Composite of PLA Nanofiber and Hexadecyl Trimethyl-Ammonium Chloride-Modified Montmorillonite Clay: Fabrication and Morphology
Abstract
1. Introduction
2. Materials and Method
2.1. Materials
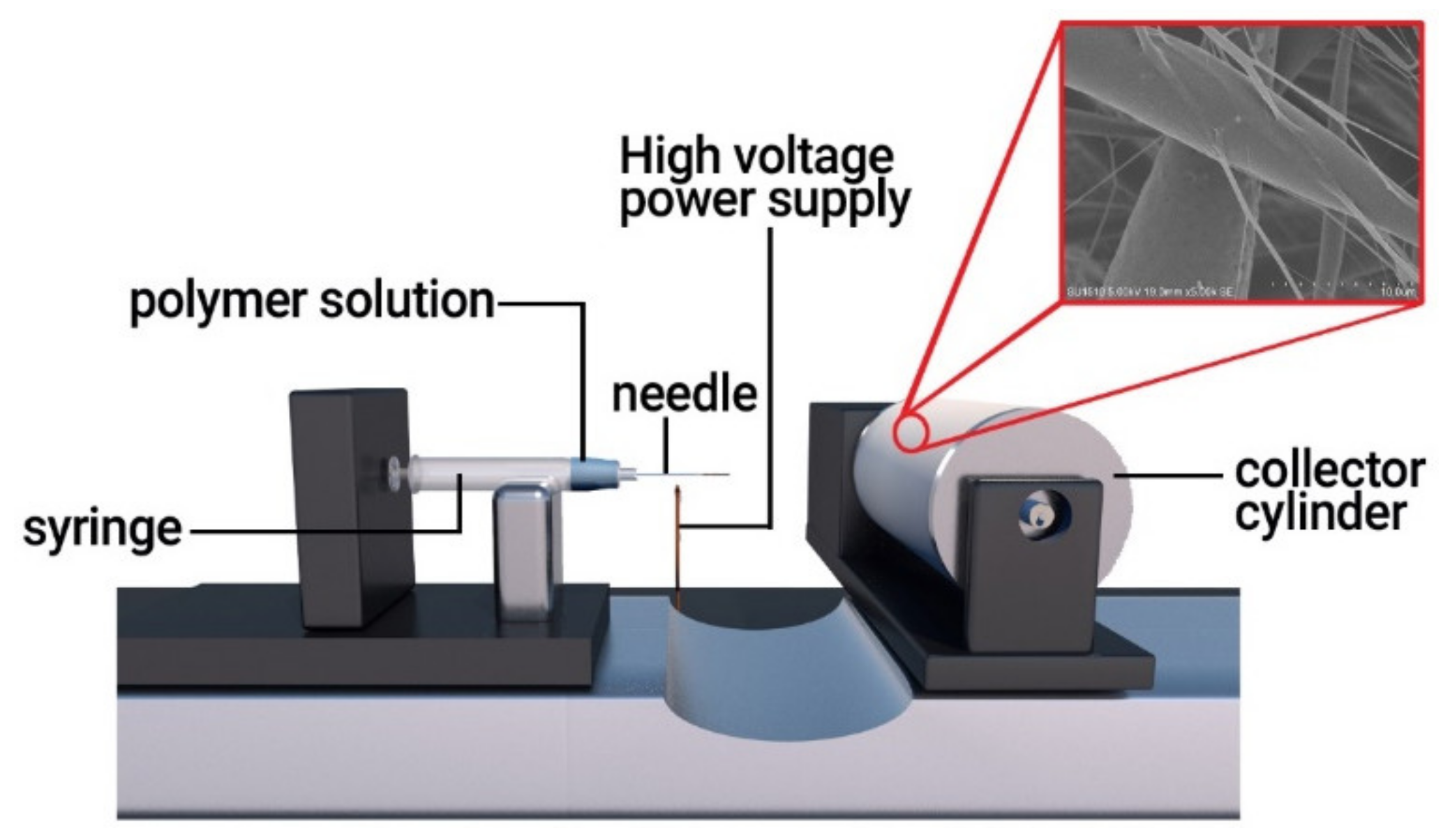
2.2. Preparation of PLA Nanofiber
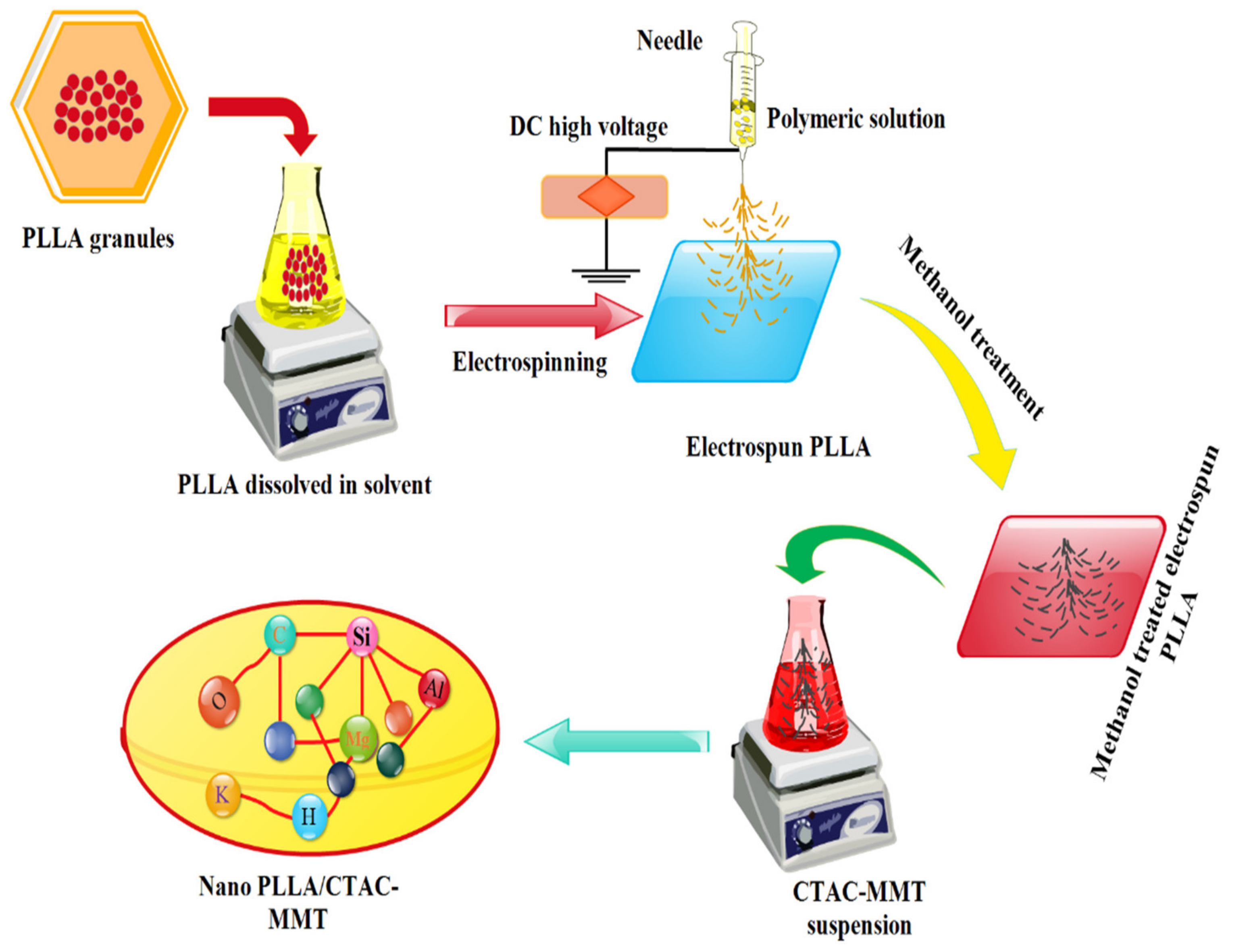
2.3. Synthesis of PLA/CTAC-MMT Composites
2.4. Characterization
2.5. Mechanical Performance
2.6. Contact Angle
3. Results and Discussion
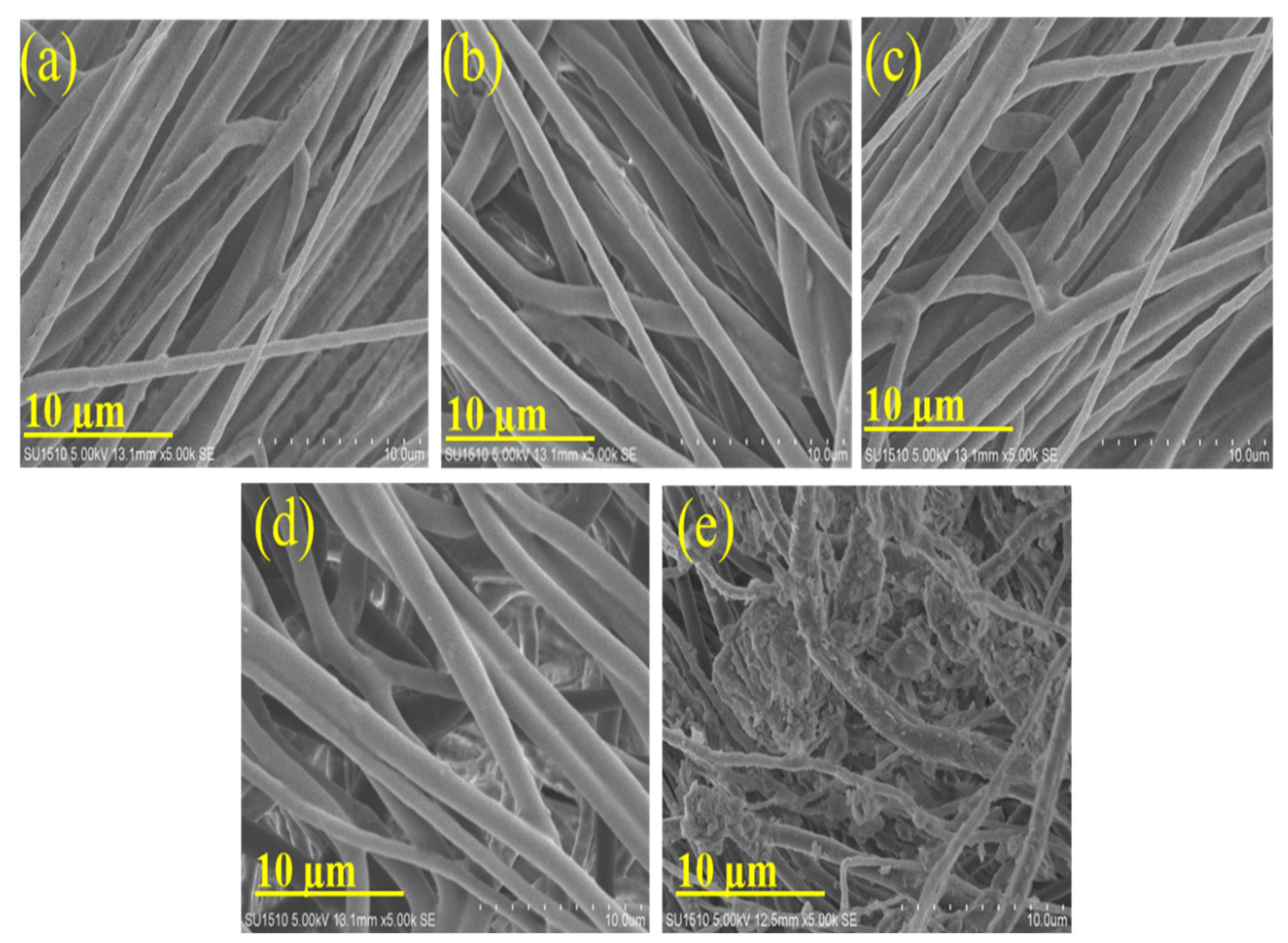
3.1. Concentration Effect of PLA/DCM:THF:DFM Solution and Application
3.2. FT-IR Spectroscopy
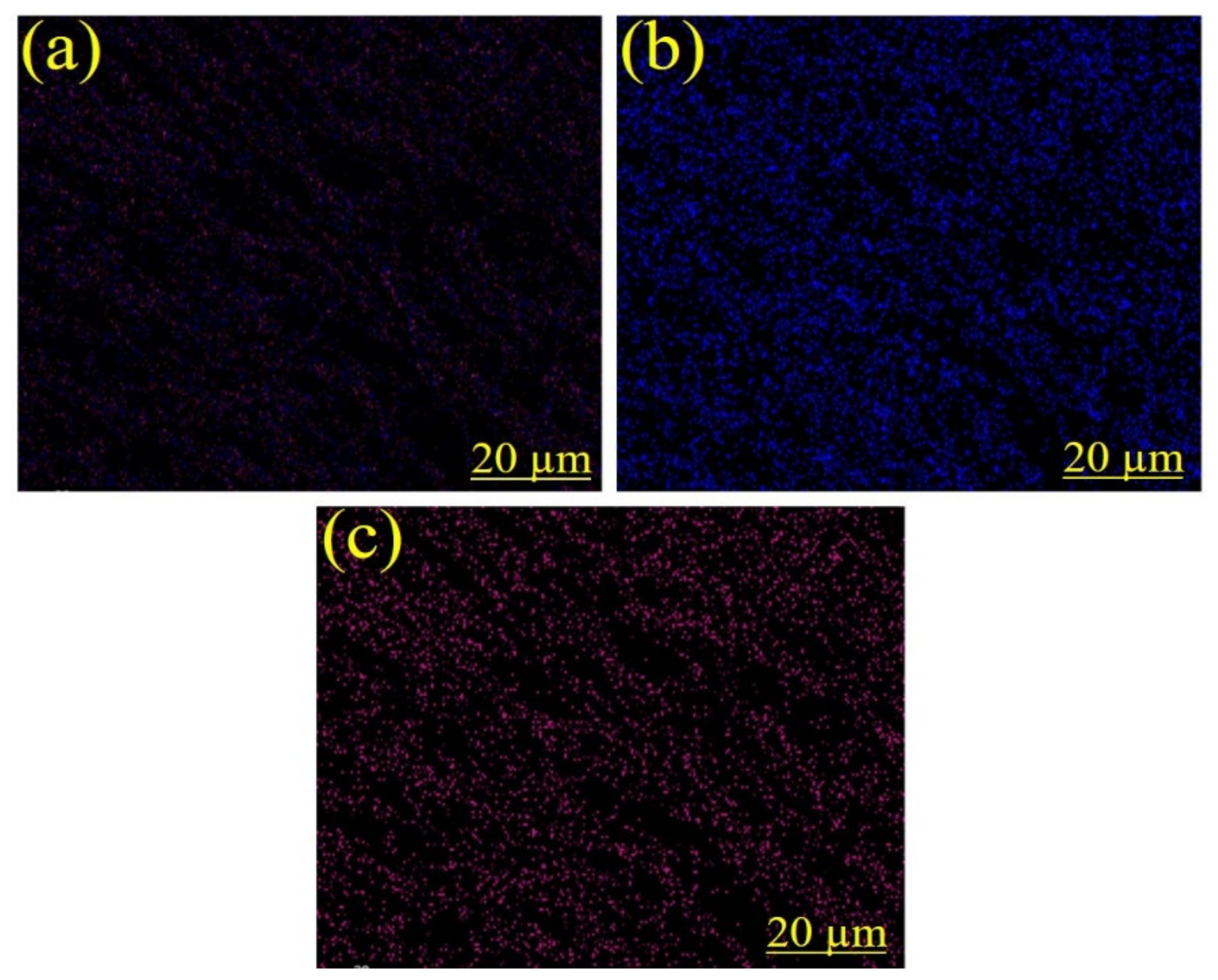
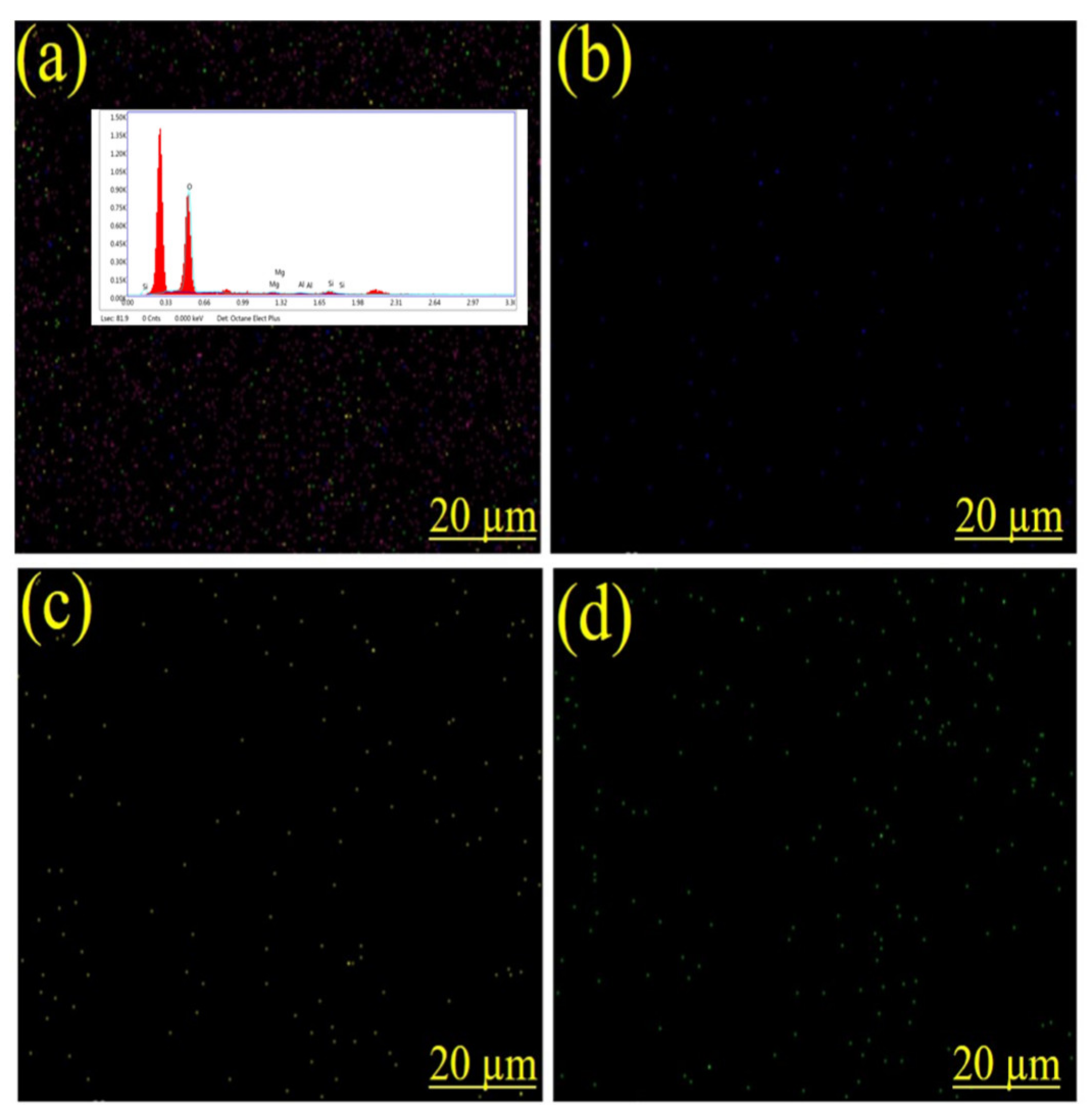
3.3. Energy Dispersive X-ray (EDX) Spectroscopy Analysis
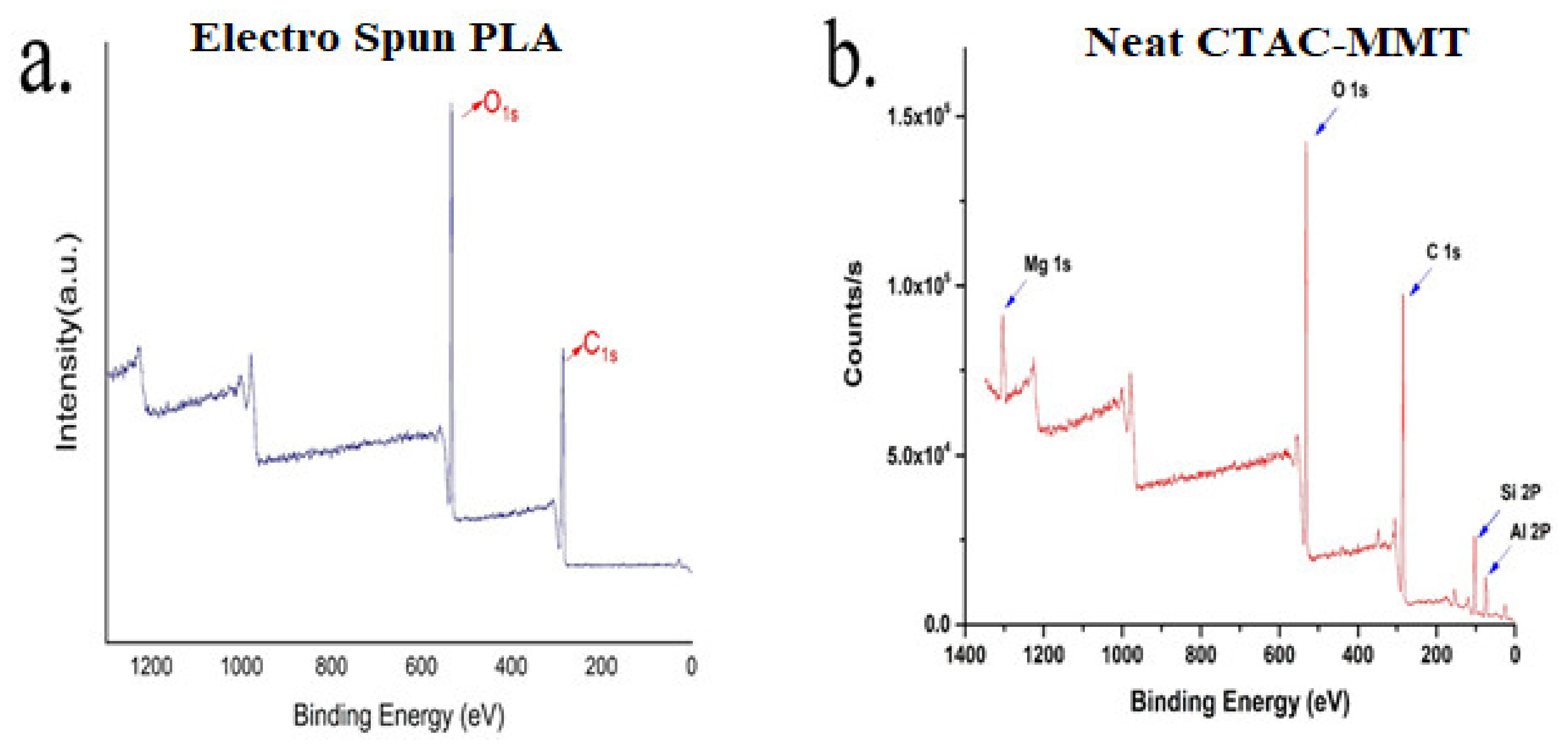
3.4. X-ray Photoelectron Spectroscopy Analysis
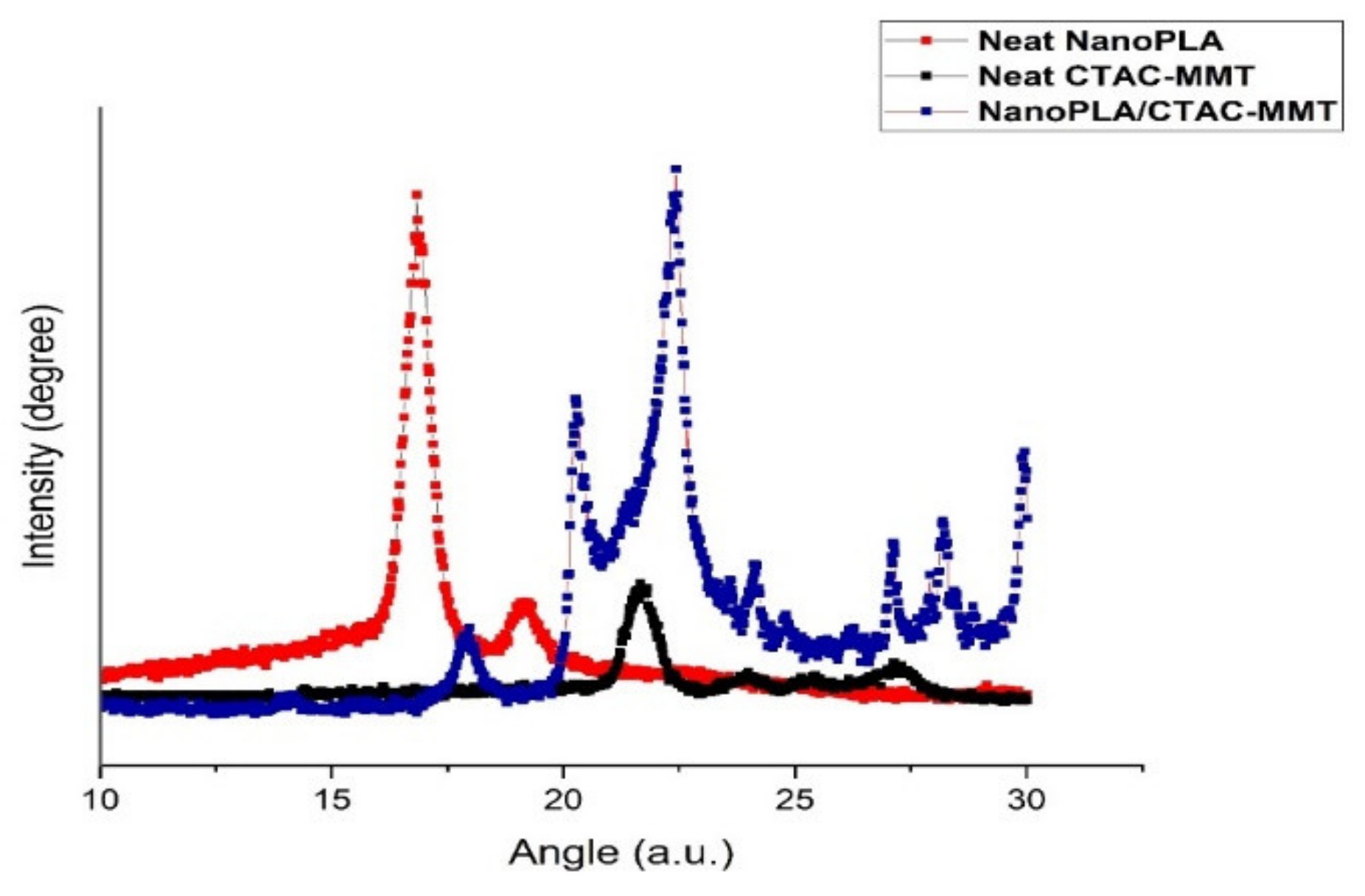
3.5. X-ray Diffraction (XRD) Analysis
3.6. Contact Angle
3.7. Mechanical Testing
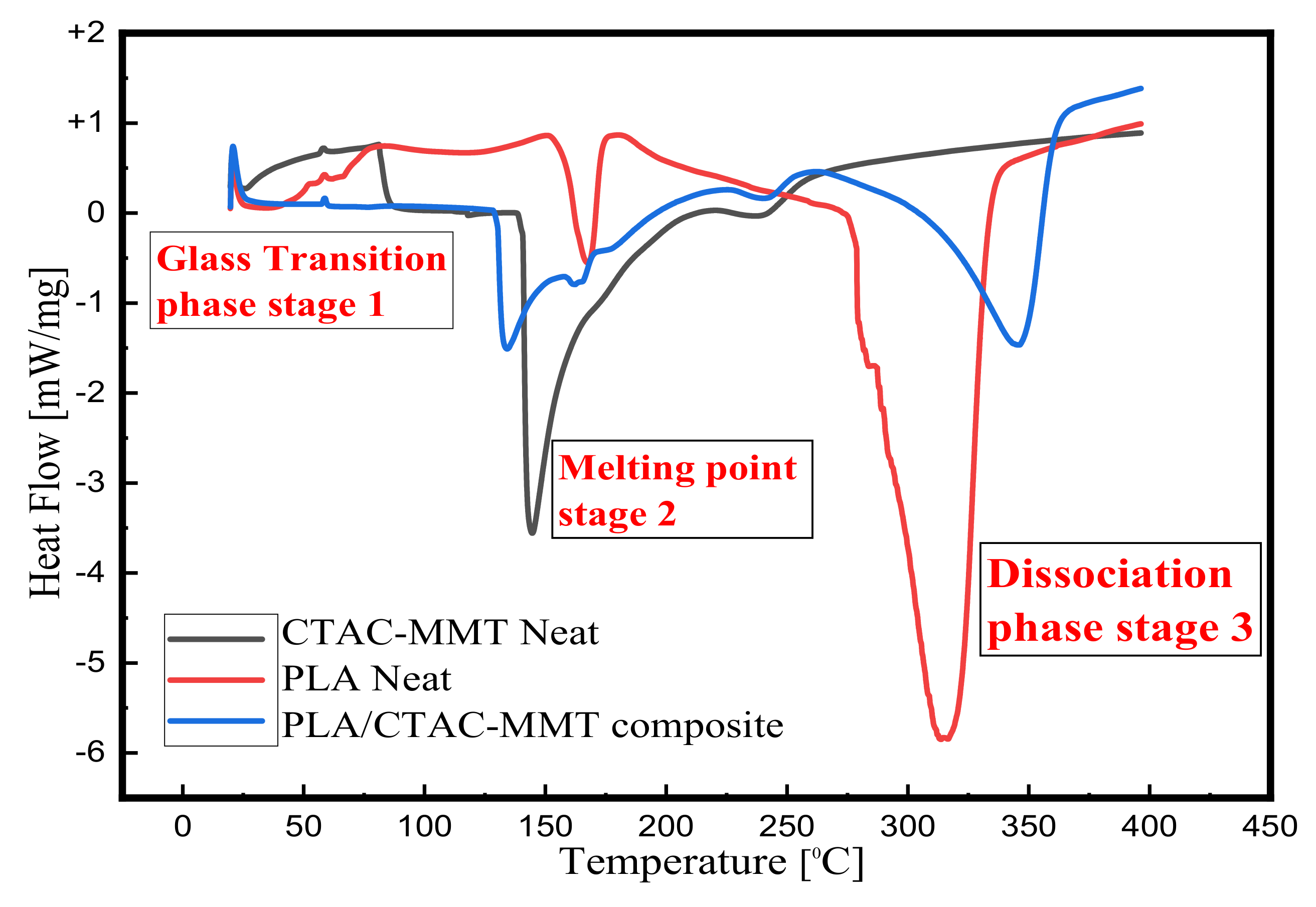
3.8. Differential Scanning Calorimeter Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhu, Y.; Liu, X.; Hu, Y.; Wang, R.; Chen, M.; Wu, J.; Wang, Y.; Kang, S.; Sun, Y.; Zhu, M. Behavior, remediation effect, and toxicity of nanomaterials in water environments. Environ. Res. 2019, 174, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Shahrin, S.; Lau, W.J.; Goh, P.S.; Ismail, A.F.; Jaafar, J. Adsorptive mixed matrix membrane incorporating graphene oxide-manganese ferrite (GMF) hybrid nanomaterial for efficient As(V) ions removal. Compos. Part B Eng. 2019, 175, 107150. [Google Scholar] [CrossRef]
- Ullah, N.; Mansha, M.; Khan, I.; Qurashi, A. Nanomaterial-based optical chemical sensors for the detection of heavy metals in water: Recent advances and challenges. Trac Trends Anal. Chem. 2018, 100, 155–166. [Google Scholar] [CrossRef]
- Shi, J.; Zhu, Y.; Zhang, X.; Baeyens, W.R.G.; Garcia-Campaña, A.M. Recent developments in nanomaterial optical sensors. Trac Trends Anal. Chem. 2004, 23, 351–360. [Google Scholar] [CrossRef]
- Jia, H.-R.; Zhu, Y.-X.; Duan, Q.-Y.; Chen, Z.; Wu, F.-G. Nanomaterials meet zebrafish: Toxicity evaluation and drug delivery applications. J. Control. Release 2019, 311, 301–318. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, Z.; Li, N.; Pu, Y.; Wang, B.; Zhang, T.; Tao, J. Advanced review of graphene-based nanomaterials in drug delivery systems: Synthesis, modification, toxicity and application. Mater. Sci. Eng. C 2017, 77, 1363–1375. [Google Scholar] [CrossRef]
- Du, J.; Wong, K.K.Y. 9-Nanomaterials for Wound Healing: Scope and Advances, in Theranostic Bionanomaterials; Cui, W., Zhao, X., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 211–230. [Google Scholar]
- Joseph, B.; Augustine, R.; Kalarikkal, N.; Thomas, S.; Seantier, B.; Grohens, Y. Recent advances in electrospun polycaprolactone based scaffolds for wound healing and skin bioengineering applications. Mater. Today Commun. 2019, 19, 319–335. [Google Scholar] [CrossRef]
- Papkov, D.; Beese, A.M.; Goponenko, A.; Zou, Y.; Naraghi, M.; Espinosa, H.D.; Saha, B.; Schatz, G.C.; Moravsky, A.; Loutfy, R. Extraordinary improvement of the graphitic structure of continuous carbon nanofibers templated with double wall carbon nanotubes. Acs Nano 2013, 7, 126–142. [Google Scholar] [CrossRef]
- Young, K.; Blighe, F.M.; Vilatela, J.J.; Windle, A.H.; Kinloch, I.A.; Deng, L.; Young, R.J.; Coleman, J.N. Strong dependence of mechanical properties on fiber diameter for polymer− nanotube composite fibers: Differentiating defect from orientation effects. Acs Nano 2010, 4, 6989–6997. [Google Scholar] [CrossRef]
- Rehman, A.; Ahmad, T.; Aadil, R.M.; Spotti, M.J.; Bakry, A.M.; Khan, I.M.; Zhao, L.; Riaz, T.; Tong, Q. Pectin polymers as wall materials for the nano-encapsulation of bioactive compounds. Trends Food Sci. Technol. 2019, 90, 35–46. [Google Scholar] [CrossRef]
- An, T.; Pant, B.; Kim, S.Y.; Park, M.; Park, S.-J.; Kim, H.-Y. Mechanical and optical properties of electrospun nylon-6, 6 nanofiber reinforced cyclic butylene terephthalate composites. J. Ind. Eng. Chem. 2017, 55, 35–39. [Google Scholar] [CrossRef]
- Pham, Q.P.; Sharma, U.; Mikos, A.G. Electrospinning of polymeric nanofibers for tissue engineering applications: A review. Tissue Eng. 2006, 12, 1197–1211. [Google Scholar] [CrossRef] [PubMed]
- Ghalia, M.A.; Dahman, Y. Chapter 6–Advanced nanobiomaterials in tissue engineering: Synthesis, properties, and applications. In Nanobiomaterials in Soft Tissue Engineering; Grumezescu, A.M., Ed.; William Andrew Publishing: Amsterdam, The Netherlands, 2016; pp. 141–172. [Google Scholar]
- Nunes, D.; Pimentel, A.; Santos, L.; Barquinha, P.; Pereira, L.; Fortunato, E.; Martins, R. 2—Synthesis, design, and morphology of metal oxide nanostructures. In Metal Oxide Nanostructures; Nunes, D., Pimentel, A., Santos, L., Barquinha, P., Pereira, L., Fortunato, E., Martins, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 21–57. [Google Scholar]
- Casasola, R.; Thomas, N.L.; Trybala, A.; Georgiadou, S. Electrospun poly lactic acid (PLA) fibres: Effect of different solvent systems on fibre morphology and diameter. Polymer 2014, 55, 4728–4737. [Google Scholar] [CrossRef]
- Wen-Jie, C.; Bin-Jie, X.; Xiang-Ji, W. Fabrication and characterization of PSA nanofibers via electrospinning. J. Ind. Text. 2013, 44, 159–179. [Google Scholar] [CrossRef]
- Pant, B.; Park, M.; Park, S.-J. Drug delivery applications of core-sheath nanofibers prepared by coaxial electrospinning: A review. Pharmaceutics 2019, 11, 305. [Google Scholar] [CrossRef]
- Doshi, J.; Reneker, D.H. Electrospinning process and applications of electrospun fibers. J. Electrost. 1995, 35, 151–160. [Google Scholar] [CrossRef]
- Wang, H.; Cui, Y.; Wen, M.; E, Y.; Cheng, B. Release behavior under proteinase K and existing position of Domiphen® in its complexed poly-L-lactic acid micro-fibers electrospun from solution and W/O emulsion. J. Ind. Text. 2018, 48, 1024–1043. [Google Scholar] [CrossRef]
- Theron, S.A.; Zussman, E.; Yarin, A.L. Experimental investigation of the governing parameters in the electrospinning of polymer solutions. Polymer 2004, 45, 2017–2030. [Google Scholar] [CrossRef]
- Kim, G.-T.; Lee, J.-S.; Shin, J.-H.; Ahn, Y.-C.; Hwang, Y.-J.; Shin, H.-S.; Lee, J.-K.; Sung, C.-M. Investigation of pore formation for polystyrene electrospun fiber: Effect of relative humidity. Korean J. Chem. Eng. 2005, 22, 783–788. [Google Scholar] [CrossRef]
- Rehman, A.; Tong, Q.; Jafari, S.M.; Assadpour, E.; Shehzad, Q.; Aadil, R.M.; Iqbal, M.W.; Rashed, M.M.; Mushtaq, B.S.; Ashraf, W. Carotenoid-loaded nanocarriers: A comprehensive review. Adv. Colloid Interface Sci. 2020, 275, 102048. [Google Scholar] [CrossRef]
- Jayaramudu, J.; Reddy, G.S.M.; Varaprasad, K.; Sadiku, E.R.; Ray, S.S.; Rajulu, A.V. Structure and properties of poly (lactic acid)/Sterculia urens uniaxial fabric biocomposites. Carbohydr. Polym. 2013, 94, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Mikes, P.; Horakova, J.; Saman, A.; Vejsadova, L.; Topham, P.; Punyodom, W.; Dumklang, M.; Jencova, V. Comparison and characterization of different polyester nano/micro fibres for use in tissue engineering applications. J. Ind. Text. 2019, 1528083719848155. [Google Scholar] [CrossRef]
- Ray, S.S.; Okamoto, M. Biodegradable polylactide and its nanocomposites: Opening a new dimension for plastics and composites. Macromol. Rapid Commun. 2003, 24, 815–840. [Google Scholar] [CrossRef]
- Yang, D.; Li, Y.; Nie, J. Preparation of gelatin/PVA nanofibers and their potential application in controlled release of drugs. Carbohydr. Polym. 2007, 69, 538–543. [Google Scholar] [CrossRef]
- Uddin, F. Montmorillonite: An introduction to properties and utilization. Curr. Top. Util. Clay Ind. Med. Appl. 2018, 1, 5–10. [Google Scholar]
- Yu, Y.-H.; Lin, C.-Y.; Yeh, J.-M.; Lin, W.-H. Preparation and properties of poly (vinyl alcohol)–clay nanocomposite materials. Polymer 2003, 44, 3553–3560. [Google Scholar] [CrossRef]
- Gilman, J.W.; Jackson, C.L.; Morgan, A.B.; Harris, R.; Manias, E.; Giannelis, E.P.; Wuthenow, M.; Hilton, D.; Phillips, S.H. Flammability properties of polymer− layered-silicate nanocomposites. Polypropylene and polystyrene nanocomposites. Chem. Mater. 2000, 12, 1866–1873. [Google Scholar] [CrossRef]
- Roy, A.; Joshi, M.; Butola, B.S.; Srivastava, A. Silver-loaded HDPE/clay nanocomposites with antibacterial property: A potential replacement for commodity polyethylene plastic. Polym. Compos. 2018, 39, E366–E377. [Google Scholar] [CrossRef]
- Sanchez-Valdes, S. Influence of maleated elastomer on filler dispersion, mechanical and antimicrobial properties of hybrid HDPE/clay/silver nanocomposites. J. Adhes. Sci. Technol. 2016, 30, 1006–1016. [Google Scholar] [CrossRef]
- Ibarra-Alonso, M.; Sánchez-Valdes, S.; Ramírez-Vargas, E.; Fernandez-Tavizón, S.; Romero-Garcia, J.; Ledezma-Perez, A.; de Valle, L.R.; Rodriguez-Fernandez, O.; Espinoza-Martinez, A.; Martinez-Colunga, J. Preparation and characterization of Polyethylene/Clay/Silver nanocomposites using functionalized polyethylenes as an adhesion promoter. J. Adhes. Sci. Technol. 2015, 29, 1911–1923. [Google Scholar] [CrossRef]
- Paul, D.R.; Robeson, L.M. Polymer nanotechnology: Nanocomposites. Polymer 2008, 49, 3187–3204. [Google Scholar] [CrossRef]
- Ogata, N.; Jimenez, G.; Kawai, H.; Ogihara, T. Structure and thermal/mechanical properties of poly(l-lactide)-clay blend. J. Polym. Sci. Part B: Polym. Phys. 1997, 35, 389–396. [Google Scholar] [CrossRef]
- Ray, S.S.; Maiti, P.; Okamoto, M.; Yamada, K.; Ueda, K. New Polylactide/Layered Silicate Nanocomposites. 1. Preparation, Characterization, and Properties. Macromolecules 2002, 35, 3104–3110. [Google Scholar]
- Pluta, M.; Jeszka, J.K.; Boiteux, G. Polylactide/montmorillonite nanocomposites: Structure, dielectric, viscoelastic and thermal properties. Eur. Polym. J. 2007, 43, 2819–2835. [Google Scholar] [CrossRef]
- Mushtaq, M.; Saba, H.; Wang, W.; Naeem, M.A.; Wei, Q. Fabrication and characterization of electrospun membranes from poly (lactic acid) and hexadecyl trimethyl ammonium chloride-modified montmorillonite clay. Journal of Industrial Textiles 2019, 2019, 1528083719831083. [Google Scholar] [CrossRef]
- Elshof, J.E.t. Chemical solution deposition techniques for epitaxial growth of complex oxides. In Epitaxial Growth of Complex Metal Oxides; Elsevier: Amsterdam, The Netherlands, 2015; pp. 69–93. [Google Scholar]
- Buapool, S.; Thavarungkul, N.; Srisukhumbowornchai, N.; Termsuksawad, P. Modeling and Analysis of the Effect of Dip-Spin Coating Process Parameters on Coating Thickness Using Factorial Design Method. Adv. Mater. Sci. Eng. 2017, 2017, 9639306. [Google Scholar]
- Lim, L.T.; Auras, R.; Rubino, M. Processing technologies for poly(lactic acid). Prog. Polym. Sci. 2008, 33, 820–852. [Google Scholar] [CrossRef]
- Garlotta, D. A literature review of poly (lactic acid). J. Polym. Environ. 2001, 9, 63–84. [Google Scholar] [CrossRef]
- Naeem, M.A.; Lv, P.; Zhou, H.; Naveed, T.; Wei, Q. A novel in situ self-assembling fabrication method for bacterial cellulose-electrospun nanofiber hybrid structures. Polymers 2018, 10, 712. [Google Scholar] [CrossRef]
- Jun, Z.; Hou, H.Q.; Schaper, A.; Wendorff, J.H.; Greiner, A. Poly-L-lactide nanofibers by electrospinning—Influence of solution viscosity and electrical conductivity on fiber diameter and fiber morphology. E-Polym. 2003, 3, 1618–7229. [Google Scholar] [CrossRef]
- Zong, X.H.; Kim, K.; Fang, D.F.; Ran, S.F.; Hsiao, B.S.; Chu, B. Structure and process relationship of electrospun bioabsorbable nanofiber membranes. Polymer 2002, 43, 4403–4412. [Google Scholar] [CrossRef]
- Arrieta, M.P.; López, J.; López, D.; Kenny, J.; Peponi, L. Development of flexible materials based on plasticized electrospun PLA–PHB blends: Structural, thermal, mechanical and disintegration properties. Eur. Polym. J. 2015, 73, 433–446. [Google Scholar] [CrossRef]
- Oliveira, J.E.; Mattoso, L.H.C.; Orts, W.J.; Medeiros, E.S. Structural and Morphological Characterization of Micro and Nanofibers Produced by Electrospinning and Solution Blow Spinning: A Comparative Study. Adv. Mater. Sci. Eng. 2013, 2013, 40957. [Google Scholar] [CrossRef]
- Jahangir, A.; Rumi, T.; Wahab, M.; Khan, M.; Rahman, M.; Sayed, Z. Poly Lactic Acid (PLA) Fibres: Different Solvent Systems and Their Effect on Fibre Morphology and Diameter. Am. J. Chem. 2017, 2017, 177–186. [Google Scholar]
- Kim, S.H.; Nam, Y.S.; Lee, T.S.; Park, W.H. Silk fibroin nanofiber. Electrospinning, properties, and structure. Polym. J. 2003, 35, 185–190. [Google Scholar] [CrossRef]
- Chieng, B.W.; Ibrahim, N.; Yunus, W.; Hussein, M. Effects of Graphene Nanopletelets on Poly(Lactic Acid)/Poly(Ethylene Glycol) Polymer Nanocomposites. Polymers 2013, 6, 93–104. [Google Scholar] [CrossRef]
- Kirbay, F.O.; Yalcinkaya, E.E.; Atik, G.; Evren, G.; Unal, B.; Demirkol, D.O.; Timur, S. Biofunctionalization of PAMAM-montmorillonite decorated poly (Ɛ-caprolactone)-chitosan electrospun nanofibers for cell adhesion and electrochemical cytosensing. Biosens. Bioelectron. 2018, 109, 286–294. [Google Scholar] [CrossRef]
- Tunc, S.; Duman, O.; Polat, T.G. Effects of montmorillonite on properties of methyl cellulose/carvacrol based active antimicrobial nanocomposites. Carbohydr. Polym. 2016, 150, 259–268. [Google Scholar] [CrossRef]
- Wei, Q.-B.; Fu, F.; Zhang, Y.-Q.; Tang, L. Preparation, characterization, and antibacterial properties of pH-responsive P(MMA-co-MAA)/silver nanocomposite hydrogels. J. Polym. Res. 2014, 21, 349. [Google Scholar] [CrossRef]
- Klika, Z.; Pustkova, P.; Praus, P.; Kovar, P.; Pospisil, M.; Maly, P.; Grygar, T.; Kulhankova, L.; Capkova, P. Fluorescence of reduced charge montmorillonite complexes with methylene blue: Experiments and molecular modeling. J. Colloid Interface Sci. 2009, 339, 416–423. [Google Scholar] [CrossRef]
- Huang, L. Generation of synthetic elastin-mimetic small diameter fibers and fiber networks. Macromolecules 2000, 33, 2989–2997. [Google Scholar] [CrossRef]
- Jamshidian, M.; Tehrany, E.A.; Imran, M.; Jacquot, M.; Desobry, S. Poly-Lactic Acid: Production, Applications, Nanocomposites, and Release Studies. Compr. Rev. Food. Sci. Food Saf. 2010, 9, 552–571. [Google Scholar] [CrossRef]
- Floody, M.C.; Theng, B.K.G.; Reyes, P.; Mora, M.L. Natural nanoclays: Applications and future trends—A Chilean perspective. Clay Miner. 2009, 44, 161–176. [Google Scholar] [CrossRef]
- Ying, Z.; Wu, D.; Wang, Z.; Xie, W.; Qiu, Y.; Wei, X. Rheological and mechanical properties of polylactide nanocomposites reinforced with the cellulose nanofibers with various surface treatments. Cellulose 2018, 25, 3955–3971. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, C.; Li, J.; Wang, X. Preparation of montmorillonite@carbon composite and its application for U(VI) removal from aqueous solution. Appl. Surf. Sci. 2015, 349, 129–137. [Google Scholar] [CrossRef]
- Thirumalairajan, S.; Girija, K.; Mastelaro, V.R.; Ponpandian, N. Surface Morphology-Dependent Room-Temperature LaFeO3 Nanostructure Thin Films as Selective NO2 Gas Sensor Prepared by Radio Frequency Magnetron Sputtering. Acs Appl. Mater. Interfaces 2014, 6, 13917–13927. [Google Scholar] [CrossRef]
- De Azeredo, H.M. Nanocomposites for food packaging applications. Food Res. Int. 2009, 42, 1240–1253. [Google Scholar] [CrossRef]
- Reddy, M.M.; Vivekanandhan, S.; Misra, M.; Bhatia, S.K.; Mohanty, A.K. Biobased plastics and bionanocomposites: Current status and future opportunities. Prog. Polym. Sci. 2013, 38, 1653–1689. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, D.; Li, B.; Wan, G.; Zhao, G.; Zhang, A. Strong and thermal-resistance glass fiber-reinforced polylactic acid (PLA) composites enabled by heat treatment. Int. J. Biol. Macromol. 2019, 129, 448–459. [Google Scholar] [CrossRef]
- Frone, A.N.; Panaitescu, D.M.; Chiulan, I.; Nicolae, C.A.; Vuluga, Z.; Vitelaru, C.; Damian, C.M. The effect of cellulose nanofibers on the crystallinity and nanostructure of poly(lactic acid) composites. J. Mater. Sci. 2016, 51, 9771–9791. [Google Scholar] [CrossRef]
- Lai, J.C.H.; Rahman, M.; Hamdan, S. Physical, mechanical, and thermal analysis of polylactic acid/fumed silica/clay (1.28 E) nanocomposites. Int. J. Polym. Sci. 2015, 2015, 698738. [Google Scholar] [CrossRef]
- Eng, C.C.; Ibrahim, N.; Zainuddin, N.; Ariffin, H.; Yunus, W.; Then, Y.Y.; Teh, C. Enhancement of Mechanical and Thermal Properties of Polylactic Acid/Polycaprolactone Blends by Hydrophilic Nanoclay. Indian J. Mater. Sci. 2013, 2013, 816503. [Google Scholar] [CrossRef]
- Mujeeb, A.; Lobo, A.G.; Antony, A.; Ramis, M. An experimental study on the thermal properties and electrical properties of polylactide doped with nano aluminium oxide and nano cupric oxide. Ina. Lett. 2017, 2, 145–151. [Google Scholar] [CrossRef]
- Tábi, T.; Suplicz, A.; Czigány, T.; Kovács, J.G. Thermal and mechanical analysis of injection moulded poly (lactic acid) filled with poly (ethylene glycol) and talc. J. Anal. Calorim. 2014, 118, 1419–1430. [Google Scholar] [CrossRef][Green Version]
- Ohkita, T.; Lee, S.H. Thermal degradation and biodegradability of poly (lactic acid)/corn starch biocomposites. J. Appl. Polym. Sci. 2006, 100, 3009–3017. [Google Scholar] [CrossRef]
- Pyda, M.; Bopp, R.C.; Wunderlich, B. Heat capacity of poly(lactic acid). J. Chem. 2004, 36, 731–742. [Google Scholar] [CrossRef]
- Wang, G.; Li, A. Thermal Decomposition and Kinetics of Mixtures of Polylactic Acid and Biomass during Copyrolysis. Chin. J. Chem. Eng. 2008, 16, 929–933. [Google Scholar] [CrossRef]


| Solvent | Boiling Point (°C) | H (mPa·s) | Electrical Conductivity (μS·cm−1) | ε | σ (mN/m) | δ (cal1/2 cm−3/2) |
|---|---|---|---|---|---|---|
| DCM | 40 | 0.449 | 4.3 × 10−5 | 9.1 | 28 | 9.7 |
| DMF | 153 | 0.920 | 6.0 × 10−2 | 36.7 | 35 | 12.1 |
| THF | 66 | 0.480 | 4.5 × 101 | 7.6 | 28 | 9.1 |
| PLA Concentration % w/v | Solution Viscosity mPa·s | Surface Tension mN·m−1 | Conductivity μS·cm−1 | Mean Nanofiber Diameter |
|---|---|---|---|---|
| 5 | 22 ± 9 | 29 ± 0.3 | 1.98 | Few nanofibers, mainly beads |
| 7.5 | 72 ± 7 | 29 ± 0.2 | 2.93 | 223 ± 57 |
| 10 | 213 ± 7 | 29 ± 0.6 | 3.40 | 303 ± 88 |
| 12.5 | 667 ± 62 | 29.6 ± 0.9 | 3.87 | 462 ± 125 |
| 15 | 1457 ± 147 | 29.9 ± 0.9 | 3.60 | 685 ± 206 |
| Samples | Water Absorption % | CV wa % | Contact Angle | CV.cv % |
|---|---|---|---|---|
| PLA | 0.45 | 0.040 | 141 ± 2.8 | 2.8 |
| PLA/O-MMT 0.1% | 0.55 | 0.062 | 139 ± 1.7 | 1.7 |
| PLA/O-MMT 0.5% | 0.60 | 0.076 | 135 ± 1.7 | 1.7 |
| PLA/O-MMT 1.0% | 0.70 | 0.05 | 131 ± 1.9 | 1.9 |
| Samples | Tensile Strength (MPA) | Standard (σ) | Tensile Modulus | Standard (σ) | Elongation at Break, % | Standard (σ) |
|---|---|---|---|---|---|---|
| PLA-0 | 2.5 | 0.1 | 14.50 | 1.25 | 80.20 | 1.00 |
| PLA/CTAC-MMT 0.1% | 3.0 | 0.98 | 15.0 | 1.32 | 78.0 | 0.80 |
| PLA/CTAC-MMT 0.5% | 3.4 | 0.15 | 15.6 | 1.15 | 79.2 | 1.05 |
| PLA/CTAC-MMT 1.0% | 3.6 | 0.2 | 16.0 | 1.52 | 78.5 | 1.15 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mushtaq, M.; Wasim, M.; Naeem, M.A.; Khan, M.R.; Yue, S.; Saba, H.; Hussain, T.; Siddiqui, M.Q.; Farooq, A.; Wei, Q. Composite of PLA Nanofiber and Hexadecyl Trimethyl-Ammonium Chloride-Modified Montmorillonite Clay: Fabrication and Morphology. Coatings 2020, 10, 484. https://doi.org/10.3390/coatings10050484
Mushtaq M, Wasim M, Naeem MA, Khan MR, Yue S, Saba H, Hussain T, Siddiqui MQ, Farooq A, Wei Q. Composite of PLA Nanofiber and Hexadecyl Trimethyl-Ammonium Chloride-Modified Montmorillonite Clay: Fabrication and Morphology. Coatings. 2020; 10(5):484. https://doi.org/10.3390/coatings10050484
Chicago/Turabian StyleMushtaq, Muhammad, Muhammad Wasim, Muhammad Awais Naeem, Muhammad Rafique Khan, Sun Yue, Hina Saba, Tanveer Hussain, Muhammad Qasim Siddiqui, Amjad Farooq, and Qufu Wei. 2020. "Composite of PLA Nanofiber and Hexadecyl Trimethyl-Ammonium Chloride-Modified Montmorillonite Clay: Fabrication and Morphology" Coatings 10, no. 5: 484. https://doi.org/10.3390/coatings10050484
APA StyleMushtaq, M., Wasim, M., Naeem, M. A., Khan, M. R., Yue, S., Saba, H., Hussain, T., Siddiqui, M. Q., Farooq, A., & Wei, Q. (2020). Composite of PLA Nanofiber and Hexadecyl Trimethyl-Ammonium Chloride-Modified Montmorillonite Clay: Fabrication and Morphology. Coatings, 10(5), 484. https://doi.org/10.3390/coatings10050484





