Abstract
This paper presents an analysis of natural mineral waters recognized by EU member states (on the basis of being announced in the Official Journal of the European Union) and originating in Polish territory. For each of these waters, calculations were made in relation to the Langelier saturation index, Ryznar stability index, and indices S1 (effect of chloride and sulfate ions on iron and steel), S2 (effect of nitrate ions on zinc), and S3 (effect of bicarbonate/sulfate ratio on copper). The impact of mineral waters on copper, hot-dip galvanized iron, stainless steel, acid-resistant steel, cast iron, low-alloy steel, and nonalloy steel was assessed on the basis of the indices calculated. The analysis allowed determination of the possibility of these measures being used in assessing the performance of mineral water installations.
1. Introduction
Mineral waters, like tap water, are transported to places of collection or use via water pipes. However, they actually demand more from the installation materials used, given the need for the latter to be resistant to aggressive impacts that lead to corrosion. This in turn is reflected by the presence in mineral waters of a higher content of aggressive ions, such as bicarbonate, sulfate, and chloride, as well as free carbon dioxide. Under such conditions, there is a high probability that metal will start to corrode, thereby also causing a deterioration in the quality of the water flowing through it. Where the reaction of water is neutral, metals may be subject to general, pitting, selective, bimetallic, erosive, stress, fatigue, and crevice corrosion [1,2,3].
Although more and more mineral water bottling plants are being built and more and more spas being established where sources of highly mineralized waters are located, there remains a lack of awareness about the need for mineral waters to be treated differently from tap water when it comes to the installation materials used. Therefore, to help designers choose the right installation for a given mineral water, many indices have been developed to help determine, to a greater or lesser extent, water’s corrosive properties and its impact on individual metallic installation materials [4,5,6].
Mineral water was first defined at the International Balneological Congress in Bad Nauheim (Germany) in 1911 [7]. For water to qualify as mineral water, its level of mineralization has to be at least 1000 mg/L. The water may also contain natural dissolved gases, such as hydrogen sulfide or carbon dioxide. In most cases, the sources involved lie below impermeable layers (so-called submersible water), with water mineralized through the dissolution of minerals and rocks that it is in contact with.
However, the wide array of mineral waters in terms of composition means that the design of the associated water supply network should involve an analysis determining corrosion resistance of the installation materials. The type of installation material will first of all affect the capacity for water pollution to be prevented. Key analysis to allow an appropriate choice of material relates to the corrosiveness of water in relation to metals [8,9,10]. This is known to depend on the composition, notably the content of compounds with catalytic or corrosion-inhibiting properties. In line with this relationship, several indices have been adopted to predict the extent to which the corrosiveness of water will affect a given installation material in terms of its safety and reliability [11,12,13,14,15,16].
In the context of the study presented here, the five most common indices were considered, with determinations made for natural mineral waters referred to in European Parliament announcements [17,18]. An analysis of indices relative to installation materials was also carried out to give an indication as to how suitable these would prove for use in installations involving the given mineral water.
2. Legal Regulations Regarding the Corrosive Properties of Selected Mineral Waters
The basic standard in the field of corrosion with regard to the impact on the water supply system and corrosion protection is ISO 1885 [19]. This sets out the basic concepts regarding corrosion and, more specifically, general terms, types of corrosion, corrosion protection, corrosion testing, and electrochemical terms. Other standards [20,21] in this area relate to corrosion protection and indoor installations of buildings supplying water for human consumption.
Another standard specifying ways to protect materials used in the construction of the water supply network against corrosion is EN 12502, with its five parts relating to seven types of metal, i.e., copper and its alloys, hot-dip galvanized iron, steel (stainless or acid-resistant), iron, and nonalloy and low-alloy steels. Each section describes the type of corrosion that can threaten a given metal under certain conditions and also determines the impact of various factors (such as water parameters, design and performance, and temperature effects) on water distribution systems. In some cases, possibilities are also given for the corrosion process to be inhibited or eliminated entirely [22,23,24,25,26].
Characterized to assess the corrosive effect of mineral waters on tap water materials, the now obligatory standard N-72/C-04609 entails an initial qualitative assessment of the corrosive effect of cold natural waters on pipes made of cast iron or ordinary or galvanized steel. The standard describes the method by which the saturation index is determined as well as permissible parameter values for cold water under which corrosive properties of water are weakened.
Guidelines for the design of water supply systems are also included in EN 806, which has information relating to requirements and recommendations for the design, manufacture, reconstruction, testing, operation, and use of installations intended for the transport of water for human consumption [27,28,29,30,31].
The indices introduced by the German standard DIN 50930 dating back to 1993 has now been replaced by a standard analogous to EN 12502. The indices involved concern the impact of the installation of chloride and sulfate ions (S1) and nitrate ions (S2) as well as the content of bicarbonate and sulfate ions in relation to copper installations (S3).
Under Directive 2009/54/EC of the European Parliament and of the Council of 18 June 2009 on the exploitation and marketing of natural mineral waters, “natural mineral water” means a microbiologically wholesome water, originating in an underground water table or deposit and emerging from a spring tapped at one or more natural or bore exits [32]. The regulation specifies the exact requirements to be met by waters considered as mineral, spring, or table waters. These are microbiological requirements, maximum levels for minerals in water, and conditions under which waters can be saturated or have components or carbon dioxide removed from them. The ordinance also sets out the scope of basic research and methodologies for its implementation and qualification in relation to a given group as well as hygiene requirements with respect to the extraction, transport, and bottling of water.
The classification of mineral waters is as follows [32]:
- Low mineral content—a mineral salt content, calculated as fixed residue, not greater than 500 mg/L;
- Very low mineral content—a mineral salt content, calculated as fixed residue, not greater than 50 mg/L;
- Rich in mineral salts—a mineral salt content, calculated as fixed residue, greater than 1500 mg/L.
Basic regulations on the supply to consumers of water of adequate quantity and quality are transpositions of the Drinking Water Directive (Council Directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption), with its latest amendment including Commission Directive (EU) 2015/1787 of 6 October 2015 [33]. This specifies permissible concentrations of individual elements that classify water from taps in terms of drinking water.
Bottled mineral waters analyzed in this work are contained in the aforementioned Official Journal of the European Union [17]. This posting is in compliance with Article 1 of Directive 2009/54/EC of the Parliament and of the Council dated 18 June 2009 [32].
3. Materials and Methods
This section provides details of the proposed methods to assess the corrosive properties of selected mineral waters. The indices used to assess the stability of water were as follows: the value of pH at saturation, the Langelier index IL, the Ryznar index IR, the Larson and Skold index S1, the S2 index of selective corrosion in iron pipes that are hot-dip galvanized, and the S3 index for the assessment of pitting corrosion in pipes made of copper [22,23,24,25,26]. The analysis was carried out with Statsoft software [34].
3.1. Description of the Study Region
The mineral waters considered in this study are mined in Poland, a country located in Central Europe and on the Baltic Sea. It has a humid continental climate, though one that is also defined as transitional between warm and rainy temperate and snow-forest boreal. Poland is inhabited by 38.5 million people over an area of 312,700 km2, with an average population density of 123 people/km2. Poland occupies the area between 54°50′ and 49°00′ N and 14°07′ and 24°09′ E (Figure 1). Because lowland areas (below 200 m a.s.l.) predominate in Poland (accounting for as much as 75% of the country’s area), the average height is only 173 m a.s.l., and the median height is 149 m a.s.l. The lowlands occur in the north and in the center, with mountain and upland areas in the south.
Figure 1.
Location of the study region.
3.2. Characteristics of the Research Object
Data on the content of individual ions were collected from individual bottling plants extracting a given water, with about 78 samples taken into account.
Table 1 presents some of the analyzed bottled mineral waters available in Poland for retail and wholesale. These are extracted from both spa areas and other places in Poland. All properties of selected bottled mineral waters are contained in the table in Appendix A (Table A1). During calculation, the following values of standard deviation were obtained: pH (0.75), Ca2+ (104.78 mg/L), HCO3− (571.32 mg/L), Cl− (204.33 mg/L), SO42− (168.45), and NO3− (0.0123 mg/L).

Table 1.
Properties of selected bottled mineral waters—some results from all of the analyzed waters.
3.3. Methods
3.3.1. Indices Assessing the Corrosive Effect of Water on Water Installations
The corrosive effect of water on water installations was assessed using indices as follows:
- Water stability, i.e., the pH value at saturation, defined as follows:where A is a value determined on the basis of dry residue, B is a value determined on the basis of temperature, C is a value determined in reference to calcium content, and D is a value determined in reference to total alkalinity.pHs = (9.3 + A + B) − (C + D)
The values necessary to determine the pH of water at saturation are contained in the table in Appendix A (Table A2).
- The Langelier index, as used to assess the corrosive effect of water on steel and galvanized installations, calculated in line with a formula in the standard [34] as follows:where IL is the saturation index, pH0 is the pH of the examined water sample, and pHs is the pH in the saturated state.IL = pH0 − pHs
The saturation index often refers to the likelihood of determining limestone carbonate formation. The index assumes that, in the case of sediment formation, the rate of the corrosion process is low, meaning that water is only slightly corrosive. Sedimentation will occur if the indicator is positive. However, if the index is negative, it means that the calcium compounds in the water are dissolved and the corrosiveness of the water is high. If the index value is zero, the water has poor corrosion properties and there is a possibility of lime scale.
- The Ryznar index, based on the same assumptions as IL and otherwise known as the Ryznar stability index. The method of calculation combines analytical data with theories about the saturation of water in calcium carbonate, with the aim of predicting the tendency for sediment to form as well as the corrosiveness of water relative to carbon steel. The Ryznar index is calculated in line with the following formula:where IR is the stability index.IR = 2 pHS − pH0
Different values for the index correspond to different levels of stability of water [35]. When the value is below 6, the stability of water is deemed to be lower given the associated deposition of CaCO3 sediment and hence has more limited corrosiveness [36]. In turn, when the indicator value is in the 6–8 range, water is probably unstable and there is a possibility of underlying corrosion. However, if the IR value exceeds 8, the water is stable and its corrosiveness increases. To summarize, under the Ryznar index, stable water is that characterized by a value maintained in the range 6.25–6.75.
- The Larson and Skold index, used to assess the effect of chloride and sulfate ions on an installation, is expressed by the following formula:where [Cl−] is chloride concentration (mol/m3), [SO42−] is sulfate concentration (mol/m3), and [HCO3−] is bicarbonate concentration (mol/m3).
If the value of S1 is greater than 1, there is a likelihood of accelerated local corrosion of low-alloy iron materials. This is due to oxygen content greater than 0.1 g O2/m3. If the coefficient attains a value greater than 3, this means that the water has corrosive properties vis-à-vis galvanized steel, with possible local corrosion.
- The S2 indicator assessing the occurrence of selective corrosion in hot-dip galvanized iron pipes, as used to assess the possibility of intercrystalline corrosion of zinc, where water has an elevated content of NO3− ions, and based on the following formula:where [NO3−] is nitrate concentration (mol/m3).
Selective leaching of zinc is present when the value of this ratio is below 2. The phenomenon occurs along the grain boundaries of zinc.
- The S3 evaluation index for the occurrence of pitting corrosion in pipes made of copper, used to assess corrosiveness in the case of an installation made of copper and described by the following formula:
The risk of copper-pitting corrosion is high when the value of the indicator is under 2.
3.3.2. Criteria for Assessing the Corrosion Resistance of Individual Materials
Table 2 summarizes the criteria used in assessing the corrosion resistance of individual materials.

Table 2.
Values of indicators at which corrosion of individual installation materials will not occur (based on [22,23,24,25,26,35,36]).
Acid-resistant steel has the most limited requirements, with indices being basically of no significance as only the content of chloride ions is important. At the other extreme, hot-dip galvanized iron is problematic, given the need for matches in the case of 4 of the 6 indices.
An additional criterion for assessing the corrosion resistance of materials is their alkalinity. The water of alkalinity below 1.36 mol/m3 is aggressive, regardless of the values assumed by other indices. In the case of the H2S parameter, there is a lack of guidelines as to the value for the indices at which corrosion of individual installation materials will not occur.
4. Results and Discussion
4.1. Corrosiveness Indices for Bottled Mineral Waters
Using the formulae presented in Section 3.3.1. The following corrosion indices were calculated and are presented in Figure 2.
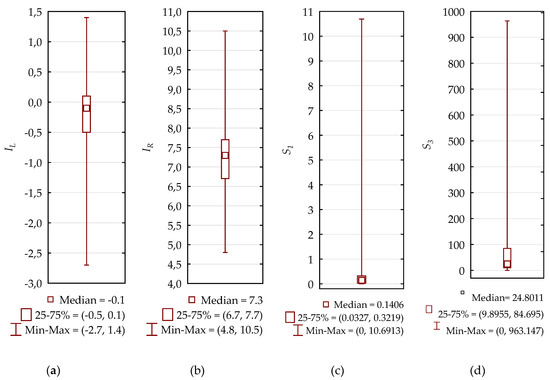
Figure 2.
Indices for the corrosiveness of bottled mineral waters. Box plots for (a) the Langelier index—IL, (b) the Ryznar index—IR, (c) S1, and (d) S3.
In the case of the (S2) index assessing the occurrence of selective corrosion in hot-dip galvanized iron pipes, 12.8% of all tested waters obtained results above 1.84, with a maximum of 1300.35 reached.
4.2. Results for Assessment of the Resistance of Installation Materials to the Corrosive Effects of Mineral Waters
Based on the collected data and the results of corrosion indicators, an assessment was made of the resistance of installation materials to the corrosive effects of the mineral waters discussed in this work. The materials evaluated were copper, galvanized iron, stainless steel, acid-resistant steel, cast iron, low-alloy steel, and nonalloy steel.
Table 3 presents the assessment of the resistance of installation materials to bottled mineral waters. All values for the indices are contained in the table in Appendix A (Table A3).

Table 3.
Values for indices at which corrosion of individual installation materials will not occur—a snapshot of all the waters analyzed.
As can be seen in Appendix A (Table A3), the results showed that it would be possible to use all types of installation materials in only five of the analyzed cases, namely, Podkarpackie (pH = 9.2, with general mineralization 837 mg/L); Malopolskie, source: T-III, T-IX, P-VI (pH = 7.5, 1249); Malopolskie, source: Z-2, Z-3, Z-3a, Z-8 (pH = 7.89, 1560); Dolnoslaskie, source: P-300a (pH = 7.6, 2190); Malopolskie, source 2, (pH = 7.54, 924) and Malopolskie, source: Z-3, Z-3A (pH = 7.89, 1023). There were only two cases where none of the installations examined would be appropriate for use, namely, Kujawsko-Pomorskie, 19a (pH = 6.31, 3417 mg/L) and Malopolskie, source: W-12, W-24 (pH = 6.06, 1973 mg/L).
Figure 3 presents in bar form the number of cases in which it is possible to use a given material, and the number in which use is (or should be) impossible.
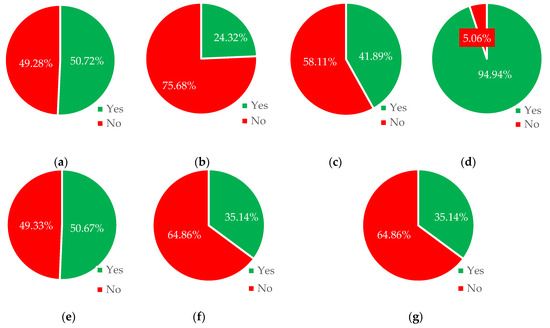
Figure 3.
Percentage of the possibility of using installation materials at which corrosion of individual installation materials will not occur. Type of installation material: (a) copper, (b) galvanized iron, (c) stainless steel, (d) acid-resistant steel, (e) cast iron, (f) low-alloy steel, and (g) nonalloy steel.
Acid-proof steel emerged as the most comprehensive material as it could be used in 33% of all cases. Following it in the list were iron (17%) and copper (15%). The materials with the lowest use were nonalloy steel (3%) and hot-dip galvanized iron (8%).
The most typical installation materials for water supply systems are polyvinyl chloride (PVC), polyethylene (PE), and polybutylene (PB) plastics. Fiberglass pipes are also used in the case of thermal waters. Plastics have gained widespread use due to the absence of corrosiveness as a criterion underpinning their selection. However, components of the above polymer installations are seen as a source of nutrients for bacteria found in water transmission systems. The result of their metabolic activity is the formation of a biofilm in the internal parts of ducts, which then assume the appearance of having been affected by microbial corrosion [37,38]. Previous studies [15,39] have indicated the importance of the selection of pipe material in water systems with high corrosiveness, among others, on the basis of water quality parameters, such as pH, alkalinity, and calcium hardness. The presented approach has been proven to be suitable for assessing the corrosive properties of mineral waters, as confirmed by analysis shown in [40,41]. The mentioned research shows the need for the broad characterization of mineral water compositions, including their aggressiveness concerning different materials, using the analyzed indices, which in turn will help to establish criteria for categorizing stability of the water [42,43]. The development of material science is dynamic, and its effect is the production of increasingly new materials, which are systematically introduced in the construction of water supply networks [44,45]. When introducing new materials for the construction of water supply networks, it should be taken into account that these materials require a completely new approach at the planning stage of construction, the construction itself, or during testing of the condition of the water supply network in comparison with the materials used so far [12]. Such tests take time and only after a long period of use will it be possible to determine exactly to what extent they affect the quality of the transported water.
Plastics are prone to fouling by microorganisms, so those choosing this type of installation for aggressive mineral waters will need to recall the possibility of secondary microbial contamination and the appearance of corrosion.
5. Conclusions and Perspectives
To this date, there have been no legal recommendations regarding assessment of the corrosiveness of water by supply companies, spas, and bottling plants. Such a procedure could do much to help designers choose the right installation without exposing the client to additional operating costs and even the need to replace corrosion-damaged pipes. There would certainly be a positive effect on the lifespan of pipes as well as the quality of the water itself.
This work makes it clear that a duty to assess corrosiveness should be imposed by law on institutions distributing mineral waters. Polish mineral waters are aggressive waters, as evidenced by the fact that 8% of the types considered were too aggressive to gain use in association with the materials analyzed. Equally, it needs to be recalled that the use of cheaper and theoretically noncorrosive materials may emerge to be uneconomical and may indeed give rise to entirely unexpected phenomena.
Operators should be involved in the design of water distribution systems and installations using appropriate materials to ensure an adequate level of safety from the water source to the recipient. It should be noted that it is necessary to adapt the internal material of the water supply system to the water parameters. There is currently no correlation between the design phase and the water parameters. It was found that in order to protect the water infrastructure, which is a critical infrastructure, water supply company should place more emphasis on the distribution of stable water that has no potentially corrosive properties. In perspective, some suggestions will be made regarding protection of the water distribution system and its safe operation as well as the long-term durability of water supply pipes.
Author Contributions
All authors equally contributed to the development of this manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We thank the reviewers for their feedback, which helped to improve the manuscript quality.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Properties of selected bottled mineral waters—some results from all of the analyzed waters.
Table A1.
Properties of selected bottled mineral waters—some results from all of the analyzed waters.
| No. of Sample | pH | Ca2+ | HCO3− | Cl− | SO42− | NO3− |
|---|---|---|---|---|---|---|
| - | mg/L | mg/L | mg/L | mg/L | mg/L | |
| 1 | 7.1 | 110.92 | 1216 | 48 | 160 | 0.00000 |
| 2 | 7.6 | 94.2 | 260.1 | 15.5 | 24.5 | 0.00645 |
| 3 | 7.4 | 72.14 | 421.5 | 180.7 | 17.28 | 0.00000 |
| 4 | 7.1 | 119.28 | 378.31 | 31.91 | 140.4 | 0.00000 |
| 5 | 7 | 70.86 | 514.07 | 17.73 | 40 | 0.00000 |
| 6 | 6.49 | 222.8 | 1256 | 6.5 | 29.5 | 0.00000 |
| 7 | 7.1 | 148 | 522 | 2.1 | 1.3 | 0.04677 |
| 8 | 7.6 | 44.7 | 0 | 17.7 | 125 | 0.00000 |
| 9 | 5.5 | 90.18 | 512 | 1.56 | 19.8 | 0.00000 |
| 10 | 6.7 | 68.3 | 271 | 3.2 | 29.2 | 0.00161 |
| 11 | 7.5 | 17 | 237.97 | 41.47 | 122 | 0.00000 |
| 12 | 5.4 | 97.19 | 311.19 | 13.1 | 50.2 | 0.00000 |
| 13 | 7 | 159.1 | 613 | 28.23 | 51.7 | 0.00000 |
| 14 | 6.31 | 210 | 1500 | 8.5 | 19 | 0.00000 |
| 15 | 7.4 | 59.12 | 190.6 | 5.3 | 14 | 0.00000 |
| 16 | 6.5 | 84.17 | 415.53 | 5.3 | 0.00000 | |
| 17 | 6.64 | 104.3 | 897 | 146 | 16.14 | 0.00000 |
| 18 | 7.7 | 90.68 | 270 | 8.2 | 57.3 | 0.00000 |
| 19 | - | 157.31 | 450.5 | 496.3 | 179 | 0.00000 |
| 20 | 6.5 | 60.12 | 107.39 | 42.89 | 160.48 | 0.00000 |
| 21 | 9.2 | 10 | 357.8 | 26.4 | 7.81 | 0.00000 |
| 22 | 7.2 | 66.9 | 342 | 7.5 | 37.3 | 0.00161 |
| 23 | - | 71.14 | 331.9 | 10.3 | 52.05 | 0.00000 |
| 24 | 7.2 | 87.7 | 387.5 | 7.4 | - | 0.00000 |
| 25 | 5.7 | 86.97 | 360.01 | 5.32 | 12.6 | 0.04032 |
| 26 | 8.32 | 42.08 | 378.5 | 8.86 | 46.5 | 0.00000 |
| 27 | 7.6 | 41 | 219 | 2 | 37 | 0.04677 |
| 28 | 7.5 | 160 | 1074 | 6.3 | 16.2 | 0.00000 |
| 29 | 6.1 | 340.4 | 1510.2 | 7.3 | 4.5 | 0.00000 |
| 30 | 6.16 | 436.87 | 1818.34 | 8.86 | 19.58 | 0.00000 |
| 31 | 6.31 | 174.11 | 470.44 | 1659.2 | 55 | 0.00000 |
| 32 | 7.3 | 76.15 | 500.3 | 11.3 | 10.58 | 0.00323 |
| 33 | 6.5 | 152.3 | 222.59 | 89.3 | 192.38 | 0.00000 |
| 34 | 8 | 196.39 | 499.7 | 301.3 | 85.08 | 0.00000 |
| 35 | 7.4 | 456.5 | 1836 | 5.6 | 3 | 0.00000 |
| 36 | 6.5 | 657.3 | 2440.7 | 5.3 | 50.9 | 0.00000 |
| 37 | 6.5 | 302.6 | 1280.6 | 12.9 | 40.53 | 0.00000 |
| 38 | 5.6 | 206.1 | 1158 | - | 3 | 0.00000 |
| 39 | 6.3 | 208 | 1289 | 12.9 | 21.8 | 0.00000 |
| 40 | 6.5 | 228.6 | 1479.9 | 17.7 | 16.3 | 0.00000 |
| 41 | 6.4 | 234.7 | 3060 | 16 | 5.2 | 0.00000 |
| 42 | 7.5 | 110.2 | 453.7 | 9.2 | - | 0.00000 |
| 43 | 7 | 96.2 | 396.6 | - | - | 0.06452 |
| 44 | 7.96 | 66 | 251 | 5 | 8 | 0.00000 |
| 45 | 7.3 | 68.14 | 253.71 | 10.98 | 11.32 | 0.00000 |
| 46 | 6.17 | 155 | 1147 | 7.8 | 17 | 0.00000 |
| 47 | 6.62 | 123 | 330 | 38 | 91 | 0.00000 |
| 48 | 7 | 130.3 | 539.1 | - | - | 0.00000 |
| 49 | 7.5 | 88.98 | 414.92 | 5.32 | 29.24 | 0.00000 |
| 50 | 7.59 | 46.67 | 187.9 | 115.2 | 1425 | 0.00000 |
| 51 | 6.3 | 180 | 1260 | - | 30 | 0.00000 |
| 52 | 6.4 | 161.1 | 625 | 28.61 | 51.6 | 0.00000 |
| 53 | 6.81 | 120 | 927 | 27 | 100 | 0.00000 |
| 54 | 6.5 | 218 | 866 | 2 | 14 | 0.00000 |
| 55 | 7.55 | 92.18 | 418.95 | 3.8 | - | 0.00000 |
| 56 | 7.52 | 94.2 | 246 | 11.6 | 90.12 | 0.00000 |
| 57 | 7 | 97.2 | 440 | 36.2 | 20 | 0.00000 |
| 58 | 7.5 | 103 | 403 | 7.1 | 35 | 0.00000 |
| 59 | 7.89 | 152.7 | 1141 | 7 | 17.4 | 0.00000 |
| 60 | 7.4 | 107.2 | 334.8 | 39.7 | 97.68 | 0.00000 |
| 61 | 5.82 | 165.38 | 737 | 7 | 32 | 0.00000 |
| 62 | 6.3 | 124 | 529 | 6.6 | 32 | 0.00000 |
| 63 | 6.09 | 220.4 | 1147.4 | 6.9 | 20.2 | 0.00000 |
| 64 | 6.71 | 57.9 | 148 | 9.48 | 29 | 0.00000 |
| 65 | 5.5 | 319 | 1639 | 2.7 | 30 | 0.00000 |
| 66 | 7.6 | 309 | 1590 | 2.9 | 30 | 0.00000 |
| 67 | 7.6 | 82.16 | 344.3 | 41.48 | 84.48 | 0.00000 |
| 68 | 7.6 | 84.17 | 325.8 | 6.75 | 37.04 | 0.00000 |
| 69 | 6.4 | 166 | 613 | 31 | 55 | 0.00000 |
| 70 | 7.4 | 117 | 295 | 26.7 | 48.8 | 0.04677 |
| 71 | 7.4 | 76.95 | 390 | 10.3 | 1.6 | 0.00000 |
| 72 | 7.54 | 184.4 | 705.6 | 6.4 | 27.8 | 0.00000 |
| 73 | 7.4 | 62.12 | 416.8 | 131.2 | 15.43 | 0.00000 |
| 74 | 7.59 | 98.2 | 365.1 | 32.3 | 84.97 | 0.00000 |
| 75 | 6.06 | 142.5 | 1665.8 | 319.1 | 11.5 | 0.00710 |
| 76 | 5.1 | 50.2 | 341.7 | 7 | 22.8 | 0.01135 |
| 77 | 6.74 | 40.08 | 183.05 | 37.23 | 19.1 | 0.00000 |
| 78 | 7.89 | 114 | 729.5 | 9 | 26 | 0.00000 |

Table A2.
Values of A–D coefficients and for calculating pHs.
Table A2.
Values of A–D coefficients and for calculating pHs.
| Dry Residue [mg/dm3] | A | Temperature [°C] | B | The Content Of Calcium [mgCa2+/dm3] | C | General Alkalinity [mval/dm3] | D |
|---|---|---|---|---|---|---|---|
| 50–400 | 0.1 | 0–1.1 | 2.6 | 4.0–4.4 | 0.6 | 0.20–0.22 | 1.0 |
| 400–1000 | 0.2 | 2.2–5.6 | 2.5 | 4.8–5.2 | 0.7 | 0.22–0.26 | 1.1 |
| - | - | 6.7–8.9 | 2.4 | 5.6–6.8 | 0.8 | 0.28–0.34 | 1.2 |
| - | - | 10.0–13.3 | 2.3 | 7.2–8.8 | 0.9 | 0.36–0.44 | 1.3 |
| - | - | 14.5–16.7 | 2.2 | 9.2–10.8 | 1.0 | 0.46–0.54 | 1.4 |
| - | - | 17.8–21.1 | 2.1 | 11.2–13.6 | 1.1 | 0.56–0.70 | 1.5 |
| - | - | 22.2–26.7 | 2.0 | 14.0–17.2 | 1.2 | 0.72–0.88 | 1.6 |
| - | - | 27.8–31.1 | 1.9 | 17.6–22.0 | 1.3 | 0.90–1.10 | 1.7 |
| - | - | 32.2–36.5 | 1.8 | 22.4–27.6 | 1.4 | 1.12–1.38 | 1.8 |
| - | - | 37.8–43.3 | 1.7 | 28.0–34.8 | 1.5 | 1.40–1.76 | 1.9 |
| - | - | 44.4–50.0 | 1.6 | 35.2–44.0 | 1.6 | 1.78–2.20 | 2.0 |
| - | - | - | - | 44.4–55.2 | 1.7 | 2.22–2.78 | 2.1 |
| - | - | - | - | 55.6–69.6 | 1.8 | 2.81–3.52 | 2.2 |
| - | - | - | - | 70.0–88.0 | 1.9 | 3.54–4.40 | 2.3 |
| - | - | - | - | 92.0–109 | 2.0 | 4.60–5.40 | 2.4 |
| - | - | - | - | 112–136 | 2.1 | 5.60–7.00 | 2.5 |
| - | - | - | - | 140–172 | 2.2 | 7.20–8.80 | 2.6 |
| - | - | - | - | 176–220 | 2.3 | 9.00–11.0 | 2.7 |
| - | - | - | - | 224–276 | 2.4 | 11.2–13.8 | 2.8 |
| - | - | - | - | 280–348 | 2.5 | 14.0–17.6 | 2.9 |
| - | - | - | - | 352–400 | 2.6 | 17.8–20.0 | 3.0 |

Table A3.
Values for indices at which corrosion of individual installation materials will not occur—a snapshot of all the waters analyzed.
Table A3.
Values for indices at which corrosion of individual installation materials will not occur—a snapshot of all the waters analyzed.
| No. of Sample | Installation Material | ||||||
|---|---|---|---|---|---|---|---|
| Copper | Galvanized Iron | Stainless Steel | Acid-Resistant Steel | Cast Iron | Low-Alloy Steel | Nonalloy Steel | |
| 1 | ✓ | X | ✓ | ✓ | ✓ | ✓ | X |
| 2 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | X |
| 3 | ✓ | X | ✓ | ✓ | ✓ | ✓ | X |
| 4 | ✓ | X | X | ✓ | ✓ | ✓ | X |
| 5 | ✓ | X | X | ✓ | ✓ | X | X |
| 6 | X | X | X | ✓ | X | X | X |
| 7 | ✓ | X | ✓ | ✓ | ✓ | ✓ | X |
| 8 | ✓ | - | - | ✓ | ✓ | - | - |
| 9 | X | X | X | ✓ | X | X | X |
| 10 | X | X | X | ✓ | X | X | X |
| 11 | ✓ | X | X | ✓ | ✓ | X | X |
| 12 | X | X | X | ✓ | X | X | X |
| 13 | ✓ | X | ✓ | ✓ | ✓ | ✓ | X |
| 14 | X | X | X | ✓ | X | X | X |
| 15 | ✓ | X | X | ✓ | ✓ | X | X |
| 16 | - | X | X | ✓ | X | X | X |
| 17 | X | X | X | ✓ | X | X | X |
| 18 | ✓ | ✓ | X | ✓ | ✓ | X | X |
| 19 | - | - | - | X | - | - | - |
| 20 | X | X | X | ✓ | X | X | X |
| 21 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | X |
| 22 | ✓ | X | X | ✓ | ✓ | X | X |
| 23 | - | - | - | ✓ | - | - | - |
| 24 | - | X | X | ✓ | ✓ | X | X |
| 25 | X | X | X | ✓ | X | X | X |
| 26 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | X |
| 27 | ✓ | ✓ | X | ✓ | ✓ | X | X |
| 28 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 29 | X | X | X | ✓ | X | X | X |
| 30 | X | X | X | ✓ | X | X | X |
| 31 | X | X | X | X | X | X | X |
| 32 | ✓ | X | ✓ | ✓ | ✓ | ✓ | X |
| 33 | X | X | X | ✓ | X | X | X |
| 34 | ✓ | X | X | X | X | X | X |
| 35 | ✓ | X | ✓ | ✓ | ✓ | ✓ | ✓ |
| 36 | X | X | ✓ | ✓ | X | X | X |
| 37 | X | X | ✓ | ✓ | X | X | X |
| 38 | X | X | X | ✓ | X | X | X |
| 39 | X | X | X | ✓ | X | X | X |
| 40 | X | X | ✓ | ✓ | X | X | X |
| 41 | X | X | ✓ | ✓ | X | X | X |
| 42 | - | ✓ | ✓ | ✓ | ✓ | ✓ | X |
| 43 | - | X | X | ✓ | ✓ | X | X |
| 44 | ✓ | X | ✓ | ✓ | ✓ | ✓ | X |
| 45 | ✓ | X | X | ✓ | X | X | X |
| 46 | X | X | ✓ | ✓ | X | X | X |
| 47 | X | X | X | ✓ | X | X | X |
| 48 | - | X | X | ✓ | ✓ | X | X |
| 49 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | X |
| 50 | X | X | X | ✓ | X | X | X |
| 51 | X | X | X | ✓ | X | X | X |
| 52 | X | X | X | ✓ | X | X | X |
| 53 | X | X | ✓ | ✓ | X | X | X |
| 54 | X | X | X | ✓ | X | X | X |
| 55 | - | ✓ | ✓ | ✓ | ✓ | ✓ | X |
| 56 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | X |
| 57 | ✓ | X | X | ✓ | ✓ | X | X |
| 58 | - | - | - | ✓ | - | - | - |
| 59 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 60 | - | - | - | ✓ | - | - | - |
| 61 | X | X | X | ✓ | X | X | X |
| 62 | X | X | X | ✓ | X | X | X |
| 63 | X | X | X | ✓ | X | X | X |
| 64 | X | X | X | ✓ | X | X | X |
| 65 | X | X | X | ✓ | X | X | X |
| 66 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 67 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | X |
| 68 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | X |
| 69 | X | X | X | ✓ | X | X | X |
| 70 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | X |
| 71 | ✓ | X | ✓ | ✓ | ✓ | ✓ | X |
| 72 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 73 | ✓ | X | X | ✓ | ✓ | X | X |
| 74 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | X |
| 75 | X | X | X | X | X | X | X |
| 76 | X | X | X | ✓ | X | X | X |
| 77 | X | X | X | ✓ | X | X | X |
| 78 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
References
- Marcinowski, P.; Wojtkowska, M.; Sinicyn, G. Surface water monitoring in the area of the Zelazny Most waste disposal. Przem. Chem. 2008, 87, 512–519. [Google Scholar]
- Wąsowski, J.; Kowalski, D.; Kowalska, B.; Kwietniewski, M.; Zawilska, M. Water Quality Changes in Cement-Lined Water Pipe Networks. Appl. Sci. 2019, 9, 1348. [Google Scholar] [CrossRef]
- Wojtkowska, M.; Dmochowski, D. Seasonal Character of Changes in Nitrogen Forms in Waters of Korytow and Laki Korytowskie Retention Reservoirs. Environ. Prot. Eng. 2009, 35, 57–66. [Google Scholar]
- Kabsch-Korbutowicz, M.; Kutylowska, M. Use of artificial intelligence in predicting the turbidity retention coefficient during ultrafiltration of water. Environ. Prot. Eng. 2011, 2, 75–84. [Google Scholar]
- Rak, J.R.; Tchórzewska-Cieślak, B.; Pietrucha-Urbanik, K. A Hazard Assessment Method for Waterworks Systems Operating in Self-Government Units. Int. J. Environ. Res. Public Health 2019, 16, 767. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.J.; An, J.-H.; Kim, Y.-S.; Kim, W.-C.; Kim, J.-G. Effects of Corrosion on Mechanical Properties of Welded Carbon Steel Pipe in District Heating Water. Materials 2019, 12, 3682. [Google Scholar] [CrossRef]
- Bodzek, M.; Tomaszewska, B.; Rajca, M. Nanofiltration renovation of mineral water. Arch. Environ. Prot. 2017, 43, 51–59. [Google Scholar] [CrossRef][Green Version]
- Choucri, J.; Zanotto, F.; Grassi, V.; Balbo, A.; Ebn Touhami, M.; Mansouri, I.; Monticelli, C. Corrosion Behavior of Different Brass Alloys for Drinking Water Distribution Systems. Metals 2019, 9, 649. [Google Scholar] [CrossRef]
- Ondrejka Harbulakova, V.; Estokova, A.; Kovalcikova, M. Correlation Analysis between Different Types of Corrosion of Concrete Containing Sulfate Resisting Cement. Environments 2017, 4, 44. [Google Scholar] [CrossRef]
- Vertova, A.; Miani, A.; Lesma, G.; Rondinini, S.; Minguzzi, A.; Falciola, L.; Ortenzi, M.A. Chlorine Dioxide Degradation Issues on Metal and Plastic Water Pipes Tested in Parallel in a Semi-Closed System. Int. J. Environ. Res. Public Health 2019, 16, 4582. [Google Scholar] [CrossRef]
- Li, M.; Liu, Z.; Chen, Y. Physico-chemical Characteristics of Corrosion Scales from Different Pipes in Drinking Water Distribution Systems. Water 2018, 10, 931. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, Y.; Knibbe, W.J.; Feng, C.; Liu, W.; Medema, G.; der Meerae, W. Potential impacts of changing supply-water quality on drinking water distribution: A review. Water Res. 2017, 116, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Ondrejka Harbulakova, V.; Zelenakova, M.; Purcz, P.; Olejnik, A. Selection of the Best Alternative of Heating System by Environmental Impact Assessment—Case Study. Environments 2018, 5, 19. [Google Scholar] [CrossRef]
- Rodriguez, A.B.; Duranceau, S.J. Evaluating the effect of periodic disinfectant type transitions on the corrosion rate of common distribution system metals. Desal. Water Treat. 2019, 170, 11–23. [Google Scholar] [CrossRef]
- Toczyłowska, B.; Siwiec, T. Evaluation of corrosiveness of mineral and table waters from Polish health resorts. Gas Water Sanit. Eng. 2000, 7, 262–272. [Google Scholar]
- Urbanik, M.; Tchórzewska-Cieślak, B.; Pietrucha-Urbanik, K. Analysis of the Safety of Functioning Gas Pipelines in Terms of the Occurrence of Failures. Energies 2019, 12, 3228. [Google Scholar] [CrossRef]
- European Commission. List of Natural Mineral Waters Recognised by Member States; European Commission: Brussels, Belgium, 2013; OJ: JOC_2013_095_R_0038_01. [Google Scholar]
- Skowrońska, D. Assessment of the Corrosive Properties of Selected Mineral Water. Master Thesis, Rzeszow University of Technology, Rzeszow, Poland, 2017. [Google Scholar]
- ISO 1885:2017. Corrosion of Metals and Alloys. Basic Terms and Definitions; ISO: Geneva, Switzerland, 1999. [Google Scholar]
- DIN 50930-1:1993-02. Korrosion der Metalle; Korrosion Metallischer Werkstoffe im Innern von Rohrleitungen, Behältern und Apparaten bei Korrosionsbelastung durch Wässer; Allgemeines; DIN: Berlin, Germany.
- N-72/C-04609. Water and Sewage. Initial Qualitative Assessment of the Corrosive Effect of Cold Natural Waters on Pipes Made of Cast Iron, Ordinary or Galvanized Steel. Available online: https://sklep.pkn.pl/ (accessed on 29 June 2019).
- EN 12502-1:2004. Protection of Metallic Materials against Corrosion. Guidance on the Assessment of Corrosion Likelihood in Water Distribution and Storage Systems; BSI: London, UK, 2005. [Google Scholar]
- EN 12502-2:2004. Protection of Metallic Materials against Corrosion. Guidance on the Assessment of Corrosion Likelihood in Water Distribution and Storage Systems. Influencing Factors for Copper and Copper Alloys; BSI: London, UK, 2004. [Google Scholar]
- EN 12502-3:2004. Protection of Metallic Materials against Corrosion. Guidance on the Assessment of Corrosion Likelihood in Water Distribution and Storage Systems. Influencing Factors for Hot Dip Galvanised Ferrous Materials; BSI: London, UK, 2004. [Google Scholar]
- EN 12502-4:2004. Protection of Metallic Materials against Corrosion. Guidance on the Assessment of Corrosion Likelihood in Water Distribution and Storage Systems. Influencing Factors for Stainless Steels; BSI: London, UK, 2004. [Google Scholar]
- EN 12502-5:2004. Protection of Metallic Materials against Corrosion. Guidance on the Assessment of Corrosion Likelihood in Water Distribution and Storage Systems. Influencing Factors for Cast Iron, Unalloyed and Low Alloyed Steels; BSI: London, UK, 2004. [Google Scholar]
- EN 806-1:2000. Specifications for Installations inside Buildings Conveying Water for Human Consumption. General; BSI: London, UK, 2000. [Google Scholar]
- EN 806-2:2005. Specifications for Installations inside Buildings Conveying Water for Human Consumption. Design; BSI: London, UK, 2005. [Google Scholar]
- EN 806-3:2006. Specification for Installations inside Buildings Conveying Water for Human Consumption. Pipe Sizing; BSI: London, UK, 2006. [Google Scholar]
- EN 806-4:2010. Specifications for Installations inside Buildings Conveying Water for Human Consumption. Installation; BSI: London, UK, 2010. [Google Scholar]
- EN 806-5:2012. Specifications for Installations inside Buildings Conveying Water for Human Consumption. Operation and Maintenance; BSI: London, UK, 2012. [Google Scholar]
- European Union. Directive 2009/54/EC of the European Parliament and of the Council, of 18 June 2009, on the exploitation and marketing of natural mineral waters. Off. J. Eur. Union 2009, 164, 45. [Google Scholar]
- Council Directive 98/83/EC of 3 November 1998 on the Quality of Water Intended for Human Consumption. UR-Lex Web site. 1998. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A31998L0083 (accessed on 29 June 2019).
- STATISTICA—Data Analysis Software System, version 13.3; StatSoft, Inc.: Tulsa, OK, USA, 2020; Available online: www.statsoft.com (accessed on 4 May 2020).
- Dembińska, J. Assessment of corrosive aggressiveness of tap water in relation to installation materials. Gas Water Sanit. Eng. 1993, 11, 274–276. [Google Scholar]
- Falewicz, P.; Drela, I.; Kuczkowska, S. Corrosion aggressiveness of industrial waters. Corros. Prot. 2008, 4, 187–191. [Google Scholar]
- Ibekwe, A.M.; Murinda, S.E. Linking Microbial Community Composition in Treated Wastewater with Water Quality in Distribution Systems and Subsequent Health Effects. Microorganisms 2019, 7, 660. [Google Scholar] [CrossRef]
- Manuel, C.M.; Nunes, O.C.; Melo, L.F. Dynamics of drinking water biofilm in flow/non-flow conditions. Water Res. 2007, 41, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Rasheed, U.; Kong, M. A Study on the Comparison of Corrosion in Water Supply Pipes Due to Tap Water (TW) and Reclaimed Water (RW). Water 2018, 10, 496. [Google Scholar] [CrossRef]
- Vasconcelos, H.C.; Fernández-Pérez, B.M.; González, S.; Souto, R.M.; Santana, J.J. Characterization of the Corrosive Action of Mineral Waters from Thermal Sources: A Case Study at Azores Archipelago, Portugal. Water 2015, 7, 3515–3530. [Google Scholar] [CrossRef]
- Kalyani, D.S.; Rajesh, V.; Reddi, E.U.B.; Kumar, K.C.; Rao, S.S. Correlation between corrosion indices and corrosiveness of groundwater: A study with reference to selected areas of Krishna District, Andhra Pradesh, India. Environ. Earth Sci. 2017, 76, 568. [Google Scholar] [CrossRef]
- Abbasnia, A.; Alimohammadi, M.; Mahvia, A.H.; Nabizadeh, R.; Yousefi, M.; Mohammadi, A.A.; Pasalaric, H.; Mirzabeigia, M. Assessment of groundwater quality and evaluation of scaling and corrosiveness potential of drinking water samples in villages of Chabahr city, Sistan and Baluchistan province in Iran. Data Brief 2018, 16, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Cruz, J.V.; Freire, P.; Costa, A. Mineral waters characterization in the Azores archipelago (Portugal). J. Volcanol. Geotherm. Res. 2010, 90, 353–364. [Google Scholar] [CrossRef]
- Kumar, P.J.S. Assessment of corrosion and scaling potential of the groundwater in the Thanjavur district using hydrogeochemical analysis and spatial modeling techniques. SN Appl. Sci. 2019, 55, 395. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Ghione, R.; De Maio, M.; Lavy, M. Evaluation of hydrogeochemical processes and groundwater quality for suitability of drinking and irrigation purposes: A case study in the Aosta Valley region, Italy. Arab. J. Geosci. 2017, 12, 264. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).