Chlorides Entrapment Capability of Various In-Situ Grown NiAl-LDHs: Structural and Corrosion Resistance Properties
Abstract
1. Introduction
2. Experimental Section
2.1. Material and Methods
2.2. Characterization
3. Results and Discussion
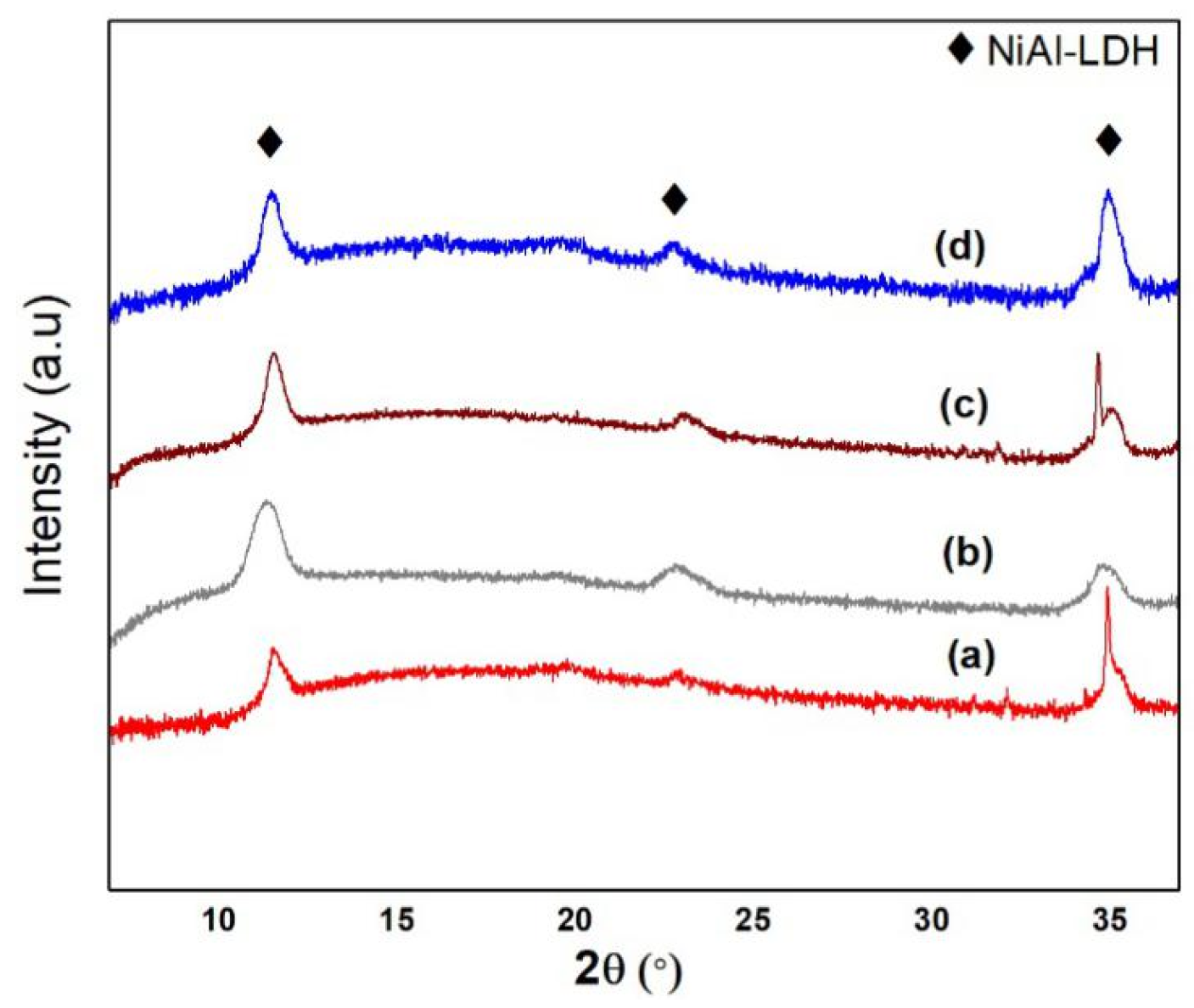
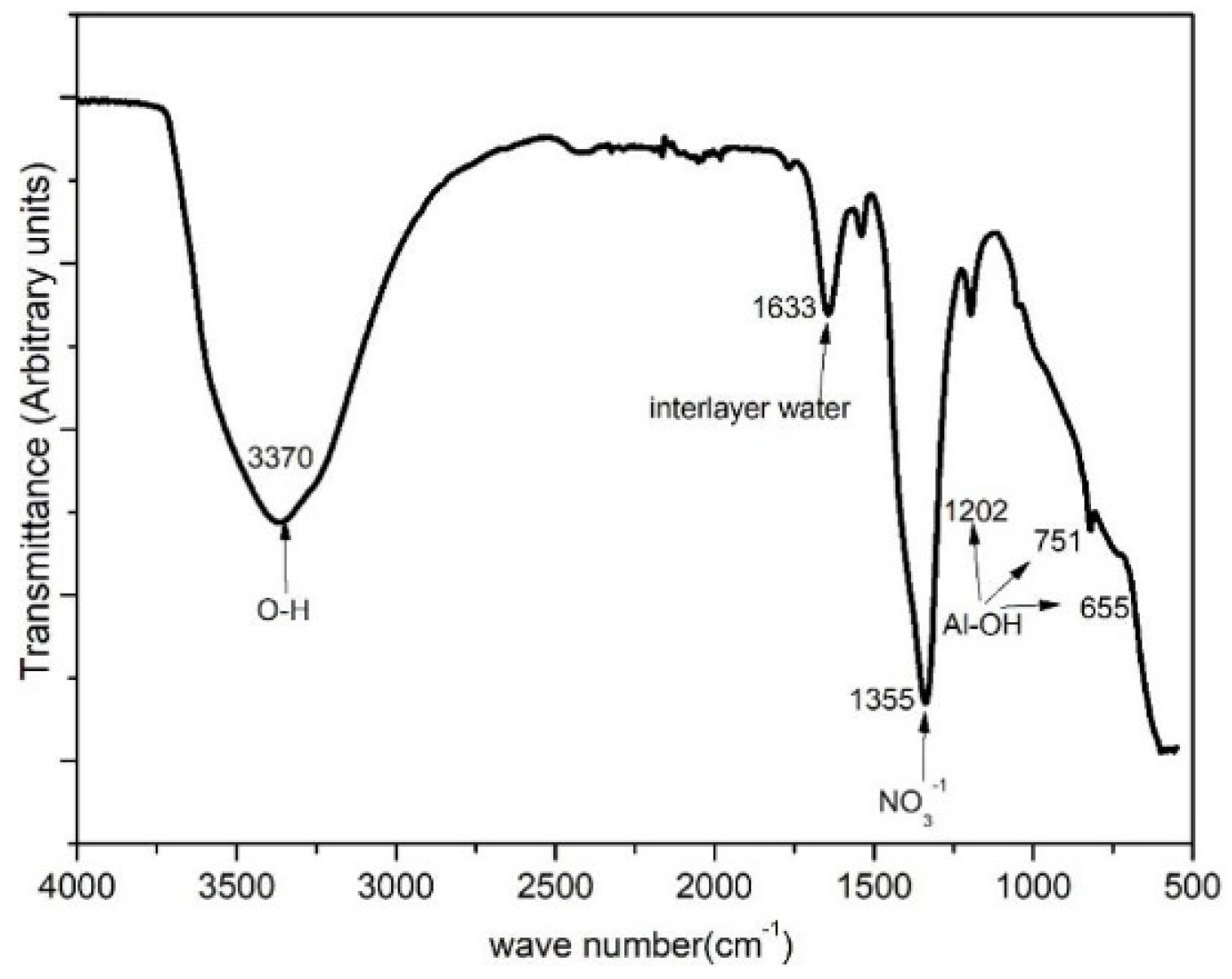
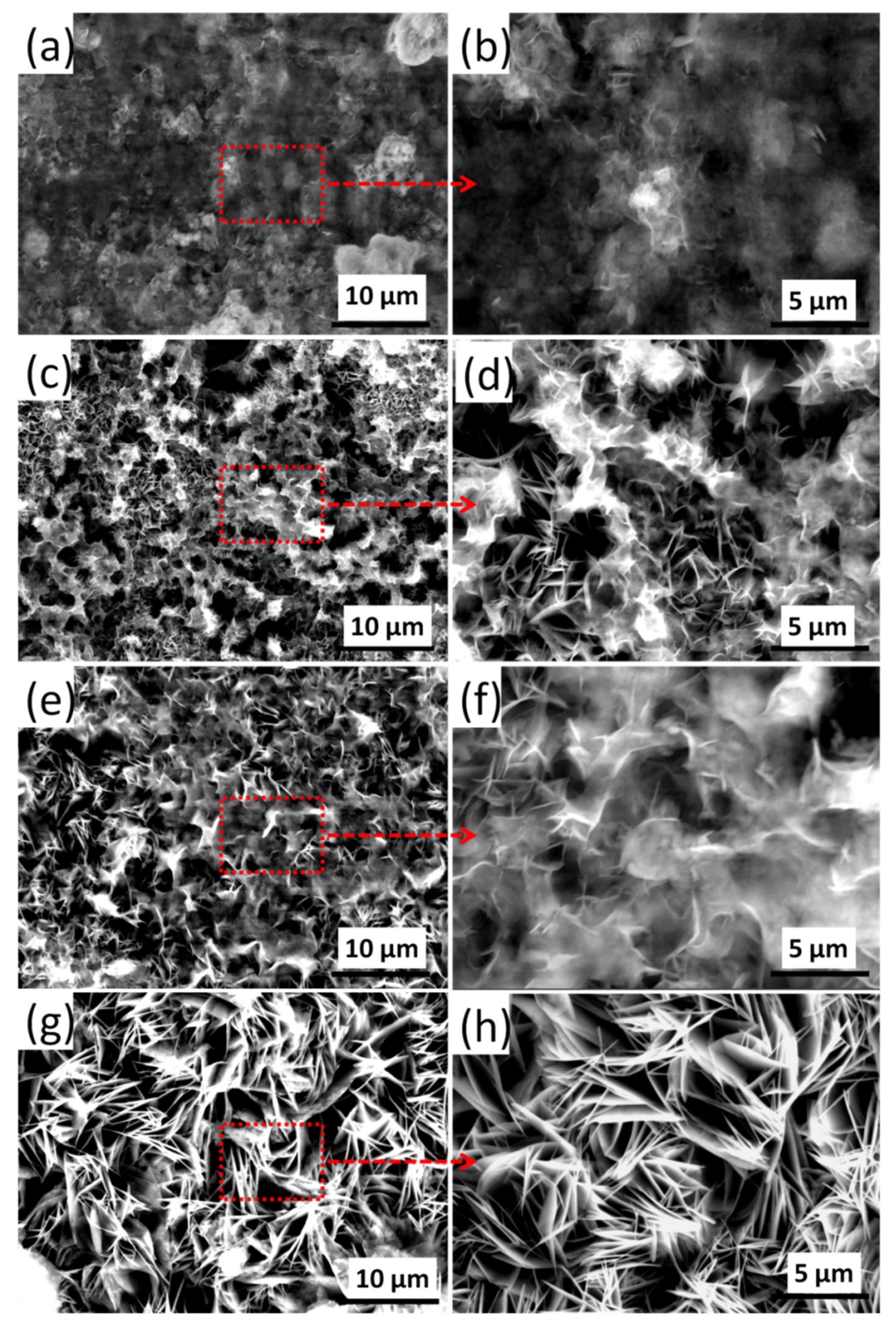
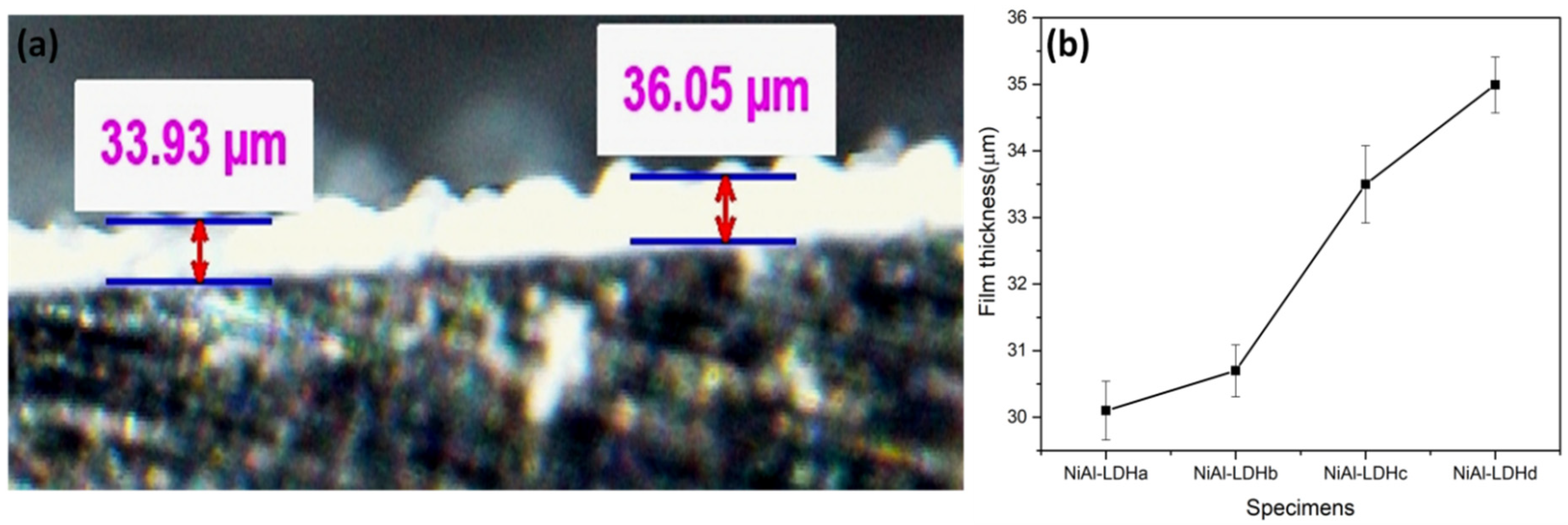
3.1. Structure and Morphology of the LDH Films
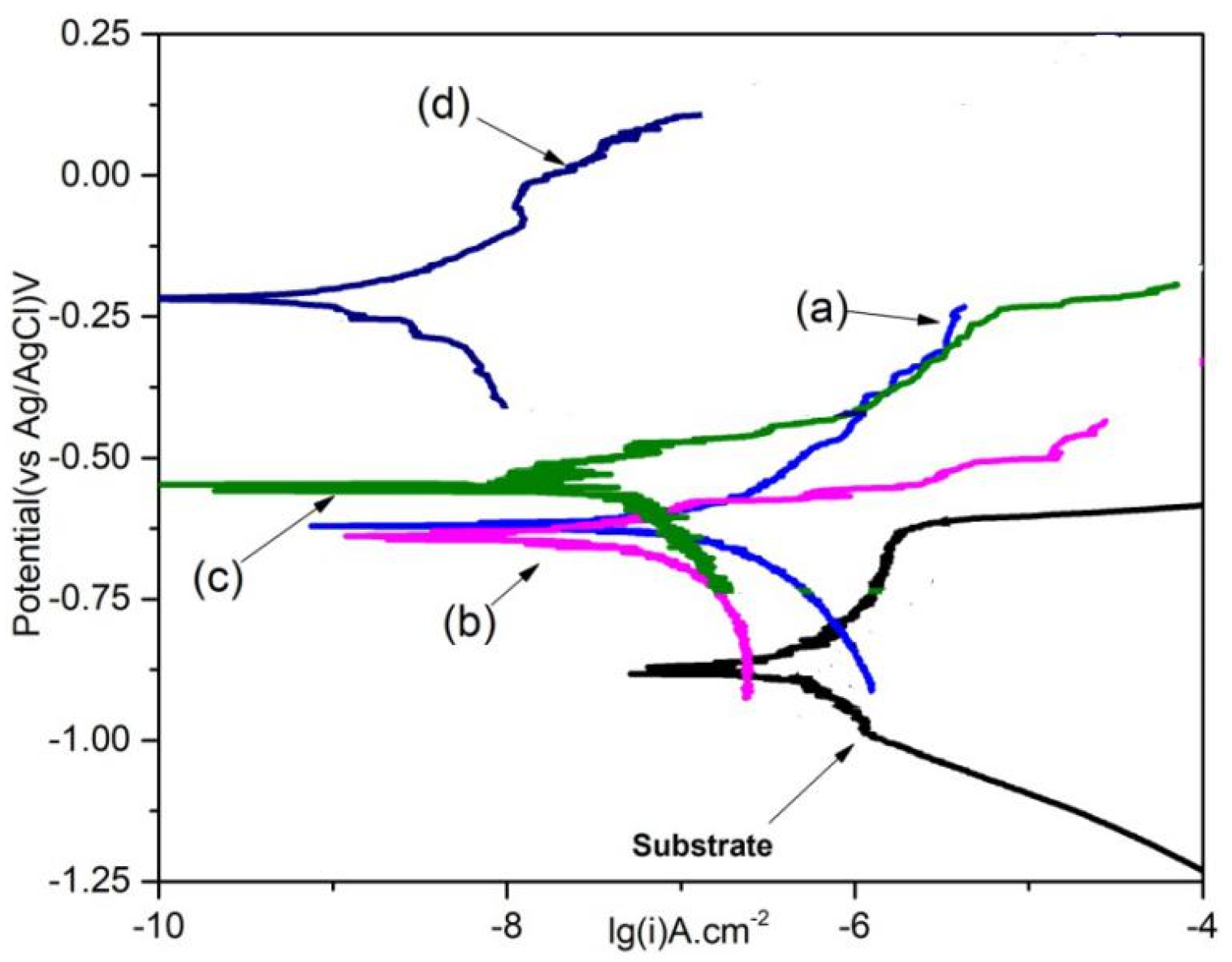
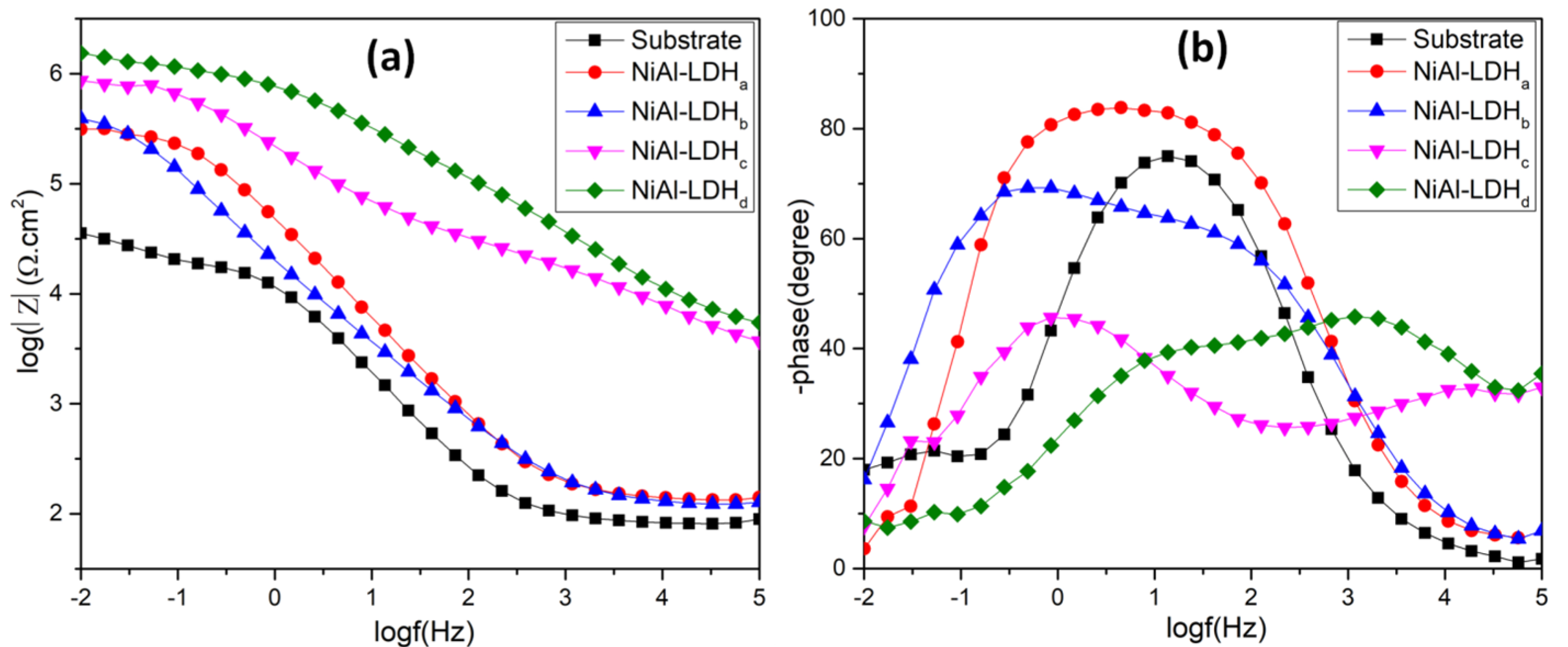
3.2. Corrosion Behavior of the LDH Films
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Schäfer, H.; Stock, H.-R. Improving the corrosion protection of aluminium alloys using reactive magnetron sputtering. Corros. Sci. 2005, 47, 953–964. [Google Scholar] [CrossRef]
- Venugopal, A.; Panda, R.; Manwatkar, S.; Sreekumar, K.; Krishna, L.R.; Sundararajan, G. Effect of micro arc oxidation treatment on localized corrosion behaviour of AA7075 aluminum alloy in 3.5% NaCl solution. Trans. Nonferrous Met. Soc. China 2012, 22, 700–710. [Google Scholar] [CrossRef]
- Qu, J.-E.; Chen, G.; Wang, H.; Nie, D.-J. Effect of water content on corrosion inhibition behavior of self-assembled TDPA on aluminum alloy surface. Trans. Nonferrous Met. Soc. China 2013, 23, 3137–3144. [Google Scholar] [CrossRef]
- Lutz, A.; Berg, O.V.D.; Van Damme, J.; Verheyen, K.; Bauters, E.; De Graeve, I.; Du Prez, F.E.; Terryn, H. A Shape-Recovery Polymer Coating for the Corrosion Protection of Metallic Surfaces. ACS Appl. Mater. Interfaces 2014, 7, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Han, E.-H.; Liu, F.; Kallip, S. Protection of 2024-T3 aluminium alloy by corrosion resistant phytic acid conversion coating. Appl. Surf. Sci. 2013, 280, 325–331. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, F.; Peng, Q.; Xu, S.; Lei, X.; Evans, D.G.; Duan, X. Layered double hydroxide/eggshell membrane: An inorganic biocomposite membrane as an efficient adsorbent for Cr(VI) removal. Chem. Eng. J. 2011, 166, 81–87. [Google Scholar] [CrossRef]
- Yang, J.-H.; Han, Y.-S.; Park, M.; Park, T.; Hwang, S.-J.; Choy, J.-H. New Inorganic-Based Drug Delivery System of Indole-3-Acetic Acid-Layered Metal Hydroxide Nanohybrids with Controlled Release Rate. Chem. Mater. 2007, 19, 2679–2685. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Asghar, H.; Iqbal, M.A.; Fedel, M. Sorption of As(V) from aqueous solution using in situ growth MgAl–NO3 layered double hydroxide thin film developed on AA6082. SN Appl. Sci. 2019, 1, 666. [Google Scholar] [CrossRef]
- Ladewig, K.; Xu, Z.P.; Lu, G. (Max) Layered double hydroxide nanoparticles in gene and drug delivery. Expert Opin. Drug Deliv. 2009, 6, 907–922. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Fedel, M. Effect of Synthesis Conditions on the Controlled Growth of MgAl–LDH Corrosion Resistance Film: Structure and Corrosion Resistance Properties. Coatings 2019, 9, 30. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Sun, L.; LaChance, A.M.; Ding, H.; Fedel, M. In situ growth of a CaAl-NO 3−-layered double hydroxide film directly on an aluminum alloy for corrosion resistance. Dalton Trans. 2020, 49, 3956–3964. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.A.; Fedel, M. The effect of the surface morphologies on the corrosion resistance of in situ growth MgAl-LDH based conversion film on AA6082. Surf. Coat. Technol. 2018, 352, 166–174. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Fedel, M. Effect of operating parameters on the structural growth of ZnAl layered double hydroxide on AA6082 and corresponding corrosion resistance properties. J. Coat. Technol. Res. 2019, 16, 1423–1433. [Google Scholar] [CrossRef]
- Sun, X.; Neuperger, E.; Dey, S. Insights into the synthesis of layered double hydroxide (LDH) nanoparticles: Part 1. Optimization and controlled synthesis of chloride-intercalated LDH. J. Colloid Interface Sci. 2015, 459, 264–272. [Google Scholar] [CrossRef]
- Israëli, Y.; Taviot-Gueho, C.; Besse, J.-P.; Morel, J.-P.; Morel-Desrosiers, N. Thermodynamics of anion exchange on a chloride-intercalated zinc–aluminum layered double hydroxide: A microcalorimetric study. J. Chem. Soc. Dalton Trans. 2000, 791–796. [Google Scholar] [CrossRef]
- Cao, Y.; Zheng, D.; Li, X.; Lin, J.; Wang, C.; Dong, S.; Lin, C. Enhanced Corrosion Resistance of Superhydrophobic Layered Double Hydroxide Films with Long-Term Stability on Al Substrate. ACS Appl. Mater. Interfaces 2018, 10, 15150–15162. [Google Scholar] [CrossRef]
- Lv, Z.; Yang, S.; Zhu, H.; Chen, L.; Alharbi, N.; Wakeel, M.; Wahid, A.; Chen, C. Highly efficient removal of As(V) by using NiAl layered double oxide composites. Appl. Surf. Sci. 2018, 448, 599–608. [Google Scholar] [CrossRef]
- Ye, X.; Jiang, Z.; Li, L.; Xie, Z. In-Situ Growth of NiAl-Layered Double Hydroxide on AZ31 Mg Alloy towards Enhanced Corrosion Protection. Nanomaterials 2018, 8, 411. [Google Scholar] [CrossRef]
- Zhang, F.; Guo, L.; Xu, S.; Zhang, R. Preparation of Nickel–Aluminum-Containing Layered Double Hydroxide Films by Secondary (Seeded) Growth Method and Their Electrochemical Properties. Langmuir 2015, 31, 6704–6712. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, T.; Cai, R.; Li, Y.; Yang, W.; Caro, J. One-pot synthesis of NiAl–CO3 LDH anti-corrosion coatings from CO2-saturated precursors. RSC Adv. 2015, 5, 29552–29557. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, F.; Fu, S.; Duan, X. In Situ Microstructure Control of Oriented Layered Double Hydroxide Monolayer Films with Curved Hexagonal Crystals as Superhydrophobic Materials. Adv. Mater. 2006, 18, 3089–3093. [Google Scholar] [CrossRef]
- Abderrazek, K.; Srasra, N.F.; Srasra, E. Synthesis and Characterization of [Zn-Al] Layered Double Hydroxides: Effect of the Operating Parameters. J. Chin. Chem. Soc. 2017, 64, 346–353. [Google Scholar] [CrossRef]
- Kameda, T.; Fubasami, Y.; Yoshioka, T. Kinetics and equilibrium studies on the treatment of nitric acid with Mg–Al oxide obtained by thermal decomposition of NO3--intercalated Mg–Al layered double hydroxide. J. Colloid Interface Sci. 2011, 362, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.A.; Fedel, M. Ordering and disordering of in situ grown MgAl-layered double hydroxide and its effect on the structural and corrosion resistance properties. Int. J. Miner. Met. Mater. 2019, 26, 1570–1577. [Google Scholar] [CrossRef]
- Aisawa, S.; Hirahara, H.; Uchiyama, H.; Takahashi, S.; Narita, E. Synthesis and Thermal Decomposition of Mn–Al Layered Double Hydroxides. J. Solid State Chem. 2002, 167, 152–159. [Google Scholar] [CrossRef]
- Wu, Q.; Olafsen, A.; Ørnulv, V.; Roots, J.; Norby, P. Delamination and restacking of a layered double hydroxide with nitrate as counter anion. J. Mater. Chem. 2005, 15, 4695. [Google Scholar] [CrossRef]
- Kloprogge, T.; Frost, R.L. Fourier Transform Infrared and Raman Spectroscopic Study of the Local Structure of Mg-, Ni-, and Co-Hydrotalcites. J. Solid State Chem. 1999, 146, 506–515. [Google Scholar] [CrossRef]
- Bukhtiyarova, M.V. A review on effect of synthesis conditions on the formation of layered double hydroxides. J. Solid State Chem. 2019, 269, 494–506. [Google Scholar] [CrossRef]
- Mikhailau, A.; Maltanava, H.; Poznyak, S.K.; Salak, A.N.; Zheludkevich, M.L.; Yasakau, K.; Ferreira, M. One-step synthesis and growth mechanism of nitrate intercalated ZnAl LDH conversion coatings on zinc. Chem. Commun. 2019, 55, 6878–6881. [Google Scholar] [CrossRef]
- Chen, J.; Song, Y.; Shan, D.; Han, E.-H. In situ growth of Mg–Al hydrotalcite conversion film on AZ31 magnesium alloy. Corros. Sci. 2011, 53, 3281–3288. [Google Scholar] [CrossRef]
- Serdechnova, M.; Mohedano, M.; Kuznetsov, B.; Mendis, C.L.; Starykevich, M.; Karpushenkov, S.; Tedim, J.; Ferreira, M.; Blawert, C.; Zheludkevich, M. PEO Coatings with Active Protection Based on In-Situ Formed LDH-Nanocontainers. J. Electrochem. Soc. 2016, 164, C36–C45. [Google Scholar] [CrossRef]
- Oestreicher, V.; Fábregas, I.; Jobbagy, M. One-Pot Epoxide-Driven Synthesis of M2Al(OH)6Cl·1.5H2O Layered Double Hydroxides: Precipitation Mechanism and Relative Stabilities. J. Phys. Chem. C 2014, 118, 30274–30281. [Google Scholar] [CrossRef]
- Chen, J.; Song, Y.; Shan, D.; Han, E.-H. Modifications of the hydrotalcite film on AZ31 Mg alloy by phytic acid: The effects on morphology, composition and corrosion resistance. Corros. Sci. 2013, 74, 130–138. [Google Scholar] [CrossRef]
- Tedim, J.; Kuznetsova, A.; Salak, A.N.; Montemor, F.; Snihirova, D.; Pilz, M.; Zheludkevich, M.; Ferreira, M. Zn–Al layered double hydroxides as chloride nanotraps in active protective coatings. Corros. Sci. 2012, 55, 1–4. [Google Scholar] [CrossRef]
- Zhang, C.; Luo, X.; Pan, X.; Liao, L.; Wu, X.; Liu, Y. Self-healing Li-Al layered double hydroxide conversion coating modified with aspartic acid for 6N01 Al alloy. Appl. Surf. Sci. 2017, 394, 275–281. [Google Scholar] [CrossRef]
- Wang, F.; Guo, Z. Insitu growth of durable superhydrophobic Mg–Al layered double hydroxides nanoplatelets on aluminum alloys for corrosion resistance. J. Alloys Compd. 2018, 767, 382–391. [Google Scholar] [CrossRef]
- Lin, K.; Luo, X.; Pan, X.; Zhang, C.; Liu, Y. Enhanced corrosion resistance of LiAl-layered double hydroxide (LDH) coating modified with a Schiff base salt on aluminum alloy by one step in-situ synthesis at low temperature. Appl. Surf. Sci. 2019, 463, 1085–1096. [Google Scholar] [CrossRef]
- Tedim, J.; Zheludkevich, M.; Bastos, A.; Salak, A.N.; Lisenkov, A.; Ferreira, M. Influence of preparation conditions of Layered Double Hydroxide conversion films on corrosion protection. Electrochim. Acta 2014, 117, 164–171. [Google Scholar] [CrossRef]
- Korkmaz, D. Precipitation titration: “Determination of Chloride by the Mohr Method”. Methods 2001, 2, 1–6. [Google Scholar]
| Specimens | NiNO3 6H2O | NH4NO3 |
|---|---|---|
| NiAl-LDHa | 0.003 M | - |
| NiAl-LDHb | 0.003 M | 0.003 M |
| NiAl-LDHc | 0.003 M | 0.009 M |
| NiAl-LDHd | 0.003 M | 0.018 M |
| Sample | Interlayer Distance “d003” (nm) | Interlayer Distance “d006” (nm) | Cell Parameter, “c” (nm) | Crystallite Size 003 “nm” |
|---|---|---|---|---|
| NiAl-LDHa | 0.886 | 0.449 | 2.675 | 19.94 |
| NiAl-LDHb | 0.881 | 0.449 | 2.668 | 18.72 |
| NiAl-LDHc | 0.878 | 0.448 | 2.659 | 17.64 |
| NiAl-LDHd | 0.872 | 0.449 | 2.656 | 17.22 |
| The Weight Percentage of NiAl-LDHs | |||||
| Sample | Ni | Al | N | O | Ni/Al |
| NiAl-LDHa | 26.2 ± 2.2 | 7.60 ± 4.1 | 4.1 ± 1.3 | 56.7 ± 5.2 | 3.44 |
| NiAl-LDHb | 26.4 ± 2.5 | 7.51 ± 4.3 | 4.8 ± 1.7 | 57.2 ± 5.3 | 3.51 |
| NiAl-LDHc | 31.8 ± 3.1 | 7.48 ± 4.2 | 4.3 ± 1.2 | 52.7 ± 5.1 | 4.25 |
| NiAl-LDHd | 34.7 ± 3.8 | 7.42 ± 4.7 | 4.6 ± 1.4 | 53.0 ± 5.6 | 4.67 |
| The Atomic Percentage of NiAl-LDHs | |||||
| Sample | Ni | Al | N | O | Ni/Al |
| NiAl-LDHa | 9.7 ± 3.2 | 6.7 ± 2.1 | 5.6 ± 1.1 | 68.5 ± 4.2 | 1.44 |
| NiAl-LDHb | 10.1 ± 3.5 | 6.4 ± 2.2 | 5.3 ± 1.8 | 69.2 ± 4.3 | 1.57 |
| NiAl-LDHc | 10.4 ± 2.9 | 6.0 ± 3.3 | 5.1 ± 1.9 | 70.7 ± 4.4 | 1.73 |
| NiAl-LDHd | 10.8 ± 2.1 | 5.9 ± 3.7 | 5.2 ± 1.4 | 69.0 ± 5.5 | 1.83 |
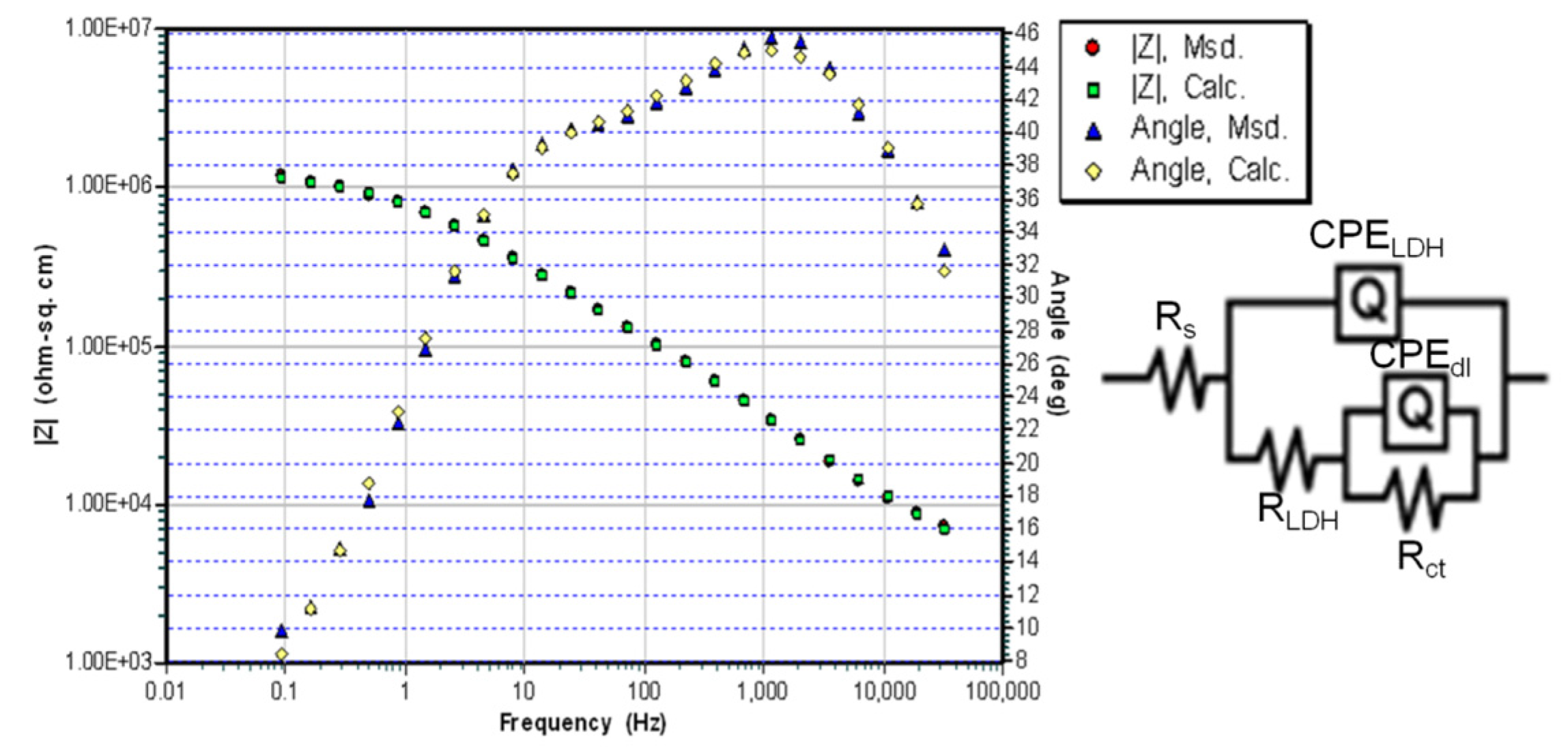
| Sample | Immersion Time | RLDH kΩ cm2 | QLDH Ω−1 cm−2 sα | αLDH | Rct kΩ cm2 | Qdl Ω−1 cm−2 sα | αdl |
|---|---|---|---|---|---|---|---|
| NiAl-LDHa | 1 day | 11.77 | 1.69 × 10−6 | 0.91 | 308 | 1.41 × 10−6 | 0.90 |
| NiAl-LDHb | 1 day | 21.87 | 7.62 × 10−6 | 0.83 | 467 | 2.04 × 10−6 | 0.90 |
| NiAl-LDHc | 1 day | 333.61 | 4.92 × 10−7 | 0.51 | 1391 | 8.60 × 10−7 | 0.70 |
| NiAl-LDHd | 1 day | 1208.8 | 5.78 × 10−7 | 0.67 | 1819 | 4.19 × 10−6 | 0.79 |
| LDH | NaCl Conc. | Time | RLDH (kΩ cm2) | Rct (kΩ cm2) | Ref. |
|---|---|---|---|---|---|
| Li/Al | 3.5 wt % | 0 h | 2.2 | 6490 | [35] |
| Mg/Al | 3.5 wt % | 1 day | n.p. | 5.88 | [36] |
| Li/Al | 3.5 wt % | 1 day | 0.8 | 0.18 × 103 | [37] |
| Zn/Al (+VOx) | 0.05 M | 1 day | 18.2 | 7.96 × 108 | [38] |
| NiAl-LDHd | 0.1 M | 1 day | 1415 | 1819.9 | This work |
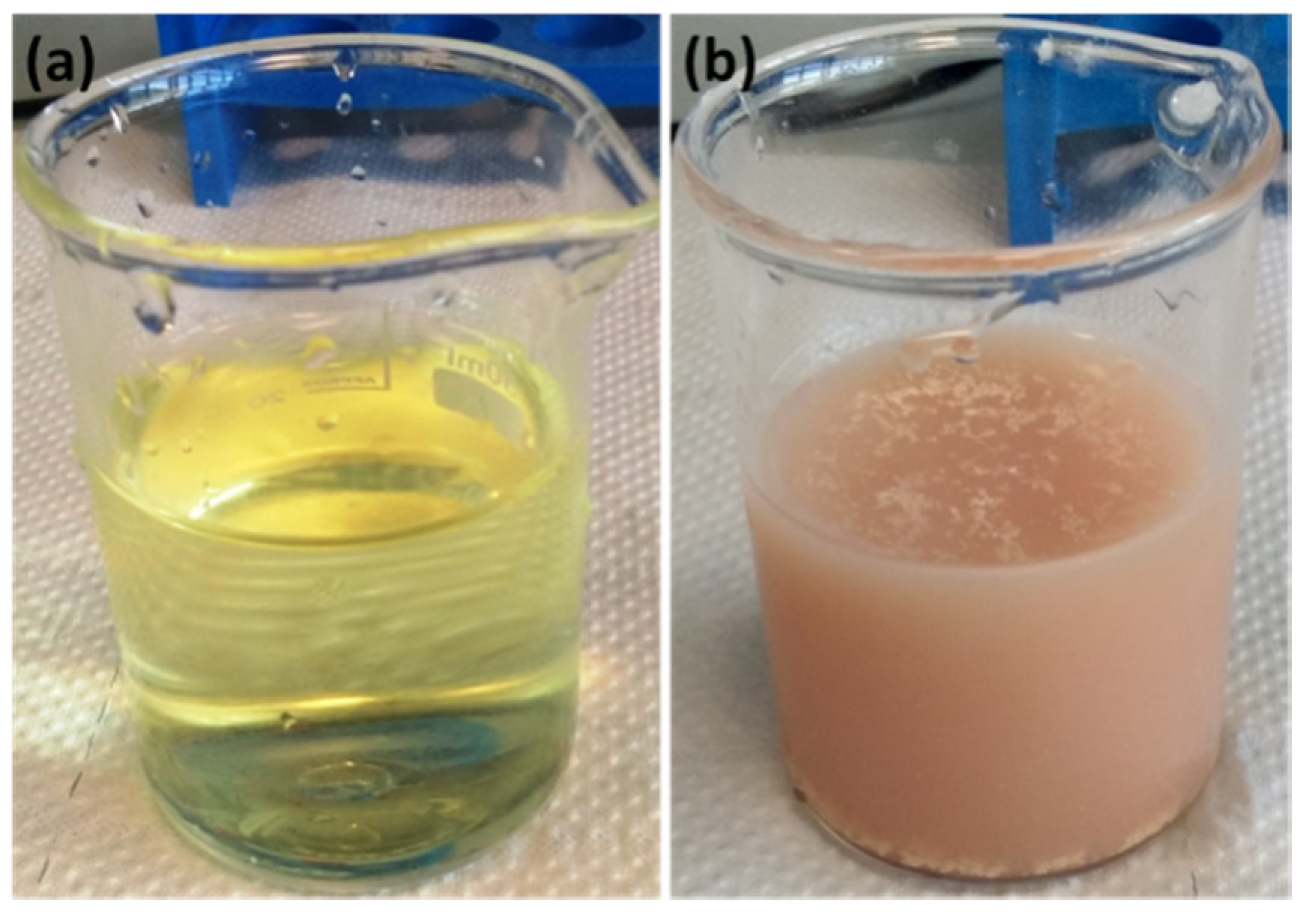
| 0.1 M NaCl (Fresh) mg·L−1 | NiAl-LDHa mg·L−1 | NiAl-LDHb mg·L−1 | NiAl-LDHc mg·L−1 | NiAl-LDHd mg·L−1 | |
|---|---|---|---|---|---|
| Chloride Conc. | 3462 | 3426 | 3388 | 3340 | 3337 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqbal, M.A.; Sun, L.; Asghar, H.; Fedel, M. Chlorides Entrapment Capability of Various In-Situ Grown NiAl-LDHs: Structural and Corrosion Resistance Properties. Coatings 2020, 10, 384. https://doi.org/10.3390/coatings10040384
Iqbal MA, Sun L, Asghar H, Fedel M. Chlorides Entrapment Capability of Various In-Situ Grown NiAl-LDHs: Structural and Corrosion Resistance Properties. Coatings. 2020; 10(4):384. https://doi.org/10.3390/coatings10040384
Chicago/Turabian StyleIqbal, Muhammad Ahsan, Luyi Sun, Humaira Asghar, and Michele Fedel. 2020. "Chlorides Entrapment Capability of Various In-Situ Grown NiAl-LDHs: Structural and Corrosion Resistance Properties" Coatings 10, no. 4: 384. https://doi.org/10.3390/coatings10040384
APA StyleIqbal, M. A., Sun, L., Asghar, H., & Fedel, M. (2020). Chlorides Entrapment Capability of Various In-Situ Grown NiAl-LDHs: Structural and Corrosion Resistance Properties. Coatings, 10(4), 384. https://doi.org/10.3390/coatings10040384






