Effects of NiSO4 Concentration on the Coloring Performance and Corrosion Resistance of the Colored Film on 304 Stainless Steel
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Coloring Experiments
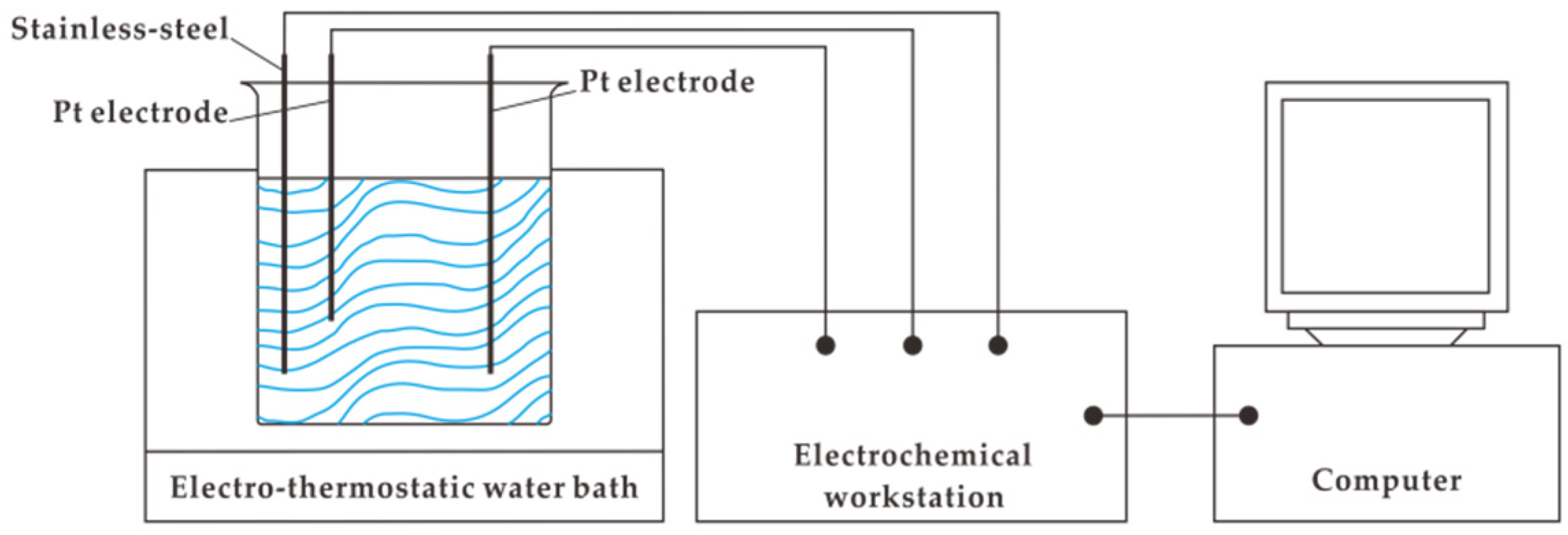
2.3. Electrochemical Measurements
2.4. Morphology
2.5. Wear Resistance Test
3. Results and Discussion
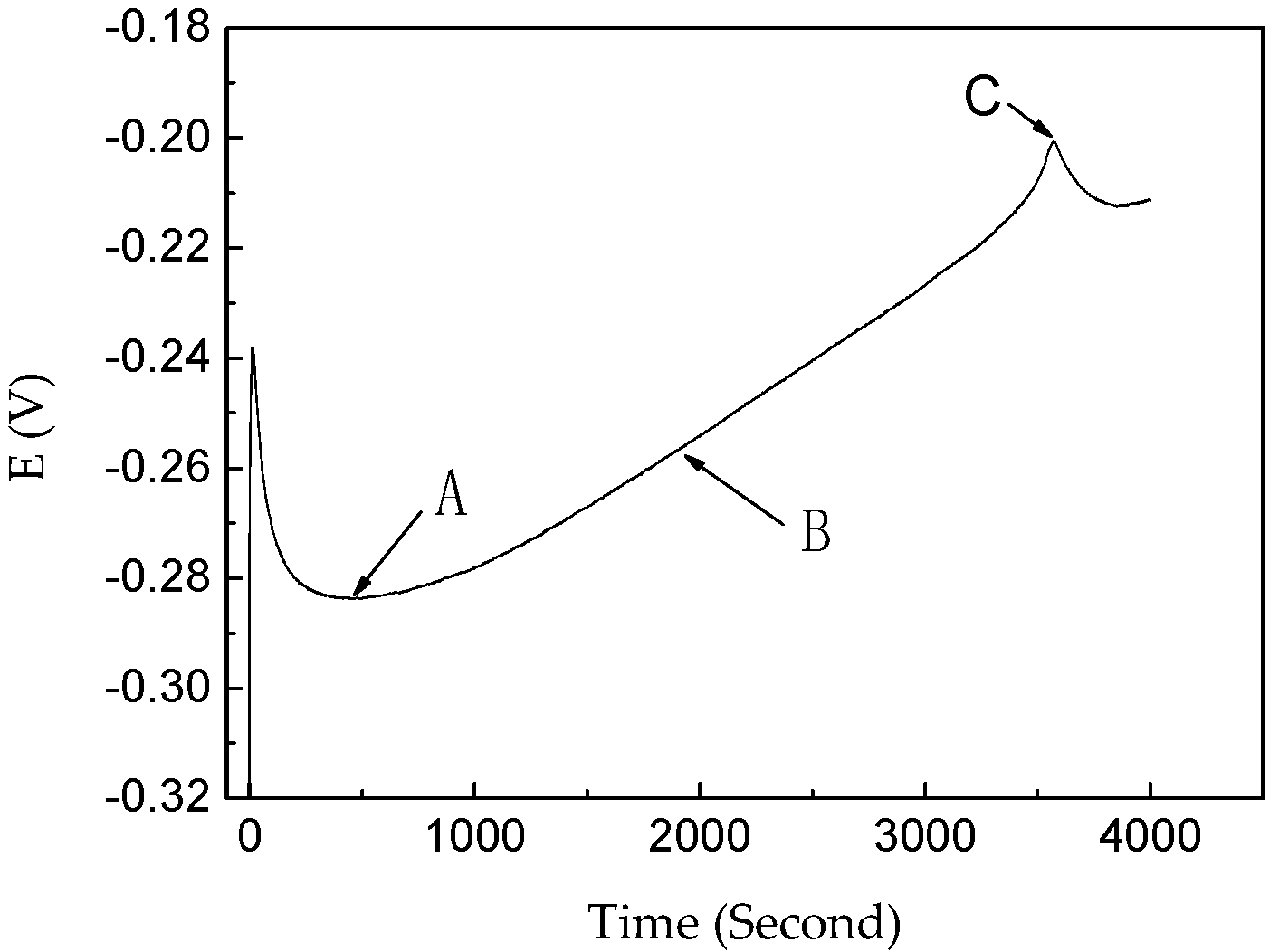
3.1. Effect of NiSO4 on the Coloring Process
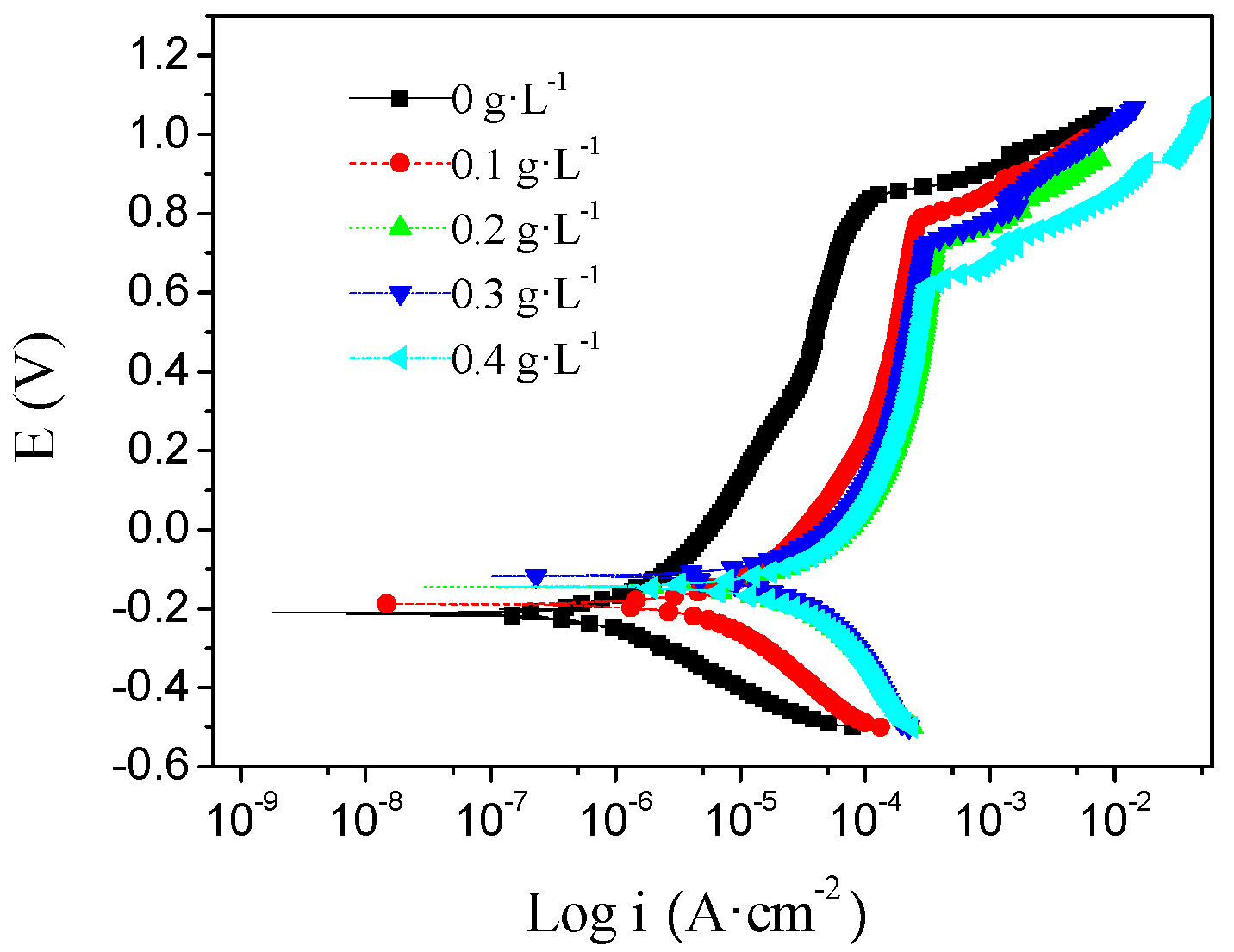
3.2. Potentiodynamic Polarization
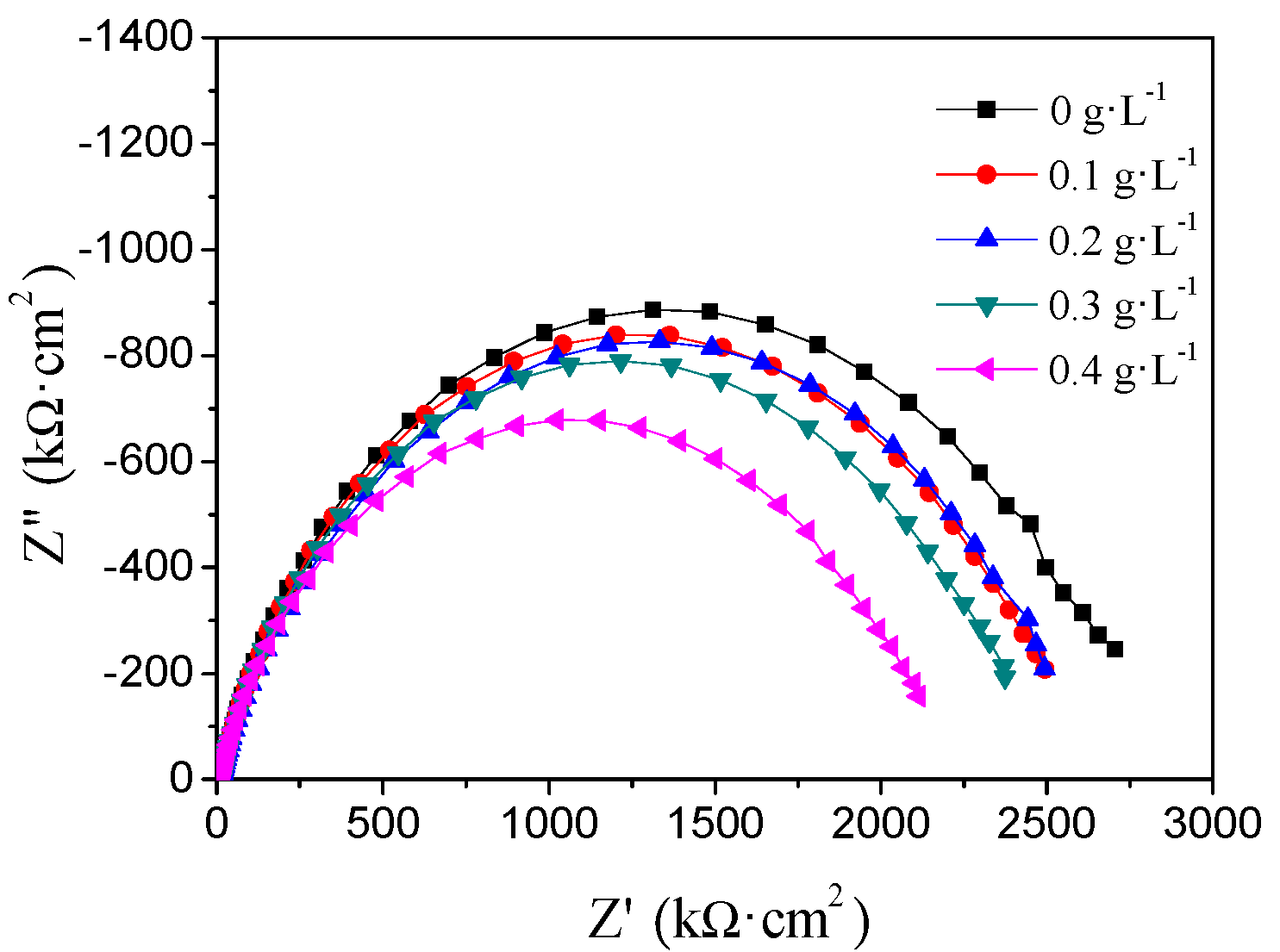
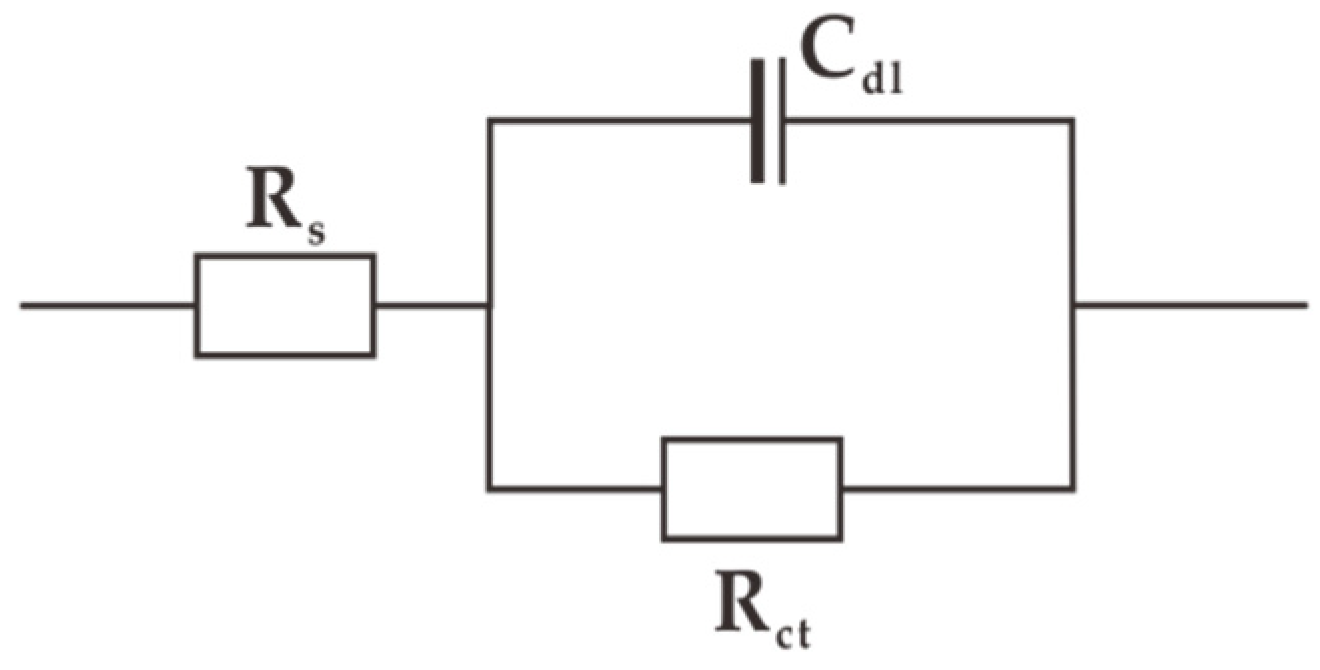
3.3. Electrochemical Impedance Spectroscopy
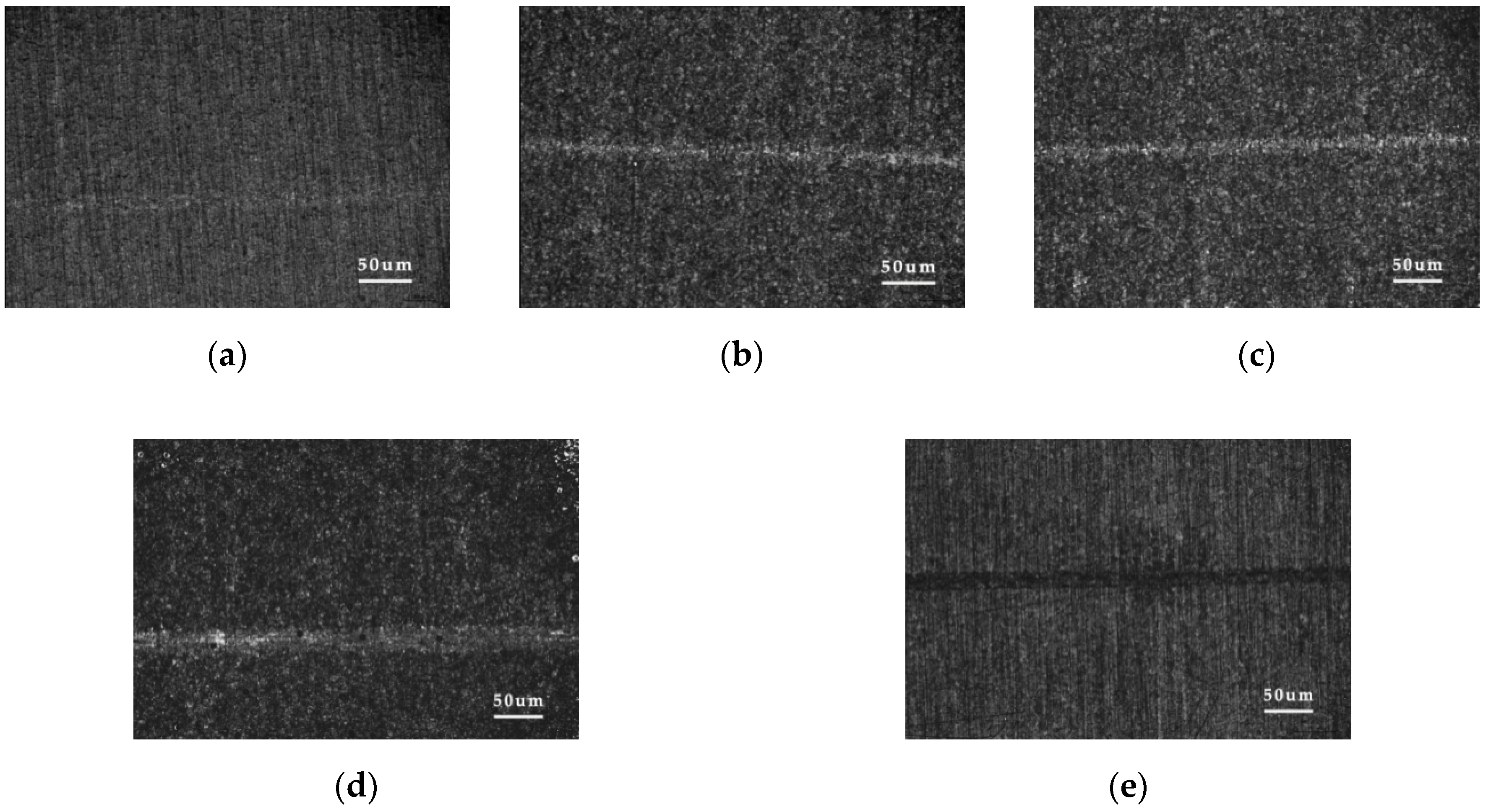
3.4. Surface Characterization
3.5. Wear Resistance Test Results
3.6. Mechanism Analysis for the Effects of NiSO4
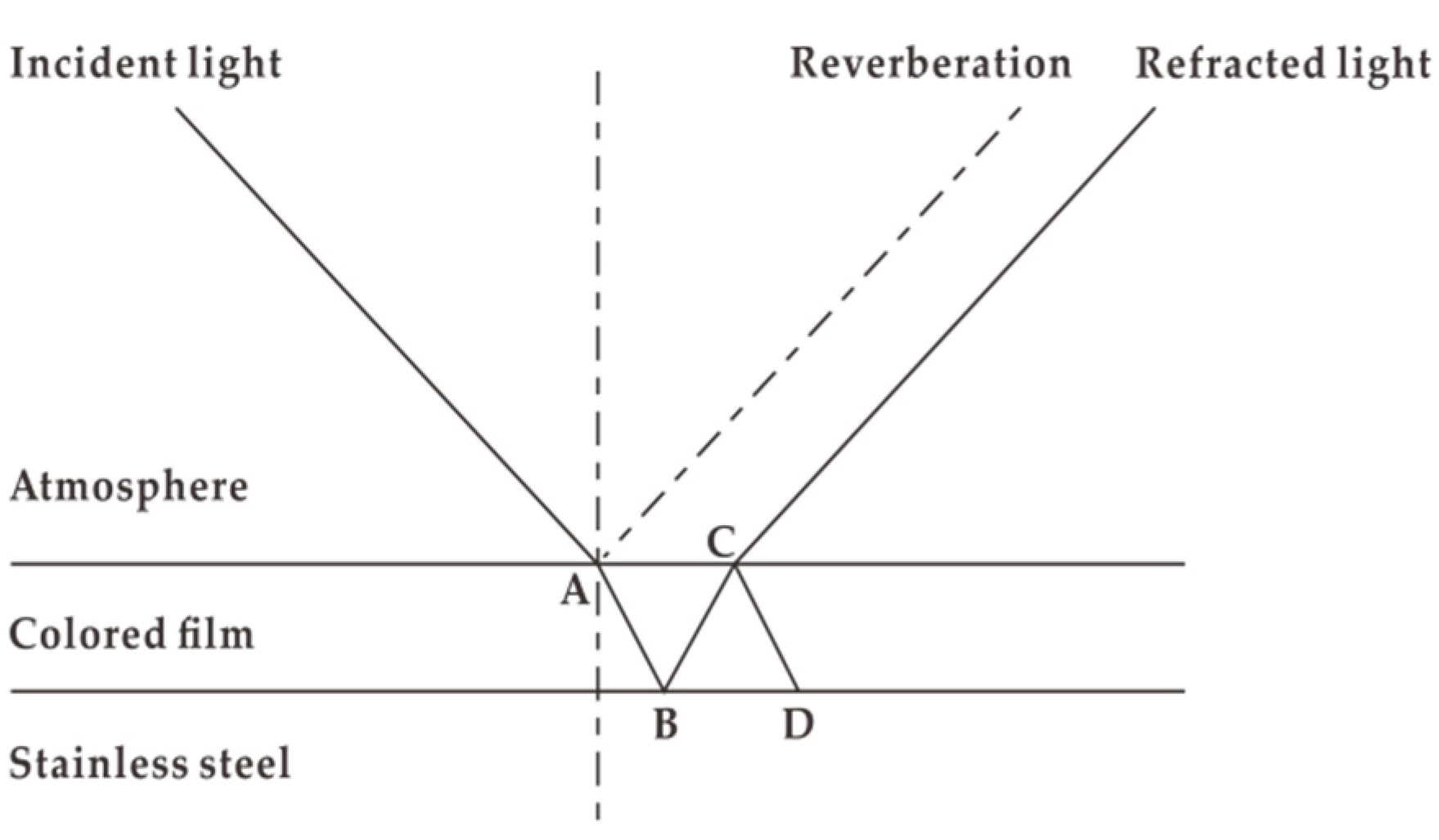
3.7. Principle of Surface Color Displaying
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Corredor, J.; Bergmann, C.P.; Pereira, M.; Dick, L.F.P. Coloring ferritic stainless steel by an electrochemical—Photochemical process under visible light illumination. Surf. Coat. Technol. 2014, 245, 125–132. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, X.; Wang, J. The influence of local glucose oxidase activity on the potential/current distribution on stainless steel: A study by the wire beam electrode method. Electrochim. Acta 2009, 54, 5598–5604. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, G.; Li, W.; Wu, D. Photocathodic protection of cobalt doped ZnO nanorod arrays for 316 stainless steel and Q235 carbon steel in 3.5 wt.% NaCl solution. Coatings 2019, 9, 803. [Google Scholar] [CrossRef]
- Somervuori, M.; Johansson, L.-S.; Heinonen, M.H.; van Hoecke, D.H.D.; Akdut, N.; Hänninen, H.E. Characterisation and corrosion of spot welds of austenitic stainless steels. Mater. Corros. 2004, 55, 421–436. [Google Scholar] [CrossRef]
- Wang, J.H.; Duh, J.G. Colour tone and chromaticity in a coloured film on stainless steel by alternating current electrolysis method. Surf. Coat. Technol. 1995, 73, 46–51. [Google Scholar] [CrossRef]
- Junqueira, R.M.R.; Andrade, M.S.; Loureiro, C.R.O.; Buono, V.T.L. Mechanical properties of interference thin films on colored stainless steel evaluated by depth-sensing nanoindentation. Surf. Coat. Technol. 2006, 201, 2431–2437. [Google Scholar] [CrossRef]
- Stoychev, D.; Stefanov, P.; Nicolova, D.; Valov, I.; Marinova, T. Chemical composition and corrosion resistance of passive chromate films formed on stainless steels 316 L and 1.4301. Mater. Chem. Phys. 2002, 73, 252–258. [Google Scholar] [CrossRef]
- Zheng, E.X. New Technology of Continuous Coloring of Stainless Steel Coil Belt. Master’s Thesis, Hubei University, Wuhan, China, 2011. [Google Scholar]
- Junqueira, R.M.R.; Loureiro, C.R.O.; Buono, V.T.L. Electrochemical coloration of stainless steel as an alternative for architectural coatings. In Proceedings of the 1st International Seminar on Industrial Innovation in Electrochemistry, Blucher Chemistry Proceedings, Blucher, Brazil, 1 June 2014. [Google Scholar]
- Li, X.; Chen, J.; Ye, J.; Feng, T.; Hu, X. Low-stress diamond films deposited on stainless steel by a two-step dropped power process in chemical vapor deposition. Diam. Relat. Mater. 2018, 81, 176–182. [Google Scholar] [CrossRef]
- Ledwig, P.; Kot, M.; Moskalewicz, T.; Dubiel, B. Electrophoretic deposition of nc-TiO2/chitosan composite coatings on X2CrNiMo17-12-2 stainless steel. Arch. Metall. Mater. 2017, 62, 405–410. [Google Scholar] [CrossRef]
- Kanazawa, K.; Nakamura, K.; Kobayashi, N. Electroswitching of emission and coloration with quick response and high reversibility in an electrochemical cell. Chem.-Asian J. 2012, 7, 2551–2554. [Google Scholar] [CrossRef]
- Conrrado, R.; Bocchi, N.; Rocha-Filho, R.C.; Biaggio, S.R. Corrosion resistance of colored films grown on stainless steel by the alternating potential pulse method. Electrochim. Acta 2003, 48, 2417–2424. [Google Scholar] [CrossRef]
- Wang, J.H.; Duh, J.G.; Shih, H.C. Corrosion characteristics of coloured films on stainless steel formed by chemical, INCO and a.c. processes. Surf. Coat. Technol. 1996, 78, 248–254. [Google Scholar] [CrossRef]
- Groeb, M.; Fritz, M. Process analysis on milled optical surfaces in hardened stainless steel. J. Manuf. Mater. Process. 2019, 3, 67. [Google Scholar] [CrossRef]
- Huang, Y.; Young, B. Design of cold-formed stainless steel circular hollow section columns using direct strength method. Eng. Struct. 2018, 163, 177–183. [Google Scholar] [CrossRef]
- Fedrizzi, L.; Crousier, J.; Bonora, P.-L.; Crousier, J.-P. Corrosion mechanisms of an AISI type 316L sintered stainless steel in sodium chloride solution. Werkst. Korros. 1991, 42, 403–409. [Google Scholar] [CrossRef]
- Zhang, T.; Zeng, C.L. Corrosion protection of 1Cr18Ni9Ti stainless steel by polypyrrole coatings in HCl aqueous solution. Electrochim. Acta 2005, 50, 4721–4727. [Google Scholar] [CrossRef]
- Strzelecki, P.; Mazurkiewicz, A.; Musiał, J.; Tomaszewski, T.; Słomion, M. Fatigue Life for Different Stress Concentration Factors for Stainless Steel 1.4301. Materials 2019, 12, 3677. [Google Scholar] [CrossRef]
- Böhm, M.; Kowalski, M.; Niesłony, A. Influence of the Elastoplastic Strain on Fatigue Durability Determined with the Use of the Spectral Method. Materials 2020, 13, 423. [Google Scholar] [CrossRef]
- Gartner, N.; Kosec, T.; Legat, A. Monitoring the Corrosion of Steel in Concrete Exposed to a Marine Environment. Materials 2020, 13, 407. [Google Scholar] [CrossRef]
- Lin, C.J.; Duh, J.G. Elemental redistribution in coloured films on SUS304 stainless steel produced by current pulse method. Surf. Coat. Technol. 1996, 85, 175–182. [Google Scholar] [CrossRef]
- Chen, Y.D.; Li, J.D.; Cui, Z.M.; Peng, B. Colouristic optical principle of colour stainless steel and technological process of chemical deposit. J. WUYI Univ. Nat. Sci. Ed. 1995, 9, 21–27. [Google Scholar]
- Lin, C.J.; Duh, J.G. Mechanical characteristics of colored film on stainless steel by the current pulse method. Thin Solid Films 1996, 287, 80–86. [Google Scholar] [CrossRef]
- Ogura, K.; Lou, W.; Nakayama, M. Coloration of stainless steel at room temperature by triangular current scan method. Electrochim. Acta 1996, 41, 2849–2853. [Google Scholar] [CrossRef]
- Kwok, C.T.; Man, H.C.; Leung, L.K. Effect of temperature, pH and sulphide on the cavitation erosion behaviour of super duplex stainless steel. Wear 1997, 211, 84–93. [Google Scholar] [CrossRef]
- Cieślik, M.; Engvall, K.; Pan, J.; Kotarba, A. Silane–parylene coating for improving corrosion resistance of stainless steel 316L implant material. Corros. Sci. 2011, 53, 296–301. [Google Scholar] [CrossRef]
- Ye, W.; Li, Y.; Wang, F. Effects of nanocrystallization on the corrosion behavior of 309 stainless steel. Electrochim. Acta 2006, 51, 4426–4432. [Google Scholar] [CrossRef]
- Evans, T.E. The mechanism of colored film on stainless steels. Corros. Sci. 1977, 17, 105–109. [Google Scholar] [CrossRef]
- Xu, J.; Bai, X.; He, F.; Fan, Y. The growth mechanism of the colored film on the stainless steel studied by 18o tracing and nuclear reaction analysis. Nucl. Instrum. Methods Phys. Res. 1999, 149, 147–152. [Google Scholar] [CrossRef]
- Zhao, J.F. University Physics, 5th ed.; Beijing University of Posts and Telecommunications Press: Beijing, China, 2017; pp. 154–156. [Google Scholar]
- Panjan, M.; Klanjšek Gunde, M.; Panjan, P.; Čekada, M. Designing the color of AlTiN hard coating through interference effect. Surf. Coat. Technol. 2014, 254, 65–72. [Google Scholar] [CrossRef]
- Aguilar-Morales, A.I.; Alamri, S.; Lasagni, A.F. Micro-fabrication of high aspect ratio periodic structures on stainless steel by picosecond direct laser interference patterning. J. Mater. Process. Technol. 2018, 252, 313–321. [Google Scholar] [CrossRef]




| Concentration (g·L−1) | Coloring End Time (s) | ΔEC-A (mV) | Color | ||
|---|---|---|---|---|---|
| NiSO4 | H2SO4 | Cr2O3 | |||
| 0 | 490 | 250 | 556 | 27 | purplish red |
| 0.1 | 490 | 250 | 548 | 30 | purplish red |
| 0.2 | 490 | 250 | 480 | 28 | purplish red |
| 0.3 | 490 | 250 | 452 | 27 | purplish red |
| 0.4 | 490 | 250 | 388 | 29 | purplish red |
| Concentration of NiSO4 (g·L−1) | io (A·cm−2) | Eo (V) | Epit (V) |
|---|---|---|---|
| 0 | 6.16 × 10−7 | −0.21 | 0.92 |
| 0.1 | 9.84 × 10−6 | −0.19 | 0.80 |
| 0.2 | 5.59 × 10−5 | −0.15 | 0.77 |
| 0.3 | 5.18 × 10−5 | −0.14 | 0.75 |
| 0.4 | 4.51 × 10−5 | −0.12 | 0.64 |
| Concentration of NiSO4 (g·L−1) | Cdl (Ω−1·cm−2·sn) | n | Rct (Ω·cm−2) |
|---|---|---|---|
| 0 | 2.03 × 10−4 | 0.80 | 2609 |
| 0.1 | 2.28 × 10−4 | 0.79 | 2451 |
| 0.2 | 2.81 × 10−4 | 0.76 | 2473 |
| 0.3 | 2.20 × 10−4 | 0.79 | 2335 |
| 0.4 | 2.16 × 10−4 | 0.78 | 2045 |
| Concentration of NiSO4 (g·L−1) | O | Si | Cr | Fe | Ni |
|---|---|---|---|---|---|
| 0 | 8.40 | 0.80 | 20.62 | 64.04 | 6.41 |
| 0.1 | 8.46 | 0.88 | 19.24 | 63.86 | 7.60 |
| 0.2 | 8.45 | 0.82 | 18.61 | 64.30 | 7.91 |
| 0.3 | 8.53 | 0.84 | 18.50 | 63.64 | 8.30 |
| 0.4 | 8.50 | 0.88 | 18.48 | 63.53 | 8.32 |
| Concentration of NiSO4 (g·L−1) | 0 | 0.1 | 0.2 | 0.3 | 0.4 |
|---|---|---|---|---|---|
| Number of Friction | 74 ± 3 | 70 ± 2 | 58 ± 4 | 43 ± 3 | 42 ± 3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Qu, J.-e.; Cao, Z.; Wang, H. Effects of NiSO4 Concentration on the Coloring Performance and Corrosion Resistance of the Colored Film on 304 Stainless Steel. Coatings 2020, 10, 598. https://doi.org/10.3390/coatings10060598
Li W, Qu J-e, Cao Z, Wang H. Effects of NiSO4 Concentration on the Coloring Performance and Corrosion Resistance of the Colored Film on 304 Stainless Steel. Coatings. 2020; 10(6):598. https://doi.org/10.3390/coatings10060598
Chicago/Turabian StyleLi, Wenwei, Jun-e Qu, Zhiyong Cao, and Hairen Wang. 2020. "Effects of NiSO4 Concentration on the Coloring Performance and Corrosion Resistance of the Colored Film on 304 Stainless Steel" Coatings 10, no. 6: 598. https://doi.org/10.3390/coatings10060598
APA StyleLi, W., Qu, J.-e., Cao, Z., & Wang, H. (2020). Effects of NiSO4 Concentration on the Coloring Performance and Corrosion Resistance of the Colored Film on 304 Stainless Steel. Coatings, 10(6), 598. https://doi.org/10.3390/coatings10060598




