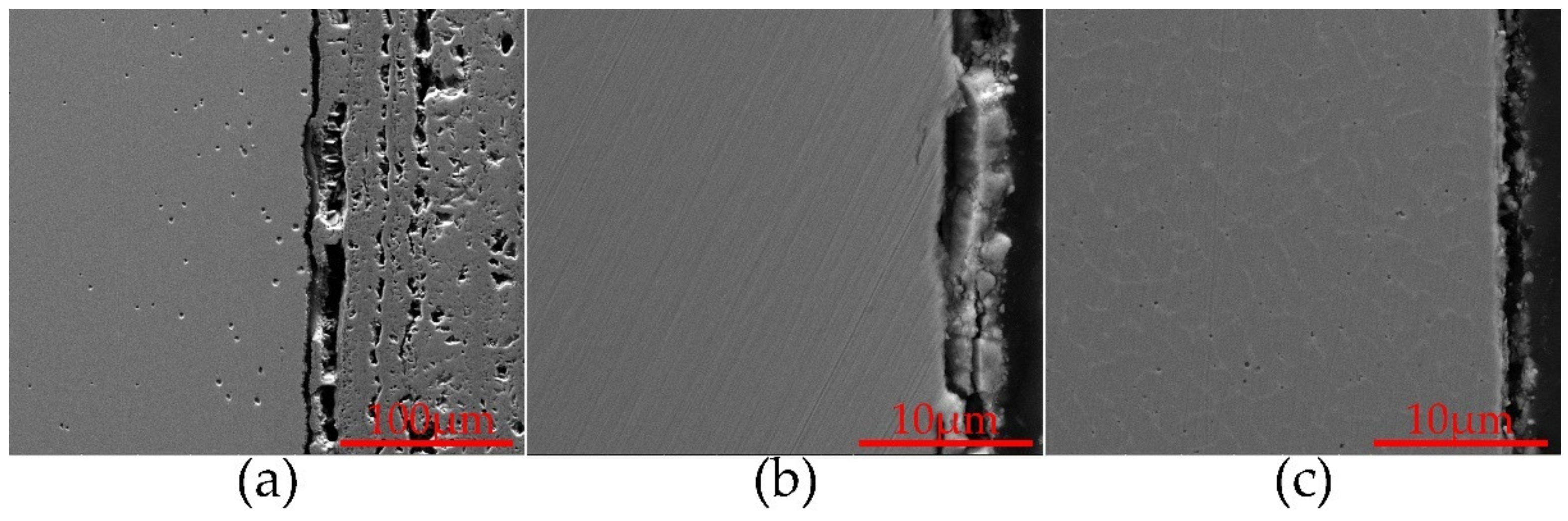
| Alloy | Element (wt%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ni | C | Cr | Mo | Mn | Fe | Si | Co | V | W | S | P | |
| C22 powder | Bal. | 0.08 | 21.3 | 13.2 | – | 2.9 | – | 2.0 | 3.0 | – | – | |
| C22 alloy | Bal. | 0.001 | 22.0 | 13.8 | 0.45 | 5.0 | 0.078 | 1.83 | 0.3 | 3.2 | 0.008 | 0.02 |
| 20G | – | 0.17–0.23 | – | – | 0.35–0.65 | Bal. | 0.17–0.37 | – | – | – | <0.015 | <0.025 |
| Title | Elements (at%) | ||
|---|---|---|---|
| S | Fe | O | |
| A | 18.78 | 27.97 | 53.24 |
| B | 21.25 | 39.64 | 39.10 |
| C | 69.30 | 39.70 | 0 |
| Point | Elements (at%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cr | S | Mn | Fe | Co | Ni | W | Mo | O | |
| A | 19.28 | 38.46 | 0.29 | 1.35 | 0.24 | 20.82 | 0.27 | 7.92 | 11.31 |
| B | 23.14 | 37.39 | 0.16 | 2.03 | 0.49 | 17.75 | – | 8.14 | 10.90 |
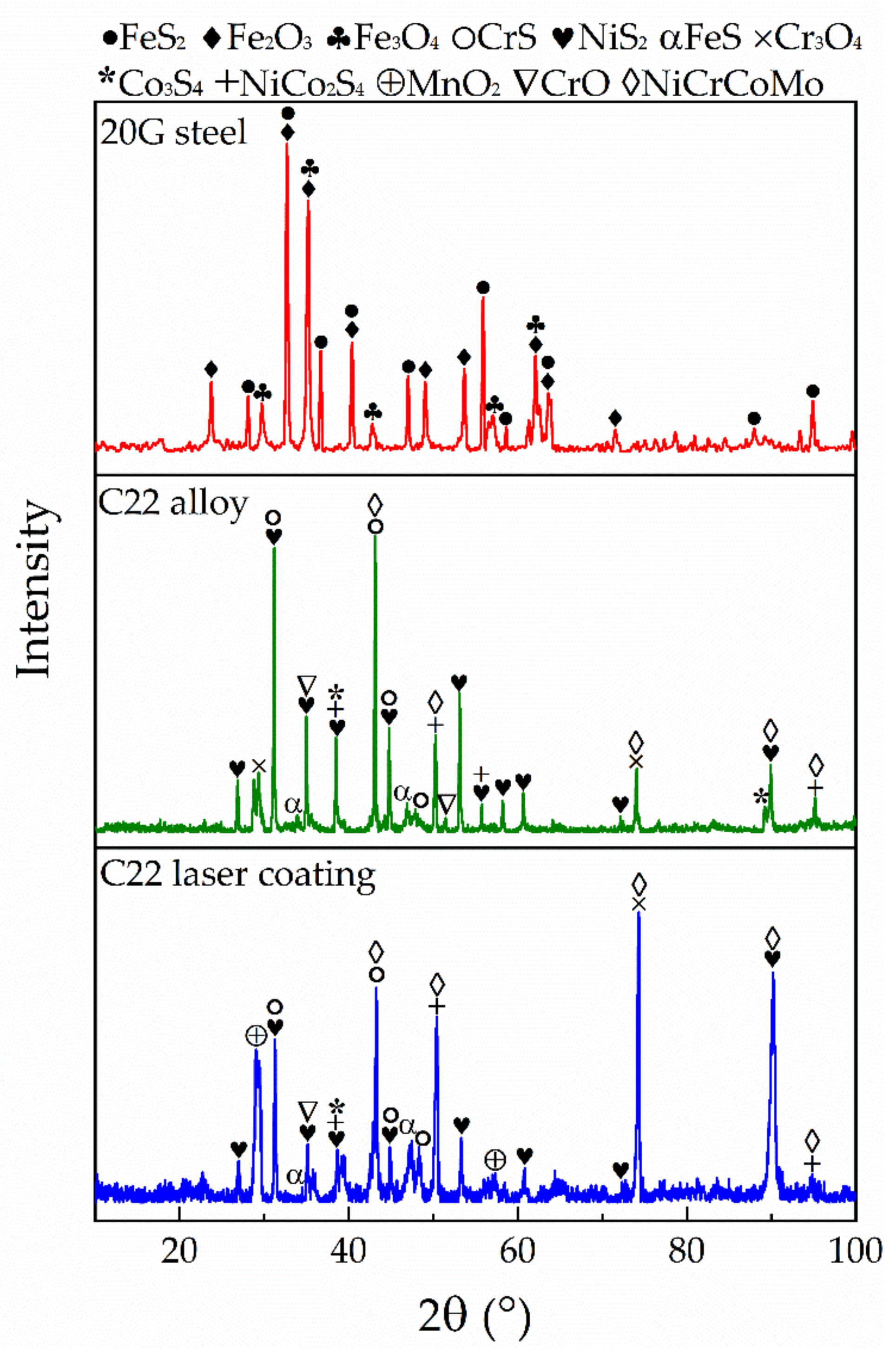
| Title 1 | Phase Identification |
|---|---|
| 20G steel | FeS2 + Fe2O3 + Fe3O4 |
| C22 alloy | CrS + NiS2 + FeS + Co3S4 + NiCo2S4 + Cr3O4 + MnO2 + CrO + NiCrCoMo |
| C22 laser coating | CrS + NiS2 + FeS + Co3S4 + NiCo2S4 + Cr3O4 + MnO2 + CrO + NiCrCoMo |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Liu, C.; Gao, Y.; Zheng, C. High Temperature Corrosion Behaviors of 20G Steel, Hastelloy C22 Alloy and C22 Laser Coating under Reducing Atmosphere with H2S. Coatings 2020, 10, 617. https://doi.org/10.3390/coatings10070617
Liu Z, Liu C, Gao Y, Zheng C. High Temperature Corrosion Behaviors of 20G Steel, Hastelloy C22 Alloy and C22 Laser Coating under Reducing Atmosphere with H2S. Coatings. 2020; 10(7):617. https://doi.org/10.3390/coatings10070617
Chicago/Turabian StyleLiu, Zongde, Congcong Liu, Yuan Gao, and Chao Zheng. 2020. "High Temperature Corrosion Behaviors of 20G Steel, Hastelloy C22 Alloy and C22 Laser Coating under Reducing Atmosphere with H2S" Coatings 10, no. 7: 617. https://doi.org/10.3390/coatings10070617
APA StyleLiu, Z., Liu, C., Gao, Y., & Zheng, C. (2020). High Temperature Corrosion Behaviors of 20G Steel, Hastelloy C22 Alloy and C22 Laser Coating under Reducing Atmosphere with H2S. Coatings, 10(7), 617. https://doi.org/10.3390/coatings10070617






