Polyethylene Glycol (PEG) Modified Porous Ca5(PO4)2SiO4 Bioceramics: Structural, Morphologic and Bioactivity Analysis
Abstract
1. Introduction
2. Materials and Methods
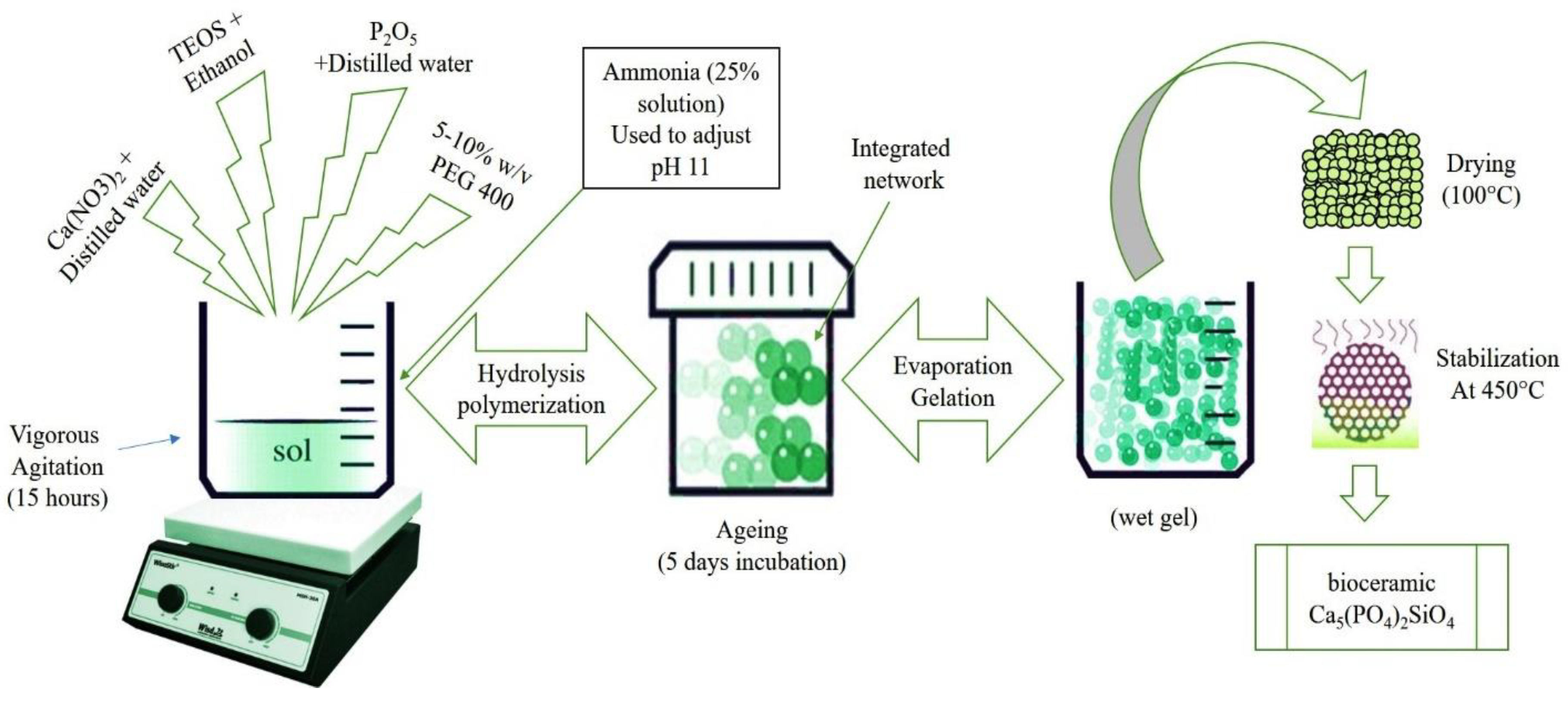
2.1. Synthesis of Porous Calcium Phosphate Silicate
2.2. Characterizations of Porous Calcium Phosphate Silicate
3. Results and Discussion
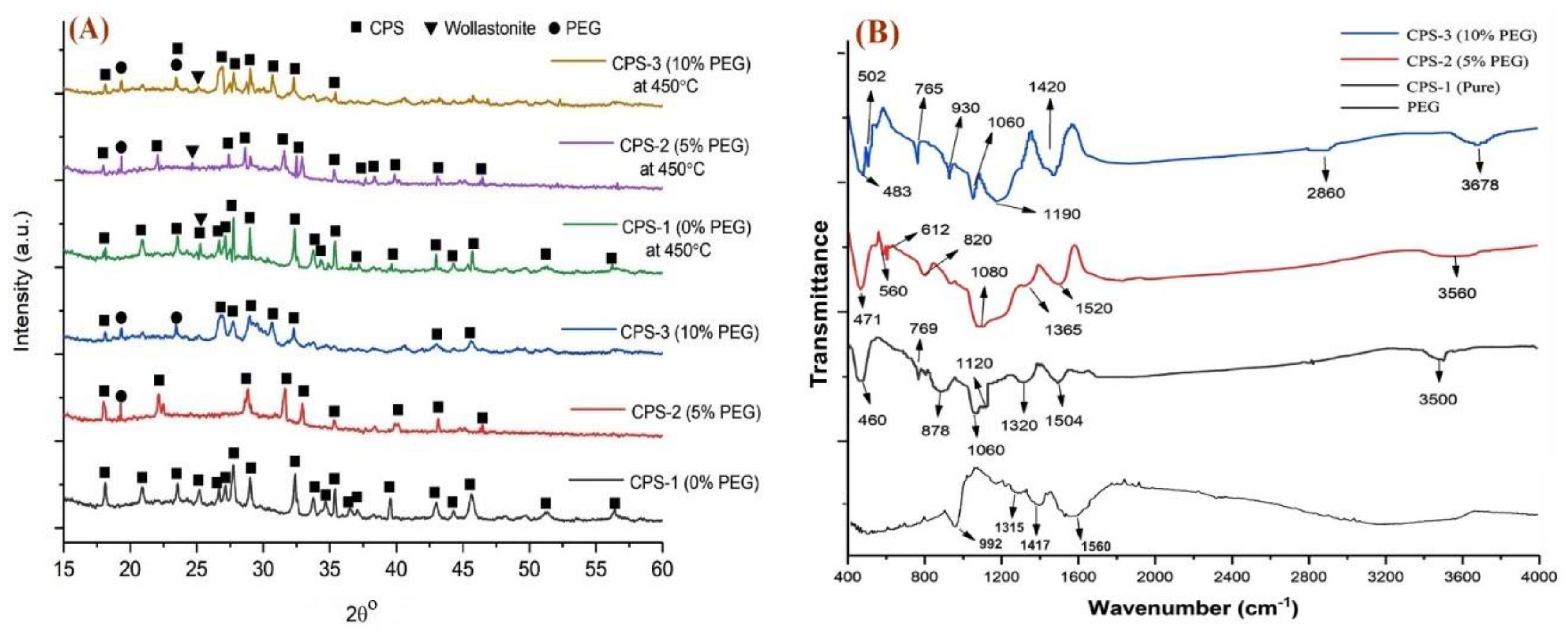
3.1. Physiochemical Analysis of Calcium Phosphate Silicate Materials
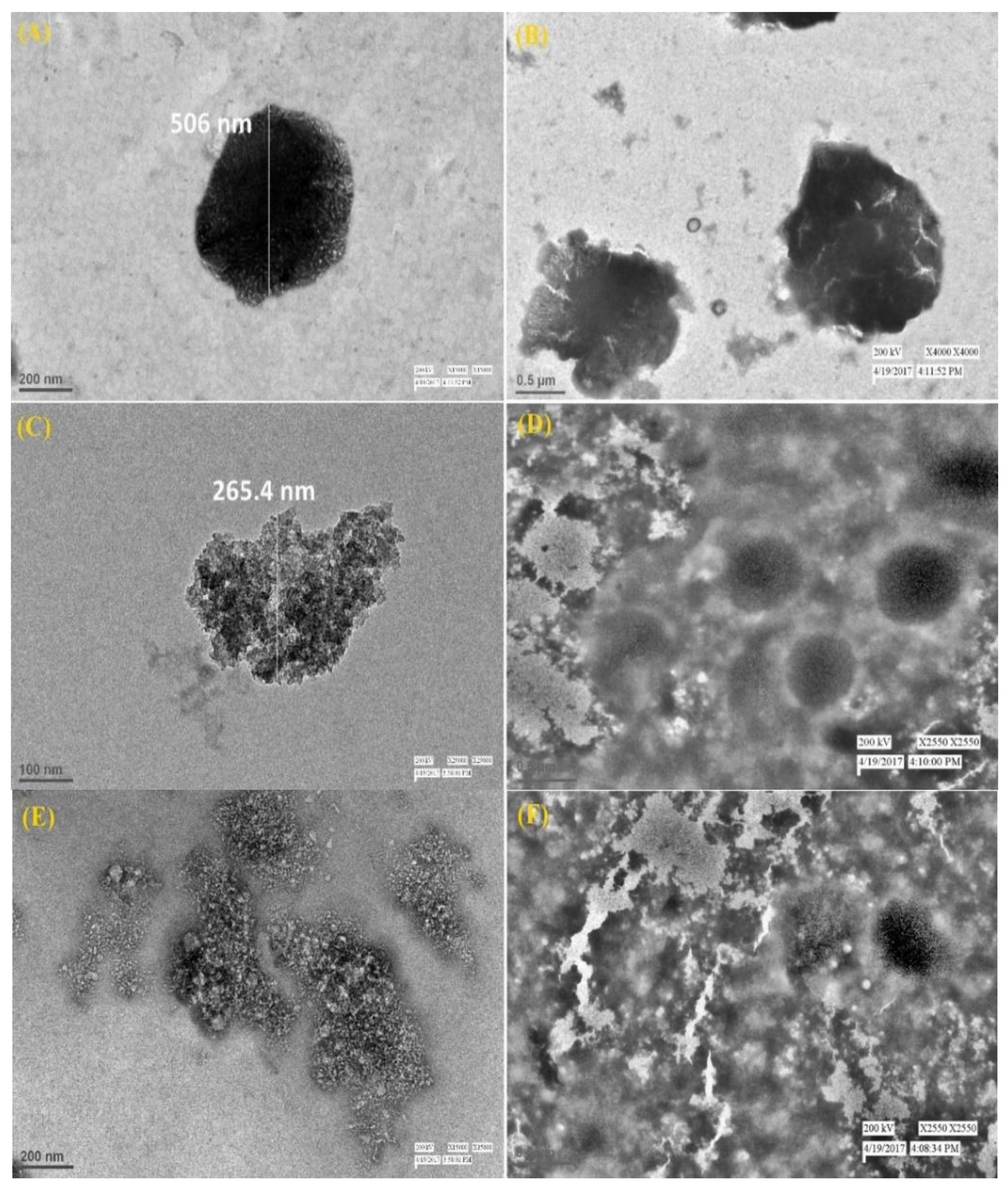
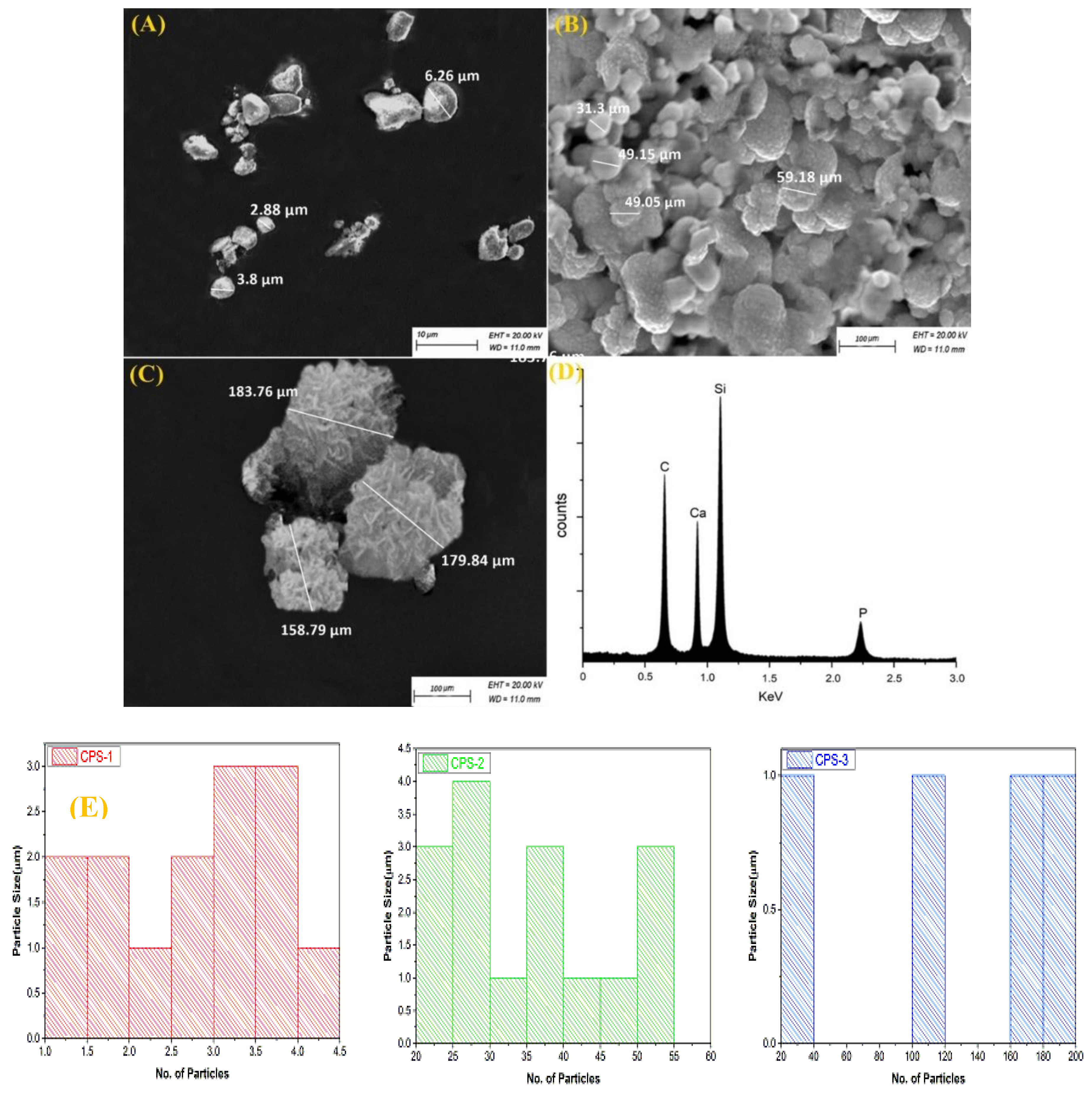
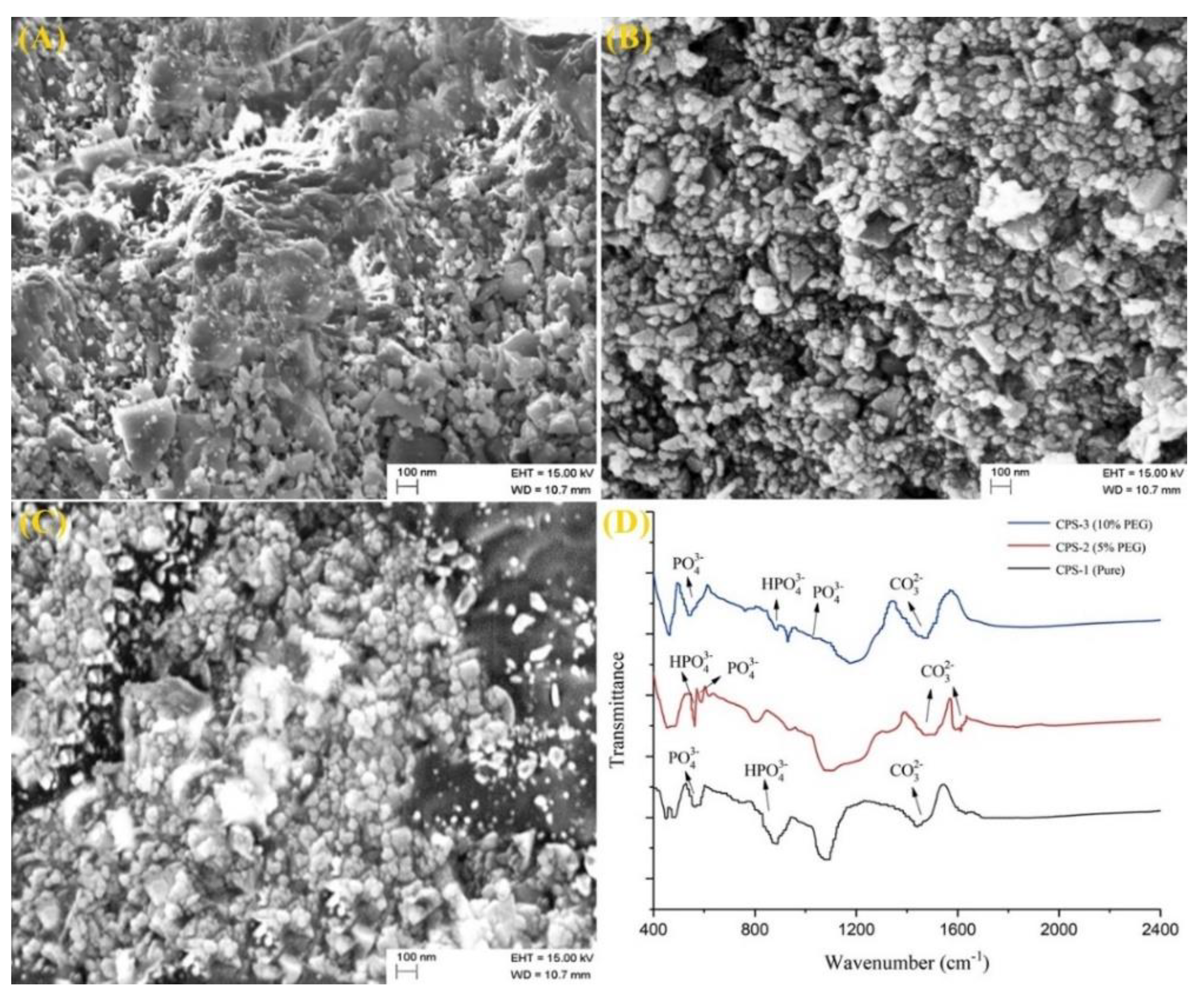
3.2. Morphologic Characterizations of Calcium Phosphate Silicate Materials
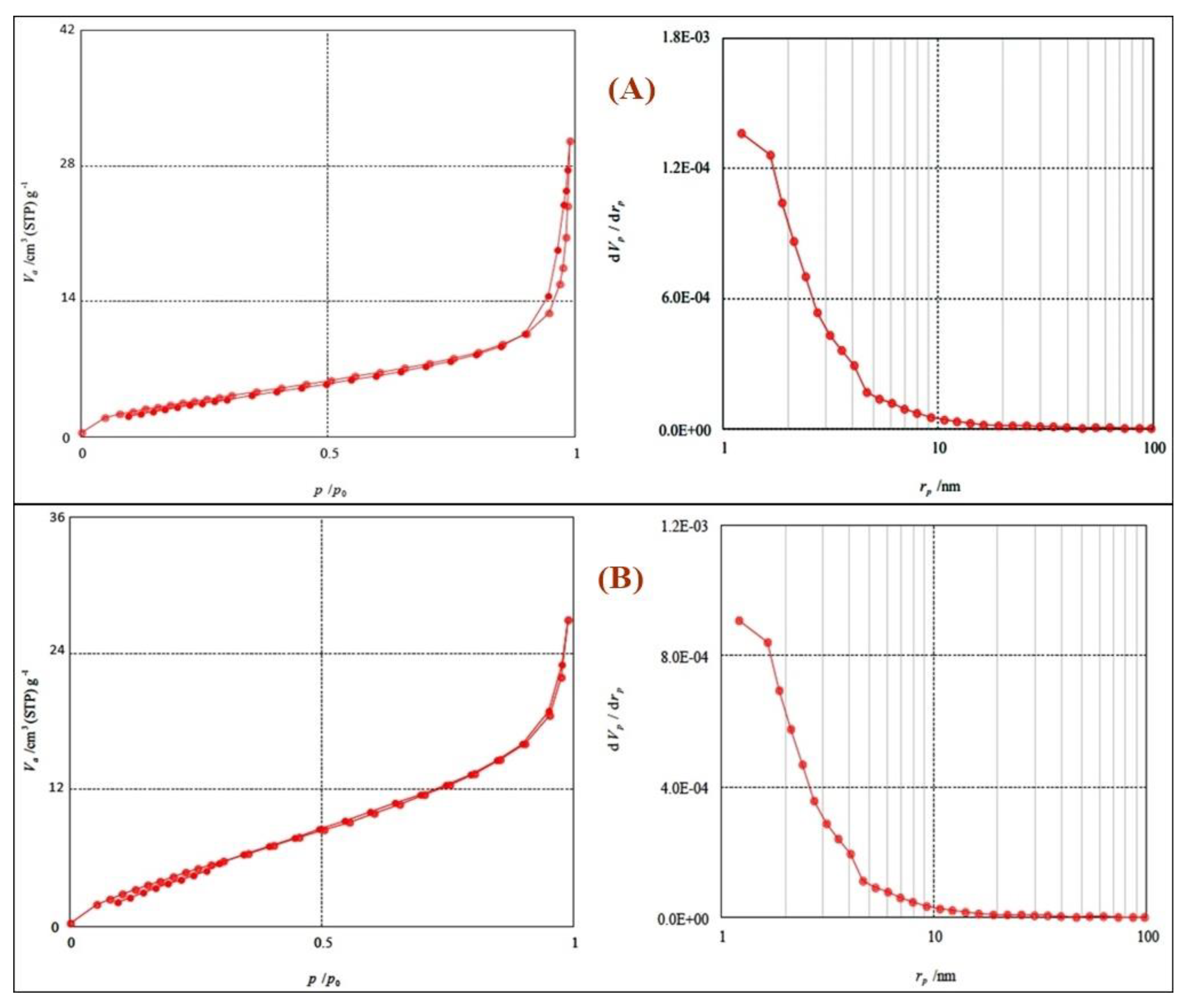
3.3. Porosity Measurements for Calcium Phosphate Silicate Materials
3.4. Bioactivity Analysis
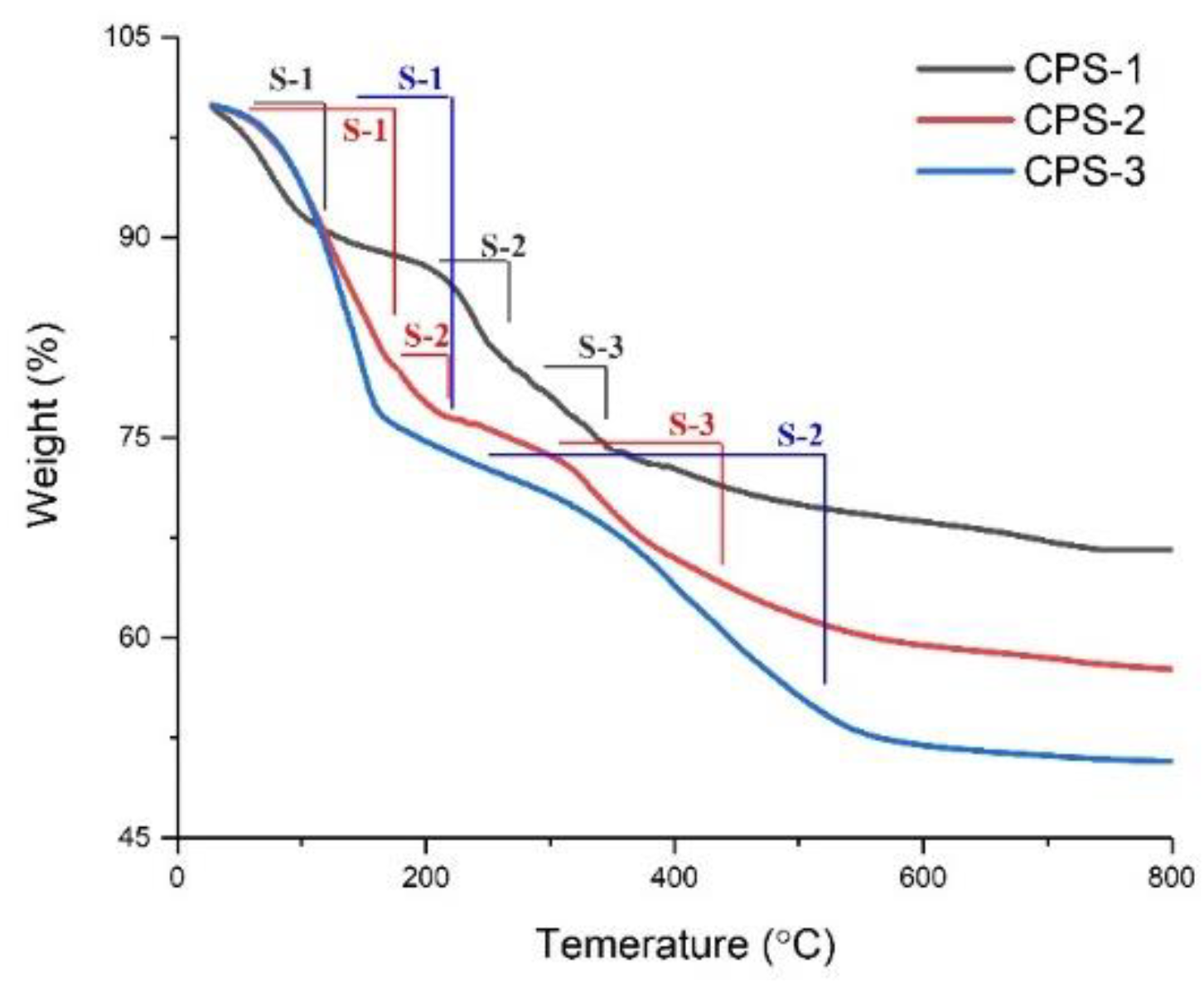
3.5. Thermal Gravimetric Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wu, C.; Chang, J. A review of bioactive silicate ceramics. Biomed. Mater. 2013, 8, 32001. [Google Scholar] [CrossRef]
- Duan, W.; Ning, C.; Tang, T. Cytocompatibility and osteogenic activity of a novel calcium phosphate silicate bioceramic: Silicocarnotite. J. Biomed. Mater. Res. Part A 2013, 101A, 1955–1961. [Google Scholar] [CrossRef] [PubMed]
- Bordbar-Khiabani, A.; Yarmand, B.; Mozafari, M. Emerging magnesium-based biomaterials for orthopedic implantation. Emerg. Mater. Res. 2019, 8, 305–319. [Google Scholar] [CrossRef]
- Lu, W.; Duan, W.; Guo, Y.; Ning, C. Mechanical properties and in vitro bioactivity of Ca5(PO4)2SiO4 bioceramic. J. Biomater. Appl. 2012, 26, 637–650. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Dehiya, B.S.; Sindhu, A. Comparative study of chitosan and chitosan-gelatin scaffold for tissue engineering. Int. Nano Lett. 2017, 7, 285–290. [Google Scholar] [CrossRef]
- Ji, D.D.; Xu, J.Y.; Gu, X.F.; Zhao, Q.M.; Gao, A.G. Preparation, characterization and biocompatibility of bioactive coating on titanium by plasma electrolytic oxidation. Sci. Adv. Mater. 2019, 11, 1411–1415. [Google Scholar] [CrossRef]
- Wang, P.; He, H.; Cai, R.; Tao, G.; Yang, M.; Zuo, H.; Umar, A.; Wang, Y. Cross-linking of dialdehyde carboxymethyl cellulose with silk sericin to reinforce sericin film for potential biomedical application. Carbohydr. Polym. 2019, 212, 403–411. [Google Scholar] [CrossRef]
- Li, J.; Wu, L.; Shen, R.; Hou, Y.; Chen, Z.; Cai, M.; Wei, Z.; Chen, X.; Gao, J. Preparation and biocompatibility of corrosion resistant micro-nano dual structures on the surface of TAx pure titanium dental implants. Sci. Adv. Mater. 2019, 11, 1656–1665. [Google Scholar] [CrossRef]
- Kumar, P.; Dehiya, B.S.; Sindhu, A. Bioceramics for hard tissue engineering applications: A review. Int. J. Appl. Eng. Res. 2018, 13, 2744–2752. [Google Scholar]
- Deng, F.; Wang, F.; Liu, Z.; Kou, H.; Cheng, G.; Ning, C. Enhanced mechanical property of Ca5(PO4)2SiO4 bioceramic by a biocompatible sintering aid of zinc oxide. Ceram. Int. 2018, 44, 18352–18362. [Google Scholar] [CrossRef]
- Chaudhary, S.; Umar, A.; Mehta, S. Surface functionalized selenium nanoparticles for biomedical applications. J. Biomed. Nanotechnol. 2014, 10, 3004–3042. [Google Scholar] [CrossRef] [PubMed]
- Shad, A.A.; Ahmad, S.; Ullah, R.; AbdEl-Salam, N.M.; Fouad, H.; Rehman, N.U.; Hussain, H.; Saeed, E. Phytochemical and biological activities of four wild medicinal plants. Sci. World J. 2014, 857363. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P. Nano-TiO2 doped chitosan scaffold for the bone tissue engineering applications. Int. J. Biomater. 2018, 6576157. [Google Scholar] [CrossRef]
- Elliott, J.E.; Macdonald, M.; Nie, J.; Bowman, C.N. Structure and swelling of poly(acrylic acid) hydrogels: Effect of pH, ionic strength, and dilution on the crosslinked polymer structure. Polymer (Guildf.) 2004, 45, 1503–1510. [Google Scholar] [CrossRef]
- Jones, D.S.; Andrews, G.P.; Gorman, S.P. Characterization of crosslinking effects on the physicochemical and drug diffusional properties of cationic hydrogels designed as bioactive urological biomaterials. J. Pharm. Pharmacol. 2005, 57, 1251–1259. [Google Scholar] [CrossRef]
- Sun, Q.; Yuan, Y.; Zhang, H.; Cao, X.; Sun, L. Thermal properties of polyethylene glycol/carbon microsphere composite as a novel phase change material. J. Therm. Anal. Calorim. 2017, 130, 1741–1749. [Google Scholar] [CrossRef]
- Paul, W.; Sharma, C.P. Polyethylene glycol modified calcium phosphate microspheres facilitate selective adsorption of immunogobulin G from human blood. Trends Biomater. Artif. Organs 2013, 27, 20–28. [Google Scholar]
- Nagar, P.; Goyal, P.; Gupta, A.; Sharma, A.K.; Kumar, P. Synthesis, characterization and evaluation of retinoic acid-polyethylene glycol nanoassembly as efficient drug delivery system. Nano Struct. Nano Objects 2018, 14, 110–117. [Google Scholar] [CrossRef]
- Mohaisen, M.; Yildirim, R.; Yilmaz, M.; Durak, M. Production of functional yogurt drink, apple and orange juice using nano-encapsulated l. brevis within sodium alginate-based biopolymers. Sci. Adv. Mater. 2019, 11, 1788–1797. [Google Scholar] [CrossRef]
- Dave, V.; Gupta, A.; Singh, P.; Gupta, C.; Sadhu, V.; Reddy, K.R. Synthesis and characterization of celecoxib loaded PEGylated liposome nanoparticles for biomedical applications. Nano Struct. Nano Objects 2019, 18, 100288. [Google Scholar]
- Zhang, X.; Zeng, D.; Li, N.; Wen, J.; Jiang, X.; Liu, C.; Li, Y. Functionalized mesoporous bioactive glass scaffolds for enhanced bone tissue regeneration. Sci. Rep. 2016, 6, 19361. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Dehiya, B.S.; Sindhu, A. Synthesis and characterization of nHA-PEG and nBG-PEG scaffolds for hard tissue engineering applications. Ceram. Int. 2019, 45, 8370–8379. [Google Scholar] [CrossRef]
- Ansari, S.G.; Fouad, H.; Shin, H.S.; Ansari, Z.A. Electrochemical enzyme-less urea sensor based on nano tim oxide synthesized by hydrothermal technique. Chem. Biol. Interact. 2015, 242, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Algarni, H.; AlShahrani, I.; Ibrahim, E.H.; Eid, R.A.; Kilany, M.; Ghramh, H.A.; Abdellahi, M.O.; Sayed, M.A.; Yousef, E.S. In-vitro bioactivity of optical glasses containing strontium oxide (SrO). J. Nanoelectron. Optoelectron. 2019, 14, 1105–1112. [Google Scholar] [CrossRef]
- Ansari, F.; Soofivand, F.; Salavati-Niasari, M. Eco-friendly synthesis of cobalt hexaferrite and improvement of photocatalytic activity by preparation of carbonic-based nanocomposites for waste-water treatment. Compos. Part B Eng. 2019, 165, 500–509. [Google Scholar] [CrossRef]
- Ansari, F.; Sobhani, A.; Salavati-Niasari, M. Simple sol-gel synthesis and characterization of new CoTiO3/CoFe2O4 nanocomposite by using liquid glucose, maltose and starch as fuel, capping and reducing agents. J. Colloid Interface Sci. 2018, 514, 723–732. [Google Scholar] [CrossRef]
- Owens, G.J.; Singh, R.K.; Foroutan, F.; Alqaysi, M.; Han, C.-M.; Mahapatra, C.; Kim, H.-W.; Knowles, J.C. Sol-gel based materials for biomedical applications. Prog. Mater. Sci. 2016, 77, 1–79. [Google Scholar] [CrossRef]
- Algarni, H.; Alshahrani, I.; Ibrahim, E.; Eid, R.; Kilany, M.; Ghramh, H.; Sayed, M.; Reben, M.; Yousef, E. Structural, thermal stability and in vivo bioactivity properties of nanobioglasses containing ZnO. Sci. Adv. Mater. 2019, 11, 925–935. [Google Scholar] [CrossRef]
- Rao, S.H.; Harini, B.; Shadamarshan, R.P.K.; Balagangadharan, K.; Selvamurugan, N. Natural and synthetic polymers/bioceramics/bioactive compounds-mediated cell signalling in bone tissue engineering. Int. J. Biol. Macromol. 2018, 110, 88–96. [Google Scholar] [CrossRef]
- Radev, L.; Hristov, V.; Michailova, I.; Samuneva, B. Sol-gel bioactive glass-ceramics Part I: Calcium phosphate silicate/ wollastonite glass-ceramics. Cent. Eur. J. Chem. 2009, 7, 317–321. [Google Scholar] [CrossRef]
- Lombardi, M.; Gremillard, L.; Chevalier, J.; Lefebvre, L.; Cacciotti, I.; Bianco, A.; Montanaro, L. A comparative study between melt-derived and sol-gel synthesized 45S5 bioactive glasses. Key Eng. Mater. 2013, 541, 15–30. [Google Scholar] [CrossRef]
- Matsuda, A.; Matsuno, Y.; Katayama, S.; Tsuno, T.; Tohge, N.; Minami, T. Physical and chemical properties of titania-silica films derived from poly(ethylene glycol)-containing gels. J. Am. Ceram. Soc. 1990, 73, 2217–2221. [Google Scholar] [CrossRef]
- Kumar, P.; Dehiya, B.S.; Sindhu, A. Ibuprofen-loaded CTS/nHA/nBG scaffolds for the applications of hard tissue engineering. Iran. Biomed. J. 2019, 23, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Ouis, M.; Abdelghany, A.; Elbatal, H. Corrosion mechanism and bioactivity of borate glasses analogue to Hench’s bioglass. Process. Appl. Ceram. 2012, 6, 141–149. [Google Scholar] [CrossRef]
- Davar, F.; Salavati-Niasari, M.; Mir, N.; Saberyan, K.; Monemzadeh, M.; Ahmadi, E. Thermal decomposition route for synthesis of Mn3O4 nanoparticles in presence of a novel precursor. Polyhedron 2010, 29, 1747–1753. [Google Scholar] [CrossRef]
- Kumar, P.; Saini, M.; Dehiya, B.S.; Umar, A.; Sindhu, A.; Mohammed, H.; Al-Hadeethi, Y.; Guo, Z. Fabrication and in-vitro biocompatibility of freeze-dried CTS-nHA and CTS-nBG scaffolds for bone regeneration applications. Int. J. Biol. Macromol. 2020, 149, 1–10. [Google Scholar] [CrossRef]
- Salavati-Niasari, M. Ship-in-a-bottle synthesis, characterization and catalytic oxidation of styrene by host (nanopores of zeolite-Y)/guest ([bis(2-hydroxyanil)acetylacetonato manganese(III)]) nanocomposite materials (HGNM). Microporous Mesoporous Mater. 2006, 95, 248–256. [Google Scholar] [CrossRef]
- Kumar, P.; Saini, M.; Kumar, V.; Singh, M.; Dehiya, B.S.; Umar, A.; Ajmal Khan, M.; Alhuwaymel, T.F. Removal of Cr (VI) from aqueous solution using VO2(B) nanoparticles. Chem. Phys. Lett. 2019, 136934. [Google Scholar] [CrossRef]
- Sampath, U.G.T.M.; Ching, Y.C.; Chuah, C.H.; Sabariah, J.J.; Lin, P.-C. Fabrication of porous materials from natural/synthetic biopolymers and their composites. Materials 2016, 9, 991. [Google Scholar] [CrossRef]
- Fathi, M.H.; Hanifi, A.; Mortazavi, V. Preparation and bioactivity evaluation of bone-like hydroxyapatite nanopowder. J. Mater. Process. Technol. 2008, 202, 536–542. [Google Scholar] [CrossRef]
- Jiang, Y.; Lawrence, M.; Ansell, M.P.; Hussain, A. Cell wall microstructure, pore size distribution and absolute density of hemp shiv. R. Soc. Open Sci. 2018, 5, 171945. [Google Scholar] [CrossRef] [PubMed]
- Stanciu, G.A.; Sandulescu, I.; Savu, B.; Stanciu, S.G.; Paraskevopoulos, K.M.; Chatzistavrou, X.; Kontonasaki, E.; Koidis, P. Investigation of the hydroxyapatite growth on bioactive glass surface. J. Biomed. Pharm. Eng. 2007, 1, 34–39. [Google Scholar]
- Shin, K.; Acri, T.; Geary, S.; Salem, A.K. Biomimetic mineralization of biomaterials using simulated body fluids for bone tissue engineering and regenerative medicine. Tissue Eng. Part A 2017, 23, 1169–1180. [Google Scholar] [CrossRef] [PubMed]
- Radev, L.; Hristov, V.; Michailova, I.; Fernandes, H.M.V.; Salvado, M.I.M. In vitro bioactivity of biphasic calcium phosphate silicate glass-ceramic in CaO-SiO2-P2O5 system. Process. Appl. Ceram. 2010, 4, 15–24. [Google Scholar] [CrossRef]
- Algarni, H.; Shahrani, I.; Ibrahim, E.; Eid, R.; Kilany, M.; Ghramh, H.; Ali, A.; Yousef, E. Silver Modified tricalcium phosphate for biomedical application: Structural investigation and study of antimicrobial with histopathological activity. Sci. Adv. Mater. 2019, 11, 1383–1391. [Google Scholar] [CrossRef]
- Zadpoor, A.A. Relationship between in vitro apatite-forming ability measured using simulated body fluid and in vivo bioactivity of biomaterials. Mater. Sci. Eng. C 2014, 35, 134–143. [Google Scholar] [CrossRef]
- Meejoo, S.; Maneeprakorn, W.; Winotai, P. Phase and thermal stability of nanocrystalline hydroxyapatite prepared via microwave heating. Thermochim. Acta 2006, 447, 115–120. [Google Scholar] [CrossRef]
- Destainville, A.; Champion, E.; Bernache-Assollant, D.; Laborde, E. Synthesis, characterization and thermal behavior of apatitic tricalcium phosphate. Mater. Chem. Phys. 2003, 80, 269–277. [Google Scholar] [CrossRef]
- Algarni, H.; Alshahrani, I.; Ibrahim, E.; Eid, R.; Kilany, M.; Ghramh, H.; Ali, A.; Yousef, E. Fabrication and biocompatible characterizations of bio-glasses containing oxyhalides ions. J. Nanoelectron. Optoelectron. 2019, 14, 328–334. [Google Scholar] [CrossRef]
- Clupper, D.C.; Hench, L.L. Bioactive response of Ag-doped tape cast Bioglass® 45S5 following heat treatment. J. Mater. Sci. Mater. Med. 2001, 12, 917–921. [Google Scholar] [CrossRef]
- Algarni, H.; AlShahrani, I.; Ibrahim, E.; Eid, R.; Kilany, M.; Ghramh, H.; Abdellahi, M.; Shaaban, E.; Reben, M.; Yousef, E. Synthesis, Mechanical, In Vitro and In vivo bioactivity and preliminary biocompatibility studies of bioglasses. Sci. Adv. Mater. 2019, 11, 1458–1466. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, P.; Saini, M.; Kumar, V.; Dehiya, B.S.; Sindhu, A.; Fouad, H.; Ahmad, N.; Mahmood, A.; Hashem, M. Polyethylene Glycol (PEG) Modified Porous Ca5(PO4)2SiO4 Bioceramics: Structural, Morphologic and Bioactivity Analysis. Coatings 2020, 10, 538. https://doi.org/10.3390/coatings10060538
Kumar P, Saini M, Kumar V, Dehiya BS, Sindhu A, Fouad H, Ahmad N, Mahmood A, Hashem M. Polyethylene Glycol (PEG) Modified Porous Ca5(PO4)2SiO4 Bioceramics: Structural, Morphologic and Bioactivity Analysis. Coatings. 2020; 10(6):538. https://doi.org/10.3390/coatings10060538
Chicago/Turabian StyleKumar, Pawan, Meenu Saini, Vinod Kumar, Brijnandan S. Dehiya, Anil Sindhu, H. Fouad, Naushad Ahmad, Amer Mahmood, and Mohamed Hashem. 2020. "Polyethylene Glycol (PEG) Modified Porous Ca5(PO4)2SiO4 Bioceramics: Structural, Morphologic and Bioactivity Analysis" Coatings 10, no. 6: 538. https://doi.org/10.3390/coatings10060538
APA StyleKumar, P., Saini, M., Kumar, V., Dehiya, B. S., Sindhu, A., Fouad, H., Ahmad, N., Mahmood, A., & Hashem, M. (2020). Polyethylene Glycol (PEG) Modified Porous Ca5(PO4)2SiO4 Bioceramics: Structural, Morphologic and Bioactivity Analysis. Coatings, 10(6), 538. https://doi.org/10.3390/coatings10060538





