Non-Fluorinated, Sustainable, and Durable Superhydrophobic Microarrayed Surface for Water-Harvesting
Abstract
1. Introduction
2. Materials and Methods
2.1. Electrodeposition
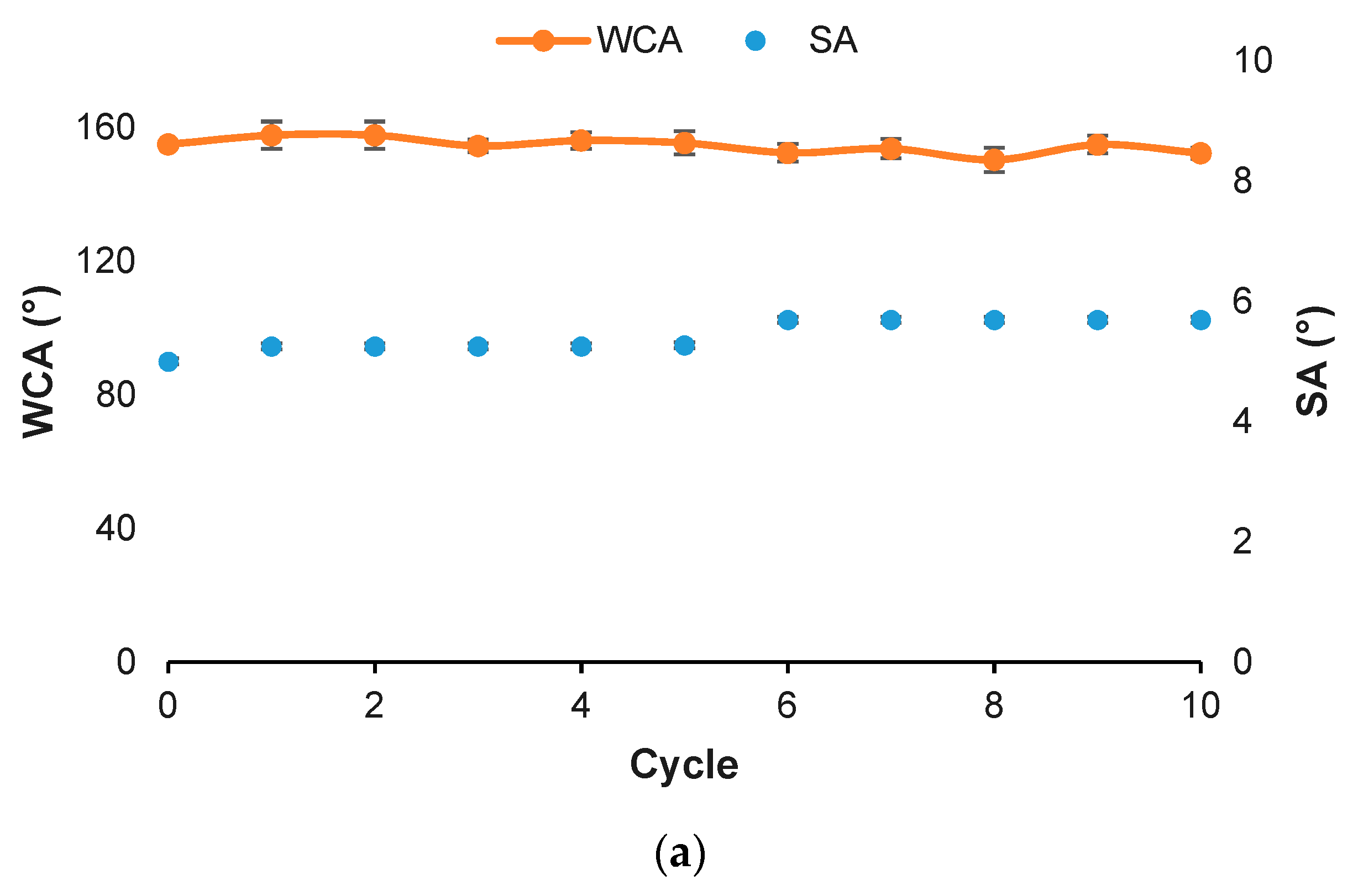
2.2. Durability Tests
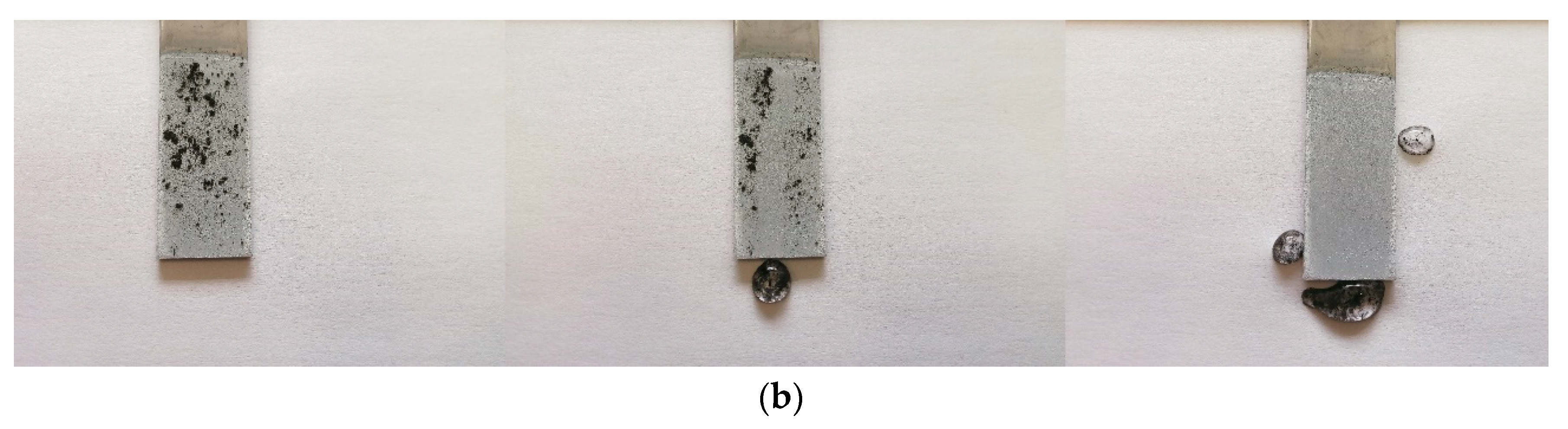
2.3. Water-Harvesting Test
2.4. Characterization Techniques
3. Results and Discussion
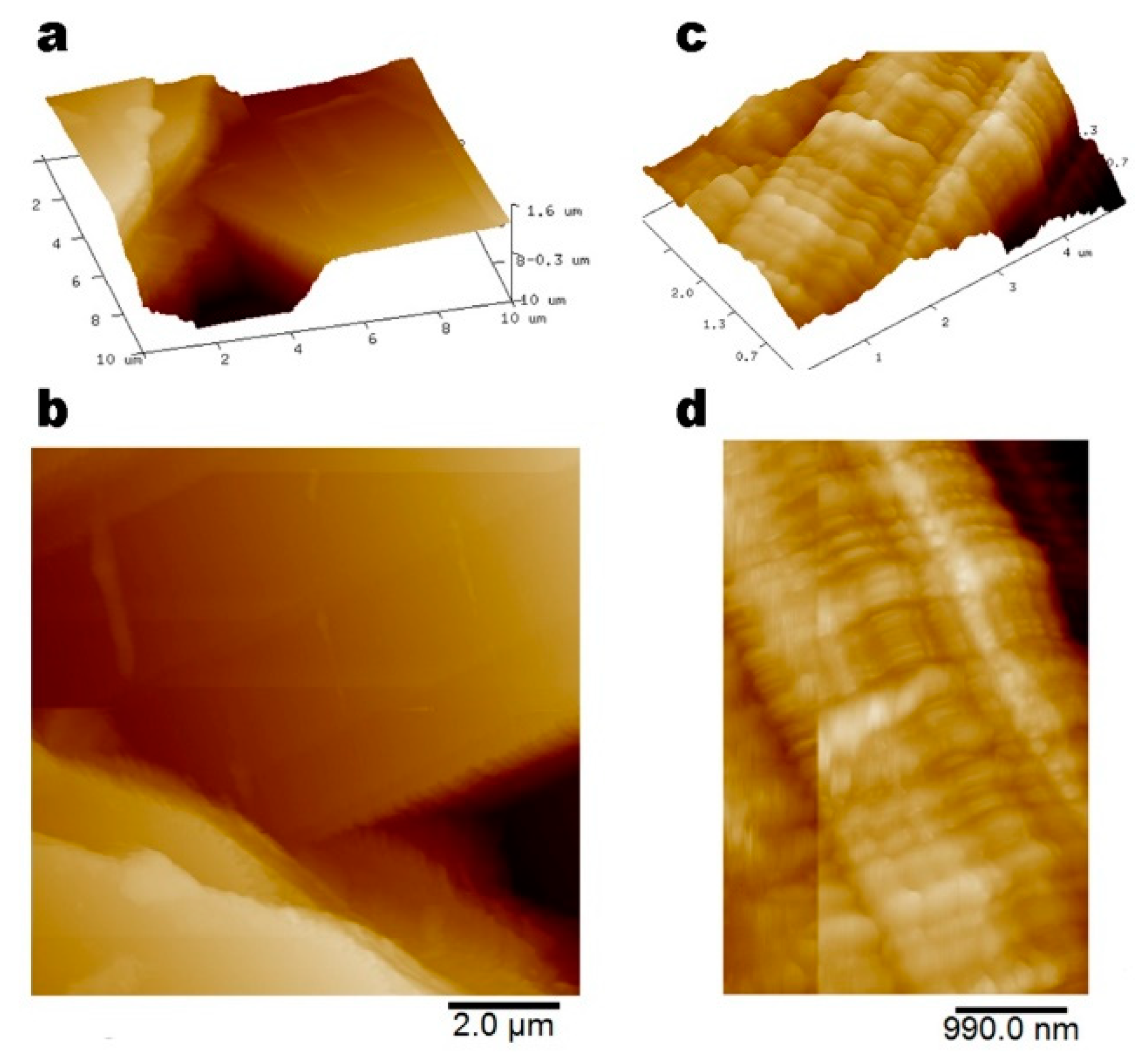
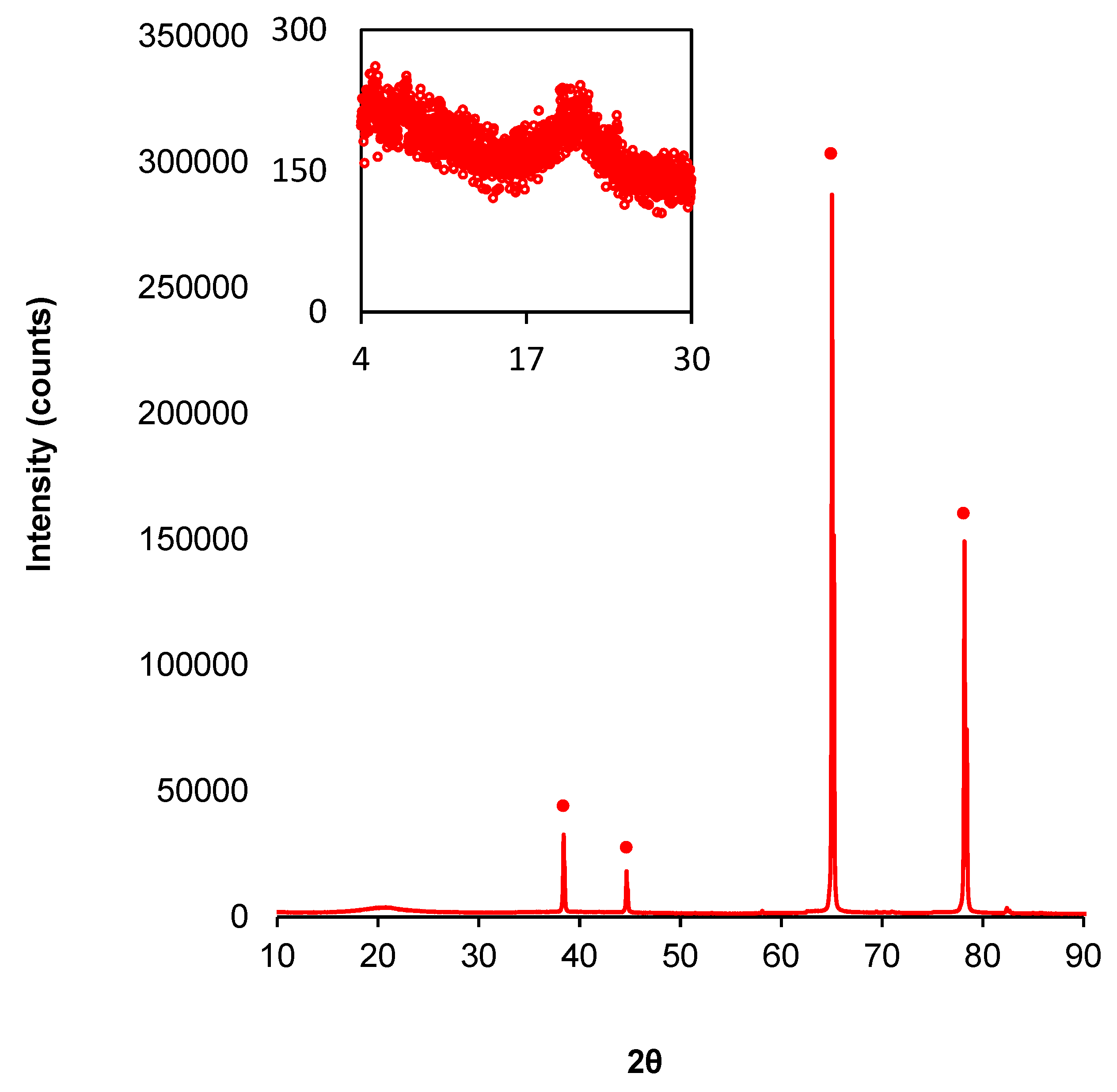
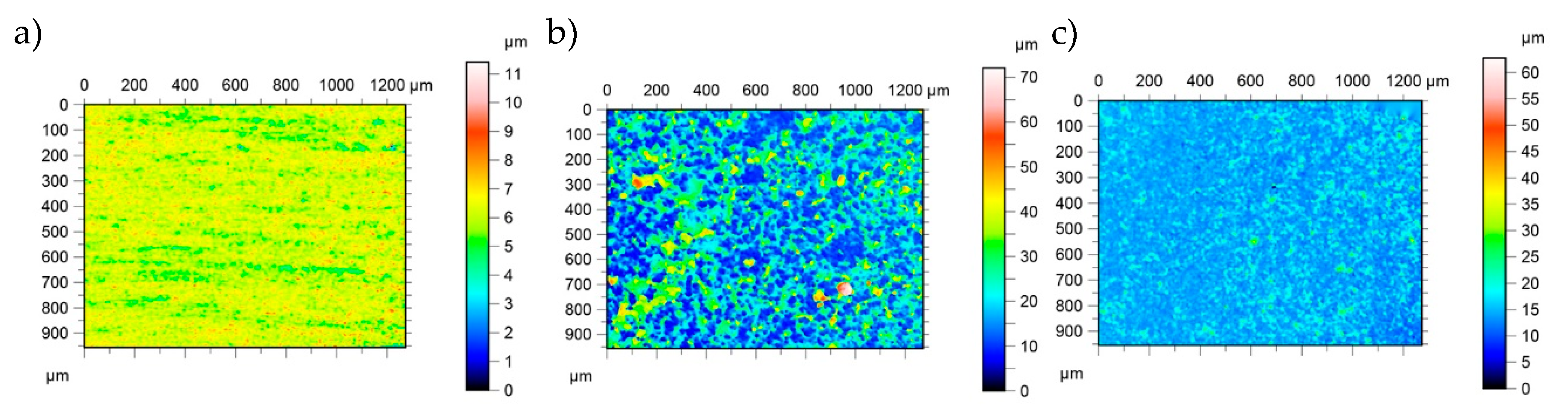
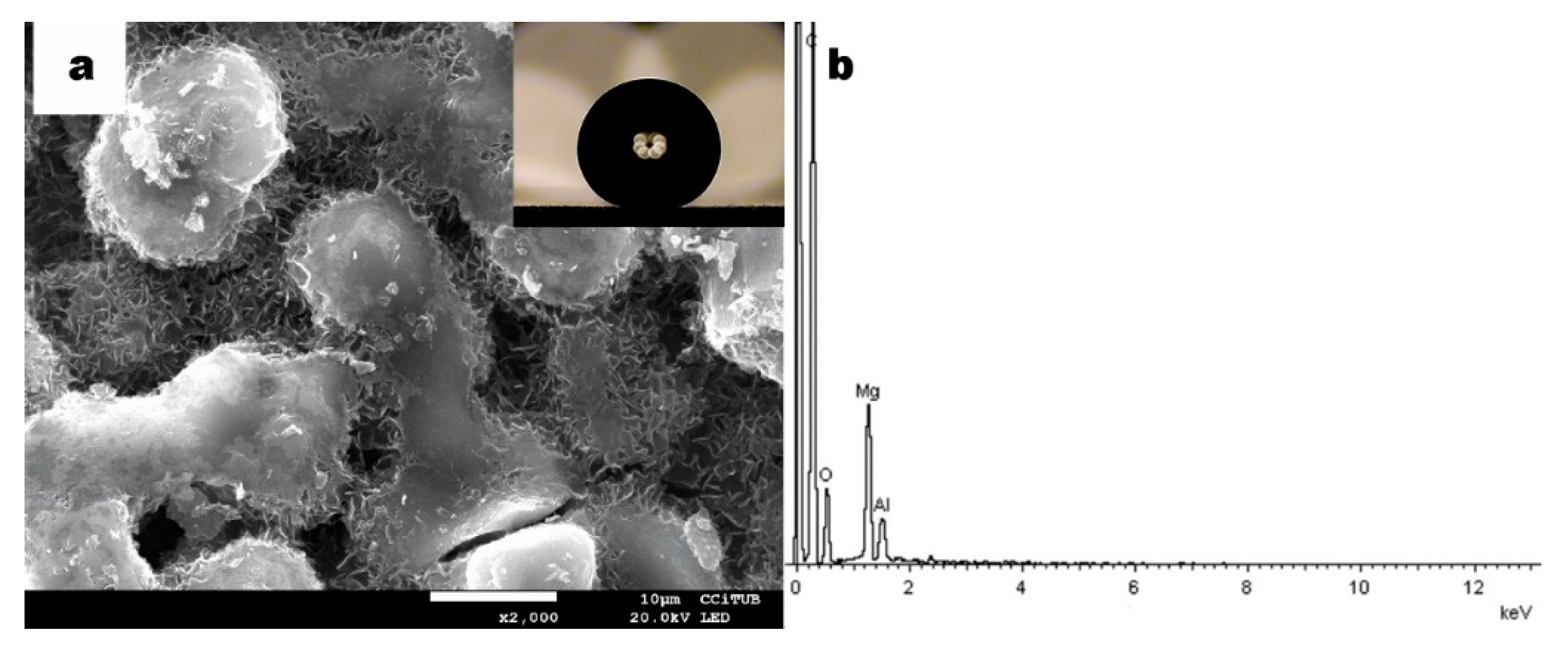
3.1. Structural Characterization
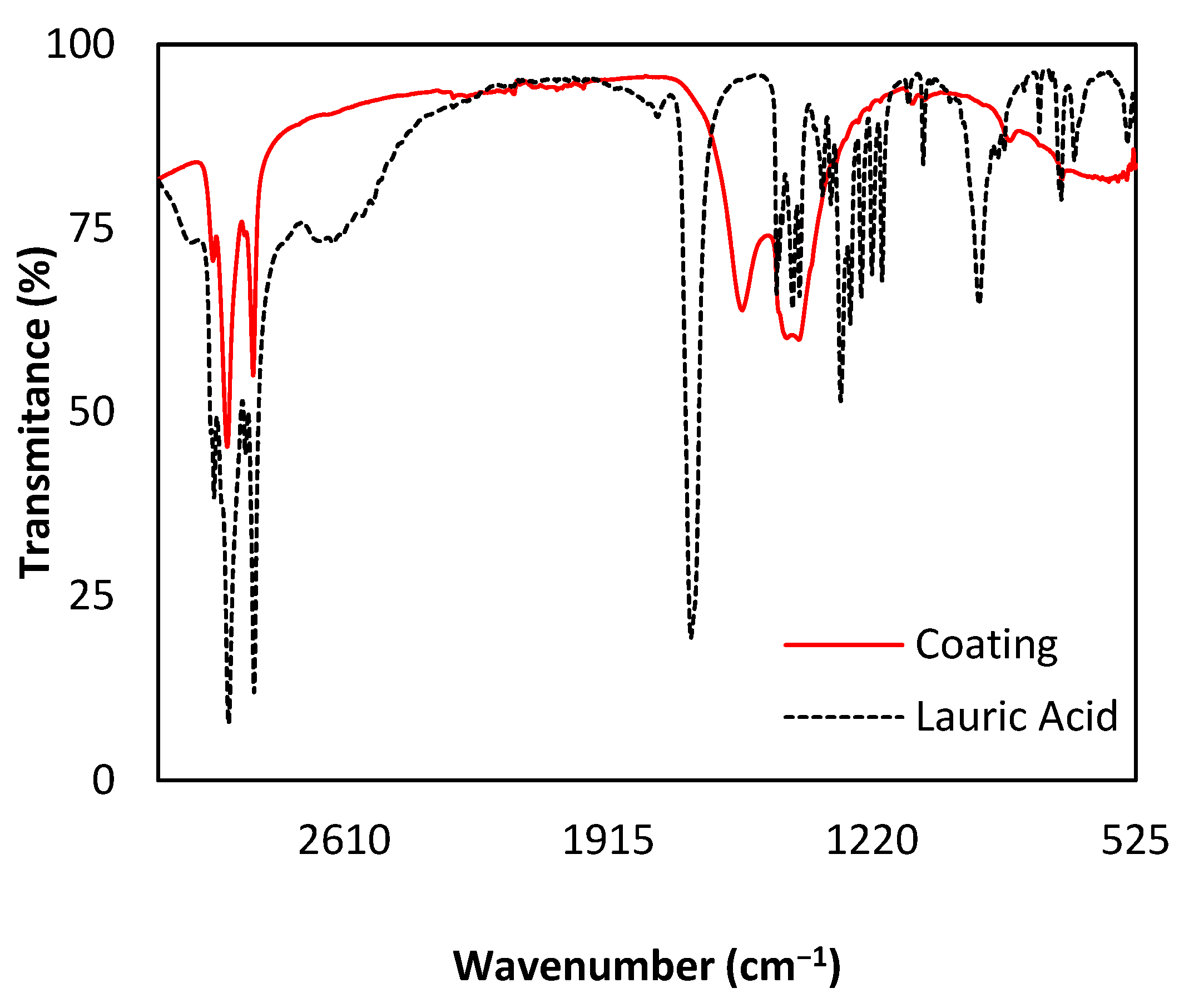
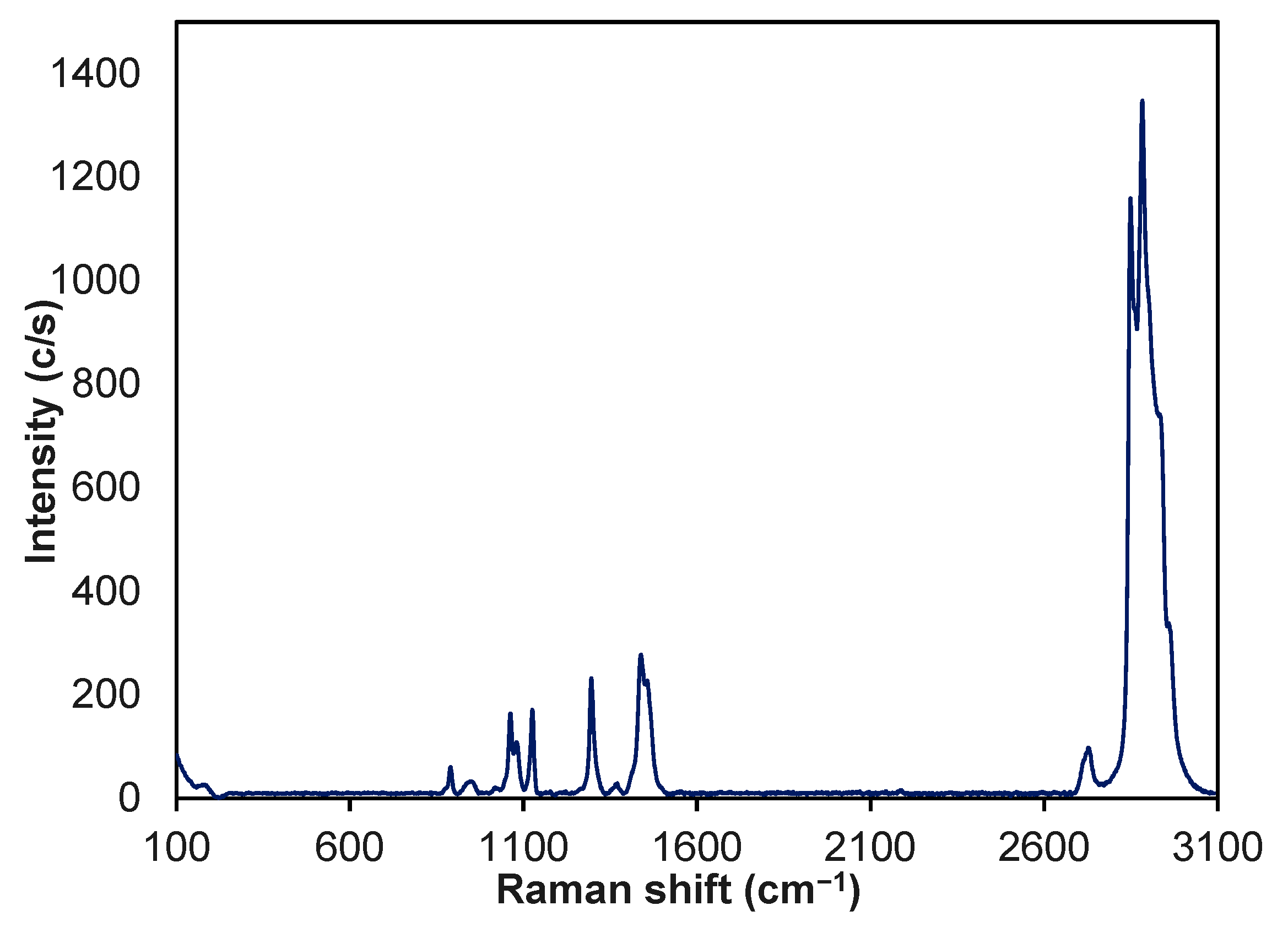
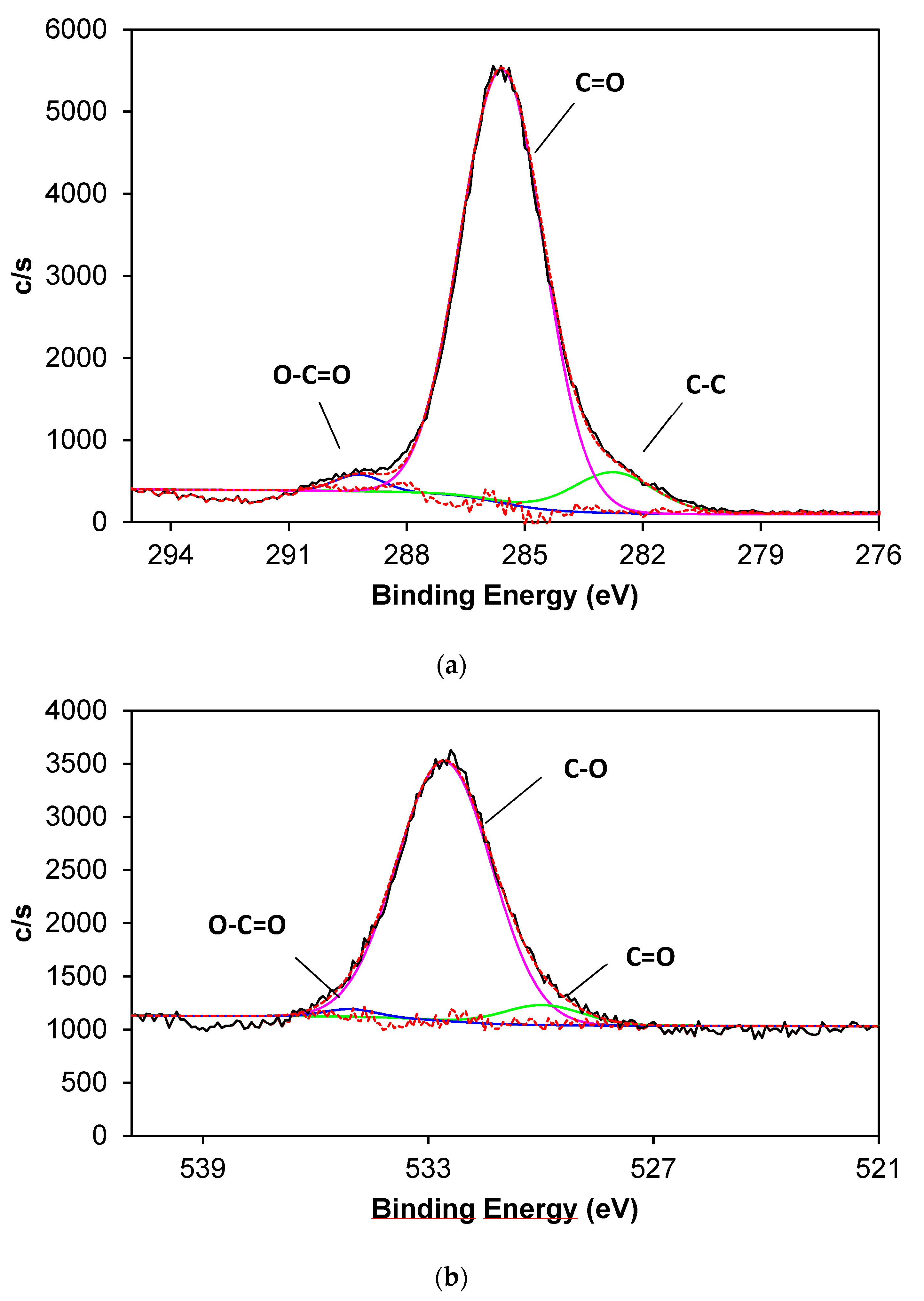
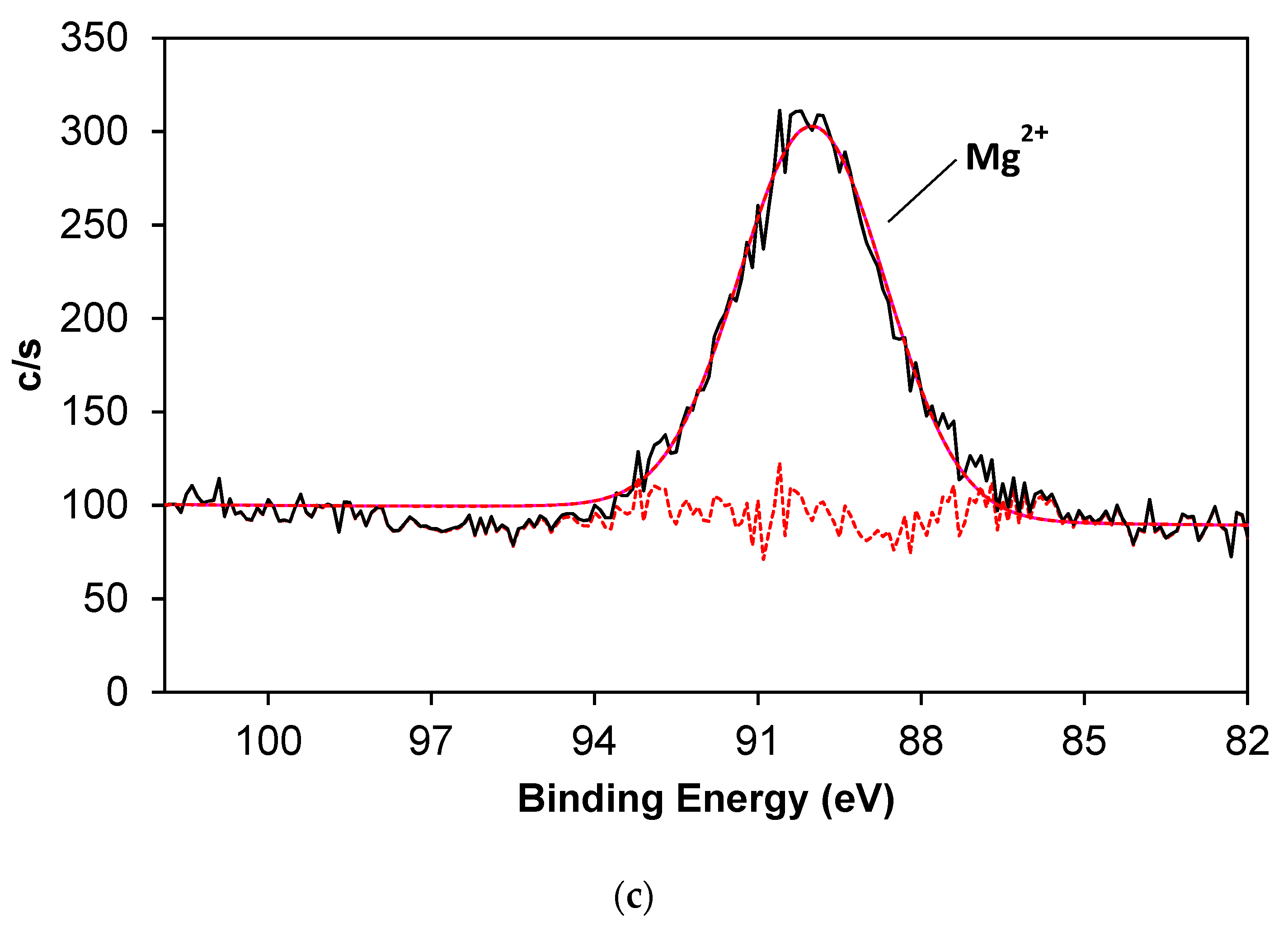
3.2. Chemical Characterization
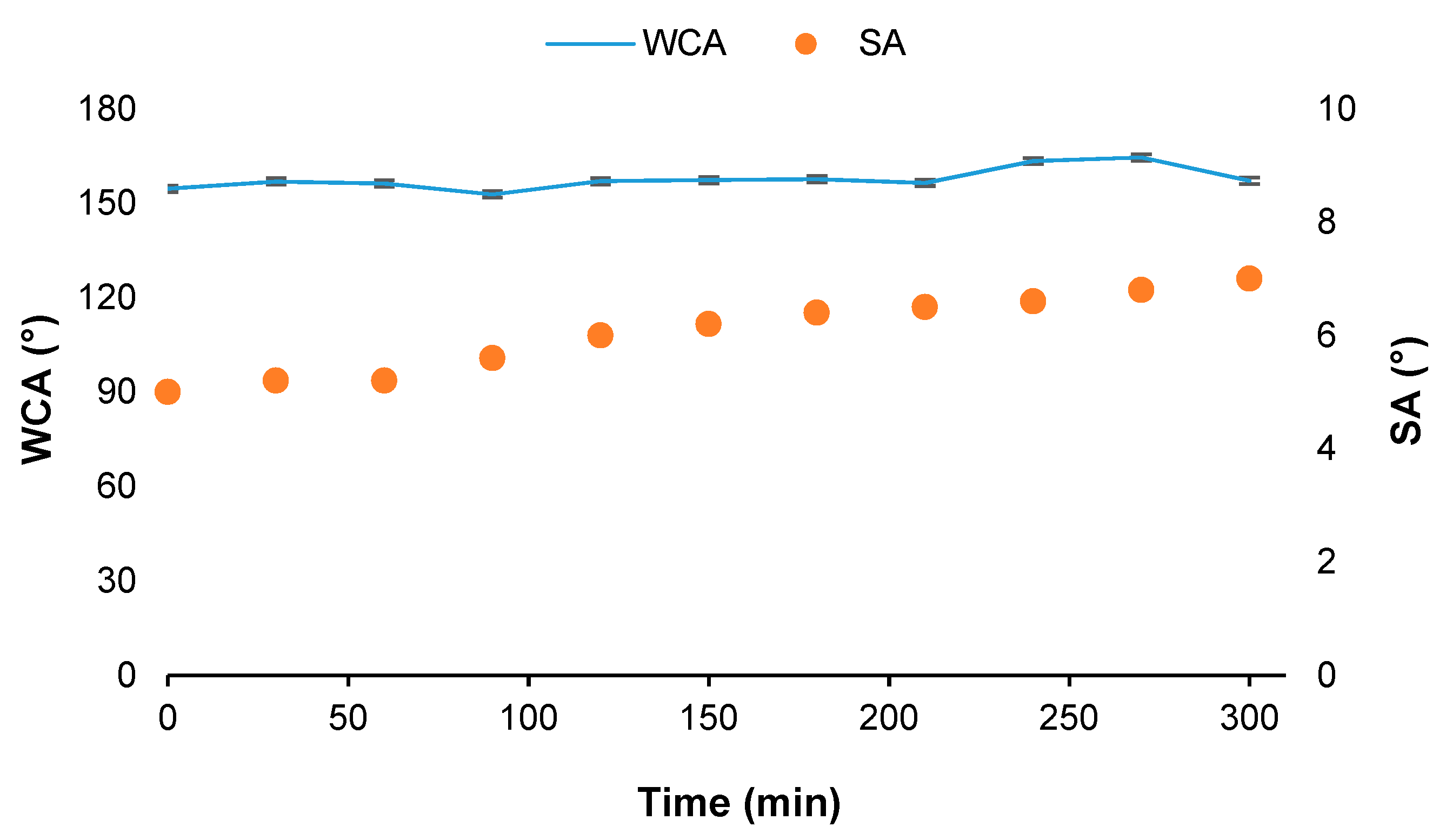
3.3. Wettability Properties
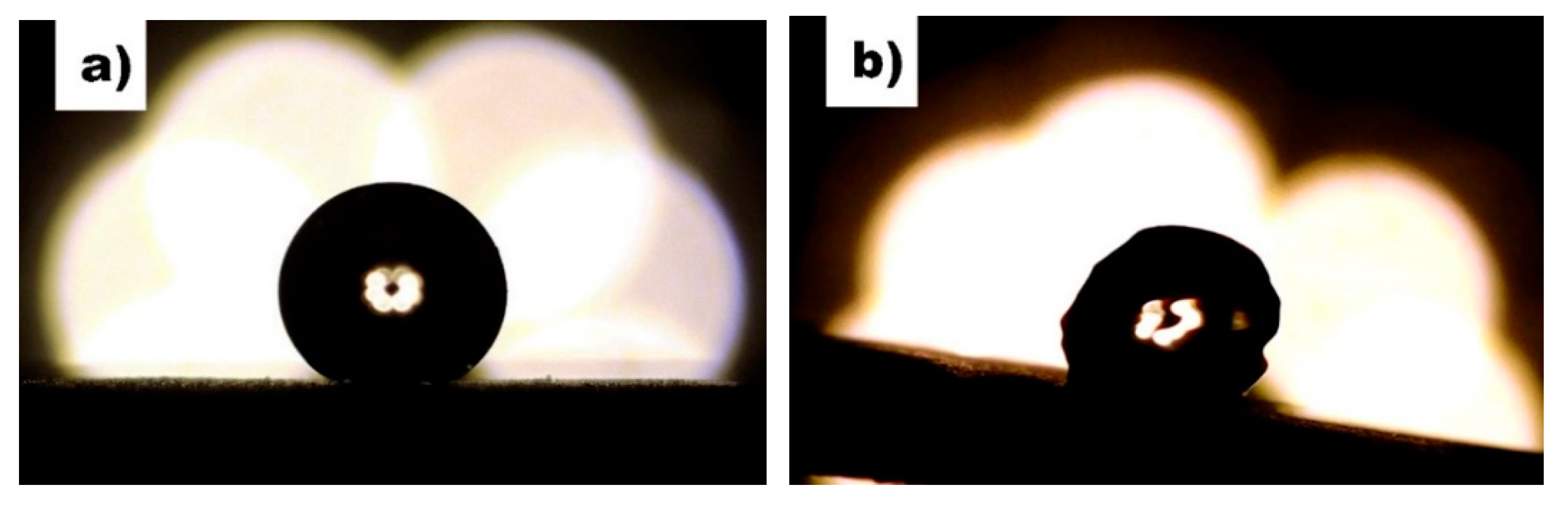
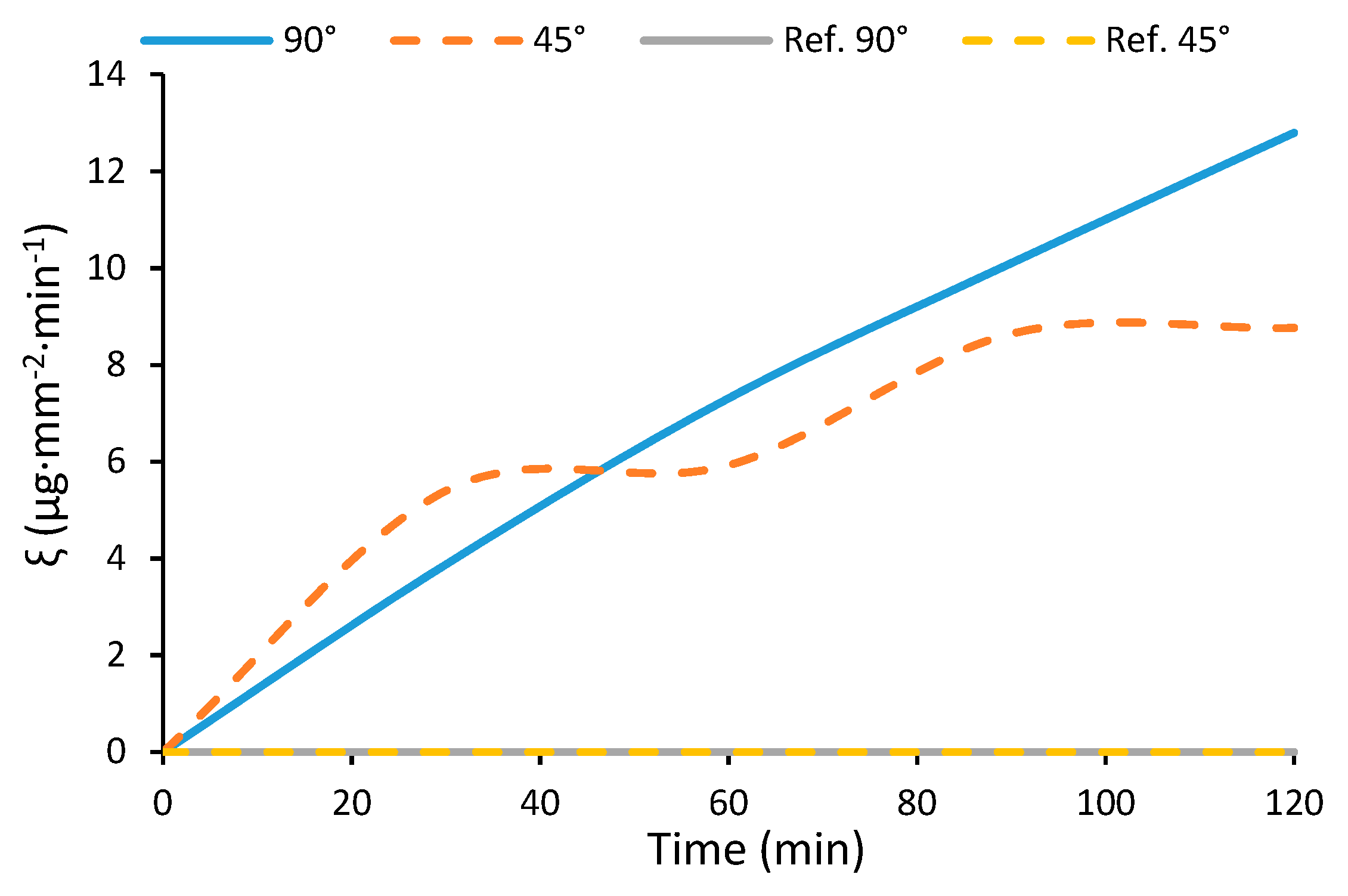
3.4. Water-Harvesting
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bhushan, B.; Jung, Y.C. Natural and biomimetic artificial surfaces for superhydrophobicity, self-cleaning, low adhesion, and drag reduction. Prog. Mater. Sci. 2011, 56, 1–108. [Google Scholar] [CrossRef]
- Gao, L.; McCarthy, T.J. Contact Angle Hysteresis Explained. Langmuir 2006, 22, 6234–6237. [Google Scholar] [CrossRef] [PubMed]
- Nosonovsky, M.; Bhushan, B. Superhydrophobic surfaces and emerging applications: Non-adhesion, energy, green engineering. Curr. Opin. Colloid Interface Sci. 2009, 14, 270–280. [Google Scholar] [CrossRef]
- Liu, M.; Wang, S.; Jiang, L. Nature-inspired superwettability systems. Nat. Rev. Mater. 2017, 2, 17036. [Google Scholar] [CrossRef]
- Su, B.; Tian, Y.; Jiang, L. Bioinspired Interfaces with Superwettability: From Materials to Chemistry. J. Am. Chem. Soc. 2016, 138, 1727–1748. [Google Scholar] [CrossRef]
- Guix, M.; Orozco, J.; Garcia, M.; Gao, W.; Sattayasamitsathit, S.; Merkoçi, A.; Escarpa, A.; Wang, J. Superhydrophobic Alkanethiol-Coated Microsubmarines for Effective Removal of Oil. ACS Nano 2012, 6, 4445–4451. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Parbat, D.; Shome, A.; Manna, U. Sustainable Biomimicked Oil/Water Wettability That Performs Under Severe Challenges. ACS Sustain. Chem. Eng. 2019, 7, 11350–11359. [Google Scholar] [CrossRef]
- Mobarakeh, L.F.; Jafari, R.; Farzaneh, M. Robust icephobic, and anticorrosive plasma polymer coating. Cold Reg. Sci. Technol. 2018, 151, 89–93. [Google Scholar] [CrossRef]
- Vazirinasab, E.; Jafari, R.; Momen, G. Application of superhydrophobic coatings as a corrosion barrier: A review. Surf. Coat. Technol. 2018, 341, 40–56. [Google Scholar] [CrossRef]
- Zhuo, Y.; Li, T.; Wang, F.; Håkonsen, V.; Xiao, S.; He, J.; Zhang, Z. An ultra-durable icephobic coating by a molecular pulley. Soft Matter 2019, 15, 3607–3611. [Google Scholar] [CrossRef]
- Brassard, J.-D.; Laforte, J.-L.; Blackburn, C.; Perron, J.; Sarkar, D.K. Silicone based superhydrophobic coating efficient to reduce ice adhesion and accumulation on aluminum under offshore arctic conditions. Ocean Eng. 2017, 144, 135–141. [Google Scholar] [CrossRef]
- Masood, M.T.; Zahid, M.; Goldoni, L.; Ceseracciu, L.; Athanassiou, A.; Bayer, I.S. Highly Transparent Polyethylcyanoacrylates from Approved Eco-Friendly Fragrance Materials Demonstrating Excellent Fog-Harvesting and Anti-Wear Properties. ACS Appl. Mater. Interfaces 2018, 10, 34573–34584. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Sun, N.; Nielsen, S.O.; Stogin, B.B.; Wang, J.; Yang, S.; Wong, T.-S. Hydrophilic directional slippery rough surfaces for water harvesting. Sci. Adv. 2018, 4, eaaq0919. [Google Scholar] [CrossRef]
- Suvindran, N.; Li, F.; Pan, Y.; Zhao, X. Characterization and Bioreplication of Tradescantia pallida Inspired Biomimetic Superwettability for Dual Way Patterned Water Harvesting. Adv. Mater. Interfaces 2018, 5, 1–10. [Google Scholar] [CrossRef]
- Raut, H.; Ranganath, A.; Baji, A.; Wood, K.L. Bio-inspired hierarchical topography for texture driven fog harvesting. Appl. Surf. Sci. 2019, 465, 362–368. [Google Scholar] [CrossRef]
- Anastas, P.T.; Werner, J. Green Chemistry: Theory and Practice; OUP: Oxford, UK, 1998. [Google Scholar]
- Erythropel, H.C.C.; Zimmerman, J.B.; De Winter, T.M.; Petitjean, L.; Melnikov, F.; Lam, C.H.; Lounsbury, A.W.; Mellor, K.; Janković, N.Z.; Tu, Q.; et al. The Green ChemisTREE: 20 years after taking root with the 12 principles. Green Chem. 2018, 20, 1929–1961. [Google Scholar] [CrossRef]
- Clark, J.H.; Farmer, T.; Herrero-Davila, L.; Sherwood, J. Circular economy design considerations for research and process development in the chemical sciences. Green Chem. 2016, 18, 3914–3934. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Bohn, A.; Fink, H.-P. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Calvo-Flores, F.G.; Monteagudo-Arrebola, M.J.; Dobado, J.A.; Isac-García, J. Green and Bio-Based Solvents. Top. Curr. Chem. 2018, 376, 18. [Google Scholar] [CrossRef]
- Spiridon, I.; Darie-Nita, R.N.; Bele, A. New opportunities to valorize biomass wastes into green materials. II. Behaviour to accelerated weathering. J. Clean. Prod. 2018, 172, 2567–2575. [Google Scholar] [CrossRef]
- Zanoletti, A.; Bilo, F.; Depero, L.; Zappa, D.; Bontempi, E. The first sustainable material designed for air particulate matter capture: An introduction to Azure Chemistry. J. Environ. Manag. 2018, 218, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Dichiarante, V.; Milani, R.; Metrangolo, P. Natural surfactants towards a more sustainable fluorine chemistry. Green Chem. 2018, 20, 13–27. [Google Scholar] [CrossRef]
- Schlaich, C.; Yu, L.; Haag, R.; Camacho, L.C.; Wei, Q. Fluorine-free superwetting systems: Construction of environmentally friendly superhydrophilic, superhydrophobic, and slippery surfaces on various substrates. Polym. Chem. 2016, 7, 7446–7454. [Google Scholar] [CrossRef]
- Escobar, A.M.; Llorca-Isern, N. Superhydrophobic coating deposited directly on aluminum. Appl. Surf. Sci. 2014, 305, 774–782. [Google Scholar] [CrossRef]
- Moosavi, S.S.; Norouzbeigi, R.; Velayi, E. Fabrication of flower-like micro/nano dual scale structured copper oxide surfaces: Optimization of self-cleaning properties via Taguchi design. Appl. Surf. Sci. 2017, 422, 787–797. [Google Scholar] [CrossRef]
- Razavi, S.M.R.; Oh, J.; Sett, S.; Feng, L.; Yan, X.; Hoque, M.J.; Liu, A.; Haasch, R.; Masoomi, M.; Bagheri, R.; et al. Superhydrophobic Surfaces Made from Naturally Derived Hydrophobic Materials. ACS Sustain. Chem. Eng. 2017, 5, 11362–11370. [Google Scholar] [CrossRef]
- Selim, M.S.; Elmarakbi, A.; Azzam, A.M.; Shenashen, M.A.; El-Saeed, A.M.; El-Safty, S.A. Eco-friendly design of superhydrophobic nano-magnetite/silicone composites for marine foul-release paints. Prog. Org. Coat. 2018, 116, 21–34. [Google Scholar] [CrossRef]
- Davis, A.; Surdo, S.; Caputo, G.; Bayer, I.S.; Athanassiou, A. Environmentally Benign Production of Stretchable and Robust Superhydrophobic Silicone Monoliths. ACS Appl. Mater. Interfaces 2018, 10, 2907–2917. [Google Scholar] [CrossRef]
- Yue, X.; Zhang, T.; Yang, D.; Qiu, F.; Li, Z. Hybrid aerogels derived from banana peel and waste paper for efficient oil absorption and emulsion separation. J. Clean. Prod. 2018, 199, 411–419. [Google Scholar] [CrossRef]
- Moreno, E.; Cordobilla, R.; Calvet, T.; Cuevas-Diarte, M.A.; Gbabode, G.; Negrier, P.; Mondieig, D.; Oonk, H.A.J. Polymorphism of even saturated carboxylic acids from n-decanoic to n-eicosanoic acid. New J. Chem. 2007, 31, 947. [Google Scholar] [CrossRef]
- Nelson, P.N.; Taylor, R.A. Powder X-ray diffraction, infrared and 13C NMR spectroscopic studies of the homologous series of some solid-state zinc(II) and sodium(I) n-alkanoates. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2015, 138, 800–806. [Google Scholar] [CrossRef]
- Hartman, P. Structural morphology of organic compounds having two centrosymmetric molecules in a monoclinic unit cell. J. Cryst. Growth 1991, 110, 559–570. [Google Scholar] [CrossRef]
- Bond, A.D. On the crystal structures and melting point alternation of the n-alkyl carboxylic acids. New J. Chem. 2004, 28, 104–114. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546. [Google Scholar] [CrossRef]
- Gadermann, M.; Preston, T.C.; Troster, C.; Signorell, R. Characterization of palmitic and lauric acid aerosols from rapid expansion of supercritical CO2 solutions. Mol. Phys. 2008, 106, 945–953. [Google Scholar] [CrossRef]
- Caroline, M.L.; Vasudevan, S. Growth and characterization of an organic nonlinear optical material: L-alanine alaninium nitrate. Mater. Lett. 2008, 62, 2245–2248. [Google Scholar] [CrossRef]
- Jiesheng, L.; Yuanyuan, Y.; Xiang, H. Research on the preparation and properties of lauric acid/expanded perlite phase change materials. Energy Build. 2016, 110, 108–111. [Google Scholar] [CrossRef]
- Yin, W.Z.; Tan, Q.; Liu, L.; Li, X.L. Synthesis and Characterization of Mg-Al Layered Double Hydroxide. Adv. Mater. Res. 2012, 454, 101–104. [Google Scholar] [CrossRef]
- Dang, L.; Nai, X.; Zhu, D.; Jing, Y.; Liu, X.; Dong, Y.; Li, W. Study on the mechanism of surface modification of magnesium oxysulfate whisker. Appl. Surf. Sci. 2014, 317, 325–331. [Google Scholar] [CrossRef]
- Robinet, L.; Corbeil-A2, M.-C. The Characterization of Metal Soaps. Stud. Conserv. 2003, 48, 23–40. [Google Scholar] [CrossRef]
- Otero, V.; Sanches, D.; Montagner, C.; Vilarigues, M.; Carlyle, L.; Lopes, J.; Melo, M.J. Characterisation of metal carboxylates by Raman and infrared spectroscopy in works of art. J. Raman Spectrosc. 2014, 45, 1197–1206. [Google Scholar] [CrossRef]
- Saggu, M.; Liu, J.; Patel, A.R. Identification of Subvisible Particles in Biopharmaceutical Formulations Using Raman Spectroscopy Provides Insight into Polysorbate 20 Degradation Pathway. Pharm. Res. 2015, 32, 2877–2888. [Google Scholar] [CrossRef]
- De Gelder, J.; De Gussem, K.; Vandenabeele, P.; Moens, L. Reference database of Raman spectra of biological molecules. J. Raman Spectrosc. 2007, 38, 1133–1147. [Google Scholar] [CrossRef]
- Czamara, K.; Majzner, K.; Pacia, M.; Kochan, K.; Kaczor, A.; Baranska, M. Raman spectroscopy of lipids: A review. J. Raman Spectrosc. 2014, 46, 4–20. [Google Scholar] [CrossRef]
- De Veij, M.; Vandenabeele, P.; De Beer, T.; Remon, J.P.; Moens, L. Reference database of Raman spectra of pharmaceutical excipients. J. Raman Spectrosc. 2009, 40, 297–307. [Google Scholar] [CrossRef]
- Men, S.; Jiang, X.; Xiang, X.; Sun, G.; Yan, Y.; Lyu, Z.; Jin, Y. Synthesis of Cellulose Long-Chain Esters in 1-Butyl-3-methylimidazolium Acetate: Structure-Property Relations. Polym. Sci. Ser. B 2018, 60, 349–353. [Google Scholar] [CrossRef]
- Cocco, F.; Elsener, B.; Fantauzzi, M.; Atzei, D.; Rossi, A. Nanosized surface films on brass alloys by XPS and XAES. RSC Adv. 2016, 6, 31277–31289. [Google Scholar] [CrossRef]
- Wu, P.-Y.; Jiang, Y.-P.; Zhang, Q.-Y.; Jia, Y.; Peng, D.-Y.; Xu, W. Comparative study on arsenate removal mechanism of MgO and MgO/TiO2 composites: FTIR and XPS analysis. New J. Chem. 2016, 40, 2878–2885. [Google Scholar] [CrossRef]
- Dobrovolsky, V.; Khyzhun, O.; Sinelnichenko, A.; Ershova, O.; Solonin, Y. XPS study of influence of exposure to air on thermal stability and kinetics of hydrogen decomposition of MgH 2 films obtained by direct hydrogenation from gaseous phase of metallic Mg. J. Electron Spectrosc. Relat. Phenom. 2017, 215, 28–35. [Google Scholar] [CrossRef]
- Zhang, B.; Li, Y.; Hou, B. One-step electrodeposition fabrication of a superhydrophobic surface on an aluminum substrate with enhanced self-cleaning and anticorrosion properties. RSC Adv. 2015, 5, 100000–100010. [Google Scholar] [CrossRef]
- Chen, L.; Meng, H.; Jiang, L.; Wang, S. Fatty-Acid-Metal-Ion Complexes as Multicolor Superhydrophobic Coating Materials. Chem.–Asian J. 2011, 6, 1757–1760. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Elimelech, M.; Lin, S. Environmental Applications of Interfacial Materials with Special Wettability. Environ. Sci. Technol. 2016, 50, 2132–2150. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Sarkar, D.K.; Chen, X.G. Ultraviolet-Durable Superhydrophobic Nanocomposite Thin Films Based on Cobalt Stearate-Coated TiO2 Nanoparticles Combined with Polymethylhydrosiloxane. ACS Omega 2017, 2, 8198–8204. [Google Scholar] [CrossRef]
- Verho, T.; Bower, C.; Andrew, P.; Franssila, S.; Ikkala, O.; Ras, R.H.A. Mechanically Durable Superhydrophobic Surfaces. Adv. Mater. 2010, 23, 673–678. [Google Scholar] [CrossRef]
- Milionis, A.; Loth, E.; Bayer, I.S. Recent advances in the mechanical durability of superhydrophobic materials. Adv. Colloid Interface Sci. 2016, 229, 57–79. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, V.; Khonsari, M. On the degradation of superhydrophobic surfaces: A review. Wear 2017, 372, 145–157. [Google Scholar] [CrossRef]
- Welsh, A.; Hou, M.; Meriki, N.; Stevenson, G. Use of Four-Dimensional Analysis of Power Doppler Perfusion Indices to Demonstrate Cardiac Cycle Pulsatility in Fetoplacental Flow. Ultrasound Med. Boil. 2012, 38, 1345–1351. [Google Scholar] [CrossRef]
- De Gennes, P.-G.; Brochard-Wyart, F.; Quéré, D. Capillarity and Wetting Phenomena, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Guan, Y.; Cheng, F.; Pan, Z. Superwetting polymeric three dimensional (3D) porous materials for Oil/Water separation: A Review. Coatings 2019, 11, 806. [Google Scholar] [CrossRef]
- Lin, J.; Tan, X.; Shi, T.; Tang, Z.; Liao, G. Leaf Vein-Inspired Hierarchical Wedge-Shaped Tracks on Flexible Substrate for Enhanced Directional Water Collection. ACS Appl. Mater. Interfaces 2018, 10, 44815–44824. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, J.; Chen, Z.; Lai, Y. Bioinspired Special Wettability Surfaces: From Fundamental Research to Water Harvesting Applications. Small 2016, 13, 1602992. [Google Scholar] [CrossRef]
- Gurera, D.; Bhushan, B. Bioinspired conical design for efficient water collection from fog. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2019, 377, 20190125. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-C.; Kim, P.; Grinthal, A.; He, N.; Fox, D.; Weaver, J.C.; Aizenberg, J. Condensation on slippery asymmetric bumps. Nature 2016, 531, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Nosonovsky, M.; Bhushan, B. Patterned Nonadhesive Surfaces: Superhydrophobicity and Wetting Regime Transitions. Langmuir 2008, 24, 1525–1533. [Google Scholar] [CrossRef] [PubMed]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rius-Ayra, O.; Fiestas-Paradela, S.; Llorca-Isern, N. Non-Fluorinated, Sustainable, and Durable Superhydrophobic Microarrayed Surface for Water-Harvesting. Coatings 2020, 10, 314. https://doi.org/10.3390/coatings10040314
Rius-Ayra O, Fiestas-Paradela S, Llorca-Isern N. Non-Fluorinated, Sustainable, and Durable Superhydrophobic Microarrayed Surface for Water-Harvesting. Coatings. 2020; 10(4):314. https://doi.org/10.3390/coatings10040314
Chicago/Turabian StyleRius-Ayra, Oriol, Sheila Fiestas-Paradela, and Nuria Llorca-Isern. 2020. "Non-Fluorinated, Sustainable, and Durable Superhydrophobic Microarrayed Surface for Water-Harvesting" Coatings 10, no. 4: 314. https://doi.org/10.3390/coatings10040314
APA StyleRius-Ayra, O., Fiestas-Paradela, S., & Llorca-Isern, N. (2020). Non-Fluorinated, Sustainable, and Durable Superhydrophobic Microarrayed Surface for Water-Harvesting. Coatings, 10(4), 314. https://doi.org/10.3390/coatings10040314




