Effect of Laser Processing on Surface Properties of Additively Manufactured 18-Percent Nickel Maraging Steel Parts
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials for Samples Manufacturing
2.2. SLM Process Details
2.3. Surface Laser Processing Details
2.4. Characterization Methods
3. Results and Discussion
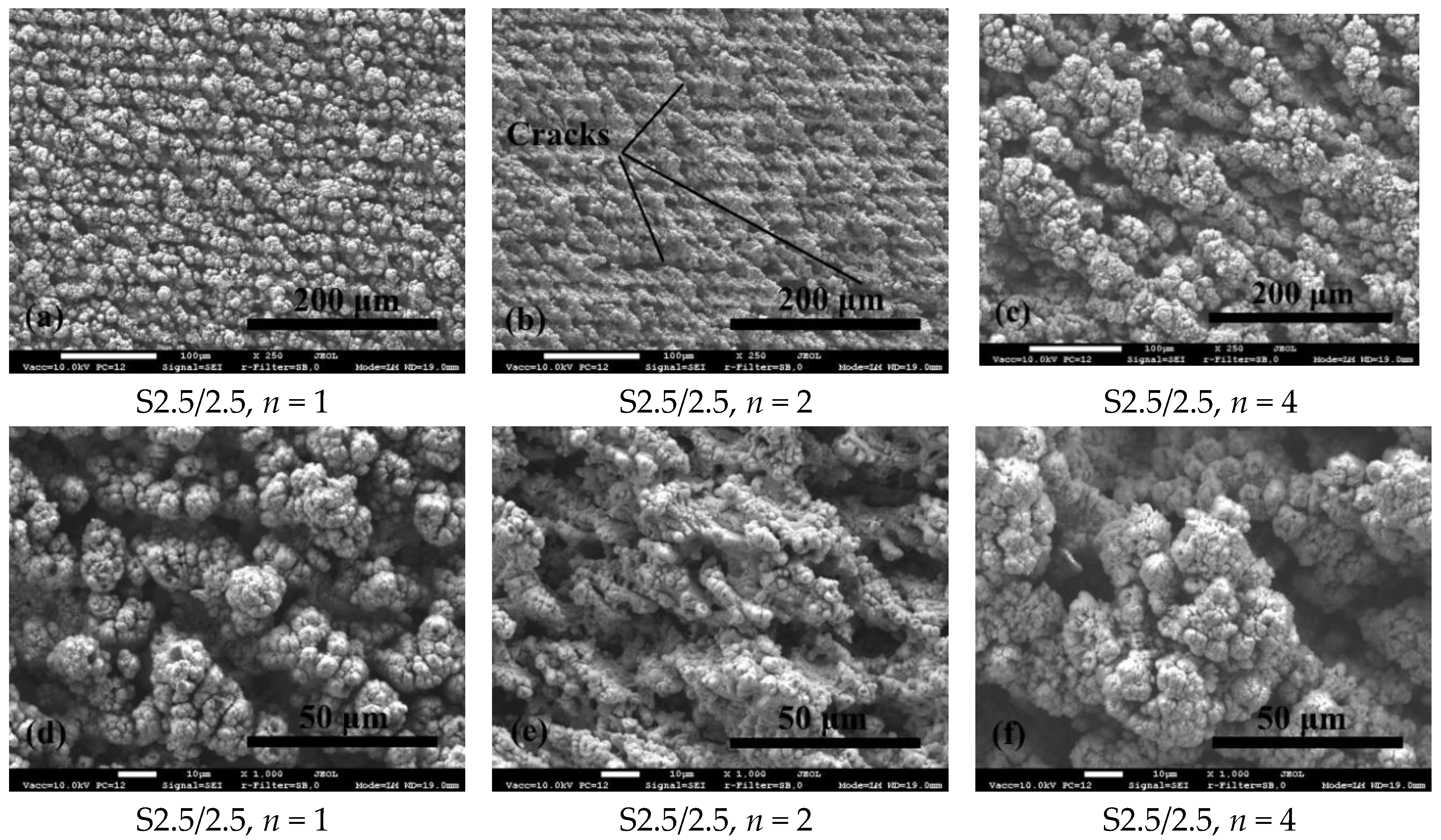
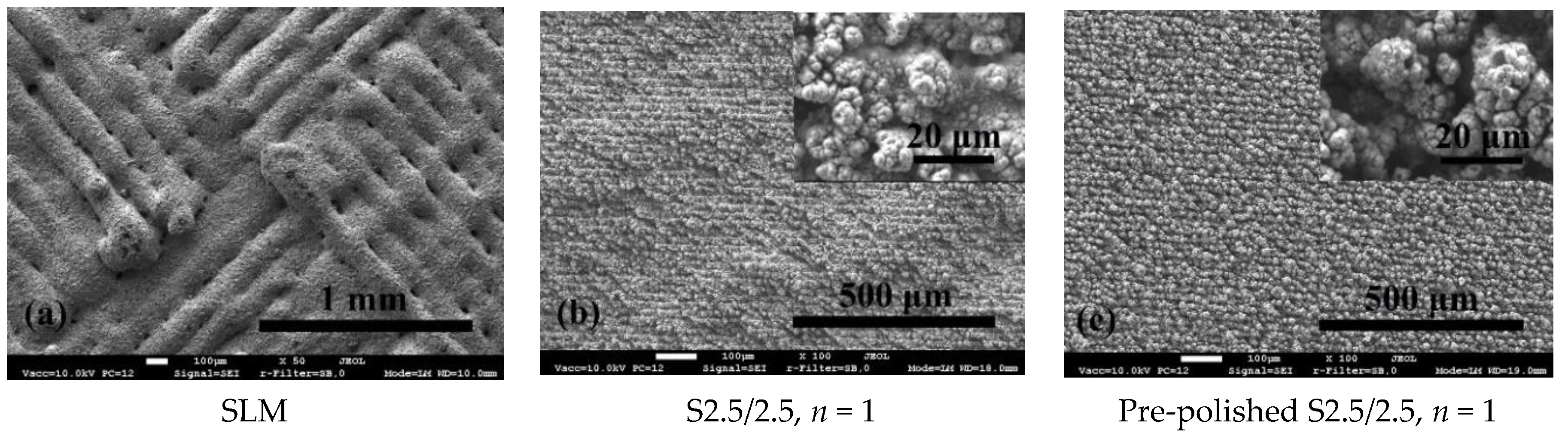
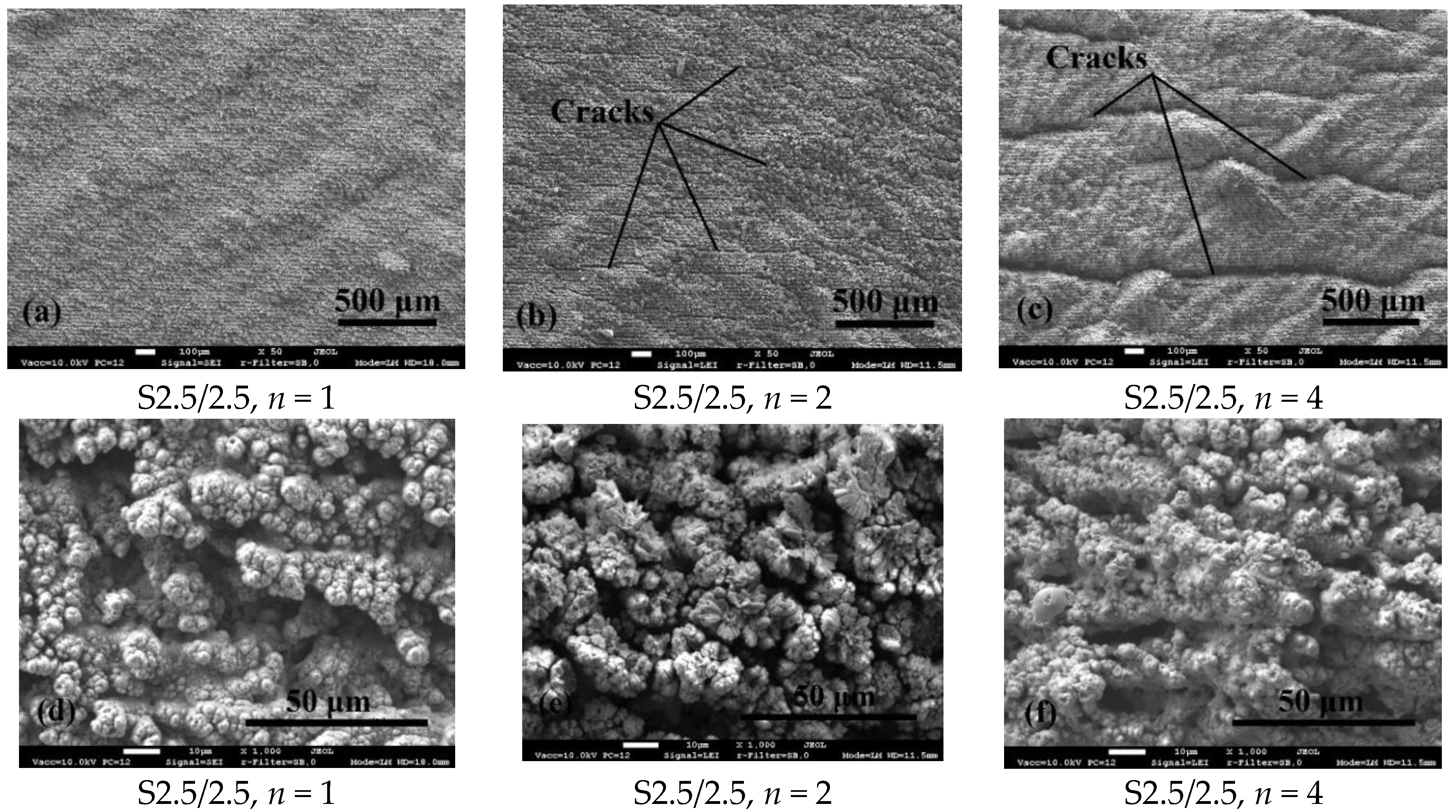
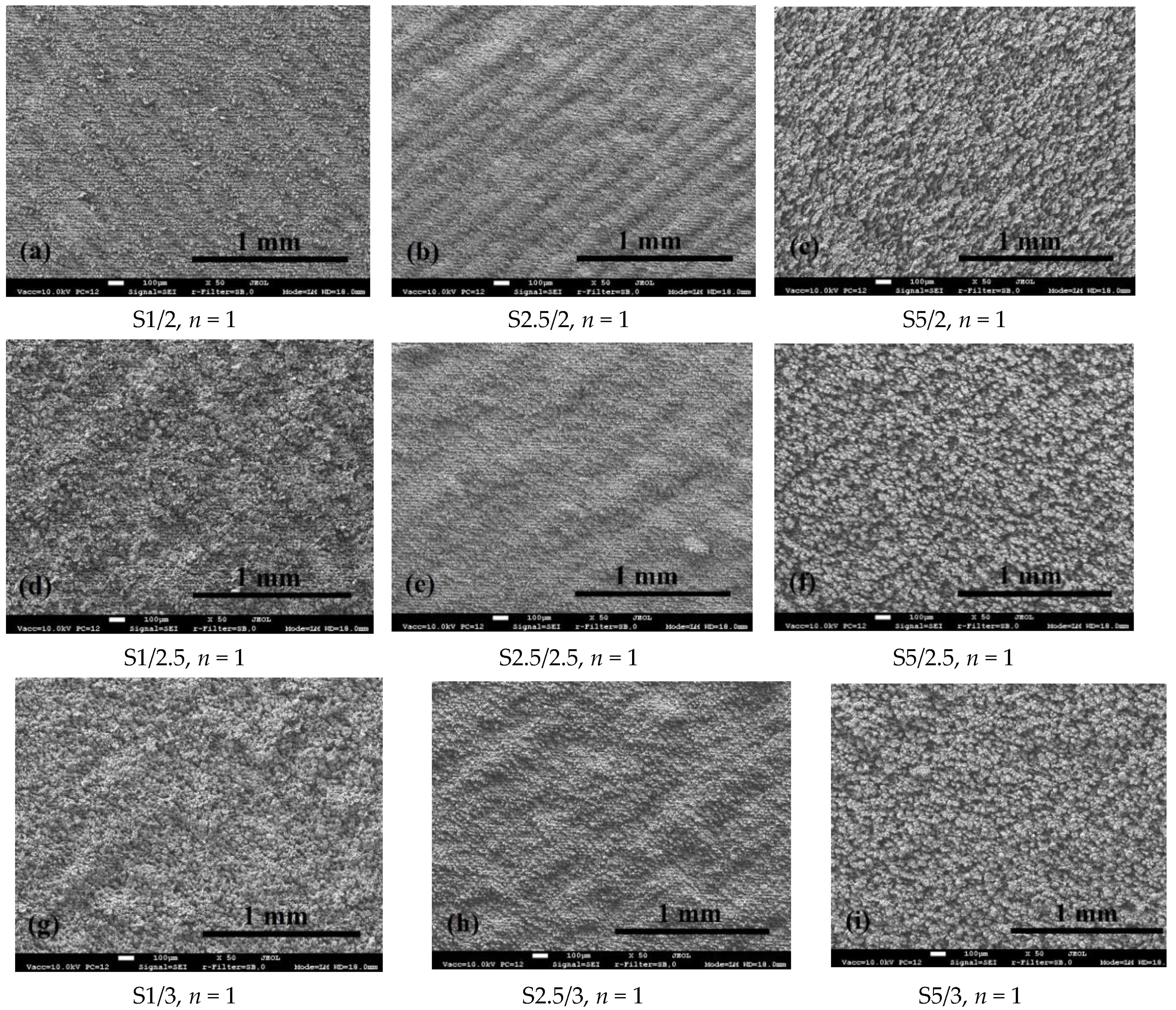
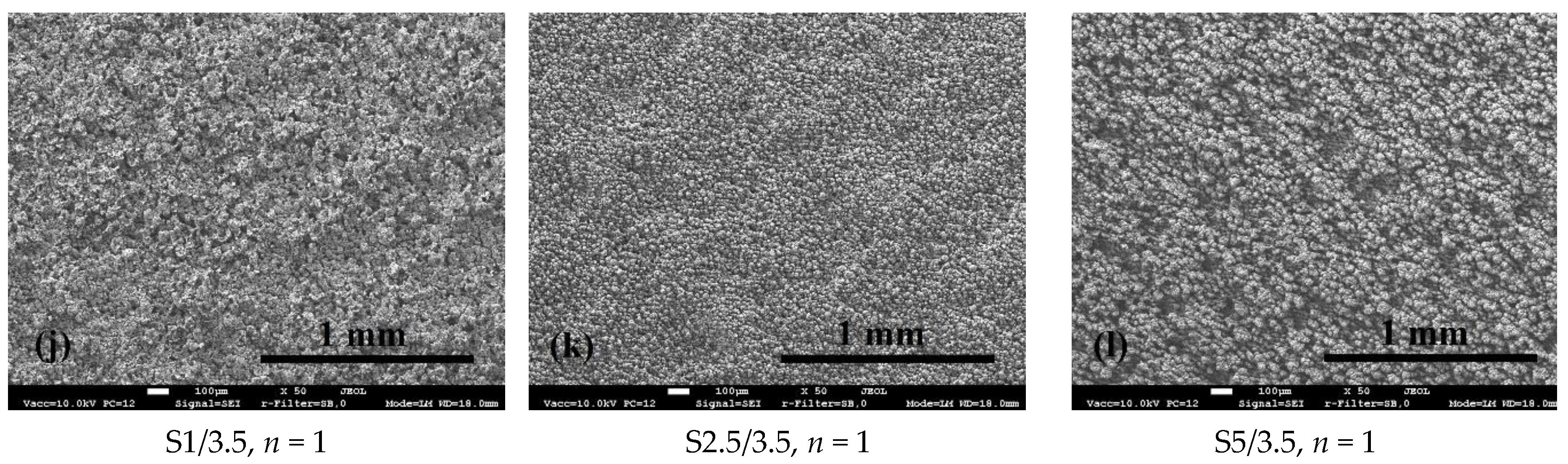
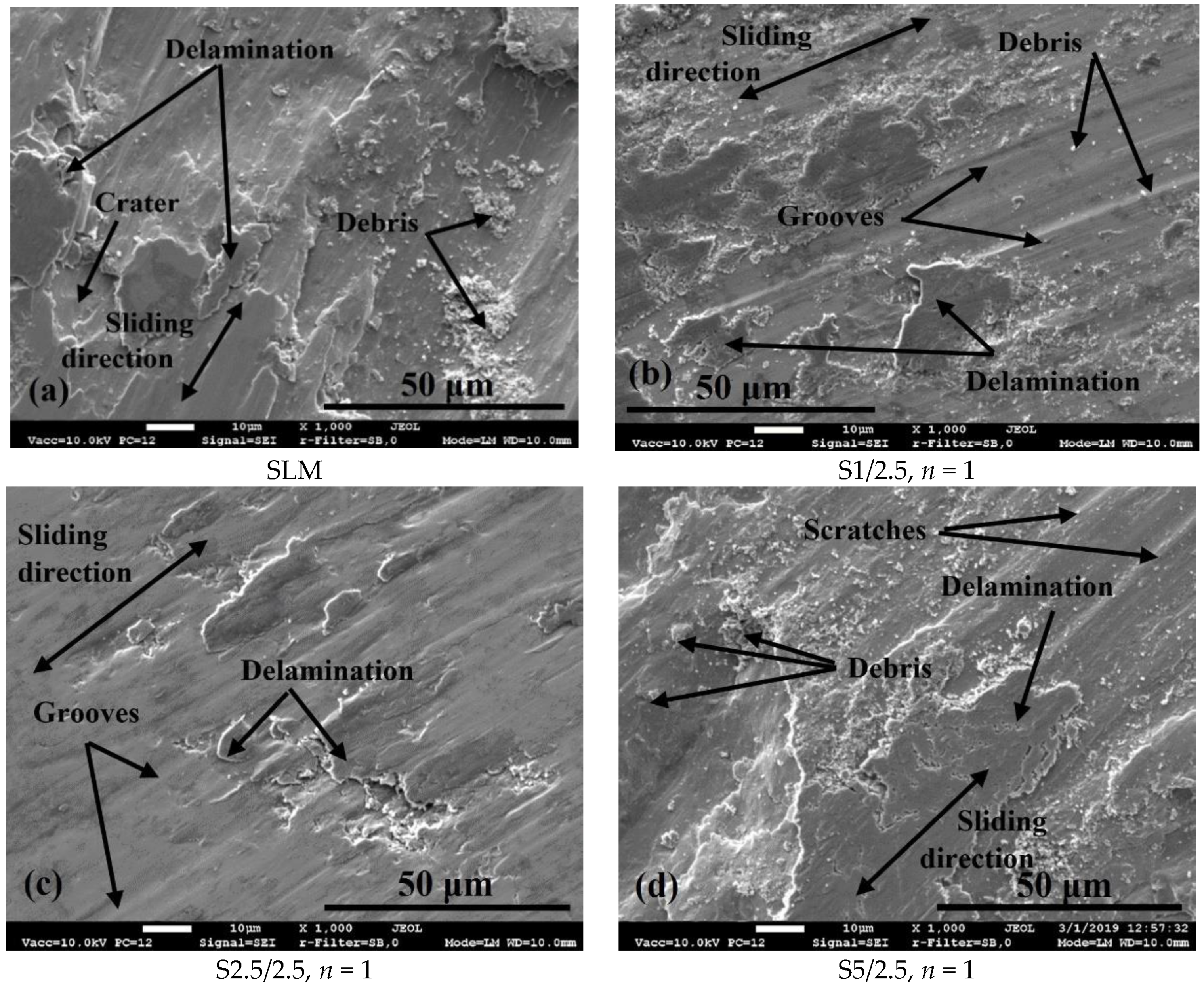
3.1. Surface Morphology
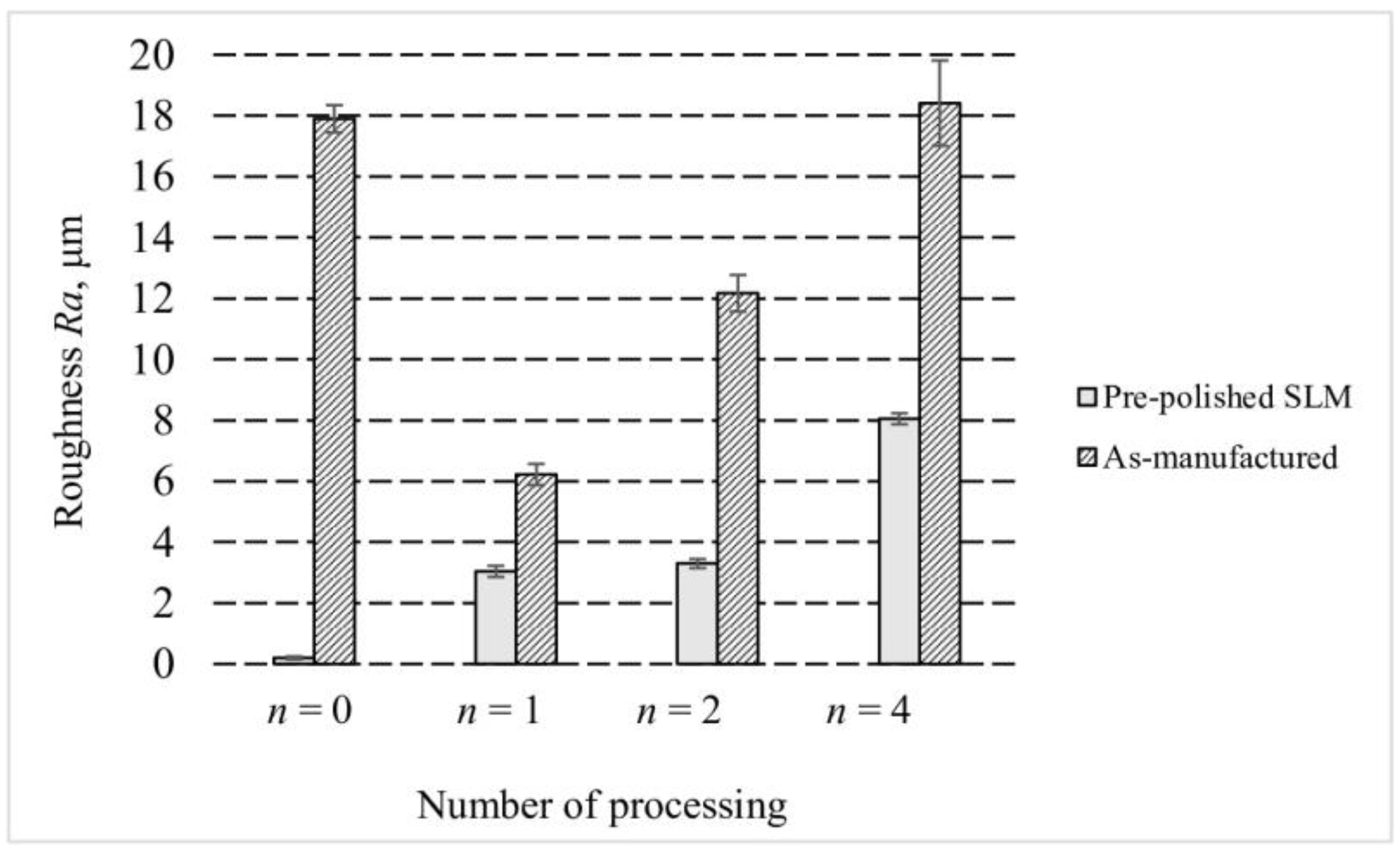
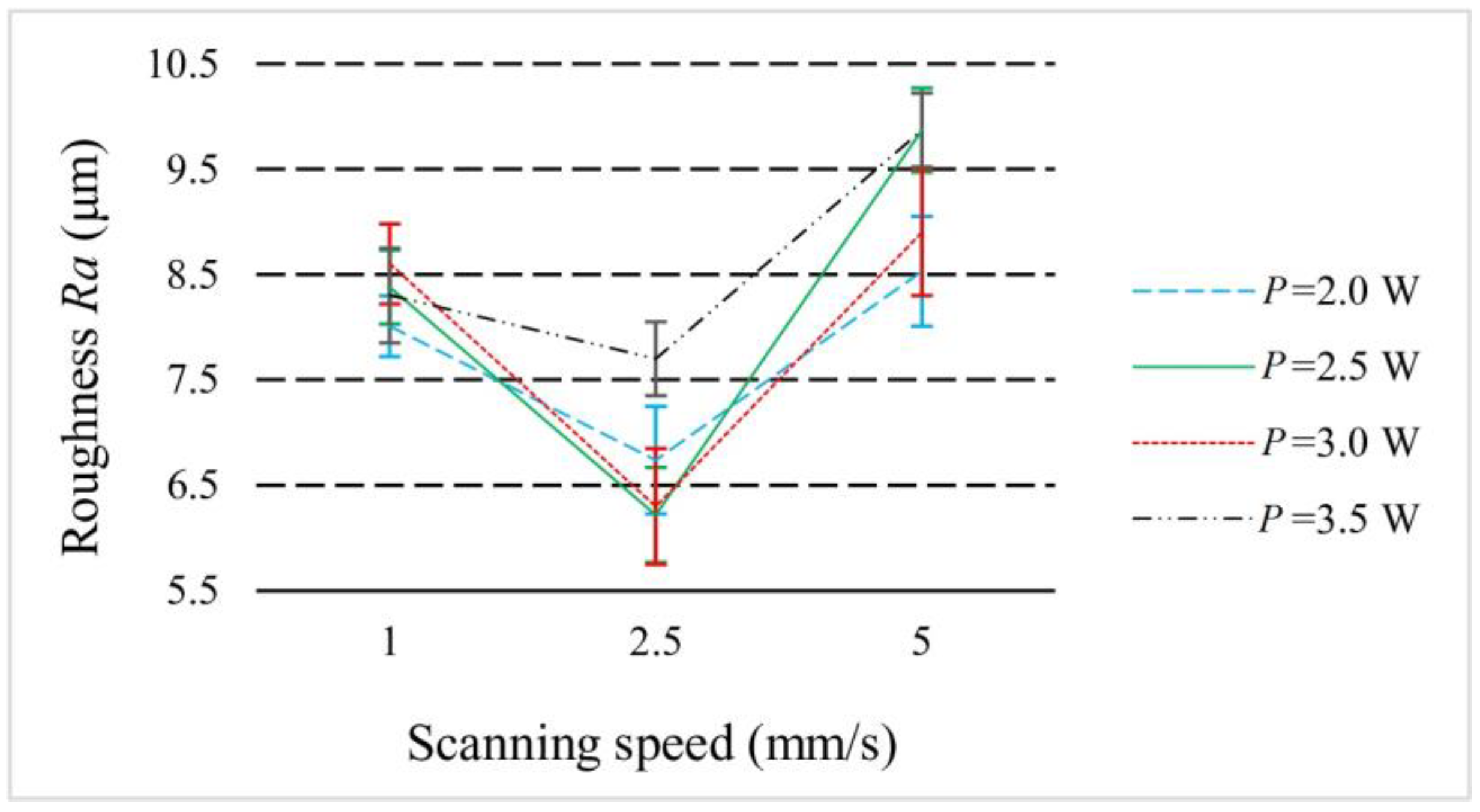
3.2. Surface Roughness
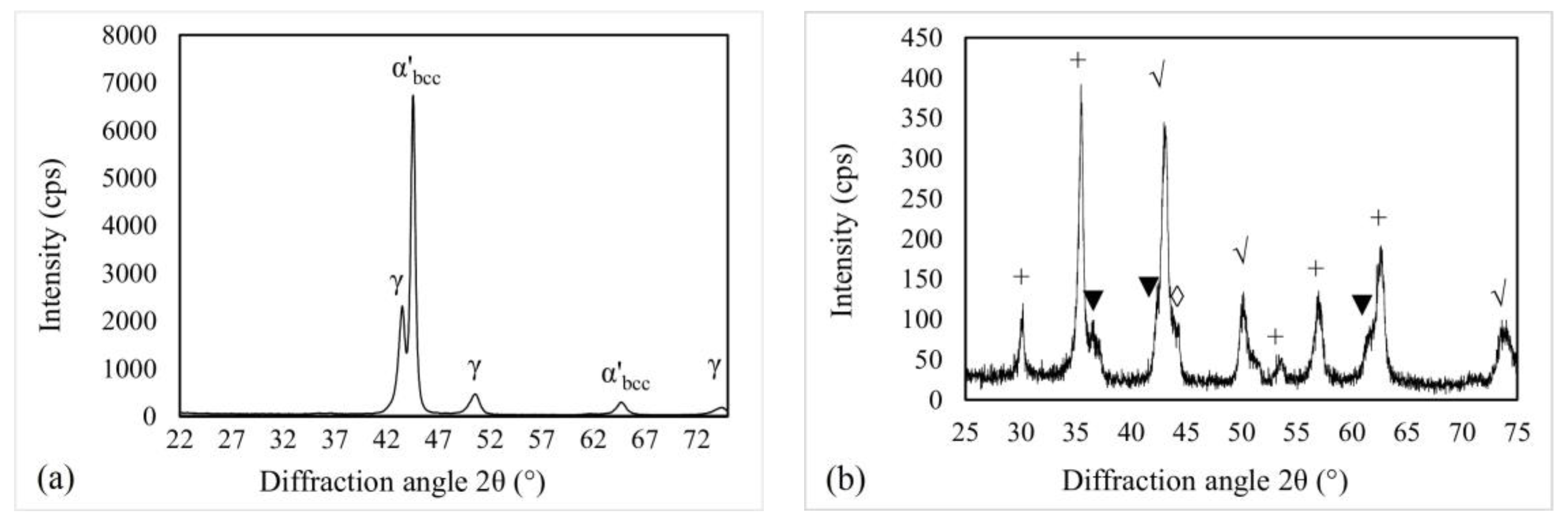
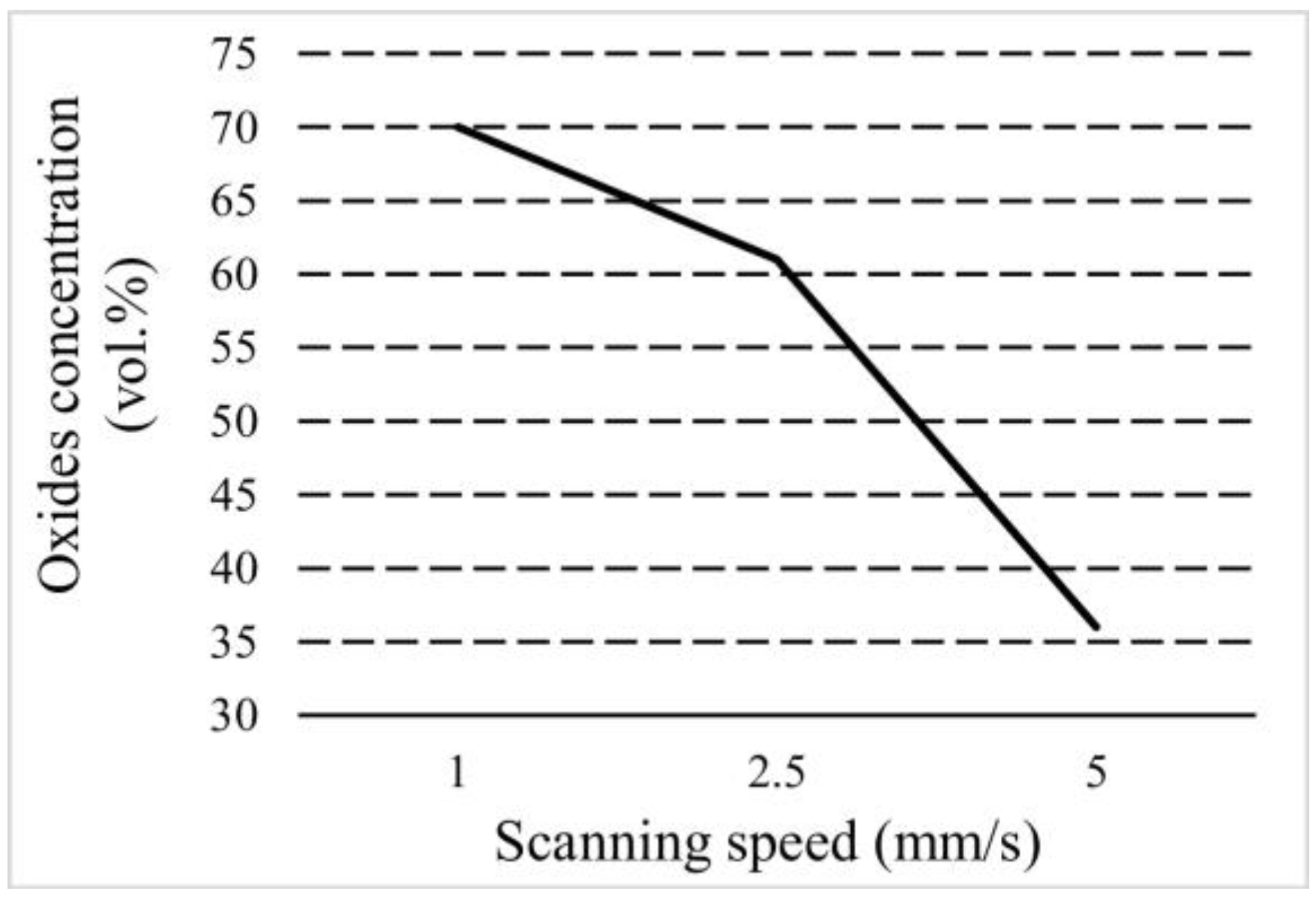
3.3. Surface Elemental and Phase Composition
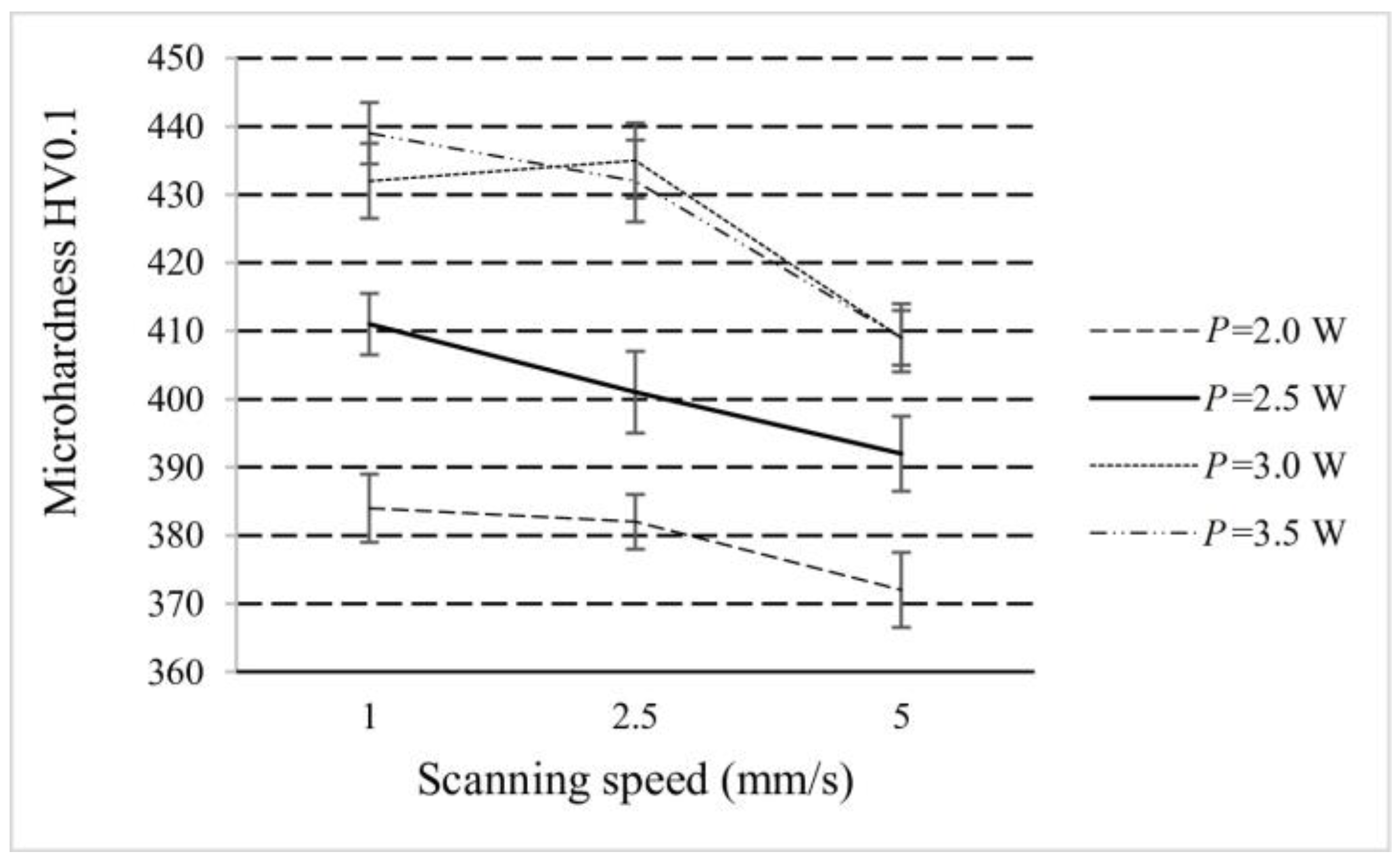
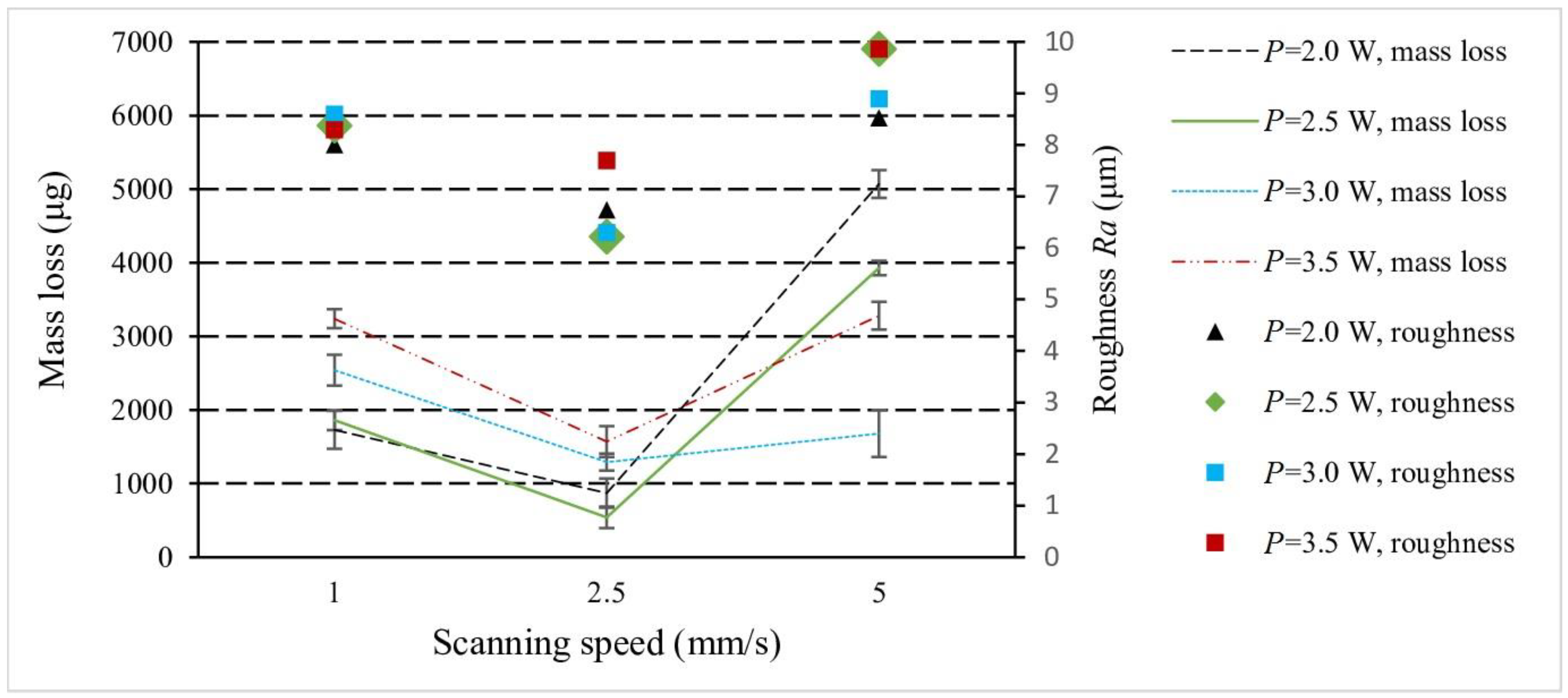
3.4. Surface Microhardness and Tribology
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Eyers, D.R.; Potter, A.T. Industrial additive manufacturing: A manufacturing systems perspective. Comput. Ind. 2017, 92–93, 208–218. [Google Scholar] [CrossRef]
- Bhavar, V.; Kattire, P.; Patil, V.; Khot, S.; Gujar, K.; Singh, R. A review on powder bed fusion technology of metal additive manufacturing. In Proceedings of the 4th International Conference and Exhibition on Additive Manufacturing Technologies AM-2014, Bangalore, India, 1–2 September 2014; pp. 1–7. [Google Scholar]
- Deckard, C. Method and Apparatus for Producing Parts by Selective Sintering. U.S. Patent US4863538, 17 October 1986. [Google Scholar]
- ASM International. Maraging steels. In ASM Handbook, Volume 1: Properties and Selection: Irons, Steels, and High-Performance Alloys, 10th ed.; ASM International: Materials Park, OH, USA, 1990; Volume 1, pp. 1869–1887. [Google Scholar]
- 18 Per Cent Maraging Steels. Engineering Properties. INCO Databooks. 1976. Available online: https://www.nickelinstitute.org/media/1598/18_nickelmaragingsteel_engineeringproperties_4419_.pdf (accessed on 29 May 2020).
- Hall, A.M. The Metallurgy, Behavior, and Application of the 18-Percent Nickel Maraging Steels. A Survey; Technology Utilization Division, Office of Technology Utilization, National Aeronautics and Space Admin: Washington, DC, USA, 1968; p. 143. [Google Scholar]
- Klocke, F.; Arntz, K.; Teli, M.; Winands, K.; Wegener, M.; Oliari, S. State-of-the-art laser additive manufacturing for hot-work tool steels. Procedia CIRP 2017, 63, 58–63. [Google Scholar] [CrossRef]
- Popov, V.; Fleisher, A. Hybrid additive manufacturing of steels and alloys. Manuf. Rev. 2020, 7, 6. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Mohammadi, M. Numerical tools to investigate mechanical and fatigue properties of additively manufactured MS1-H13 hybrid steels. Addit. Manuf. 2018, 23, 381–393. [Google Scholar] [CrossRef]
- Decker, R.F.; Eash, J.T.; Goldman, A.J. Eighteen percent nickel maraging steel. Trans. ASM 1962, 55, 58–76. [Google Scholar]
- Azizi, H.; Ghiaasiaan, R.; Prager, R.; Ghoncheh, M.H.; Samk, K.A.; Lausic, A.; Byleveld, W.; Phillion, A.B. Metallurgical and mechanical assessment of hybrid additively-manufactured maraging tool steels via selective laser melting. Addit. Manuf. 2019, 27, 389–397. [Google Scholar] [CrossRef]
- Seabrook, J.B. Working with maraging steels-nitriding. Met. Progr. 1963, 84, 78–80. [Google Scholar]
- Haynes, A.G. Maraging steels-surface protection and surface effects. In Metallurgical Developments in High Alloy Steels; Special report 86; The Iron and Steel Institute: London, UK, 1964; pp. 125–133. [Google Scholar]
- Imrie, W.M. Assessment of maraging steel for aircraft applications. J. R. Aeronaut. Soc. 1966, 70, 776–777. [Google Scholar] [CrossRef]
- Graae, A. How to nitride maraging steels. Met. Prog. 1967, 92, 74–76. [Google Scholar]
- Tsyrlin, E.S. Nitriding of maraging steel N18K9M5T. Met. Sci. Heat Treat. 1971, 4, 22–25. [Google Scholar] [CrossRef]
- Steen, W.; Mazumder, J. Laser Material Processing, 4th ed.; Springer-Verlag: London, UK, 2010; p. 558. [Google Scholar]
- Lamikiz, A.; Sánchez, J.A.; López de Lacalle, L.N.; Arana, J.L. Laser polishing of parts built up by selective laser sintering. Int. J. Mach. Tool. Manuf. 2007, 47, 2040–2050. [Google Scholar] [CrossRef]
- Bhaduri, D.; Penchev, P.; Batal, A.; Dimov, S.; Soo, S.L.; Sten, S.; Harrisson, U.; Zhang, Z.; Dong, H. Laser polishing of 3D printed mesoscale components. Appl. Surf. Sci. 2017, 405, 29–46. [Google Scholar] [CrossRef]
- Yung, K.C.; Wang, W.J.; Xiao, T.Y.; Choy, H.S.; Mo, X.Y.; Zhang, S.S.; Cai, Z.X. Laser polishing of additive manufactured CoCr components for controlling their wettability characteristics. Surf. Coat. Technol. 2018, 351, 89–98. [Google Scholar] [CrossRef]
- Ma, C.P.; Guan, Y.C.; Zhou, W. Laser polishing of additive manufactured Ti alloys. Opt. Lasers Eng. 2017, 93, 171–177. [Google Scholar] [CrossRef]
- Zhihao, F.; Libin, L.; Longfei, C.; Yingchun, G. Laser polishing of additive manufactured superalloy. Procedia CIRP 2018, 71, 150–154. [Google Scholar] [CrossRef]
- Li, Y.-H.; Wang, B.; Ma, C.-P.; Fang, Z.-H.; Chen, L.-F.; Guan, Y.-C.; Yang, S.-F. Material characterization, thermal analysis, and mechanical performance of a laser-polished Ti alloy prepared by selective laser melting. Metals 2019, 9, 112. [Google Scholar] [CrossRef]
- Afkhami, S.; Dabiri, M.; Alavi, S.H.; Björk, T.; Salminen, A. Fatigue characteristics of steels manufactured by selective laser melting. Int. J. Fatigue 2019, 122, 72–83. [Google Scholar] [CrossRef]
- Nanai, L.; Vajtai, R.; George, T.G. Laser-induced oxidation of metals: State of the art. Thin Solid Films 1997, 298, 160–164. [Google Scholar] [CrossRef]
- Schaaf, P. Laser nitriding of metals. Prog. Mater. Sci. 2002, 47, 1–161. [Google Scholar] [CrossRef]
- Černašėjus, O.; Škamat, J.; Markovič, V.; Višniakos, N.; Indrišiūnas, S. Surface laser processing of additive manufactured 1.2709 steel parts: Preliminary study. Adv. Mater. Sci. Eng. 2019, 9. [Google Scholar] [CrossRef]
- Parfenov, V.A. Laser Micromachnining of Materials: Textbook; Publishing House of the Saint Petersburg Electrotechnical University ETU: Saint Petersburg, Russia, 2011. [Google Scholar]
- Yan, J.; Zhou, Y.; Gu, R.; Zhang, X.; Quach, W.-M.; Yan, M.A. Comprehensive study of steel powders (316L, H13, P20 and 18Ni300) for their selective laser melting additive manufacturing. Metals 2019, 9, 86. [Google Scholar] [CrossRef]
- Maraging 300 Alloy Steel (UNS K93120). Available online: https://www.azom.com/article.aspx?ArticleID=6751 (accessed on 19 May 2020).
- Gursel, A.; Akca, E. 3D surface morphology and roughness on treated surface of Ti–6Al–4V alloy by Nd:YAG laser: Effect of spot size. In Proceedings of the 5th International Conference on Welding Technologies and Exhibition (ICWET’18), Sarajevo, Bosnia and Herzegovina, 26–28 September 2018; pp. 324–335. [Google Scholar]
- Tzeng, Y.F. Process characterisation of pulsed Nd:YAG laser seam welding. Int. J. Adv. Manuf. Tech. 2000, 16, 10–18. [Google Scholar] [CrossRef]
- Naeem, K. Developments in laser microwelding technology. In Handbook of Laser Welding Technologies, 1st ed.; Katayama, S., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2013; pp. 163–212. [Google Scholar]
- Reye, T. Zur theorie der zapfenreibung. Der Civil. 1860, 6, 235–255. [Google Scholar]
- Popov, V.L. Contact Mechanics and Friction. Physical Principles and Applications; Springer: Berlin, Germany, 2010; p. 22. [Google Scholar]
- Bayer, R.G.; Sirico, J.L. The influence of surface roughness on wear. Wear 1975, 35, 251–260. [Google Scholar] [CrossRef]


| Laser | Thickness of Layer, mm | Laser Operating Rate, mm/s | Shielding Gas | |||
|---|---|---|---|---|---|---|
| Wave Length, nm | Power, W | Spot Size, mm | Type | Consumption, l/h | ||
| 1064 | 100 | Ø 0.2 | 0.03 | 0.2 | Ar | 0.75 |
| Laser Scanning Speed v, mm/s | Laser Power, W | |||
|---|---|---|---|---|
| 2 | 2.5 | 3 | 3.5 | |
| 1 | S1/2 | S1/2.5 | S1/3 | S1/3.5 |
| 2.5 | S2.5/2 | S2.5/2.5 | S2.5/3 | S2.5/3.5 |
| 5 | S5/2 | S5/2.5 | S5/3 | S5/3.5 |
| Energy Parameters of the Process | Average Laser Power, W | |||
|---|---|---|---|---|
| 2.0 | 2.5 | 3.0 | 3.5 | |
| Ep, J | 2.0 × 10−4 | 2.5 × 10−4 | 3.0 × 10−4 | 3.5 × 10−4 |
| Pp, W | 2.0 × 104 | 2.5 × 104 | 3.0 × 104 | 3.5 × 104 |
| Pd, W∙m−2 | 4.07 × 1013 | 5.10 × 1013 | 6.11 × 1032 | 7.13 × 1013 |
| Laser Processing Speed, mm∙s−1 | 1 | 2.5 | 5 |
|---|---|---|---|
| Overlap coefficient, % | 99.60 | 99.00 | 98.00 |
| Specimen | Element | |||||
|---|---|---|---|---|---|---|
| Fe | Ni | Co | Mo | Ti | O | |
| Initial powder | Balance | 20.0 | 6.9 | 4.5 | 0.7 | 2.0 |
| SLM specimen | 23.0 | 8.2 | 4.1 | 0.7 | 5.1 | |
| S5/2 (v = 5 mm/s/P = 2.0 W) | 15.3 | 11.0 | 3.7 | 1.2 | 24.1 | |
| S1/3.5 (v = 1 mm/s/P = 3.5 W) | 11.9 | 8.8 | 3.3 | 1.3 | 28.8 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Černašėjus, O.; Škamat, J.; Markovič, V.; Višniakov, N.; Indrišiūnas, S. Effect of Laser Processing on Surface Properties of Additively Manufactured 18-Percent Nickel Maraging Steel Parts. Coatings 2020, 10, 600. https://doi.org/10.3390/coatings10060600
Černašėjus O, Škamat J, Markovič V, Višniakov N, Indrišiūnas S. Effect of Laser Processing on Surface Properties of Additively Manufactured 18-Percent Nickel Maraging Steel Parts. Coatings. 2020; 10(6):600. https://doi.org/10.3390/coatings10060600
Chicago/Turabian StyleČernašėjus, Olegas, Jelena Škamat, Vladislav Markovič, Nikolaj Višniakov, and Simonas Indrišiūnas. 2020. "Effect of Laser Processing on Surface Properties of Additively Manufactured 18-Percent Nickel Maraging Steel Parts" Coatings 10, no. 6: 600. https://doi.org/10.3390/coatings10060600
APA StyleČernašėjus, O., Škamat, J., Markovič, V., Višniakov, N., & Indrišiūnas, S. (2020). Effect of Laser Processing on Surface Properties of Additively Manufactured 18-Percent Nickel Maraging Steel Parts. Coatings, 10(6), 600. https://doi.org/10.3390/coatings10060600





