Surface Immobilization Chemistry of a Laminin-Derived Peptide Affects Keratinocyte Activity
Abstract
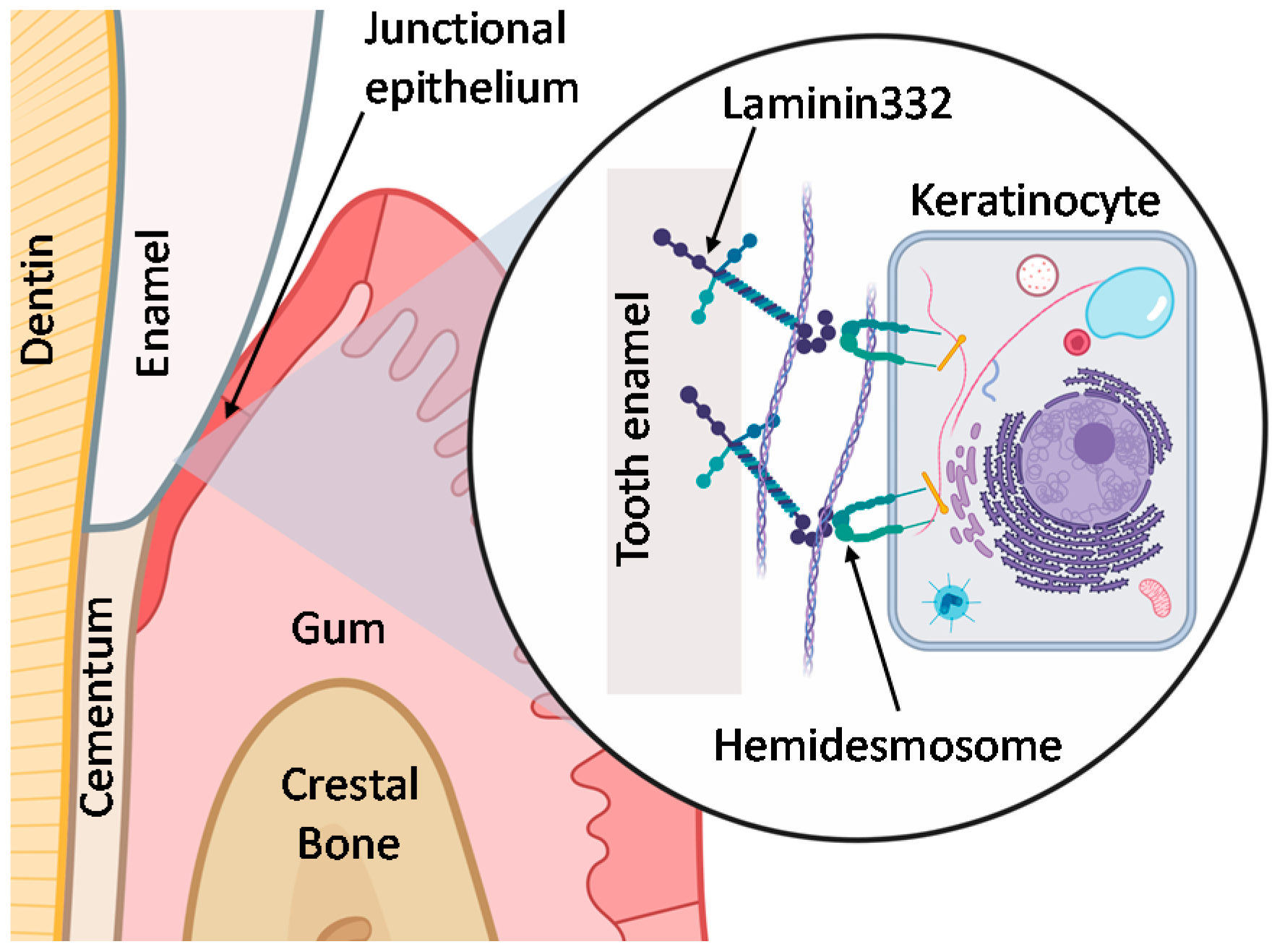
:1. Introduction
2. Materials and Methods
2.1. Surface Synthesis
2.2. Surface Characterization
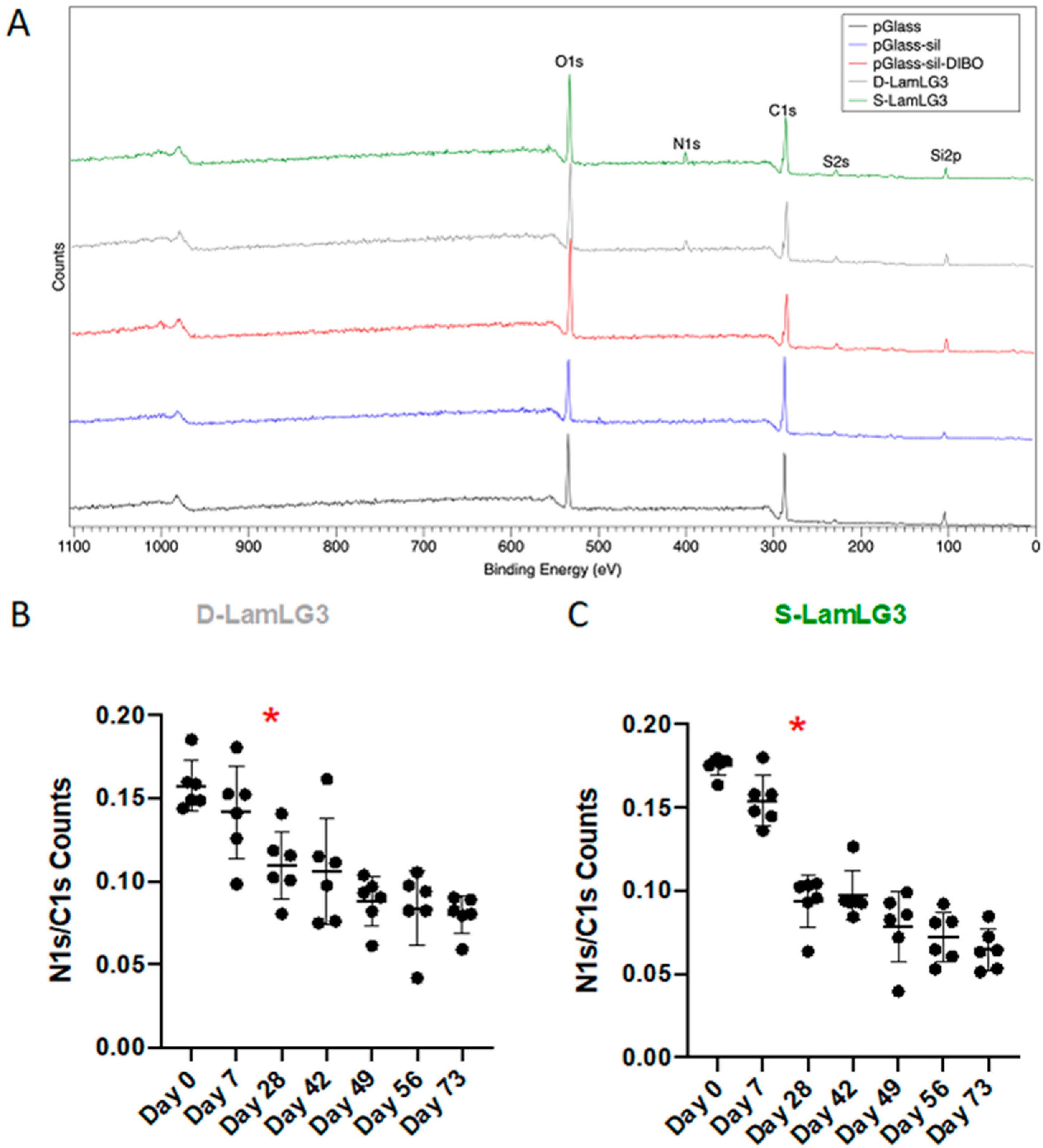
2.2.1. X-ray Photoelectron Spectroscopy (XPS)
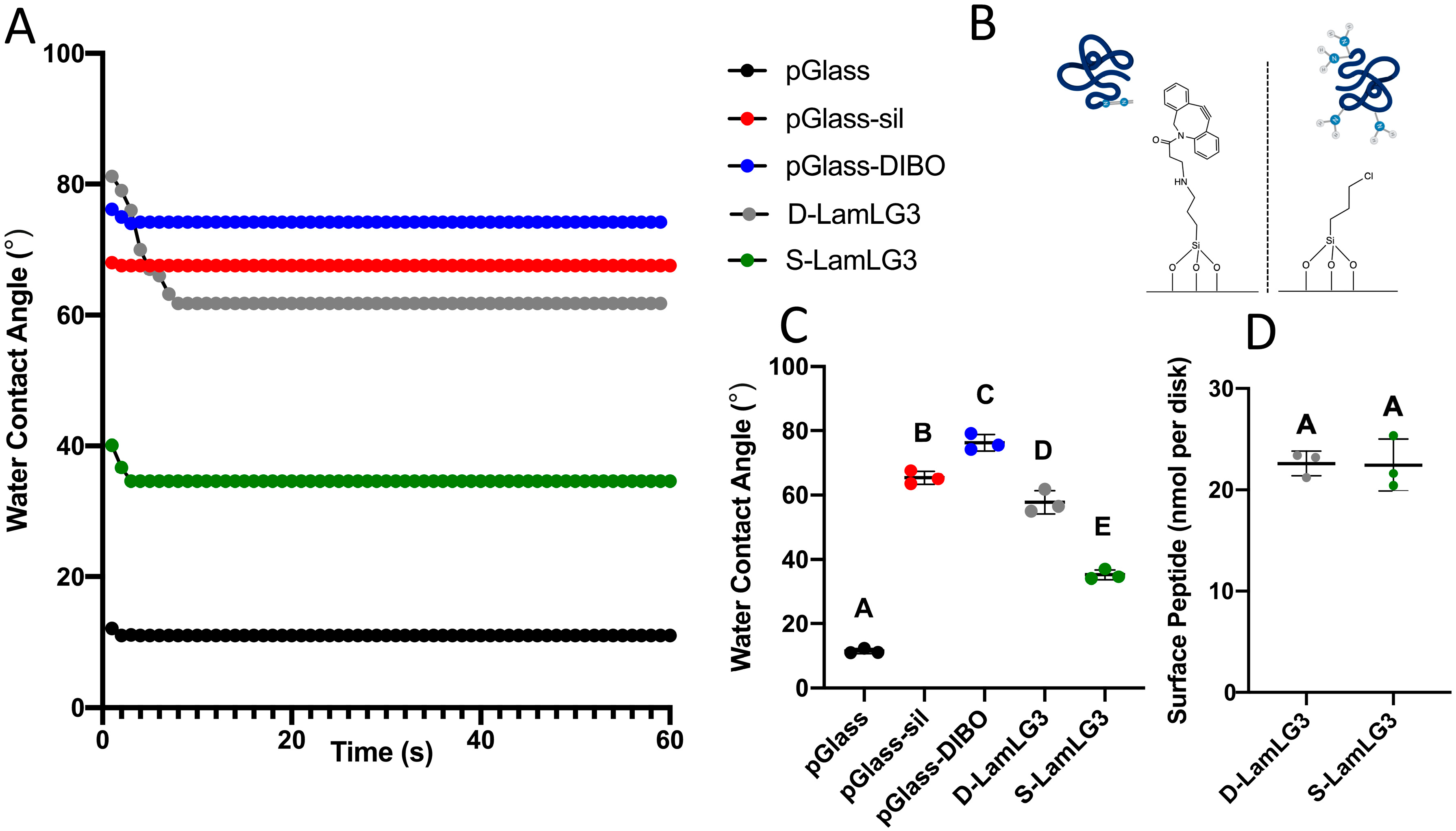
2.2.2. Water Contact Angle (WCA)
2.2.3. Coating Durability
2.2.4. Relative Amount of Surface Peptide
2.3. Oral Keratinocyte Response
2.3.1. Oral Keratinocyte Proliferation
2.3.2. Lactase Dehydrogenase (LDH) Release
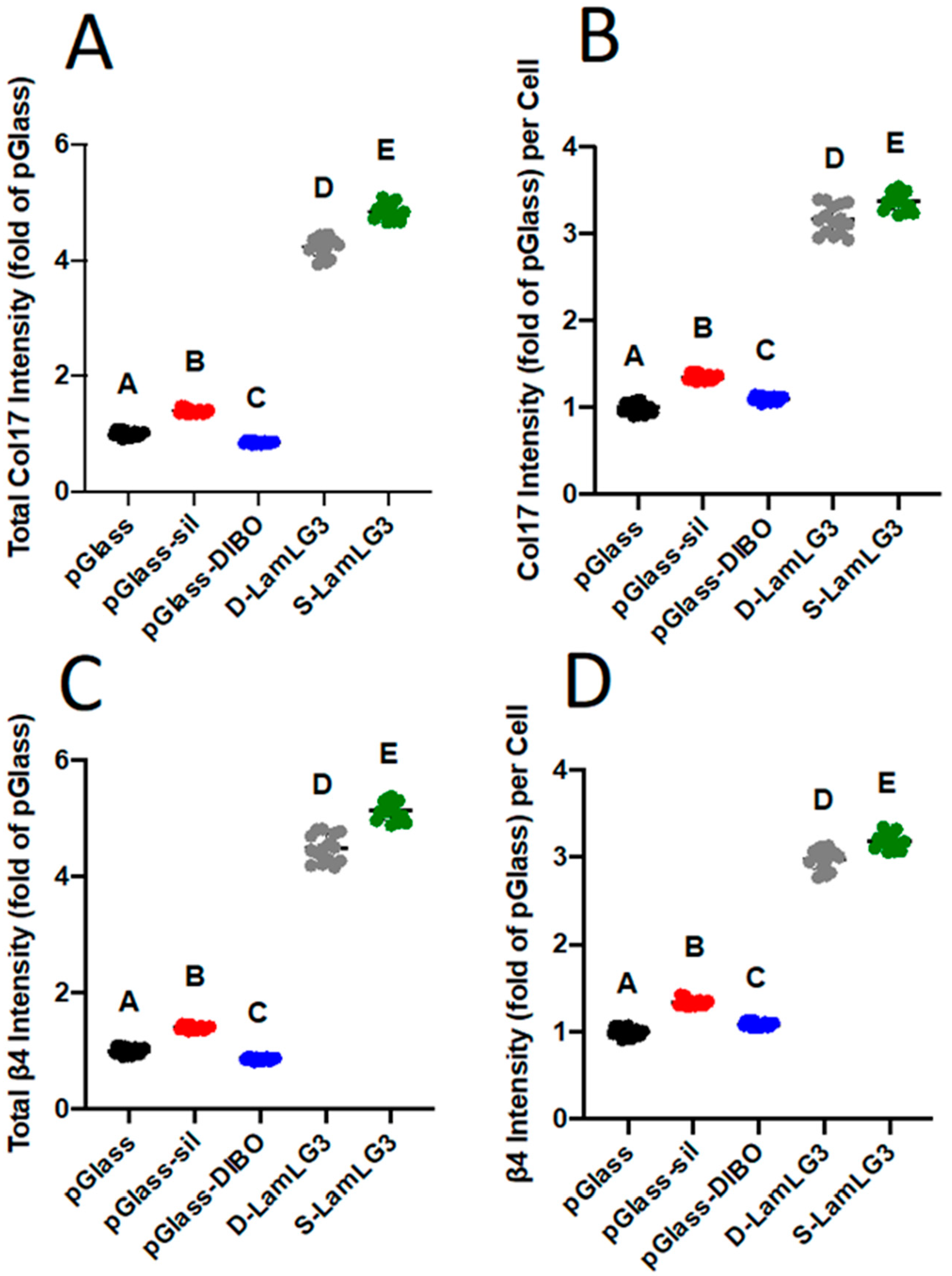
2.3.3. Immunofluorescence and Oral Keratinocyte Hemidesmosome Formation
2.4. Statistical Analysis
3. Results
3.1. Physical and Chemical Characterization of Peptide Coatings
3.2. Durability of Peptide Coatings
3.3. Keratinocytes Reponse on Peptide Coatings with Different Peptide Immobilization Chemistry
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Collier, J.H.; Segura, T. Evolving the use of peptides as components of biomaterials. Biomaterials 2011, 32, 4198–4204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamley, I.W. Small bioactive peptides for biomaterials design and therapeutics. Chem. Rev. 2017, 117, 14015–14041. [Google Scholar] [CrossRef] [Green Version]
- Spicer, C.D.; Pashuck, E.T.; Stevens, M.M. Achieving controlled biomolecule–biomaterial conjugation. Chem. Rev. 2018, 118, 7702–7743. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, N.R.; Marzuki, N.H.C.; Buang, N.A.; Huyop, F.; Wahab, R.A. An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnol. Biotechnol. Equip. 2015, 29, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Koidou, V.P.; Argyris, P.P.; Skoe, E.P.; Mota Siqueira, J.; Chen, X.; Zhang, L.; Hinrichs, J.E.; Costalonga, M.; Aparicio, C. Peptide coatings enhance keratinocyte attachment towards improving the peri-implant mucosal seal. Biomater. Sci. 2018, 6, 1936–1945. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Sevilla, P.; Aparicio, C. Surface biofunctionalization by covalent co-immobilization of oligopeptides. Colloids Surf. B Biointerfaces 2013, 107, 189–197. [Google Scholar] [CrossRef]
- Huettner, N.; Dargaville, T.R.; Forget, A. Discovering cell-adhesion peptides in tissue engineering: Beyond RGD. Trends Biotechnol. 2018, 36, 372–383. [Google Scholar] [CrossRef]
- LeBaron, R.G.; Athanasiou, K.A. Extracellular matrix cell adhesion peptides: Functional applications in orthopedic materials. Tissue Eng. 2000, 6, 85–103. [Google Scholar] [CrossRef]
- Holmberg, K.V.; Abdolhosseini, M.; Li, Y.; Chen, X.; Gorr, S.U.; Aparicio, C. Bio-inspired stable antimicrobial peptide coatings for dental applications. Acta Biomater. 2013, 9, 8224–8231. [Google Scholar] [CrossRef] [Green Version]
- Costa, F.; Carvalho, I.F.; Montelaro, R.C.; Gomes, P.; Martins, M.C.L. Covalent immobilization of antimicrobial peptides (AMPs) onto biomaterial surfaces. Acta Biomater. 2011, 7, 1431–1440. [Google Scholar] [CrossRef] [Green Version]
- Mas-Moruno, C.; Su, B.; Dalby, M.J. Multifunctional coatings and nanotopographies: Toward cell instructive and antibacterial implants. Adv. Healthc. Mater. 2019, 8, 1801103. [Google Scholar] [CrossRef] [PubMed]
- Hoyos-Nogués, M.; Velasco, F.; Ginebra, M.P.; Manero, J.M.; Gil, F.J.; Mas-Moruno, C. Regenerating bone via multifunctional coatings: The blending of cell integration and bacterial inhibition properties on the surface of biomaterials. ACS Appl. Mater. Interfaces 2017, 9, 21618–21630. [Google Scholar] [CrossRef] [PubMed]
- Hoyos-Nogués, M.; Buxadera-Palomero, J.; Ginebra, M.P.; Manero, J.M.; Gil, F.J.; Mas-Moruno, C. All-in-one trifunctional strategy: A cell adhesive, bacteriostatic and bactericidal coating for titanium implants. Colloids Surf. B Biointerfaces 2018, 169, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Hoyos-Nogués, M.; Brosel-Oliu, S.; Abramova, N.; Muñoz, F.X.; Bratov, A.; Mas-Moruno, C.; Gil, F.J. Impedimetric antimicrobial peptide-based sensor for the early detection of periodontopathogenic bacteria. Biosens. Bioelectron. 2016, 86, 377–385. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.; Zuo, X.; Shen, T.; Duan, Y.; Mao, Z.; Gao, C. A density gradient of VAPG peptides on a cell-resisting surface achieves selective adhesion and directional migration of smooth muscle cells over fibroblasts. Acta Biomater. 2018, 72, 70–81. [Google Scholar] [CrossRef]
- Kam, L.; Shain, W.; Turner, J.N.; Bizios, R. Selective adhesion of astrocytes to surfaces modified with immobilized peptides. Biomaterials 2002, 23, 511–515. [Google Scholar] [CrossRef]
- Brooks, K.; Yatvin, J.; McNitt, C.D.; Reese, R.A.; Jung, C.; Popik, V.V.; Locklin, J. Multifunctional surface nanipulation using orthogonal click chemistry. Langmuir 2016, 32, 6600–6605. [Google Scholar] [CrossRef]
- Sakala, G.P.; Reches, M. Peptide-Based Approaches to Fight Biofouling. Adv. Mater. Interfaces 2018, 5, 1–26. [Google Scholar] [CrossRef]
- Boskey, A.L.; Villarreal-Ramirez, E. Intrinsically disordered proteins and biomineralization. Matrix Biol. 2016, 52–54, 43–59. [Google Scholar] [CrossRef] [Green Version]
- Gungormus, M.; Fong, H.; Kim, I.W.; Evans, J.S.; Tamerler, C.; Sarikaya, M. Regulation of in vitro calcium phosphate mineralization by combinatorially selected hydroxyapatite-binding peptides. Biomacromolecules 2008, 9, 966–973. [Google Scholar] [CrossRef]
- Sevilla, P.; Gil, J.; Aparicio, C. Relevant properties for immobilizing short peptides on biosurfaces. IRBM 2017, 38, 256–265. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Aparicio, C. Biofunctional coatings for dental implants. In Thin Films and Coatings in Biology; Springer: Berlin/Heidelberg, Germany, 2013; pp. 105–143. ISBN 978-94-007-2591-1. [Google Scholar]
- Shadish, J.A.; DeForest, C.A. Site-Selective Protein Modification: From Functionalized Proteins to Functional Biomaterials. Matter 2020, 2, 50–77. [Google Scholar] [CrossRef]
- Saha, B.; Saikia, J.; Das, G. Correlating enzyme density, conformation and activity on nanoparticle surfaces in highly functional bio-nanocomposites. Analyst 2015, 140, 532–542. [Google Scholar] [CrossRef]
- Sevilla, P.; Vining, K.V.; Dotor, J.; Rodriguez, D.; Gil, F.J.; Aparicio, C. Surface immobilization and bioactivity of TGF-β1 inhibitor peptides for bone implant applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 385–394. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, J.; Avaltroni, M.J.; Danahy, M.P.; Silverman, B.M.; Hanson, E.L.; Schwarzbauer, J.E.; Midwood, K.S.; Gawalt, E.S. Cell attachment and spreading on metal implant materials. Mater. Sci. Eng. C 2003, 23, 395–400. [Google Scholar] [CrossRef]
- Pagel, M.; Hassert, R.; John, T.; Braun, K.; Wießler, M.; Abel, B.; Beck-Sickinger, A.G. Multifunctional coating improves cell adhesion on titanium by using cooperatively acting peptides. Angew. Chem. Int. Ed. Engl. 2016, 55, 4826–4830. [Google Scholar] [CrossRef]
- Lummerstorfer, T.; Hoffmann, H. Click chemistry on surfaces: 1,3-dipolar cycloaddition reactions of azide-terminated monolayers on silica. J. Phys. Chem. B 2004, 108, 3963–3966. [Google Scholar] [CrossRef]
- Buxadera-Palomero, J.; Calvo, C.; Torrent-Camarero, S.; Gil, F.J.; Mas-Moruno, C.; Canal, C.; Rodríguez, D. Biofunctional polyethylene glycol coatings on titanium: An in vitro-based comparison of functionalization methods. Colloids Surf. B Biointerfaces 2017, 152, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Godoy-Gallardo, M.; Mas-Moruno, C.; Yu, K.; Manero, J.M.; Gil, F.J.; Kizhakkedathu, J.N.; Rodriguez, D. Antibacterial properties of hLf1-11 peptide onto titanium surfaces: A comparison study between silanization and surface initiated polymerization. Biomacromolecules 2015, 16, 483–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.T.; Huang, Y.W.; Zhu, L.; Weltman, R. Prevalences of peri-implantitis and peri-implant mucositis: Systematic review and meta-analysis. J. Dent. 2017, 62, 1–12. [Google Scholar] [CrossRef]
- Overmann, A.L.; Aparicio, C.; Richards, J.T.; Mutreja, I.; Fischer, N.G.; Wade, S.M.; Potter, B.K.; Davis, T.A.; Bechtold, J.E.; Forsberg, J.A.; et al. Orthopaedic osseointegration: Implantology and future directions. J. Orthop. Res. 2020, jor.24576. [Google Scholar] [CrossRef] [PubMed]
- O’Niel, M.B.; Runge, C.L.; Friedland, D.R.; Kerschner, J.E. Patient outcomes in magnet-based implantable auditory assist devices. JAMA Otolaryngol. Head Neck Surg. 2014, 140, 513–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atallah, R.; Leijendekkers, R.A.; Hoogeboom, T.J.; Frölke, J.P. Complications of bone-anchored prostheses for individuals with an extremity amputation: A systematic review. PLoS ONE 2018, 13, e0201821. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.M.; Park, W.H.; Min, B.M. The PPFLMLLKGSTR motif in globular domain 3 of the human laminin-5 α3 chain is crucial for integrin α3β1 binding and cell adhesion. Exp. Cell Res. 2005, 304, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ma, S.; Lu, X.; Zhang, T.; Sun, Y.; Feng, W.; Zheng, G.; Sui, L.; Wu, X.; Zhang, X.; et al. Reinforcement of epithelial sealing around titanium dental implants by chimeric peptides. Chem. Eng. J. 2019, 356, 117–129. [Google Scholar] [CrossRef]
- Werner, S.; Huck, O.; Frisch, B.; Vautier, D.; Elkaim, R.; Voegel, J.C.; Brunel, G.; Tenenbaum, H. The effect of microstructured surfaces and laminin-derived peptide coatings on soft tissue interactions with titanium dental implants. Biomaterials 2009, 30, 2291–2301. [Google Scholar] [CrossRef]
- Atsuta, I.; Ayukawa, Y.; Kondo, R.; Oshiro, W.; Matsuura, Y.; Furuhashi, A.; Tsukiyama, Y.; Koyano, K. Soft tissue sealing around dental implants based on histological interpretation. J. Prosthodont. Res. 2016, 60, 3–11. [Google Scholar] [CrossRef]
- Iorio, V.; Troughton, L.D.; Hamill, K.J. Laminins: Roles and utility in wound repair. Adv. Wound Care 2015, 4, 250–263. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.; Koo, H. Biomedical applications of copper-free click chemistry: In vitro, in vivo, and ex vivo. Chem. Sci. 2019, 10, 7835–7851. [Google Scholar] [CrossRef] [Green Version]
- Meldal, M.; Tornøe, C.W. Cu-Catalyzed Azide−Alkyne Cycloaddition. Chem. Rev. 2008, 108, 2952–3015. [Google Scholar] [CrossRef]
- Zhang, N.; Weir, M.D.; Romberg, E.; Bai, Y.; Xu, H.H.K. Development of novel dental adhesive with double benefits of protein-repellent and antibacterial capabilities. Dent. Mater. 2015, 31, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Chen, C.; Melo, M.A.; Bai, Y.-X.; Cheng, L.; Xu, H.H. A novel protein-repellent dental composite containing 2-methacryloyloxyethyl phosphorylcholine. Int. J. Oral Sci. 2015, 7, 103–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dickson, M.A.; Hahn, W.C.; Ino, Y.; Ronfard, V.; Wu, J.Y.; Weinberg, R.A.; Louis, D.N.; Li, F.P.; Rheinwald, J.G. Human keratinocytes that express hTERT and also bypass a p16INK4a-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Mol. Cell. Biol. 2000, 20, 1436–1447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dowling, J.; Yu, Q.C.; Fuchs, E. β4 Integrin is required for hemidesmosome formation, cell adhesion and cell survival. J. Cell Biol. 1996, 134, 559–572. [Google Scholar] [CrossRef] [Green Version]
- Van den Bergh, F.; Eliason, S.L.; Giudice, G.J. Type XVII collagen (BP180) can function as a cell−matrix adhesion molecule via binding to laminin 332. Matrix Biol. 2011, 30, 100–108. [Google Scholar] [CrossRef] [Green Version]
- Porté-Durrieu, M.; Guillemot, F.; Pallu, S.; Labrugère, C.; Brouillaud, B.; Bareille, R.; Amédée, J.; Barthe, N.; Dard, M.; Baquey, C. Cyclo-(DfKRG) peptide grafting onto Ti–6Al–4V: Physical characterization and interest towards human osteoprogenitor cells adhesion. Biomaterials 2004, 25, 4837–4846. [Google Scholar] [CrossRef]
- Ma, Y.; Policastro, G.M.; Li, Q.; Zheng, J.; Jacquet, R.; Landis, W.J.; Becker, M.L. Concentration-Dependent hMSC Differentiation on Orthogonal Concentration Gradients of GRGDS and BMP-2 Peptides. Biomacromolecules 2016, 17, 1486–1495. [Google Scholar] [CrossRef]
- Sharma, A.; Jain, H.; Miller, A.C. Surface modification of a silicate glass during XPS experiments. Surf. Interface Anal. 2001, 31, 369–374. [Google Scholar] [CrossRef]
- Wasserman, S.R.; Tao, Y.T.; Whitesides, G.M. Structure and reactivity of alkylsiloxane monolayers formed by reaction of alkyltrichlorosilanes on silicon substrates. Langmuir 1989, 5, 1074–1087. [Google Scholar] [CrossRef]
- Lempens, E.H.M.; Helms, B.A.; Merkx, M.; Meijer, E.W. Efficient and chemoselective surface immobilization of proteins by using aniline-catalyzed oxime chemistry. ChemBioChem 2009, 10, 658–662. [Google Scholar] [CrossRef]
- Gori, A.; Cretich, M.; Vanna, R.; Sola, L.; Gagni, P.; Bruni, G.; Liprino, M.; Gramatica, F.; Burastero, S.; Chiari, M. Multiple epitope presentation and surface density control enabled by chemoselective immobilization lead to enhanced performance in IgE-binding fingerprinting on peptide microarrays. Anal. Chim. Acta 2017, 983, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.M.T.A.; Maia, S.R.; Gomes, P.A.C.; Martins, M.C.L. Dhvar5 antimicrobial peptide (AMP) chemoselective covalent immobilization results on higher antiadherence effect than simple physical adsorption. Biomaterials 2015, 52, 531–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Cao, G.; Zhao, T.; Wang, X.; Niu, X.; Fan, Y.; Li, X. Terminal group modification of carbon nanotubes determines covalently bound osteogenic peptide performance. ACS Biomater. Sci. Eng. 2020, acsbiomaterials.9b01501. [Google Scholar] [CrossRef]
- Argos, P. An investigation of oligopeptides linking domains in protein tertiary structures and possible candidates for general gene fusion. J. Mol. Biol. 1990, 211, 943–958. [Google Scholar] [CrossRef]
- Nie, B.; Long, T.; Li, H.; Wang, X.; Yue, B. A comparative analysis of antibacterial properties and inflammatory responses for the KR-12 peptide on titanium and PEGylated titanium surfaces. RSC Adv. 2017, 7, 34321–34330. [Google Scholar] [CrossRef] [Green Version]
- Wisdom, C.; VanOosten, S.K.; Boone, K.W.; Khvostenko, D.; Arnold, P.M.; Snead, M.L.; Tamerler, C. Controlling the biomimetic implant interface: Modulating antimicrobial activity by spacer design. J. Mol. Eng. Mater. 2016, 04, 1640005. [Google Scholar] [CrossRef]
- Wisdom, E.C.; Zhou, Y.; Chen, C.; Tamerler, C.; Snead, M.L. Mitigation of Peri-implantitis by Rational Design of Bifunctional Peptides with Antimicrobial Properties. ACS Biomater. Sci. Eng. 2019, 6. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, S.; Duan, S.; Xuliang, D.; Sun, Y.; Zhang, X.; Xu, X.; Guan, B.; Wang, C.; Hu, M.; et al. Modification of titanium substrates with chimeric peptides comprising antimicrobial and titanium-binding motifs connected by linkers to inhibit biofilm formation. ACS Appl. Mater. Interfaces 2016, 8, 5124–5136. [Google Scholar] [CrossRef]
- Craig, J.A.; Rexeisen, E.L.; Mardilovich, A.; Shroff, K.; Kokkoli, E. Effect of linker and spacer on the design of a fibronectin-mimetic peptide evaluated via cell studies and AFM adhesion forces. Langmuir 2008, 24, 10282–10292. [Google Scholar] [CrossRef]
- Lan, C. A Multi-Functional st-ELR Scaffold for Dentin Regeneration; University of Minnesota: Minneapolis, MN, USA, 2016. [Google Scholar]
- Acosta, S.; Quintanilla, L.; Alonso, M.; Aparicio, C.; Rodríguez-Cabello, J.C. Recombinant AMP/Polypeptide Self-Assembled Monolayers with Synergistic Antimicrobial Properties for Bacterial Strains of Medical Relevance. ACS Biomater. Sci. Eng. 2019, 5, 4708–4716. [Google Scholar] [CrossRef]
- Gabriel, M.; Nazmi, K.; Veerman, E.C.; Amerongen, A.V.N.; Zentner, A. Preparation of LL-37-grafted titanium surfaces with bactericidal activity. Bioconjug. Chem. 2006, 17, 548–550. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wei, S.; Wu, J.; Jasensky, J.; Xi, C.; Li, H.; Xu, Y.; Wang, Q.; Marsh, E.N.G.; Brooks, C.L.; et al. Effects of peptide immobilization sites on the structure and activity of surface tethered antimicrobial peptides. J. Phys. Chem. C 2015, 119, 7146–7155. [Google Scholar] [CrossRef]
- Schroeder, M.M.; Wang, Q.; Badieyan, S.; Chen, Z.; Marsh, E.N.G. Effect of surface crowding and surface hydrophilicity on the activity, stability and molecular orientation of a covalently tethered enzyme. Langmuir 2017, 33, 7152–7159. [Google Scholar] [CrossRef] [PubMed]
- Vida, Y.; Collado, D.; Najera, F.; Claros, S.; Becerra, J.; Andrades, J.A.; Perez-Inestrosa, E. Dendrimer surface orientation of the RGD peptide affects mesenchymal stem cell adhesion. RSC Adv. 2016, 6, 49839–49844. [Google Scholar] [CrossRef]
- Li, Y.; Ogorzalek, T.L.; Wei, S.; Zhang, X.; Yang, P.; Jasensky, J.; Brooks, C.L.; Marsh, E.N.G.; Chen, Z. Effect of immobilization site on the orientation and activity of surface-tethered enzymes. Phys. Chem. Chem. Phys. 2018, 20, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Wei, S.; Badieyan, S.; Schroeder, M.; Jasensky, J.; Brooks, C.L.; Marsh, E.N.G.; Chen, Z. Investigating the effect of two-point surface attachment on enzyme stability and activity. J. Am. Chem. Soc. 2018, 140, 16560–16569. [Google Scholar] [CrossRef]
- Koo, L.Y.; Irvine, D.J.; Mayes, A.M.; Lauffenburger, D.A.; Griffith, L.G. Co-regulation of cell adhesion by nanoscale RGD organization and mechanical stimulus. J. Cell Sci. 2002, 115, 1423–1433. [Google Scholar]
- Moore, N.M.; Lin, N.J.; Gallant, N.D.; Becker, M.L. Synergistic enhancement of human bone marrow stromal cell proliferation and osteogenic differentiation on BMP-2-derived and RGD peptide concentration gradients. Acta Biomater. 2011, 7, 2091–2100. [Google Scholar] [CrossRef]
- Martin, L.J.; Akhavan, B.; Bilek, M.M.M. Electric fields control the orientation of peptides irreversibly immobilized on radical-functionalized surfaces. Nat. Commun. 2018, 9, 357. [Google Scholar] [CrossRef] [Green Version]
- Youn, Y.H.; Lee, S.J.; Choi, G.R.; Lee, H.R.; Lee, D.; Heo, D.N.; Kim, B.S.; Bang, J.B.; Hwang, Y.S.; Correlo, V.M.; et al. Simple and facile preparation of recombinant human bone morphogenetic protein-2 immobilized titanium implant via initiated chemical vapor deposition technique to promote osteogenesis for bone tissue engineering application. Mater. Sci. Eng. C 2019, 100, 949–958. [Google Scholar] [CrossRef]
- Song, I.T.; Lee, M.; Lee, H.; Han, J.; Jang, J.H.; Lee, M.S.; Koh, G.Y.; Lee, H. PEGylation and HAylation via catechol: α-Amine-specific reaction at N-terminus of peptides and proteins. Acta Biomater. 2016, 43, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Fernandes-Cunha, G.M.; Putra, I.; Koh, W.-G.; Myung, D. Tethering Growth Factors to Collagen Surfaces Using Copper-Free Click Chemistry: Surface Characterization and in Vitro Biological Response. ACS Appl. Mater. Interfaces 2017, 9, 23389–23399. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Fernandes-Cunha, G.M.; Na, K.-S.; Hull, S.M.; Myung, D. Bio-Orthogonally Crosslinked, In Situ Forming Corneal Stromal Tissue Substitute. Adv. Healthc. Mater. 2018, 7, 1800560. [Google Scholar] [CrossRef] [PubMed]
- Ashley, G.W.; Henise, J.; Reid, R.; Santi, D.V. Hydrogel drug delivery system with predictable and tunable drug release and degradation rates. Proc. Natl. Acad. Sci. USA 2013, 110, 2318–2323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schweigel, H.; Wicht, M.; Schwendicke, F. Salivary and pellicle proteome: A datamining analysis. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fischer, N.G.; He, J.; Aparicio, C. Surface Immobilization Chemistry of a Laminin-Derived Peptide Affects Keratinocyte Activity. Coatings 2020, 10, 560. https://doi.org/10.3390/coatings10060560
Fischer NG, He J, Aparicio C. Surface Immobilization Chemistry of a Laminin-Derived Peptide Affects Keratinocyte Activity. Coatings. 2020; 10(6):560. https://doi.org/10.3390/coatings10060560
Chicago/Turabian StyleFischer, Nicholas G., Jiahe He, and Conrado Aparicio. 2020. "Surface Immobilization Chemistry of a Laminin-Derived Peptide Affects Keratinocyte Activity" Coatings 10, no. 6: 560. https://doi.org/10.3390/coatings10060560
APA StyleFischer, N. G., He, J., & Aparicio, C. (2020). Surface Immobilization Chemistry of a Laminin-Derived Peptide Affects Keratinocyte Activity. Coatings, 10(6), 560. https://doi.org/10.3390/coatings10060560






