Layered Double Hydroxide Protective Films Developed on Aluminum and Aluminum Alloys: Synthetic Methods and Anti-Corrosion Mechanisms
Abstract
1. Introduction
2. Synthesis of LDH on Aluminum and Aluminum Alloys
2.1. Coprecipitated Synthesis
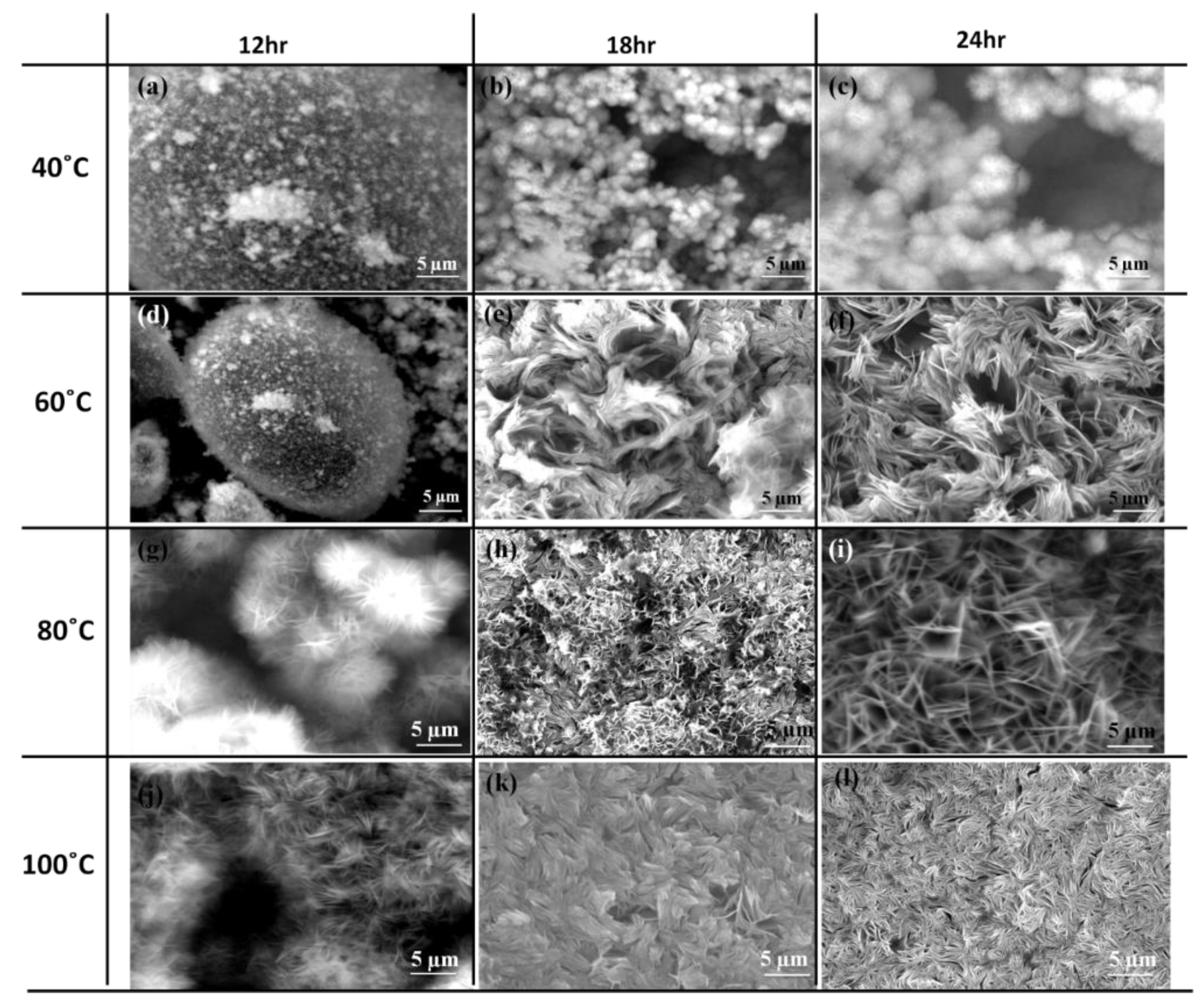
2.2. In Situ Growth Method
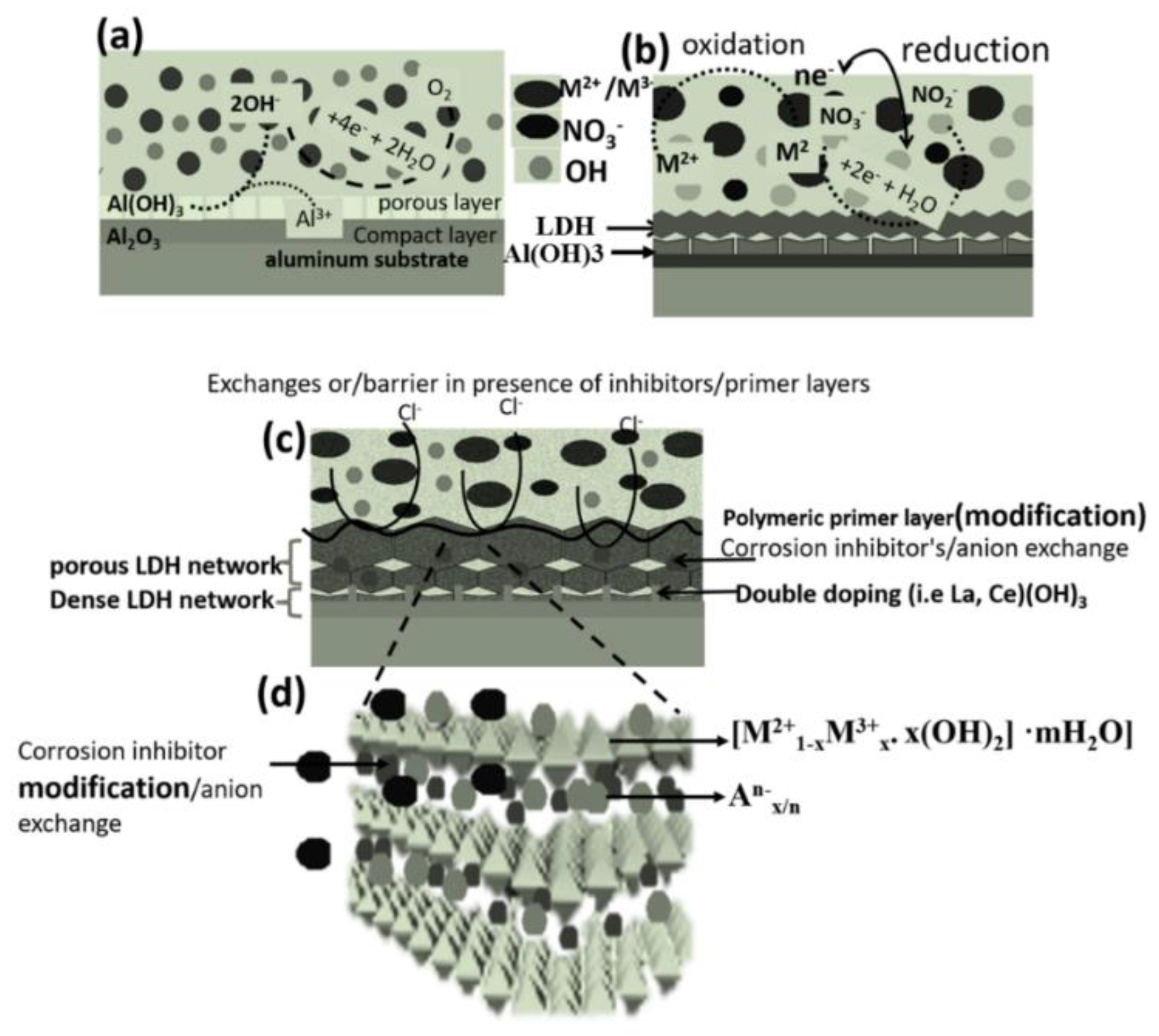
3. Corrosion Resistance of LDH Films
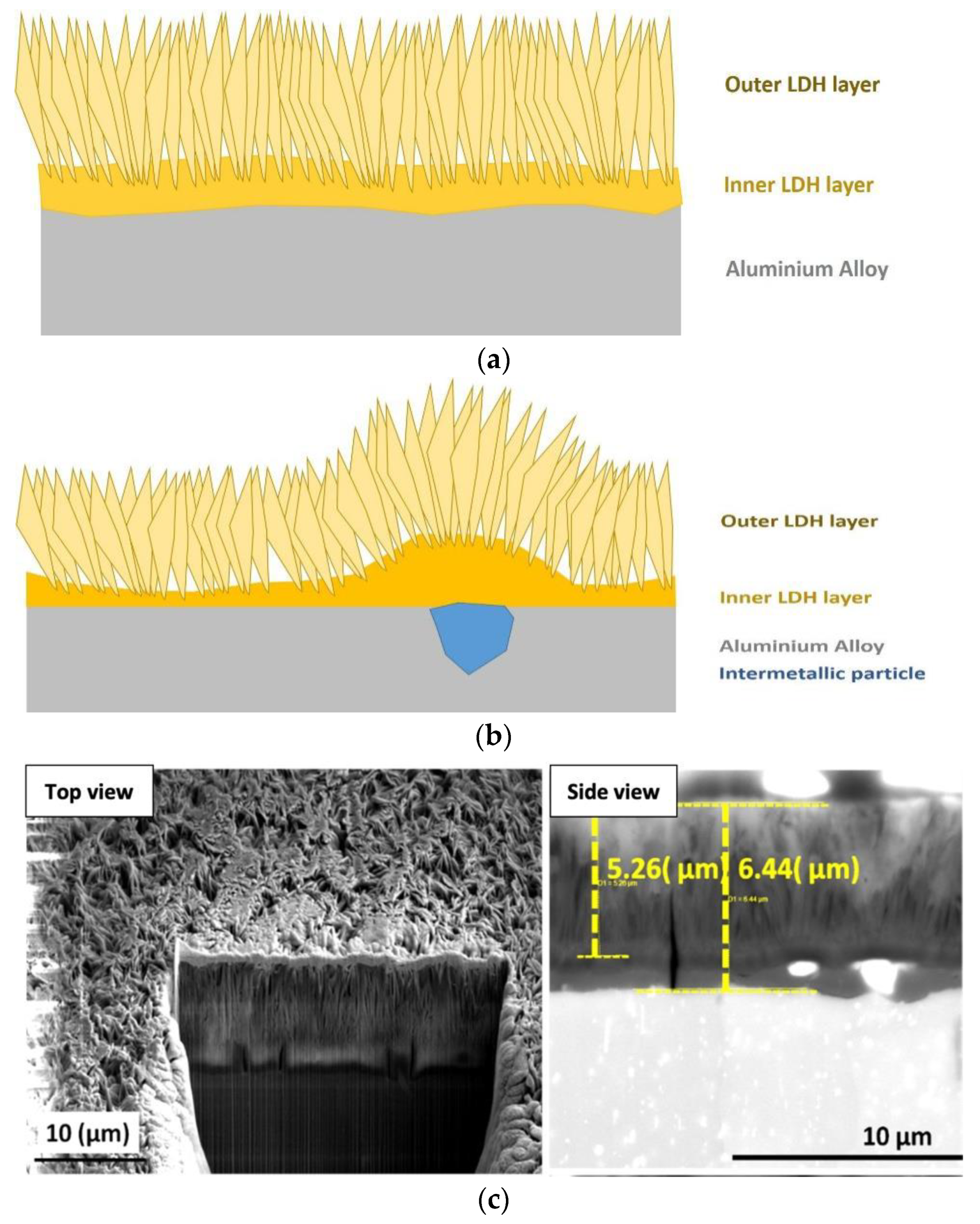
3.1. Corrosion Resistance of In-Situ Grown LDHs
3.2. Corrosion Resistance Co-Precipitated LDHs in Organic and Hybrid Matrices
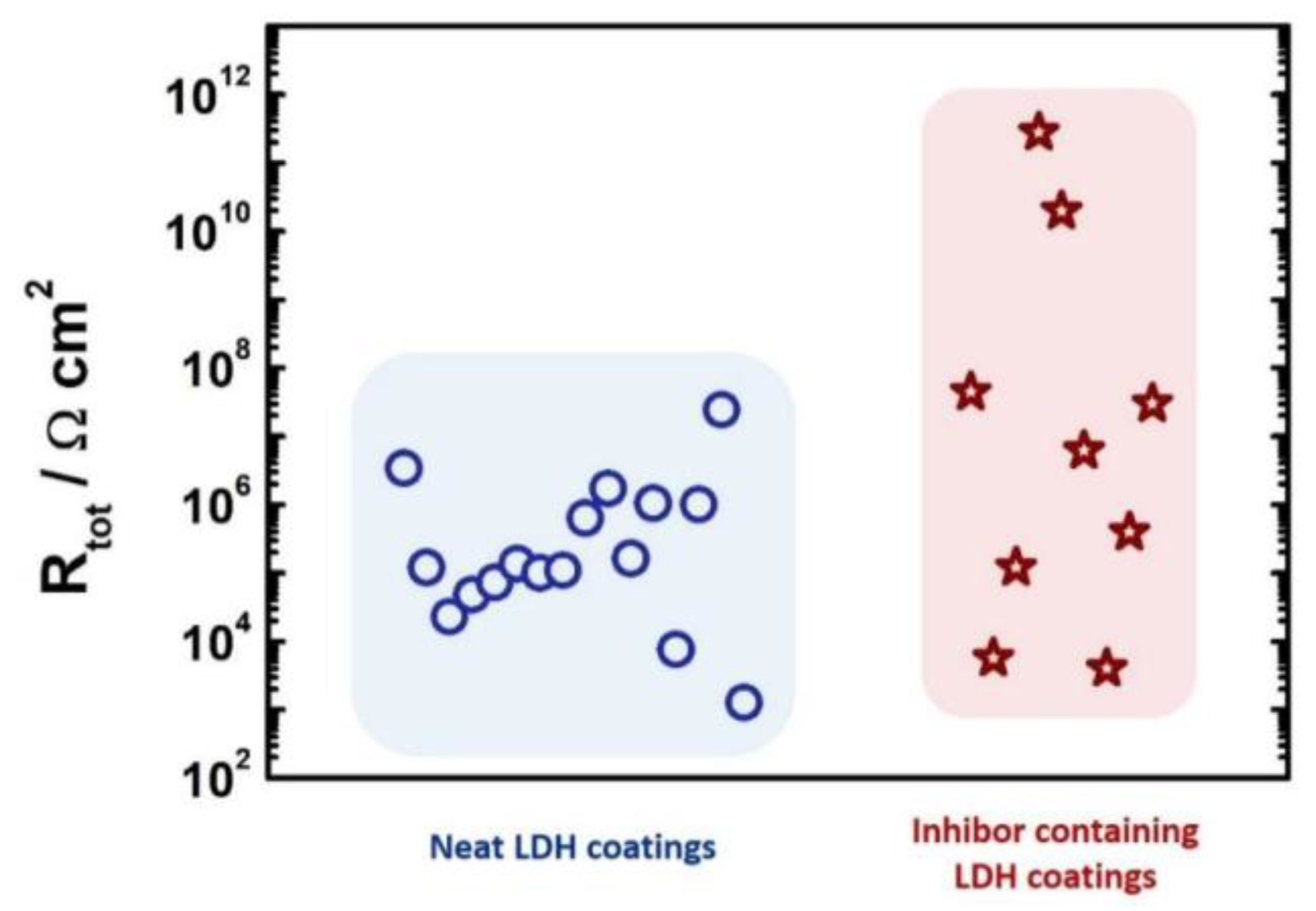
3.3. Kinetics and Controlled Release of Interlayer LDHs Corrosion Inhibitors
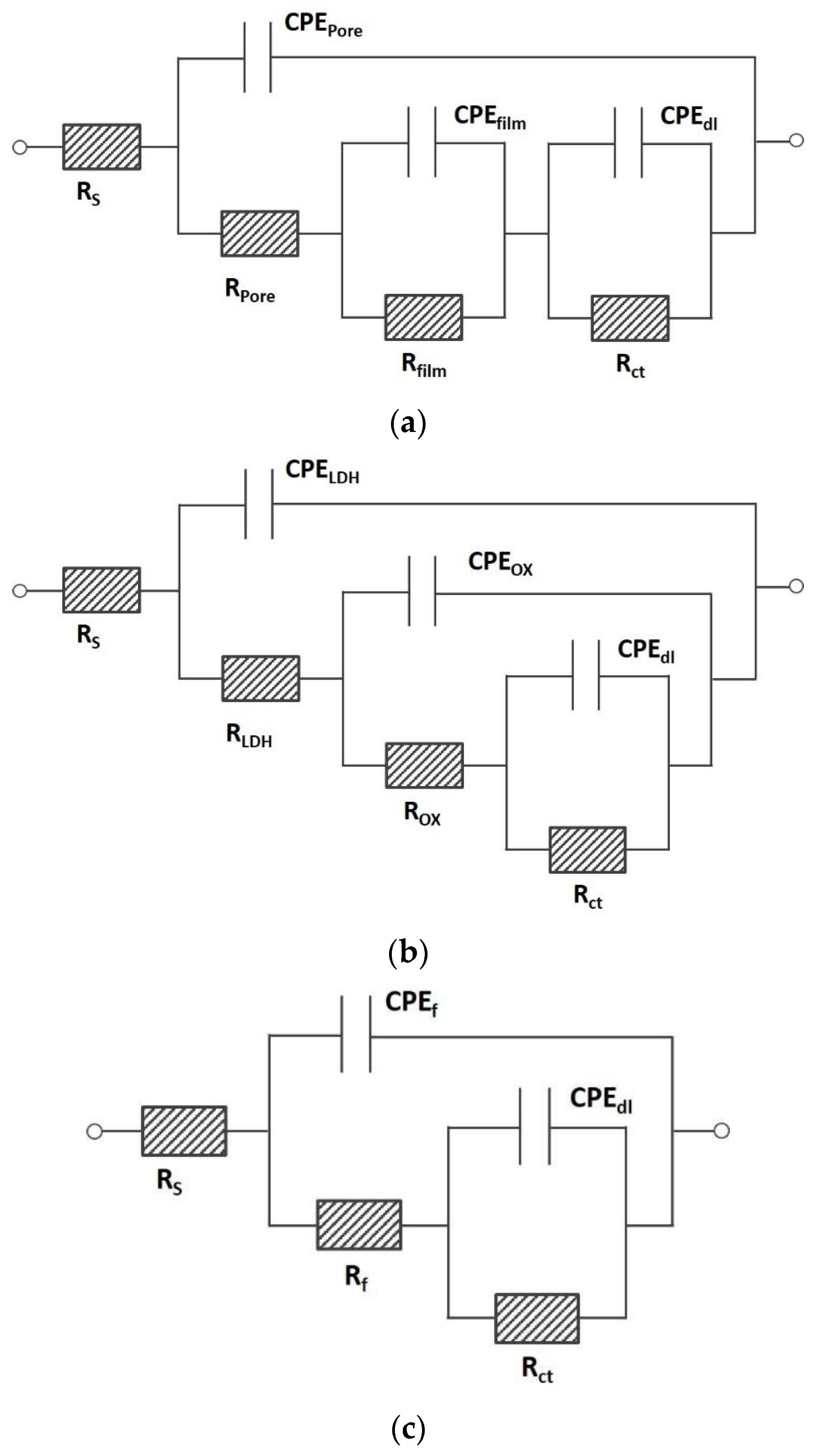
3.4. Testing and Evaluation of LDH Coatings or Co-Precipitated Particles
4. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Davis, J.R. (Ed.) Corrosion of Aluminum and Aluminum Alloys; ASM International: Geauga, OH, USA, 1999. [Google Scholar]
- Xhanari, K.; Finšgar, M. Organic corrosion inhibitors for aluminum and its alloys in chloride and alkaline solutions: A review. Arab. J. Chem. 2016, 12, 4646–4663. [Google Scholar] [CrossRef]
- Liang, W.J.; Rometsch, P.A.; Cao, L.F.; Birbilis, N. General aspects related to the corrosion of 6xxx series aluminium alloys: Exploring the influence of Mg/Si ratio and Cu. Corros. Sci. 2013, 76, 119–128. [Google Scholar] [CrossRef]
- Larsen, M.H.; Walmsley, J.C.; Lunder, O.; Mathiesen, R.H.; Nisancioglu, K. Intergranular corrosion of copper-containing AA6xxx AlMgSi aluminum alloys. J. Electrochem. Soc. 2008, 155, 550–556. [Google Scholar] [CrossRef]
- Ye, H. An Overview of the Development of Al-Si-Alloy Based Material for Engine Applications. J. Mater. Eng. Perform. 2003, 12, 288–297. [Google Scholar] [CrossRef]
- Chen, C.L.; West, G.; Thomson, R.C. Characterisation of intermetallic phases in multicomponent Al-Si casting alloys for engineering applications. Mat. Sci. Forum 2006, 521, 359–364. [Google Scholar] [CrossRef]
- Donatus, U.; Thompson, G.E.; Omotoyinbo, J.A.; Alaneme, K.K.; Aribo, S.; Agbabiaka, O.G. Corrosion pathways in aluminium alloys. Trans. Nonferrous Met. Soc. China 2017, 27, 55–62. [Google Scholar] [CrossRef]
- Tupaj, M.; Orłowicz, A.W.; Mróz, M.; Trytek, A.; Markowska, O. The Effect of Cooling Rate on Properties of Intermetallic Phase in a Complex Al-Si Alloy. Arch. Foundry Eng. 2016, 16, 125–128. [Google Scholar] [CrossRef]
- Yasakau, K.A.; Zheludkevich, M.L.; Lamaka, S.V.; Ferreira, M.G.S. Role of intermetallic phases in localized corrosion of AA5083. Electrochim. Acta 2007, 52, 7651–7659. [Google Scholar] [CrossRef]
- Linder, J. The influence of surrounding environment on the fatigue properties for a high pressure die cast AlSi9Cu3 alloy. Fatigue Fract. Eng. Mater. Struct. 2007, 30, 759–765. [Google Scholar] [CrossRef]
- Ferreira, M.G.S.; Zheludkevich, M.L.; Tedim, J.; Yasakau, K.A. Self-Healing Nanocoatings for Corrosion Control; Woodhead Publishing Limited: Sawston, UK, 2012. [Google Scholar]
- Makhlouf, A.S.H. Current and Advanced Coating Technologies for Industrial Applications; Woodhead Publishing Limited: Sawston, UK, 2011. [Google Scholar]
- Arenas, M.A.; Conde, A.; de Damborenea, J.J. Effect of acid traces on hydrothermal sealing of anodising layers on 2024 aluminium alloy. Electrochim. Acta 2010, 55, 8704–8708. [Google Scholar] [CrossRef]
- Venugopal, A.; Panda, R.; Manwatkar, S.; Sreekumar, K.; Krishna, L.R.; Sundararajan, G. Effect of micro arc oxidation treatment on localized corrosion behaviour of AA7075 aluminum alloy in 3.5 NaCl solution. Trans. Nonferrous Met. Soc. China 2012, 22, 700–710. [Google Scholar] [CrossRef]
- Dalmoro, V.; Santos, J.H.Z.D.; Armelin, E.; Alemán, C.; Azambuja, D.S. A synergistic combination of tetraethylorthosilicate and multiphosphonic acid offers excellent corrosion protection to AA1100 aluminum alloy. Appl. Surf. Sci. 2013, 273, 758–768. [Google Scholar] [CrossRef]
- Voevodin, N.N.; Kurdziel, J.W.; Mantz, R. Corrosion protection for aerospace aluminum alloys by Modified Self-assembled NAnophase Particle (MSNAP) sol-gel. Surf. Coat. Technol. 2006, 201, 1080–1084. [Google Scholar] [CrossRef]
- Johansen, H.D.; Brett, C.M.A.; Motheo, A.J. Corrosion protection of aluminium alloy by cerium conversion and conducting polymer duplex coatings. Corros. Sci. 2012, 63, 342–350. [Google Scholar] [CrossRef]
- Shi, H.; Han, E.H.; Liu, F.; Kallip, S. Protection of 2024-T3 aluminium alloy by corrosion resistant phytic acid conversion coating. Appl. Surf. Sci. 2013, 280, 325–331. [Google Scholar] [CrossRef]
- Schäfer, H.; Stock, H.R. Improving the corrosion protection of aluminium alloys using reactive magnetron sputtering. Corros. Sci. 2005, 47, 953–964. [Google Scholar] [CrossRef]
- Diesselberg, M.; Stock, H.R.; Mayr, P. Corrosion protection of magnetron sputtered TiN coatings deposited on high strength aluminium alloys. Surf. Coat. Technol. 2004, 178, 399–403. [Google Scholar] [CrossRef]
- Lutz, A.; van den Berg, O.; van Damme, J.; Verheyen, K.; Bauters, E.; de Graeve, I.; Prez, F.E.D.; Terryn, H. A shape-recovery polymer coating for the corrosion protection of metallic surfaces. ACS Appl. Mater. Interfaces 2015, 7, 175–183. [Google Scholar] [CrossRef]
- Williams, G.R.; O’Hare, D. Towards understanding, control and application of layered double hydroxide chemistry. J. Mater. Chem. 2006, 16, 3065–3074. [Google Scholar] [CrossRef]
- Qu, J.E.; Chen, G.; Wang, H.R.; Nie, D.J. Effect of water content on corrosion inhibition behavior of self-assembled TDPA on aluminum alloy surface. Trans. Nonferrous Met. Soc. China 2013, 23, 3137–3144. [Google Scholar] [CrossRef]
- Zuo, K.; Wang, X.; Liu, W.; Zhao, Y. Preparation and characterization of Ce-silane-ZrO2 composite coatings on 1060 aluminum. Trans. Nonferrous Met. Soc. China 2014, 24, 1474–1480. [Google Scholar] [CrossRef]
- Newman, S.P.; Jones, W. Synthesis, characterization and applications of layered double hydroxides containing organic guests. New J. Chem. 1998, 22, 105–115. [Google Scholar] [CrossRef]
- Oh, J.M.; Biswick, T.T.; Choy, J.H. Layered nanomaterials for green materials. J. Mater. Chem. 2009, 19, 2553–2563. [Google Scholar] [CrossRef]
- Evans, D.G.; Slade, R.C.T. Structural aspects of layered double hydroxides. Struct. Bond. 2005, 119, 1–87. [Google Scholar]
- Yu, J.; Liu, J.; Clearfield, A.; Sims, J.E.; Speiegle, M.T.; Suib, S.L.; Sun, L. Synthesis of layered double hydroxide single-layer nanosheets in formamide. Inorg. Chem. 2016, 55, 12036–12041. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.L.; Tian, D.Y.; Li, S.P.; Li, X.D.; Lu, T.H. MTX/LDHs hybrids synthesized from reverse microemulsions: Particle control and bioassay study. Int. J. Pharm. 2014, 473, 414–425. [Google Scholar] [CrossRef]
- Chitrakar, R.; Makita, Y.; Sonoda, A.; Hirotsu, T. Synthesis of a novel layered double hydroxides [MgAl4(OH)12](Cl)2·2.4H2O and its anion-exchange properties. J. Hazard. Mater. 2011, 185, 1435–1439. [Google Scholar] [CrossRef]
- Cai, X.; Shen, X.; Ma, L.; Ji, Z.; Xu, C.; Yuan, A. Solvothermal synthesis of NiCo-layered double hydroxide nanosheets decorated on RGO sheets for high performance supercapacitor. Chem. Eng. J. 2015, 268, 251–259. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Asghar, H.; Iqbal, M.A.; Fedel, M. Sorption of As(V) from aqueous solution using in situ growth MgAl–NO3 layered double hydroxide thin film developed on AA6082. SN Appl. Sci. 2019, 1, 1–9. [Google Scholar] [CrossRef]
- Laipan, M.; Fu, H.; Zhu, R.; Sun, L.; Steel, R.M.; Ye, S.; Zhu, J.; He, H. Calcined Mg/Al-LDH for acidic wastewater treatment: Simultaneous neutralization and contaminant removal. Appl. Clay Sci. 2018, 153, 46–53. [Google Scholar] [CrossRef]
- Laipan, M.; Fu, H.; Zhu, R.; Sun, L.; Zhu, J.; He, H. Converting Spent Cu/Fe Layered Double Hydroxide into Cr(VI) Reductant and Porous Carbon Material. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, W.; Chen, X.; Ge, F.; Zhu, R.; Sun, L. Self-assembled ZnAl-LDH/PMo12 nano-hybrids as effective catalysts on the degradation of methyl orange under room temperature and ambient pressure. Appl. Catal. A 2018, 550, 206–213. [Google Scholar] [CrossRef]
- Yang, J.H.; Han, Y.S.; Park, M.; Park, T.; Hwang, S.J.; Choy, J.H. New inorganic-based drug delivery system of indole-3-acetic acid-layered metal hydroxide nanohybrids with controlled release rate. Chem. Mater. 2007, 19, 2679–2685. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, F.; Peng, Q.; Xu, S.; Lei, X.; Evans, D.G.; Duan, X. Layered double hydroxide/eggshell membrane: An inorganic biocomposite membrane as an efficient adsorbent for Cr(VI) removal. Chem. Eng. J. 2011, 166, 81–87. [Google Scholar] [CrossRef]
- Guo, L.; Wu, W.; Zhou, Y.; Zhang, F.; Zeng, R.; Zeng, J. Layered double hydroxide coatings on magnesium alloys: A review. J. Mater. Sci. Technol. 2018, 34, 1455–1466. [Google Scholar] [CrossRef]
- Okamoto, K.; Iyi, N.; Sasaki, T. Factors affecting the crystal size of the MgAl-LDH (layered double hydroxide) prepared by using ammonia-releasing reagents. Appl. Clay Sci. 2007, 37, 23–31. [Google Scholar] [CrossRef]
- Iqbal, M.; Fedel, M. Effect of Synthesis Conditions on the Controlled Growth of MgAl–LDH Corrosion Resistance Film: Structure and Corrosion Resistance Properties. Coatings 2019, 9, 30. [Google Scholar] [CrossRef]
- Bukhtiyarova, M.V. A review on effect of synthesis conditions on the formation of layered double hydroxides. J. Solid State Chem. 2019, 269, 494–506. [Google Scholar] [CrossRef]
- Miyata, S. Anion-exchange properties of hydrotalcite-like compounds. Clay Clay Miner. 1983, 31, 305–311. [Google Scholar] [CrossRef]
- Bravo-Suárez, J.J.; Páez-Mozo, E.A.; Oyama, S.T. Review of the synthesis of layered double hydroxides: A thermodynamic approach. Quim. Nova 2004, 27, 601–604. [Google Scholar] [CrossRef]
- Yan, K.; Wu, G.; Jin, W. Recent Advances in the Synthesis of Layered, Double-Hydroxide-Based Materials and Their Applications in Hydrogen and Oxygen Evolution. Energy Technol. 2016, 4, 354–368. [Google Scholar] [CrossRef]
- Chubar, N.; Gilmour, R.; Gerda, V.; Mičušík, M.; Omastova, M.; Heister, K.; Man, P.; Fraissard, J.; Zaitsev, V. Layered double hydroxides as the next generation inorganic anion exchangers: Synthetic methods versus applicability. Adv. Colloid Interface Sci. 2017, 245, 62–80. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ohare, D. Recent advances in the synthesis and application of layered double hydroxide (LDH) nanosheets. Chem. Rev. 2012, 112, 4124–4155. [Google Scholar] [CrossRef] [PubMed]
- Nalawade, P.; Aware, B.; Kadam, V.J.; Hirlekar, R.S. Layered double hydroxides: A review. J. Sci. Ind. Res. 2009, 68, 267–272. [Google Scholar]
- Theiss, F.L.; Ayoko, G.A.; Frost, R.L. Synthesis of layered double hydroxides containing Mg2+, Zn2+, Ca2+ and Al3+ layer cations by co-precipitation methods—A review. Appl. Surf. Sci. 2016, 383, 200–213. [Google Scholar] [CrossRef]
- Salak, A.N.; Tedim, J.; Kuznetsova, A.I.; Ribeiro, J.L.; Vieira, L.G.; Zheludkevich, M.L.; Ferreira, M.G. Comparative X-ray diffraction and infrared spectroscopy study of Zn-Al layered double hydroxides: Vanadate vs nitrate. Chem. Phys. 2012, 397, 102–108. [Google Scholar] [CrossRef]
- Buchheit, R.G.; Guan, H.; Mahajanam, S.; Wong, F. Active corrosion protection and corrosion sensing in chromate-free organic coatings. Prog. Org. Coat. 2003, 4, 174–182. [Google Scholar] [CrossRef]
- Mahajanam, S.P.V.; Buchheit, R.G. Characterization of inhibitor release from Zn-Al-[V10 O28]6- hydrotalcite pigments and corrosion protection from hydrotalcite-pigmented epoxy coatings. Corrosion 2008, 64, 230–240. [Google Scholar] [CrossRef]
- Williams, G.; McMurray, H.N. Anion-exchange inhibition of filiform corrosion on organic coated AA2024-T3 aluminum alloy by hydrotalcite-like pigments. Electrochem. Solid-State Lett. 2003, 6, 9–11. [Google Scholar] [CrossRef]
- Williams, G.; McMurray, H.N. Inhibition of filiform corrosion on polymer coated AA2024-T3 by hydrotalcite-like pigments incorporating organic anions. Electrochem. Solid-State Lett. 2004, 7, 13–15. [Google Scholar] [CrossRef]
- Michailidou, E.; McMurray, H.N.; Williams, G. Inhibition of filiform corrosion on organic-coated magnesium-containing galvanized steel by smart-release ion exchange pigments. ECS Trans. 2017, 80, 585–591. [Google Scholar] [CrossRef]
- Poznyak, S.K.; Tedim, J.; Rodrigues, L.M.; Salak, A.N.; Zheludkevich, M.L.; Dick, L.F.; Ferreira, M.G. Novel inorganic host layered double hydroxides intercalated with guest organic inhibitors for anticorrosion applications. ACS Appl. Mater. Interfaces 2009, 1, 2353–2362. [Google Scholar] [CrossRef]
- Zheludkevich, M.L.; Poznyak, S.K.; Rodrigues, L.M.; Raps, D.; Hack, T.; Dick, L.F.; Nunes, T.; Ferreira, M.G.S. Active protection coatings with layered double hydroxide nanocontainers of corrosion inhibitor. Corros. Sci. 2010, 52, 602–611. [Google Scholar] [CrossRef]
- Stimpfling, T.; Leroux, F.; Hintze-Bruening, H. Organo-modified layered double hydroxide in coating formulation to protect AA2024 from corrosion. Colloids Surf., A 2014, 458, 147–154. [Google Scholar] [CrossRef]
- Stimpfling, T.; Leroux, F.; Hintze-Bruening, H. Unraveling EDTA corrosion inhibition when interleaved into Layered Double Hydroxide epoxy filler system coated onto aluminum AA 2024. Appl. Clay Sci. 2013, 84, 32–41. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y.; Yu, M.; Li, S.; Xue, B.; Yin, X. Influence of embedded ZnAlCe-NO3- layered double hydroxides on the anticorrosion properties of sol-gel coatings for aluminum alloy. Prog. Org. Coat. 2015, 81, 93–100. [Google Scholar] [CrossRef]
- Galvão, T.L.; Neves, C.S.; Caetano, A.P.; Maia, F.; Mata, D.; Malheiro, E.; Ferreira, M.J.; Bastos, A.C.; Salak, A.N.; Gomes, J.R.; et al. Control of crystallite and particle size in the synthesis of layered double hydroxides: Macromolecular insights and a complementary modeling tool. J. Colloid Interf. Sci. 2016, 468, 86–94. [Google Scholar] [CrossRef]
- Buchheit, R.G., Jr.; Stoner, G.E. Method for increasing the corrosion resistance of aluminum and aluminum alloys. US Patent number RE35576, 29 July 1997. [Google Scholar]
- Zhang, Y.; Li, Y.; Ren, Y.; Wang, H.; Chen, F. Double-doped LDH films on aluminum alloys for active protection. Mater. Lett. 2017, 192, 33–35. [Google Scholar] [CrossRef]
- Zhou, B.; Wei, X.; Wang, Y.; Huang, Q.; Hong, B.; Wei, Y. Effect of lanthanum addition on microstructures and corrosion behavior of ZnAl-LDHs film of 6061 aluminum alloys. Surf. Coat. Technol. 2019, 379, 125056. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Fedel, M. The effect of the surface morphologies on the corrosion resistance of in situ growth MgAl-LDH based conversion film on AA6082. Surf. Coat. Technol. 2018, 352, 166–174. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Sun, L.; Fedel, M. Synthesis of novel cone—shaped CaAl - LDH directly on aluminum alloy by a facile urea hydrolysis method. SN Appl. Sci. 2019, 1, 1415. [Google Scholar] [CrossRef]
- Serdechnova, M.; Mohedano, M.; Bouali, A.C.; Höche, D.; Kuznetsov, B.; Karpushenkov, S.; Blawert, C.; Zheludkevich, M.L. Role of Phase Composition of PEO Coatings on AA2024 for In-Situ LDH Growth. Coatings 2017, 7, 190. [Google Scholar] [CrossRef]
- Bouali, A.C.; Straumal, E.A.; Serdechnova, M.; Wieland, D.C.; Starykevich, M.; Blawert, C.; Hammel, J.U.; Lermontov, S.A.; Ferreira, M.G.; Zheludkevich, M.L. Applied Surface Science Layered double hydroxide based active corrosion protective sealing of plasma electrolytic oxidation/sol-gel composite coating on AA2024. Appl. Surf. Sci. 2019, 494, 829–840. [Google Scholar] [CrossRef]
- Mikhailau, A.; Maltanava, H.; Poznyak, S.K.; Salak, A.N.; Zheludkevich, M.L.; Yasakau, K.A.; Ferreira, M.G.S. One-step synthesis and growth mechanism of nitrate intercalated ZnAl LDH conversion coatings on zinc. Chem. Commun. 2019, 55, 6878–6881. [Google Scholar] [CrossRef] [PubMed]
- Tedim, J.; Zheludkevich, M.L.; Salak, A.N.; Lisenkov, A.; Ferreira, M.G.S. Nanostructured LDH-container layer with active protection functionality. J. Mater. Chem. 2011, 21, 15464–15470. [Google Scholar] [CrossRef]
- Zhang, F.; Zhao, L.; Chen, H.; Xu, S.; Evans, D.G.; Duan, X. Corrosion resistance of superhydrophobic layered double hydroxide films on aluminum. Angew. Chemie Int. Ed. 2008, 47, 2466–2469. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, K.; He, H.; Sun, W.; Zong, Q.; Liu, G. Enhanced corrosion resistance of MgAl hydrotalcite conversion coating on aluminum by chemical conversion treatment. Surf. Coat. Technol. 2013, 235, 484–488. [Google Scholar] [CrossRef]
- Kaseem, M.; Ko, Y.G. Benzoate intercalated Mg-Al-layered double hydroxides (LDHs) as efficient chloride traps for plasma electrolysis coatings. J. Alloys Compd. 2019, 787, 772–778. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Zhang, Y.; Yu, M.; Liu, J. Enhanced protective Zn-Al layered double hydroxide film fabricated on anodized 2198 aluminum alloy. J. Alloys Compd. 2015, 630, 29–36. [Google Scholar] [CrossRef]
- Serdechnova, M.; Mohedano, M.; Kuznetsov, B.; Mendis, C.L.; Starykevich, M.; Karpushenkov, S.; Tedim, J.; Ferreira, M.G.; Blawert, C.; Zheludkevich, M.L. PEO coatings with active protection based on in-situ formed LDH-nanocontainers. J. Electrochem. Soc. 2017, 164, C36–C45. [Google Scholar] [CrossRef]
- Kuznetsov, B.; Serdechnova, M.; Tedim, J.; Starykevich, M.; Kallip, S.; Oliveira, M.P.; Hack, T.; Nixon, S.; Ferreira, M.G.; Zheludkevich, M.L. Sealing of tartaric sulfuric (TSA) anodized AA2024 with nanostructured LDH layers. RSC Adv. 2016, 6, 13942–13952. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Fedel, M. Effect of operating parameters on the structural growth of ZnAl layered double hydroxide on AA6082 and corresponding corrosion resistance properties. J. Coat. Technol. Res. 2019, 16, 1423–1433. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Sun, L.; LaChance, A.M.; Ding, H.; Fedel, M. In situ growth of a CaAl-NO 3—layered double hydroxide film directly on an aluminum alloy for corrosion resistance. Dalton Trans. 2020, 49, 3956–3964. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zheng, D.; Li, X.; Lin, J.; Wang, C.; Dong, S.; Lin, C. Enhanced Corrosion Resistance of Superhydrophobic Layered Double Hydroxide Films with Long-Term Stability on Al Substrate. ACS Appl. Mater. Interfaces 2018, 10, 15150–15162. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.A.; Sun, M.A.L.; Asghar, H.; Fedel, M. Chlorides Entrapment Capability of Various In-Situ Grown NiAl-LDHs: Structural and Corrosion Resistance Properties. Coatings 2020, 10, 384. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, T.; Cai, R.; Li, Y.; Yang, W.; Caro, J. One-pot synthesis of NiAl-CO3 LDH anti-corrosion coatings from CO2-saturated precursors. RSC Adv. 2015, 5, 29552–29557. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, C.L.; Song, L.; Zeng, R.C.; Liu, Z.G.; Cui, H.Z. Corrosion of in-situ grown MgAl-LDH coating on aluminum alloy. Trans. Nonferrous Met. Soc. China 2015, 25, 3498–3504. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, D.; Lu, Z. Hydrophobic Mg-Al layered double hydroxide film on aluminum: Fabrication and microbiologically influenced corrosion resistance properties. Colloids Surf. A 2015, 474, 44–51. [Google Scholar] [CrossRef]
- Guo, X.; Xu, S.; Zhao, L.; Lu, W.; Zhang, F.; Evans, D.G.; Duan, X. One-step hydrothermal crystallization of a layered double hydroxide/alumina bilayer film on aluminum and its corrosion resistance properties. Langmuir 2009, 25, 9894–9897. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, C.L.; Song, L.; Zeng, R.C.; Cui, L.Y.; Cui, H.Z. Corrosion resistance of superhydrophobic mg-al layered double hydroxide coatings on aluminum alloys. Acta Metall. Sin. English Lett. 2015, 28, 1373–1381. [Google Scholar] [CrossRef]
- Luo, X.; Yuan, S.; Pan, X.; Zhang, C.; Du, S.; Liu, Y. Synthesis and Enhanced Corrosion Protection Performance of Reduced Graphene Oxide Nanosheet/ZnAl Layered Double Hydroxide Composite Films by Hydrothermal Continuous Flow Method. ACS Appl. Mater. Interfaces 2017, 9, 18263–18275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, P.; Wang, J.; Li, Y.; Chen, F.; Wei, K.; Zuo, Y. LDHs/graphene film on aluminum alloys for active protection. Appl. Surf. Sci. 2018, 433, 927–933. [Google Scholar] [CrossRef]
- Álvarez, D.; Collazo, A.; Hernández, M.; Nóvoa, X.R.; Pérez, C. Characterization of hybrid sol-gel coatings doped with hydrotalcite-like compounds to improve corrosion resistance of AA2024-T3 alloys. Prog. Org. Coat. 2010, 68, 91–99. [Google Scholar] [CrossRef]
- Li, W.; Zhang, X.; Yang, J.; Miao, F. In situ growth of superhydrophobic and icephobic films with micro/nanoscale hierarchical structures on the aluminum substrate. J. Colloid Interface Sci. 2013, 410, 165–171. [Google Scholar] [CrossRef]
- Mohedano, M.; Serdechnova, M.; Starykevich, M.; Karpushenkov, S.; Bouali, A.C. Active protective PEO coatings on AA2024: Role of voltage on in-situ LDH growth. Mater. Des. 2017, 120, 36–46. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Zhang, Y.; Yu, M.; Liu, J. Fabrication of superhydrophobic layered double hydroxides films with different metal cations on anodized aluminum 2198 alloy. Mater. Lett. 2015, 142, 137–140. [Google Scholar] [CrossRef]
- Subasri, R.; Raju, K.S.; Reddy, D.S.; Jyothirmayi, A.; Ijeri, V.S.; Prakash, O.; Gaydos, S.P. Environmentally friendly Zn–Al layered double hydroxide (LDH)-based sol–gel corrosion protection coatings on AA 2024-T3. J. Coat. Technol. Res. 2019, 16, 1447–1463. [Google Scholar] [CrossRef]
- Lin, K.; Luo, X.; Pan, X.; Zhang, C.; Liu, Y. Enhanced corrosion resistance of LiAl-layered double hydroxide (LDH) coating modified with a Schiff base salt on aluminum alloy by one step in-situ synthesis at low temperature. Appl. Surf. Sci. 2019, 463, 1085–1096. [Google Scholar] [CrossRef]
- Liang, Z.; Ma, Y.; Li, K.; Liao, Y.; Yang, B.; Liu, L.; Zhu, P. Formation of layered double hydroxides fi lm on AA2099-T83 Al-Cu-Li alloy and its e ff ect on corrosion resistance. Surf. Coat. Technol. 2019, 378, 124967. [Google Scholar] [CrossRef]
- Radha, A.V.; Kamath, P.V.; Shivakumara, C. Mechanism of the anion exchange reactions of the layered double hydroxides ( LDHs ) of Ca and Mg with Al. Solid State Sci. 2005, 7, 1180–1187. [Google Scholar] [CrossRef]
- Allada, R.; Navrotsky, A. Thermochemistry and Aqueous Solubilities of Hydrotalcite-Like Solids. Science 2002, 296, 721–723. [Google Scholar] [CrossRef] [PubMed]
- Tedim, J.; Kuznetsova, A.; Salak, A.N.; Montemor, F.; Snihirova, D.; Pilz, M.; Zheludkevich, M.L.; Ferreira, M.G. Zn-Al layered double hydroxides as chloride nanotraps in active protective coatings. Corros. Sci. 2012, 55, 1–4. [Google Scholar] [CrossRef]
- Tedim, J.; Zheludkevich, M.L.; Bastos, A.C.; Salak, A.N.; Lisenkov, A.D.; Ferreira, M.G.S. Influence of preparation conditions of Layered Double Hydroxide conversion films on corrosion protection. Electrochim. Acta 2014, 117, 164–171. [Google Scholar] [CrossRef]
- Neves, C.S.; Bastos, A.C.; Salak, A.N.; Starykevich, M.; Rocha, D.; Zheludkevich, M.L.; Cunha, A.; Almeida, A.; Tedim, J.; Ferreira, M.G. Layered Double Hydroxide Clusters as Precursors of Novel Multifunctional Layers: A Bottom-Up Approach. Coatings 2019, 9, 328. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, F.; Fu, S.; Duan, X. In situ microstructure control of oriented layered double hydroxide monolayer films with curved hexagonal crystals as superhydrophobic materials. Adv. Mater. 2006, 18, 3089–3093. [Google Scholar] [CrossRef]
- Wang, F.; Guo, Z. Insitu growth of durable superhydrophobic Mg e Al layered double hydroxides nanoplatelets on aluminum alloys for corrosion resistance. J. Alloys Compd. 2018, 767, 382–391. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Fedel, M. Ordering and disordering of in situ grown MgAl-layered double hydroxide and its effect on the structural and corrosion resistance properties. Int. J. Miner. Metall. Mater. 2019, 26, 1570–1577. [Google Scholar] [CrossRef]
- Kovanda, F.; Masatova, P.; Novotna, P.; Jratova, K. The formation of layered double hydroxides on alumina surface. Clays Clay Miner. 2009, 57, 425–432. [Google Scholar] [CrossRef]
- Chen, F.; Yu, P.; Zhang, Y. Healing effects of LDHs nanoplatelets on MAO ceramic layer of aluminum alloy. J. Alloys Compd. 2017, 711, 342–348. [Google Scholar] [CrossRef]
- Wang, L.; Li, B.; Zhao, X.; Chen, C.; Cao, J. Effect of Rare Earth Ions on the Properties of Composites Composed of Ethylene Vinyl Acetate Copolymer and Layered Double Hydroxides. PLoS ONE 2012, 7, e37781. [Google Scholar] [CrossRef]
- Van de Sanden, M.C.; Severens, R.J.; Bastiaanssen, J.; Schram, D.C. High-quality a-Si:H growth at high rate using an expanding thermal plasma. Surf. Coat. Technol. 1997, 97, 719–722. [Google Scholar] [CrossRef]
- Lyon, S.B.; Bingham, R.; Mills, D.J. Progress in Organic Coatings Advances in corrosion protection by organic coatings: What we know and what we would like to know. Prog. Org. Coat. 2017, 102, 2–7. [Google Scholar] [CrossRef]
- Imanieh, I.; Afshar, A. Corrosion protection of aluminum by smart coatings containing layered double hydroxide (LDH) nanocontainers. J. Mater. Res. Technol. 2019, 8, 3004–3023. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Li, Y.; Yu, M.; Yin, X.; Li, S. Enhancement of active anticorrosion via Ce-doped Zn-Al layered double hydroxides embedded in sol-gel coatings on aluminum alloy. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2017, 32, 1199–1204. [Google Scholar] [CrossRef]
- ISO. ISO. 8407: 2009 (E). Corrosion of Metals and Alloys—Removal of Corrosion Products from Corrosion Test Specimens; International Standards Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Bendinelli, E.V.; Aoki, I.V.; Barcia, O.; Margarit-mattos, I.C.P. Kinetic aspects of Mg-Al layered double hydroxides influencing smart corrosion protective behavior. Mater. Chem. Phys. 2019, 238, 121883. [Google Scholar] [CrossRef]
- Rojas, R.; Jimenez-kairuz, A.F.; Manzo, R.H.; Giacomelli, C.E. Colloids and Surfaces A: Physicochemical and Engineering Aspects Release kinetics from LDH-drug hybrids: Effect of layers stacking and drug solubility and polarity. Colloids Surf. A 2014, 463, 37–43. [Google Scholar] [CrossRef]
- Gao, X.; Lei, L.; Hare, D.O.; Xie, J.; Gao, P.; Chang, T. Journal of Solid State Chemistry Intercalation and controlled release properties of vitamin C intercalated layered double hydroxide. J. Solid State Chem. 2013, 203, 174–180. [Google Scholar] [CrossRef]
- Khodam, F.; Rezvani, Z.; Amani-ghadim, A.R. Journal of Industrial and Engineering Chemistry Enhanced adsorption of Acid Red 14 by co-assembled LDH/MWCNTs nanohybrid: Optimization, kinetic and isotherm. J. Ind. Eng. Chem. 2014, 21, 1286–1294. [Google Scholar] [CrossRef]
- Szauer, T. Impedance measurements for the evaluation of protective nonmetallic coatings. Prog. Org. Coat. 1982, 10, 171–183. [Google Scholar] [CrossRef]
- Mansfeld, F.; Kendig, M.W.; Tsai, S. Evaluation of corrosion behavior of coated metals with ac impedance measurements. Corrosion 1982, 38, 478–485. [Google Scholar] [CrossRef]
- MacDonald, D.D. Reflections on the history of electrochemical impedance spectroscopy. Electrochim. Acta 2006, 51, 1376–1388. [Google Scholar] [CrossRef]
- Bacon, R.C.; Smith, J.J.; Rugg, F.M. Electrolytic Resistance in Evaluating Protective Merit of Coatings on Metals. Ind. Eng. Chem. 1948, 40, 161–167. [Google Scholar] [CrossRef]
- Murray, J.N. Electrochemical test methods for evaluating organic coatings on metals: An update. Part I. Introduction and generalities regarding electrochemical testing of organic coatings. Prog. Org. Coat. 1997, 30, 225–233. [Google Scholar] [CrossRef]
- Amirudin, A.; Thierry, D. Application of electrochemical impedance spectroscopy to study the degradation of polymer-coated metals. Prog. Org. Coat. 1995, 26, 1–28. [Google Scholar] [CrossRef]
- Macdonald, J.R.; Hill, C.; Hooper, A. Company Analysis of hydrogen-doped lithium nitride admittance data. Solid State Ionics 1982, 6, 65–77. [Google Scholar] [CrossRef]
- Boukamp, B.A. A non linear leasts squares fit procedure for analysis of immittance data of electrochemical systems. Solid State Ionics 1985, 20, 31–44. [Google Scholar] [CrossRef]
- Kelly, G.J. Corrosion Engineering-Practices vs Principles. In Engineering Conference 1984: Conference Papers; Institution of Engineers: Brisbane, Australia, 1984; p. 376. [Google Scholar]
- Jorcin, J.B.; Orazem, M.E.; Pébère, N.; Tribollet, B. CPE analysis by local electrochemical impedance spectroscopy. Electrochim. Acta 2006, 51, 1473–1479. [Google Scholar] [CrossRef]
- Walter, G.W. The application of impedance spectroscopy to study the uptake of sodium chloride solution in painted metals. Corros. Sci. 1991, 32, 1041–1058. [Google Scholar] [CrossRef]
- Fréchette, E.; Compécre, C.; Ghali, E. Evaluation of the corrosion resistance of painted steels by impedance measurements. Corros. Sci. 1992, 33, 1067–1081. [Google Scholar] [CrossRef]
- Bierwagen, G.; Allahar, K.; Hinderliter, B.; Simões, A.M.P.; Tallman, D.; Croll, S. Ionic liquid enhanced electrochemical characterization of organic coatings. Prog. Org. Coat. 2008, 63, 250–259. [Google Scholar] [CrossRef]
- Amand, S.; Musiani, M.; Orazem, M.E.; Pébère, N.; Tribollet, B.; Vivier, V. Electrochimica Acta Constant-phase-element behavior caused by inhomogeneous water uptake in anti-corrosion coatings. Electrochim. Acta 2013, 87, 693–700. [Google Scholar] [CrossRef]
- Roberge, P.R. Corrosion Engineering: Principles and Practice; McGraw-Hill: New York, NY, USA, 2009. [Google Scholar]
- Montemor, M.F.; Snihirova, D.V.; Taryba, M.G.; Lamaka, S.V.; Kartsonakis, I.A.; Balaskas, A.C.; Kordas, G.C.; Tedim, J.; Kuznetsova, A.; Zheludkevich, M.L. Evaluation of self-healing ability in protective coatings modified with combinations of layered double hydroxides and cerium molibdate nanocontainers filled with corrosion inhibitors. Electrochim. Acta 2012, 60, 31–40. [Google Scholar] [CrossRef]
- Tedim, J.; Bastos, A.C.; Kallip, S.; Zheludkevich, M.L.; Ferreira, M.G.S. Corrosion protection of AA2024-T3 by LDH conversion films. Analysis of SVET results. Electrochim. Acta 2016, 210, 215–224. [Google Scholar] [CrossRef]
- Zadeh, M.A.; Tedim, J.; Zheludkevich, M.; van der Zwaag, S.; Garcia, S.J. Synergetic active corrosion protection of AA2024-T3 by 2D- anionic and 3D- cationic nanocontainers loaded with Ce and mercaptobenzothiazole. Corros. Sci. 2018, 135, 35–45. [Google Scholar] [CrossRef]
- Yasakau, K.A.; Kuznetsova, A.; Kallip, S.; Starykevich, M.; Tedim, J.; Ferreira, M.G.; Zheludkevich, M.L. A novel bilayer system comprising LDH conversion layer and sol-gel coating for active corrosion protection of AA2024. Corros. Sci. 2018, 143, 299–313. [Google Scholar] [CrossRef]

| LDH | Anion-Exchanger | Precursors | Alkaline Media | Method | Al Alloy | Synthetic Condition | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|---|
| °C | pH | Aging Time (h) | ||||||||
| ZnAl | V | ZnCl2, AlCl3 | NaOH | coprecipitation | 2024 | 55 | 6.3–6.5 | 12 | [50] | |
| ZnAl | V | ZnCl2, AlCl3 | NaOH | coprecipitation | 2024 | 55 | 6.3–6.5 | 12 | [51] | |
| MgAl | (MBT)/(QA) | Mg(NO3)2, Al(NO)3 | NaNO3 | coprecipitation | 2024 | 65 | 10 | 24 | [55] | |
| ZnAl | Zn(NO3)2, Al(NO)3 | 2024 | 65 | 10 | 24 | |||||
| ZnAlVO3 | - | Zn(NO3)2, Al(NO3)3, NaVO3 | NaOH | coprecipitated | 2024 | 65 | 9.5 | 24 | [56] | |
| MgAlVO3 | - | Mg(NO3)2, Al(NO3)3, NaVO3 | NaOH | coprecipitated | 65 | 9.5 | 24 | |||
| ZnAl | V | Zn(NO3)2, Al(NO3)3 | NaNO3 | coprecipitated | 65 | 10 | 24 | |||
| MgAl | V | Zn(NO3)2, Al(NO3)3 | NaNO3 | coprecipitated | 65 | 10 | 24 | |||
| ZnAl | 4-ABSA, 3-ABSA 3,4-HHBA | ZnCl2, AlCl3 | NaOH | coprecipitation | 2024 | 30 | 9 | 12 | [57] | |
| ZnAl | - | ZnCl2, AlCl3 | NaOH | coprecipitation | 2024 | 30 | 10 | 12 | [58] | |
| ZnAl | Na2CO3 | ZnCl2, AlCl3 | NaOH | coprecipitation | 2024 | 30 | 9 | 12 | ||
| ZnAl | K2CrO4 | ZnCl2, AlCl3 | NaOH | coprecipitation | 2024 | 30 | 10.5 | 12 | ||
| ZnAl | Na2C10H14N2O | ZnCl2, AlCl3 | NaOH | coprecipitation | 2024 | 30 | 10 | 12 | ||
| ZnAlCe | - | Zn(NO3)2, Al(NO3),Ce(NO3)3 | NaNO3 | coprecipitation | 2024 | 65 | 10 | 18 | [59] | |
| ZnAl | V | Zn(NO3)2 | - | In situ | 2024 | <100 | 7 | - | [69] | |
| ZnAl* | Laurate | Zn(NO3)2 | NH4NO3 | In situ | Al | 45 | 6.5 | 36 | [70] | |
| MgAl | 8HQ | Mg(NO3)2 | NH4NO3 | In situ | Al | 100 | 9 | 48 | [71] | |
| MgAl | C6H5COON | Mg(NO3)2, urea | NH4NO3 | In situ | 6061 | 45 | 10 | 24 | [72] | |
| ZnAl* | V | Zn(NO3)2 | NH4NO3 | In situ | 2198 | 45 | 7 | - | [73] | |
| ZnAl* | V | Zn(NO3)2 | NH4NO3 | In situ | 2024 | 95 | 6.5 | 0.5 | [74] | |
| ZnAl* | V | Zn(NO3)2 | NH4NO3 | In situ | 2024 | 95 | 6.85 | 0.5 | [75] | |
| MgAl | - | Mg(NO3)2, NH4NO3 | NH4OH | In situ | 6082 | 60/80 | 10 | 24 | [64] | |
| MgAl | - | Mg(NO3)2 | NH4OH | In situ | 6082 | 60/80/100 | 10 | 18/24 | [40] | |
| ZnAl | - | Zn(NO3)2, NH4NO3 | NH4OH | In situ | 6082 | 60/80 | 6/6.5/7 | 18/24 | [76] | |
| CaAl | - | Ca(NO3)2 | NaOH | In situ | 6082 | 140 | 10 | 18/24/72 | [77] | |
| ZnAl | Laurate | Zn(NO3)2, NH4NO3 | - | In situ | Al | 85 | 6.5 | 12 | [78] | |
| NiAl | - | Ni(NO3)2 | NaOH | In situ | 6082 | 130 | 10 | 24 | [79] | |
| NiAl | - | Ni(NO3)2, NH4NO3 | NH4OH | In situ | Al | 85 | - | 40 | [80] | |
| MgAl | - | Mg(NO3)2, NH4NO3 | - | In situ | 5005 | 125 | 8–10.5 | 1–8 | [81] | |
| MgAl | Laurate, Stearate, oleate | Mg(NO3)2, urea | - | In situ | Al | 70 | - | 24 | [82] | |
| ZnAl | - | Zn(NO3)2, NH4NO3 | NH4OH | In situ | Al | 120 | 6.5 | - | [83] | |
| MgAl | Stearic acid | Mg(NO3)2, NH4NO3 | NH4OH | In situ | 5005 | 125 | 10 | 4 | [84] | |
| ZnAl-RGO | RGO | Zn(NO3)2, NH4NO3, Al(NO3)3, RGO | NH4OH | Hydrothermal continuous flow | A6N01 | 130–80 | 5.6 | 0.75–2 | [85] | |
| ZnAl | Mo, graphene | Zn(NO3)2, NH4NO3 | - | In situ | 2024 | 45 | 8.8 | 6 | [86] | |
| MgAl | Sol gel | Mg(NO3)2, Al(NO3)3 | NaOH | coprecipitation | 2024 | 70 | 18 | [87] | ||
| ZnAl | Stearic acid | Zn(CH3COO)2 | NH4OH | In situ | Al | 60 | 4 | [88] | ||
| ZnAl | V | Zn(NO3)2 | NH4NO3 | NH4OH | In situ | 2024 | 95 | 6.5 | 0.5 | [89] |
| MgAl | PFDTMS | Mg(NO3)2 | NH4OH | In situ | 2198 | 45 | 7 | 80 | [90] | |
| CoAl | Co(NO3)2 | |||||||||
| NiAl | Ni(NO3)2 | |||||||||
| ZnAl | Zn(NO3)2 | |||||||||
| ZnAl | MBT/8HQ/V/PA/Mo | Zn(NO3)2 | - | coprecipitation | 2024 | 60 | 20 | [91] | ||
| LiAl | Vanillin, aspartic acid | Li(NO3)2 | - | In situ | A6N01 | 60 | 0.33 | [92] | ||
| LDH | Substrate | Potentiodynamic Curves | Electrochemical Impedance Spectroscopy | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|
| Electrolyte (NaCl) | icorr sub. (A cm−2) | icorr Film (A cm−2) | Electrolyte (NaCl) | Immersion Time (h) | Rpol (Ω cm2) | Rcoat (Ω cm2) | |||
| ZnAlNO3 | 2024 | - | - | - | 0.05 | 1000 | 105 | - | [69] |
| ZnAlV2O7 | - | - | - | 106 | 2 × 103 | ||||
| MgAlNO3 | Pure Al | 3.5 wt.% | 1.5 × 10−7 | 1.95 × 10−8 | 3.5 wt.% | 336 | 3.3 × 106 | 82 | [71] |
| MgAl-8HQ | 1.02 × 10−9 | 44.3 × 106 | 128 | ||||||
| MgAlNO3 | 6061 | - | 0.05M | 10 | 9.3 × 107 | 9.0 × 105 | [72] | ||
| MgAl-C6H5COO | 3.7 × 109 | 5.2 × 107 | |||||||
| AN-ZnAl | 2198 | - | 0.5M | - | 1.0 × 106 | 6.1 × 102 | [73] | ||
| AN-ZnAl-VO3 | 1.0 × 107 | 8.6 × 102 | |||||||
| AN-ZnAl-VO3 | 9.7 × 106 | 4.9 × 103 | |||||||
| PEO-ZnAlVOx | 2024 | - | 0.5 wt.% | 72 | 47 × 103 | [74] | |||
| PEO-ZnAlNO3 | 2024 | 0.05 M | 336 | 1.8 × 107 | 2.3 × 104 | [75] | |||
| PEO-ZnAlVOx | - | 3.3 × 104 | |||||||
| MgAlNO3 | 6082 | 0.1 M | 7.5 × 10−6 | 8.3 × 10−10 | 0.1 M | - | - | - | [64] |
| MgAlNO3 | 6082 | 0.1 M | 7.5 × 10−6 | 1.9 × 10−11 | 0.1 M | 1 | - | 4.2 × 109 | [76] |
| MgAlNO3 | 6.3 × 10−10 | 3.8 × 108 | 2.3 × 108 | ||||||
| CaAlNO3 | 6082 | 0.1 | 7.5 × 10−6 | 7.0 × 10−10 | - | - | - | - | [77] |
| ZnAlNO3 | Pure Al | 3.5 wt.% | 4.4 × 10−6 | 1.1 × 10−7 | 3.5 wt.% | 1 | - | 9.9 × 104 | [78] |
| ZnAlLa | 6 × 10−8 | - | 1.2 × 105 | ||||||
| NiAlNO3 | 6082 | 0.1 | 7.5 × 10−7 | 1.410−9 | 0.1 | 1 | 2.5 × 109 | 1.4 × 106 | [79] |
| NiAlCO3 | Pure Al | 3.5 wt.% | 10−6 | 10−9 | - | - | - | - | [80] |
| MgAl-oleate | Pure Al | - | - | - | 3.5 wt.% | 168 | 6.8 × 106 | 8.2 × 104 | [82] |
| MgAl Laurate | - | - | - | 7.0 × 1011 | 9.1 × 105 | ||||
| MgAl stearate | - | - | - | 6.0 × 1010 | 1.1 × 106 | ||||
| MgAl-SA | 5005 | 3.5 wt.% | 1.3 × 10−5 | 2.0 × 10−8 | - | - | - | - | [70] |
| ZnAlNO3 | 6N01 | 3.5 wt.% | 4.7 × 10−6 | 5.3 × 10−5 | 3.5 wt.% | 168 | - | 1.9 × 1010 | [85] |
| ZnAl/RGO | 4.3 × 10−8 | - | 2.4 × 1010 | ||||||
| ZnAlNO3 | 2024 | - | - | - | 3.5 wt.% | 48 | 7.4 × 103 | 2.3 × 102 | [86] |
| ZnAlMO | - | - | - | 2.9 × 104 | 9.9 × 102 | ||||
| ZnAlMO/GN | - | - | - | 3.9 × 105 | 2.4 × 103 | ||||
| LiAlNO3 | A6N01 | 3.5 wt.% | 0.32 × 10−6 | 0.19 × 10−6 | 3.5 wt.% | 120 | 3.5 × 105 | - | [92] |
| LiNO3/Vanillin | 0.32 × 10−6 | 0.03 × 10−6 | 3.5 × 107 | 3.8 × 109 | |||||
| MgAl-FAS-13 | 6061 | 3.5 wt.% | 1.5 × 10−4 | 7.9 × 10−6 | 3.5 wt.% | 408 | 3.7 × 103 | - | [100] |
| MgAl-PVA | 5054 | - | - | - | 3.5 wt.% | 480 | 3.3 × 104 | 7.4 × 104 | [107] |
| ZnAlV2O7 | 2024 | - | - | - | 0.5 M | 1 | - | 1.0 × 104 | [132] |
| LDH | Substrate | Potentiodynamic Curves | Electrochemical Impedance Spectroscopy | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Electrolyte (NaCl) | icorr sub. (A cm−2) | icorr Film (A cm−2) | Electrolyte (NaCl) | Immersion Time (h) | Rpol (Ω cm2) | Rcoat (Ω cm2) | ||||
| ZnAl-V/PVA | 2024 | - | - | - | 0.5 M | 200 | - | 1 × 107 | [50] | |
| ZnAl-V/epoxy | 2024 | 0.124 M | 1 × 10−6 | 2 × 10−8 | - | - | - | - | [51] | |
| ZnAl/3-ABSA | 2024 | 0.5 M | - | 1.8 × 10−7 | 0.005 M | 1400 | - | 780 | [57] | |
| ZnAl/3,4-HHBA | - | 6.4 × 10−7 | - | 290 | ||||||
| LDH/4-ABSA | - | 4.4 × 10−7 | - | 910 | ||||||
| ZnAl-EDTA | 2024 | 0.5 M | - | 2.9 × 10−7 | 0.5 M | 1608 | - | 8 × 105 | [58] | |
| ZnAL-CO3 | - | 3.8 × 10−7 | - | 2 × 104 | ||||||
| ZnAl-CrO4 | - | 0.69 × 10−7 | - | 4 × 105 | ||||||
| ZnAl-Cl | - | 2.4 × 10−7 | - | 2 × 103 | ||||||
| ZnAl-Ce/sol gel | 2024 | - | - | 0.05 | 336 | - | 334.9 | [59] | ||
| MgAl-sol-gel | 2024 | - | - | 0.1 M | 210 | - | 300 | [87] | ||
| ZnAl E3/E1 | 2024 | 3.5 wt.% | 4.4×10-5 | 1.4 × 10−6 | 3.5 wt.% | 120 | - | 4.1 × 103 | [91] | |
| ZnAl-sol-gel | - | - | 7.6 × 10−7 | - | - | - | 7.6 × 103 | |||
| ZnAl-sol-gel | - | - | 1.8 × 10−7 | - | - | - | 1.6 × 104 | |||
| ZnAl-sol-gel | - | - | 2.6 × 10−7 | - | - | - | 4.6 × 103 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqbal, M.A.; Sun, L.; Barrett, A.T.; Fedel, M. Layered Double Hydroxide Protective Films Developed on Aluminum and Aluminum Alloys: Synthetic Methods and Anti-Corrosion Mechanisms. Coatings 2020, 10, 428. https://doi.org/10.3390/coatings10040428
Iqbal MA, Sun L, Barrett AT, Fedel M. Layered Double Hydroxide Protective Films Developed on Aluminum and Aluminum Alloys: Synthetic Methods and Anti-Corrosion Mechanisms. Coatings. 2020; 10(4):428. https://doi.org/10.3390/coatings10040428
Chicago/Turabian StyleIqbal, Muhammad Ahsan, Luyi Sun, Allyson T. Barrett, and Michele Fedel. 2020. "Layered Double Hydroxide Protective Films Developed on Aluminum and Aluminum Alloys: Synthetic Methods and Anti-Corrosion Mechanisms" Coatings 10, no. 4: 428. https://doi.org/10.3390/coatings10040428
APA StyleIqbal, M. A., Sun, L., Barrett, A. T., & Fedel, M. (2020). Layered Double Hydroxide Protective Films Developed on Aluminum and Aluminum Alloys: Synthetic Methods and Anti-Corrosion Mechanisms. Coatings, 10(4), 428. https://doi.org/10.3390/coatings10040428






