Microstructure and Properties of Cr-Fe2B Composite Coatings Prepared by Pack-Preboronizing Combined with Electro Brush-Plating
Abstract
1. Introduction
2. Experimental
2.1. Materials and Methods
2.2. Characterization
3. Results and Discussions
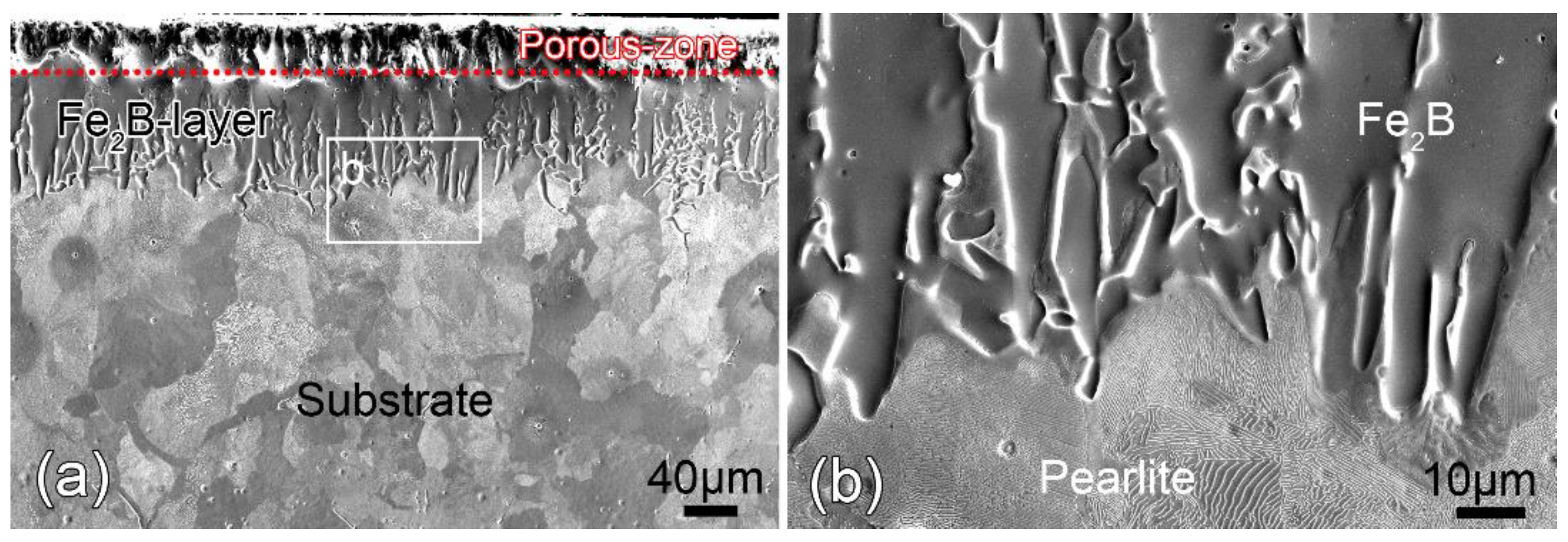
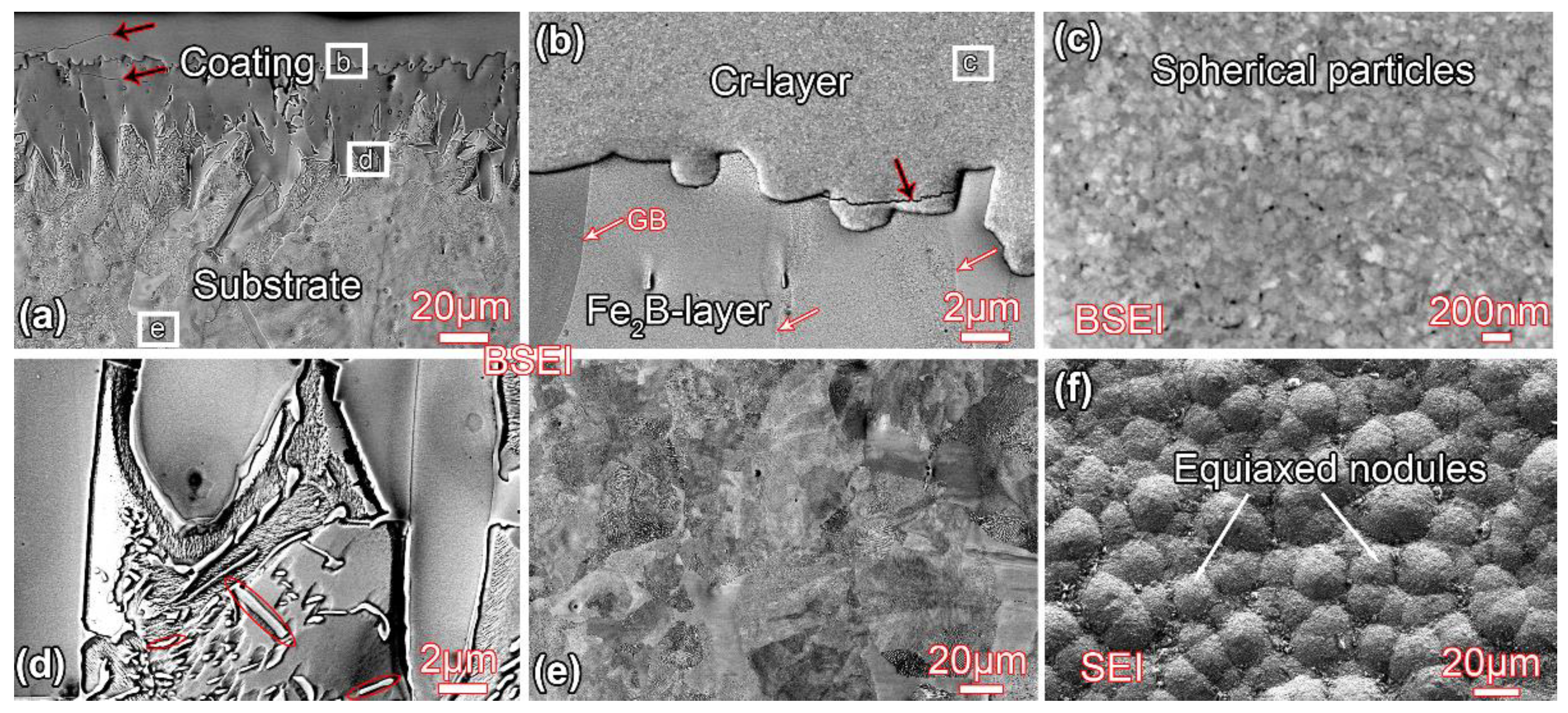
3.1. Microstructure before Annealing
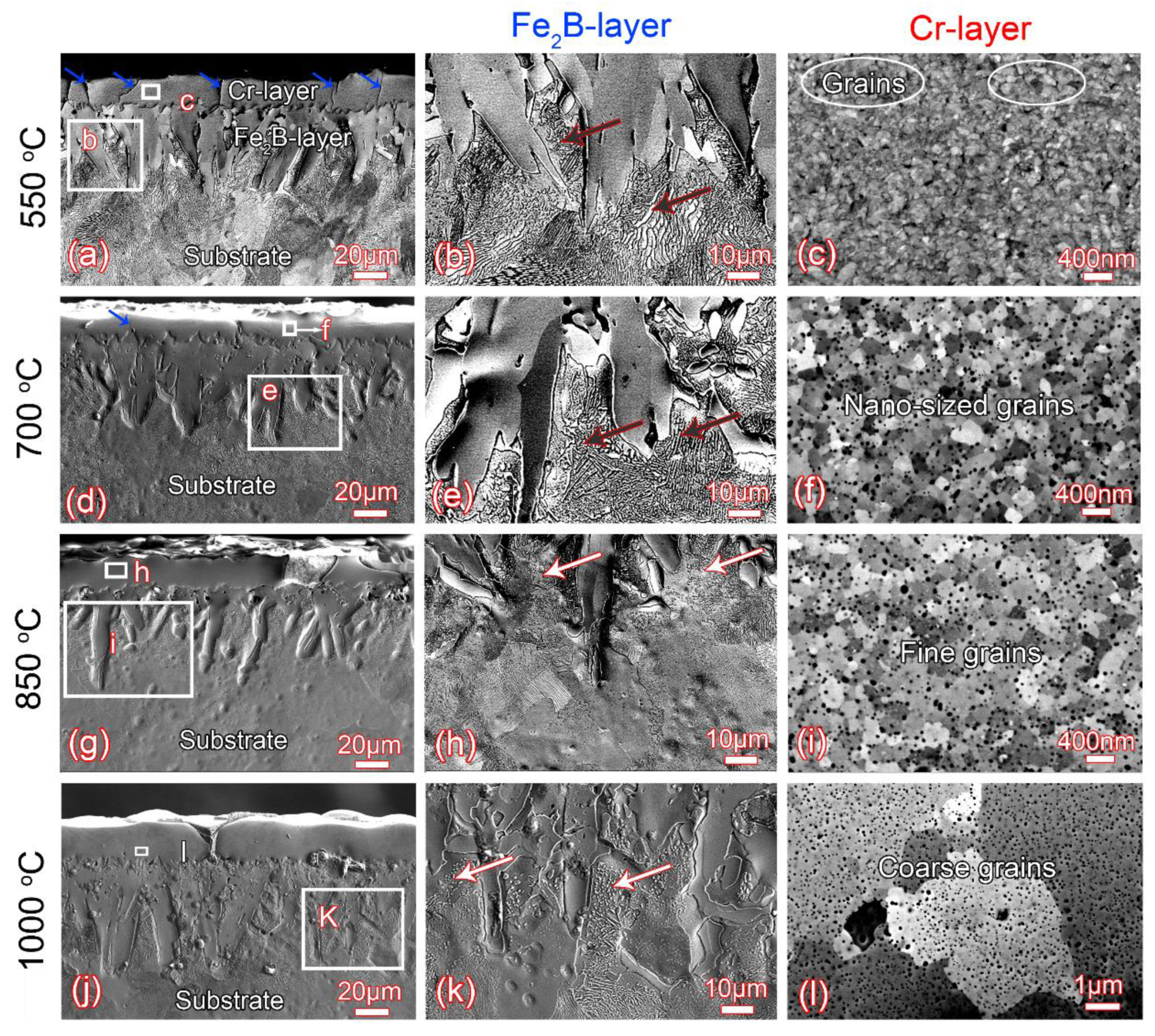
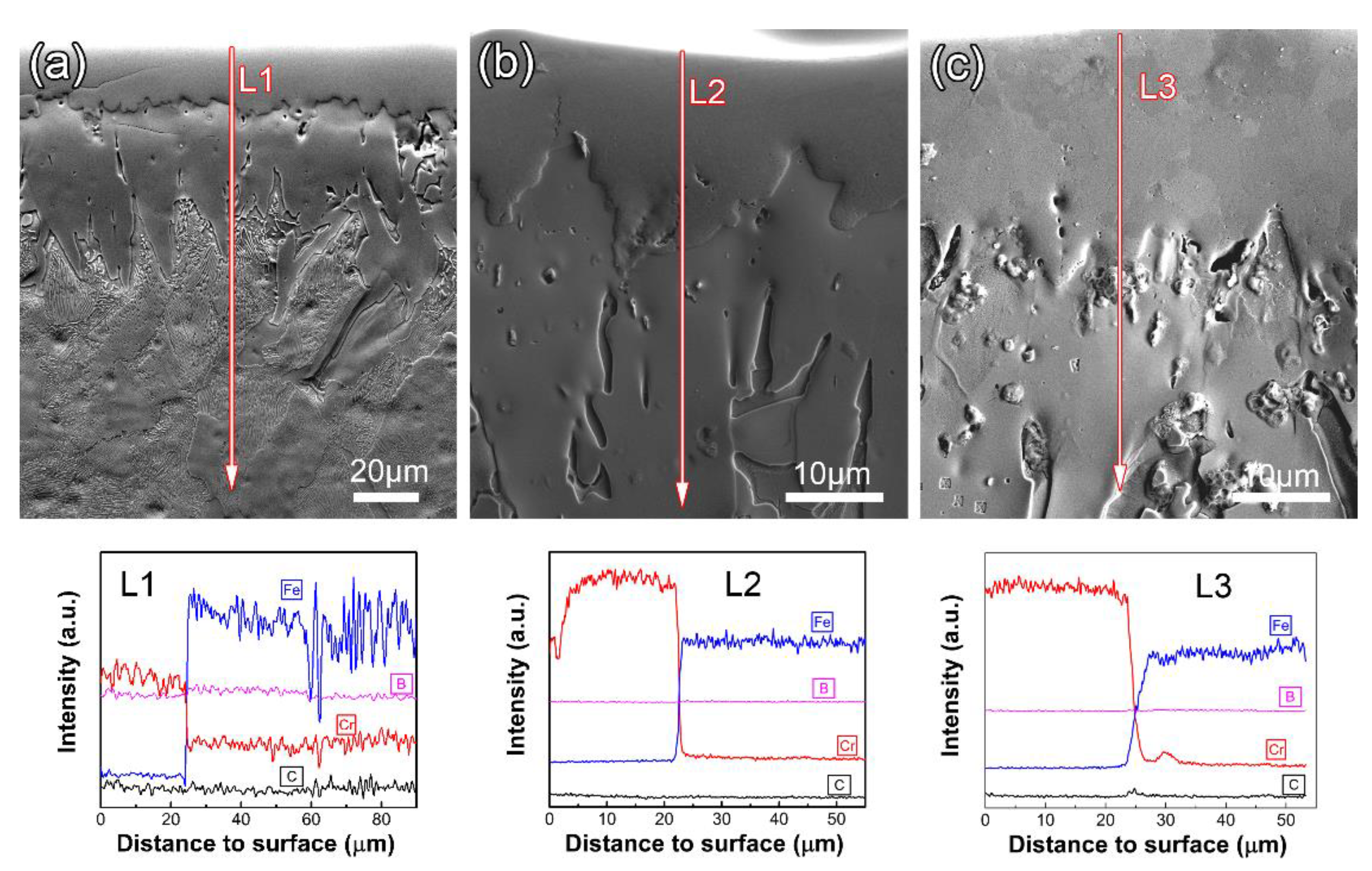
3.2. Microstructure Evolution during Annealing
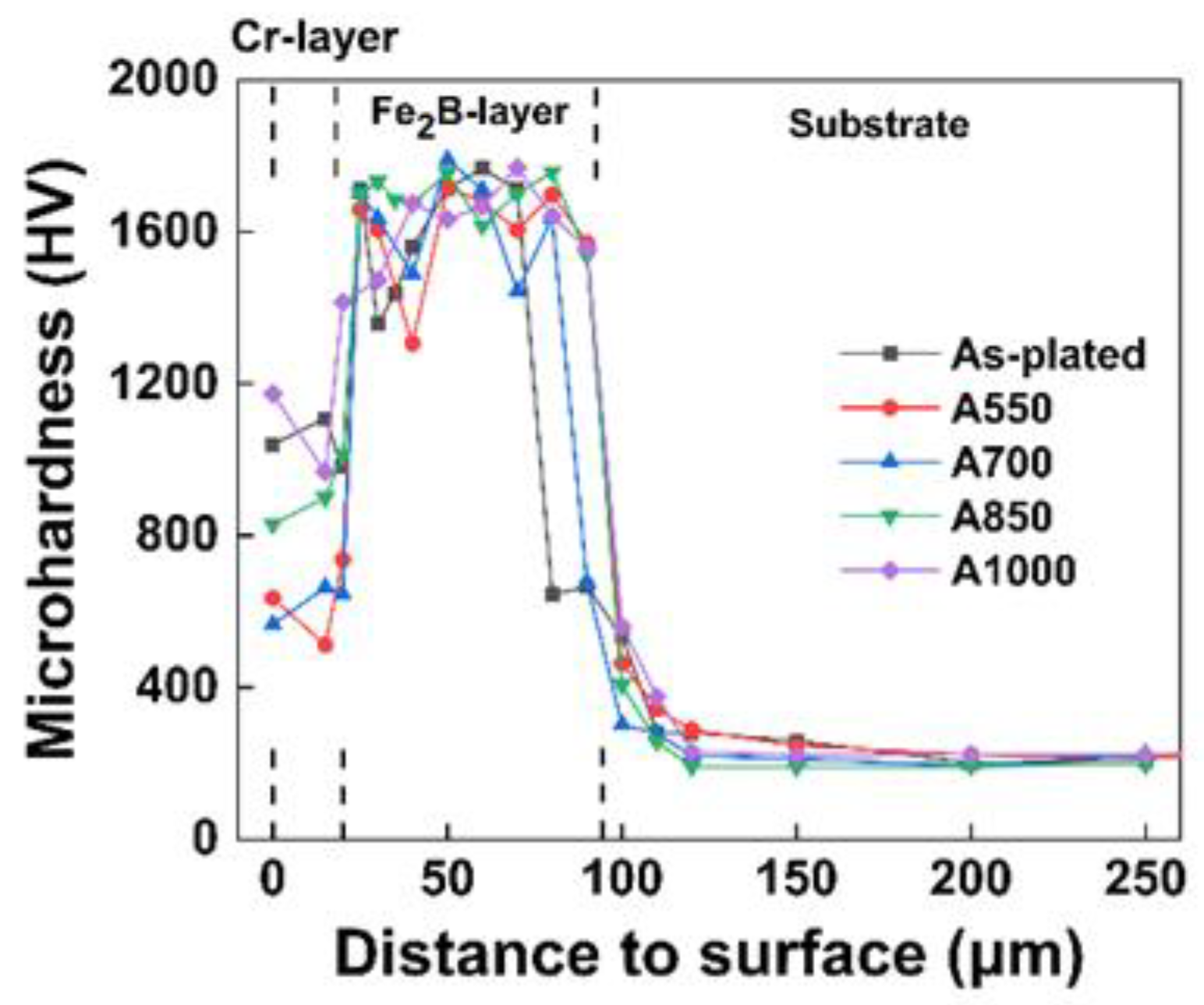
3.3. Microhardness Evolution after Annealing
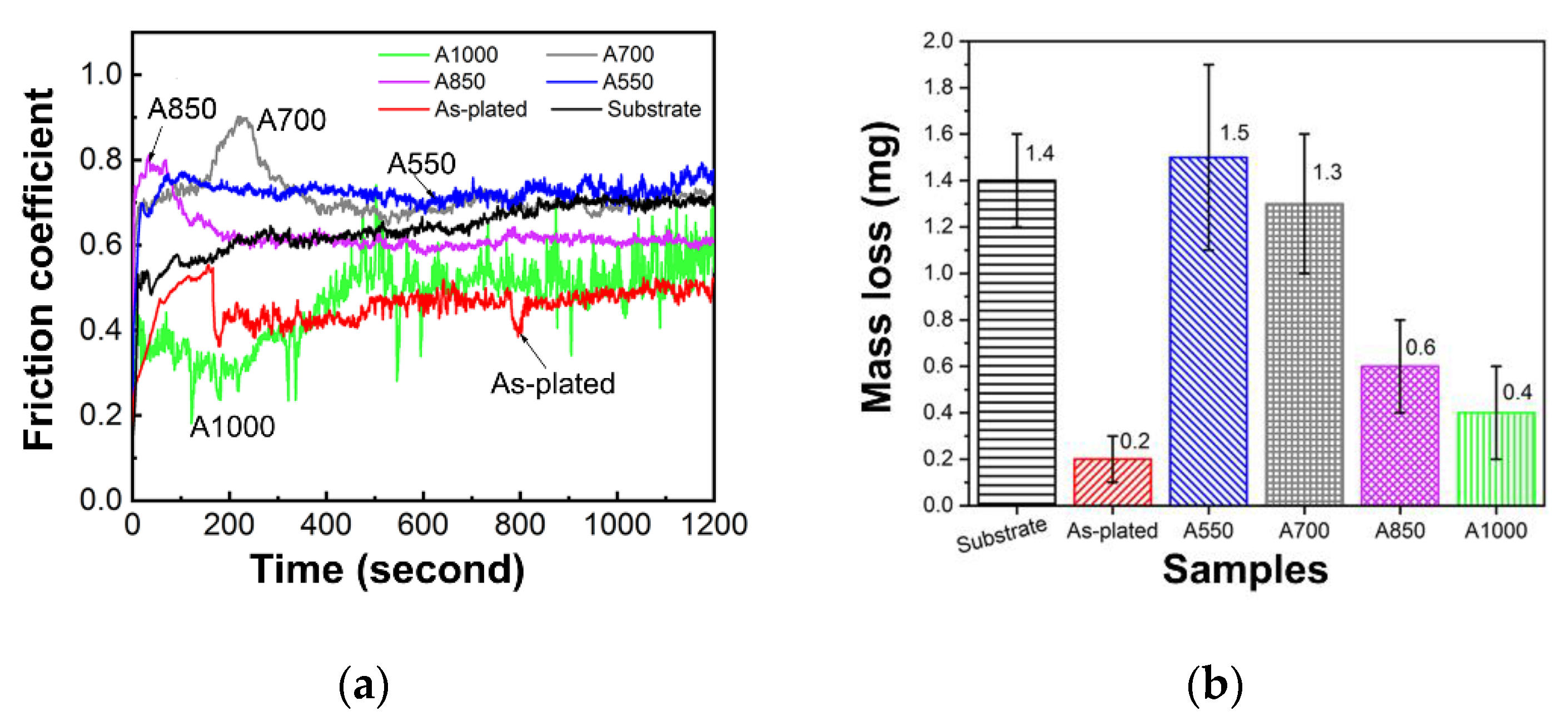
3.4. Friction and Wear Behavior
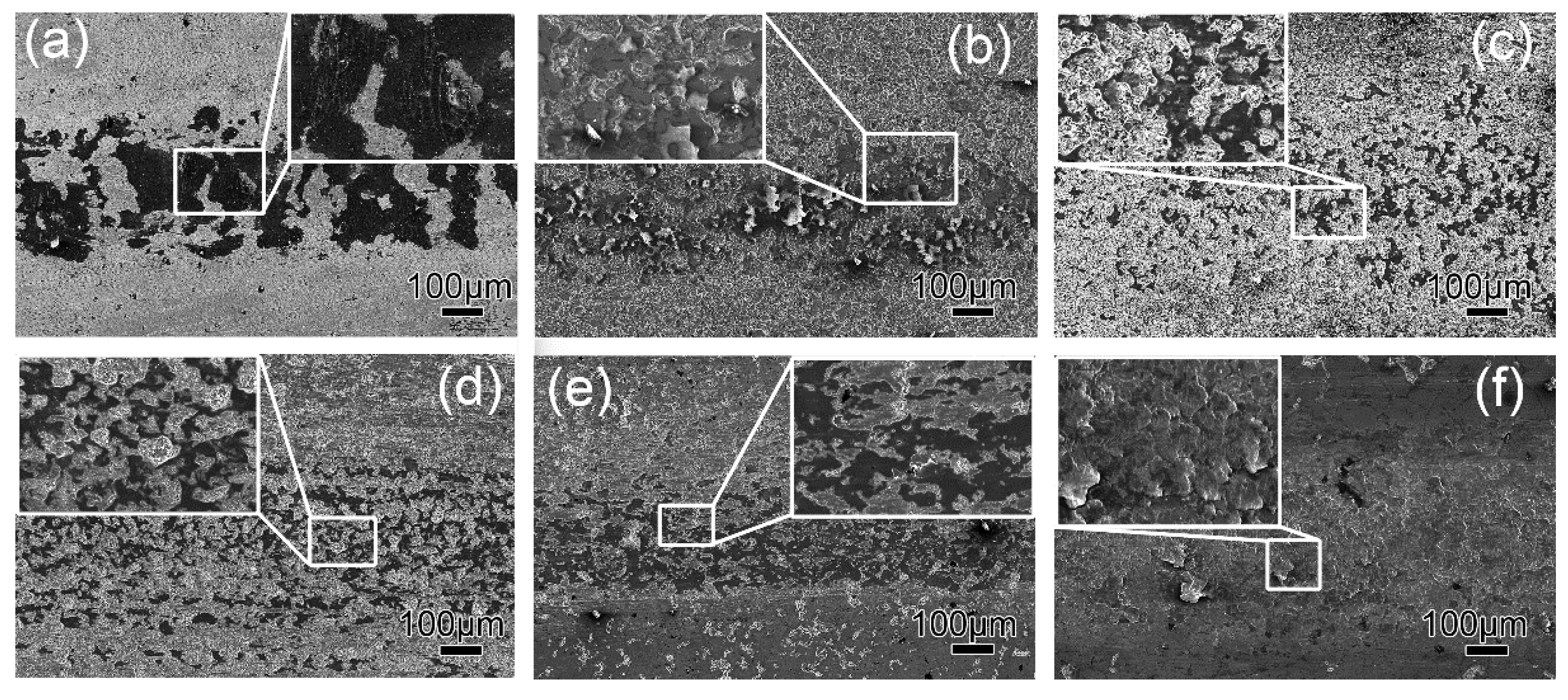
3.5. Corrosion Behavior
4. Conclusions
- The brush-plated Cr-layer has an amorphous structure which is very stable under low temperature (≤550 °C) annealing. As the annealing temperature increases to 700 °C, the amorphous structure crystallizes into nanosized grains, and the nanocrystallized grains quickly coarsen into coarse grains with further increases in the annealing temperature.
- The microcracks in the Cr-layer increase sharply after annealing at 550 °C and then decrease significantly with the further increase of the annealing temperature.
- During the annealing process, the rod-shaped Fe2B particles are gradually transformed into granular Fe2B ones. The pre-deposited Fe2B-layer prevents the formation of carbon-poor zones during annealing. The pre-deposited Fe2B-layer with high thermal stability prevents the reaction between C and Cr during annealing, so that even after high-temperature annealing, no carbon-poor zones are formed in the steel substrate.
- Annealing treatment can strengthen the metallurgical bonding strength and improve the bonding strength of the coating. It is considered that high-temperature (> 700 °C) annealing helps to eliminate coating defects and increase coating density and obtain better wear resistance and corrosion resistance.
Author Contributions
Funding
Conflicts of Interest
References
- Ouyang, T.; Huang, G.; Chen, J.; Gao, B.; Chen, N. Investigation of lubricating and dynamic performances for high-speed spur gear based on tribo-dynamic theory. Tribol. Int. 2019, 136, 421–431. [Google Scholar] [CrossRef]
- Thiagarajan, D.; Vacca, A.; Watkins, S. On the lubrication performance of external gear pumps for aerospace fuel delivery applications. Mech. Syst. Signal Pr. 2019, 129, 659–676. [Google Scholar] [CrossRef]
- Li, B.; Sun, D.; Hu, M.; Zhou, X.; Liu, J.; Wang, D. Coordinated control of gear shifting process with multiple clutches for power-shift transmission. Mech. Mach. Theory 2019, 140, 274–291. [Google Scholar] [CrossRef]
- Hu, J.; Ma, C.; Xu, H.; Guo, N.; Hou, T. Development of a composite technique for preconditioning of 41Cr4 steel used as gear material: Examination of its microstructural characteristics and properties. Sci. Technol. Nucl. Install. 2016, 2016, 1–6. [Google Scholar] [CrossRef]
- Xu, H.; Hu, J.; Ma, C.; Chai, L.; Guo, N. Influence of electron beam irradiation on surface roughness of commercially AISI 5140 steel. Mater. Trans. 2017, 58, 1519–1523. [Google Scholar] [CrossRef]
- He, Q.; Paiva, J.M.; Kohlscheen, J.; Beake, B.D.; Veldhuis, S.C. An integrative approach to coating/carbide substrate design of CVD and PVD coated cutting tools during the machining of austenitic stainless steel. Ceram. Int. 2020, 46, 5149–5158. [Google Scholar] [CrossRef]
- Chou, C.-C.; Lee, J.-W.; Chen, Y.-I. Tribological and mechanical properties of HFCVD diamond-coated WC-Co substrates with different Cr interlayers. Surf. Coat. Technol. 2008, 203, 704–708. [Google Scholar] [CrossRef]
- Zong, L.; Guo, N.; Li, R.; Yu, H. Effect of annealing treatment on microstructure and properties of Cr-coatings deposited on AISI 5140 steel by brush-plating. Coatings 2019, 9, 265. [Google Scholar] [CrossRef]
- Ettefagh, A.H.; Wen, H.; Chaichi, A.; Islam, M.I.; Lu, F.; Gartia, M.; Guo, S. Laser surface modifications of Fe-14Cr ferritic alloy for improved corrosion performance. Surf. Coat. Technol. 2020, 381, 125194. [Google Scholar] [CrossRef]
- Wu, A.; Liu, Q.; Qin, S. Influence of yttrium on laser surface alloying organization of 40Cr steel. J. Rare Earths 2011, 29, 1004–1008. [Google Scholar] [CrossRef]
- Rai, A.K.; Biswal, R.; Gupta, R.K.; Rai, S.K.; Singh, R.; Goutam, U.K.; Ranganathan, K.; Ganesh, P.; Kaul, R.; Bindra, K.S. Enhancement of oxidation resistance of modified P91 grade ferritic-martensitic steel by surface modification using laser shock peening. Appl. Surf. Sci. 2019, 495, 143611. [Google Scholar] [CrossRef]
- Grabowski, A.; Sozańska, M.; Adamiak, M.; Kępińska, M.; Florian, T. Laser surface texturing of Ti6Al4V alloy, stainless steel and aluminium silicon alloy. Appl. Surf. Sci. 2018, 461, 117–123. [Google Scholar] [CrossRef]
- Lopez-Ruiz, P.; Garcia-Blanco, M.B.; Vara, G.; Fernández-Pariente, I.; Guagliano, M.; Bagherifard, S. Obtaining tailored surface characteristics by combining shot peening and electropolishing on 316L stainless steel. Appl. Surf. Sci. 2019, 492, 1–7. [Google Scholar] [CrossRef]
- Yang, C.; Liu, Y.G.; Li, M.Q. Characteristics and formation mechanisms of defects in surface layer of TC17 subjected to high energy shot peening. Appl. Surf. Sci. 2020, 509, 144711. [Google Scholar] [CrossRef]
- Gao, S.; Dong, C.; Luo, H.; Xiao, K.; Pan, X.; Li, X. Scanning electrochemical microscopy study on the electrochemical behavior of CrN film formed on 304 stainless steel by magnetron sputtering. Electrochim. Acta 2013, 114, 233–241. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, Z.; Wang, Q.; Liu, Y.; Wang, Z.; Zhang, X. Box-behnken design to enhance the corrosion resistance of plasma sprayed Fe-based amorphous coating. Results Phys. 2019, 15, 102708. [Google Scholar] [CrossRef]
- Verhiest, K.; Mullens, S.; Graeve, I.D.; Wispelaere, N.D.; Claessens, S.; Bremaecker, A.D.; Verbeken, K. Advances in the development of corrosion and creep resistant nano-yttria dispersed ferritic/martensitic alloys using the rapid solidification processing technique. Ceram. Int. 2014, 40, 14319–14334. [Google Scholar] [CrossRef]
- Jin, G.; Lu, B.; Hou, D.; Cui, X.; Song, J.; Liu, E. Influence of rare earths addition on residual stress of Fe-based coating prepared by brush plating technology. J. Rare Earths 2016, 34, 336–340. [Google Scholar] [CrossRef]
- Zhang, D.; Cui, X.; Jin, G.; Cai, Z.; Lu, B. Microstructure and mechanical properties of electro-brush plated Fe/MWCNTs composite coatings. Surf. Coat. Technol. 2018, 348, 97–103. [Google Scholar] [CrossRef]
- Wu, B.; Xu, B.-S.; Zhang, B.; Jing, X.-D.; Liu, C.-L. Automatic brush plating: An update on brush plating. Mater. Lett. 2006, 60, 1673–1677. [Google Scholar] [CrossRef]
- Zhang, D.; Cui, X.; Jin, G.; Cai, Z.; Dong, M. Thermal stability of Ni-B/La2O3 coatings by electro-brush plating technique. Surf. Coat. Technol. 2018, 349, 1042–1047. [Google Scholar] [CrossRef]
- Han, Z.; Zuo, Y.; Ju, P.; Tang, Y.; Zhao, X.; Tang, J. The preparation and characteristics of a rare earth/nano-TiO2 composite coating on aluminum alloy by brush plating. Surf. Coat. Technol. 2012, 206, 3264–3269. [Google Scholar] [CrossRef]
- Jin, G.; Zhang, D.; Liu, M.; Cui, X.; Liu, E.; Song, Q.; Yuan, C.; Wen, X.; Fang, Y. Microstructure, deposition mechanism and tribological performance of graphene oxide reinforced Fe composite coatings by electro-brush plating technique. J. Alloys Compd. 2019, 801, 40–48. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Ji, H. Fabrication of lotus-leaf-like superhydrophobic surfaces via Ni-based nano-composite electro-brush plating. Appl. Surf. Sci. 2014, 288, 341–348. [Google Scholar] [CrossRef]
- Chen, T.; Ge, S.; Liu, H.; Sun, Q.; Zhu, W.; Yan, W.; Qi, J. Fabrication of low adhesive superhydrophobic surfaces using nano Cu/Al2O3 Ni-Cr composited electro-brush plating. Appl. Surf. Sci. 2015, 356, 81–90. [Google Scholar] [CrossRef]
- Tang, J.-L.; Zuo, Y.; Tang, Y.-M.; Xiong, J.-P. Composition and corrosion resistance of palladium film on 316L stainless steel by brush plating. Trans. Nonferrous Met. Soc. China 2012, 22, 97–103. [Google Scholar] [CrossRef]
- Zhong, Z.; Clouser, S.J. Nickel-tungsten alloy brush plating for engineering applications. Surf. Coat. Technol. 2014, 240, 380–386. [Google Scholar] [CrossRef]
- Hu, J.; Zeng, J.; Yang, Y.; Yang, X.; Li, H.; Guo, N. Microstructures and wear resistance of boron-chromium duplex-alloyed coatings prepared by a two-step pack cementation process. Coatings 2019, 9, 529. [Google Scholar] [CrossRef]
- Hu, J.J.; Chai, L.J.; Xu, H.B.; Ma, C.P.; Deng, S.B. Microstructural modification of brush-plated nanocrystalline Cr by high current pulsed electron beam irradiation. J. Nano Res. 2016, 41, 87–95. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Gao, R.; Sun, L. Study of deposition patterns of plating layers in SiC/Cu composites by electro-brush plating. Appl. Surf. Sci. 2011, 257, 10294–10299. [Google Scholar] [CrossRef]
- Türkmen, İ.; Yalamaç, E. Growth of the Fe2B layer on SAE 1020 steel employed a boron source of H3BO3 during the powder-pack boriding method. J. Alloys Compd. 2018, 744, 658–666. [Google Scholar] [CrossRef]
- Kulka, M.; Makuch, N.; Piasecki, A. Nanomechanical characterization and fracture toughness of FeB and Fe2B iron borides produced by gas boriding of Armco iron. Surf. Coat. Technol. 2017, 325, 515–532. [Google Scholar] [CrossRef]
- Kartal, G.; Timur, S.; Sista, V.; Eryilmaz, O.L.; Erdemir, A. The growth of single Fe2B phase on low carbon steel via phase homogenization in electrochemical boriding (PHEB). Surf. Coat. Technol. 2011, 206, 2005–2011. [Google Scholar] [CrossRef]
- Keddam, M.; Kulka, M.; Makuch, N.; Pertek, A.; Małdziński, L.A. Kinetic model for estimating the boron activation energies in the FeB and Fe2B layers during the gas-boriding of Armco iron: Effect of boride incubation times. Appl. Surf. Sci. 2014, 298, 155–163. [Google Scholar] [CrossRef]
- Yu, L.G.; Chen, X.J.; Khor, K.A.; Sundararajan, G. FeB/Fe2B phase transformation during SPS pack-boriding: Boride layer growth kinetics. Acta Mater. 2005, 53, 2361–2368. [Google Scholar] [CrossRef]
- Usta, M.; Glicksman, M.E.; Wright, R.N. The effect of heat treatment on Mg2Si coarsening in aluminum 6105 alloy. Metall. Mater. Trans. A 2004, 35, 435–438. [Google Scholar] [CrossRef]
- Hu, J.; Wang, J.; Jiang, J.; Yang, X.; Xu, H.; Li, H.; Guo, N. Effect of heating treatment on the microstructure and properties of Cr-Mo duplex-alloyed coating prepared by double glow plasma surface alloying. Coatings 2019, 9, 336. [Google Scholar] [CrossRef]
- Martin, C.; Palombarini, G.; Poli, G.; Prandstraller, D. Sliding and abrasive wear behaviour of boride coatings. Wear 2004, 256, 608–613. [Google Scholar] [CrossRef]
- Campos-Silva, I.; Flores-Jiménez, M.; Rodríguez-Castro, G.; Hernández-Sánchez, E.; Martínez-Trinidad, J.; Tadeo-Rosas, R. Improved fracture toughness of boride coating developed with a diffusion annealing process. Surf. Coat. Technol. 2013, 237, 429–439. [Google Scholar] [CrossRef]
- Wei, S.; Zhu, J.; Xu, L. Research on wear resistance of high speed steel with high vanadium content. Mater. Sci. Eng. A-Struct. 2005, 404, 138–145. [Google Scholar] [CrossRef]
- Souza, C.A.C.; Ribeiro, D.V.; Kiminami, C.S. Corrosion resistance of Fe-Cr-based amorphous alloys: An overview. J. Non-Cryst. Solids 2016, 442, 56–66. [Google Scholar] [CrossRef]
- Jin, L.; Cui, W.F.; Song, X.; Liu, G.; Zhou, L. Effects of surface nanocrystallization on corrosion resistance of β-type titanium alloy. Trans. Nonferrous Met. Soc. China 2014, 24, 2529–2535. [Google Scholar] [CrossRef]


| Samples | Ecorr(mV) | Icorr (μA/cm2) | CR (mm/y) |
|---|---|---|---|
| A1000 | −593 | 19.3 | 0.225 |
| A850 | −753 | 22.4 | 0.261 |
| A700 | −1040 | 27.6 | 0.322 |
| A550 | −898 | 3.10 | 0.036 |
| As-plated | −820 | 2.31 | 0.027 |
| Uncoated | −809 | 53.2 | 0.621 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Pei, Y.; Liu, Y.; Yang, X.; Li, H.; Xu, H.; Guo, N. Microstructure and Properties of Cr-Fe2B Composite Coatings Prepared by Pack-Preboronizing Combined with Electro Brush-Plating. Coatings 2020, 10, 519. https://doi.org/10.3390/coatings10060519
Hu J, Pei Y, Liu Y, Yang X, Li H, Xu H, Guo N. Microstructure and Properties of Cr-Fe2B Composite Coatings Prepared by Pack-Preboronizing Combined with Electro Brush-Plating. Coatings. 2020; 10(6):519. https://doi.org/10.3390/coatings10060519
Chicago/Turabian StyleHu, Jianjun, Yaoxin Pei, Yu Liu, Xian Yang, Hui Li, Hongbin Xu, and Ning Guo. 2020. "Microstructure and Properties of Cr-Fe2B Composite Coatings Prepared by Pack-Preboronizing Combined with Electro Brush-Plating" Coatings 10, no. 6: 519. https://doi.org/10.3390/coatings10060519
APA StyleHu, J., Pei, Y., Liu, Y., Yang, X., Li, H., Xu, H., & Guo, N. (2020). Microstructure and Properties of Cr-Fe2B Composite Coatings Prepared by Pack-Preboronizing Combined with Electro Brush-Plating. Coatings, 10(6), 519. https://doi.org/10.3390/coatings10060519






