Improving the Protectiveness of 3-Mercaptopropyl-Trimethoxysilane Coatings on Bronze by Addition of Oxidic Nano- and Microparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
- the basic silane formulation consisted of a hydro-alcoholic solution with composition 90 vol.%/5 vol.%/5 vol.% ethanol/water/silane;
- pH of the silane was set at 4 by adding some drops of diluted sulfuric acid solution;
- the coatings were prepared by dip coating, that is, by immersing the bronze electrodes in the hydro-alcoholic silane solutions for 1 h, followed by fast withdrawal;
- a RT curing was adopted to comply with cultural heritage requirements.
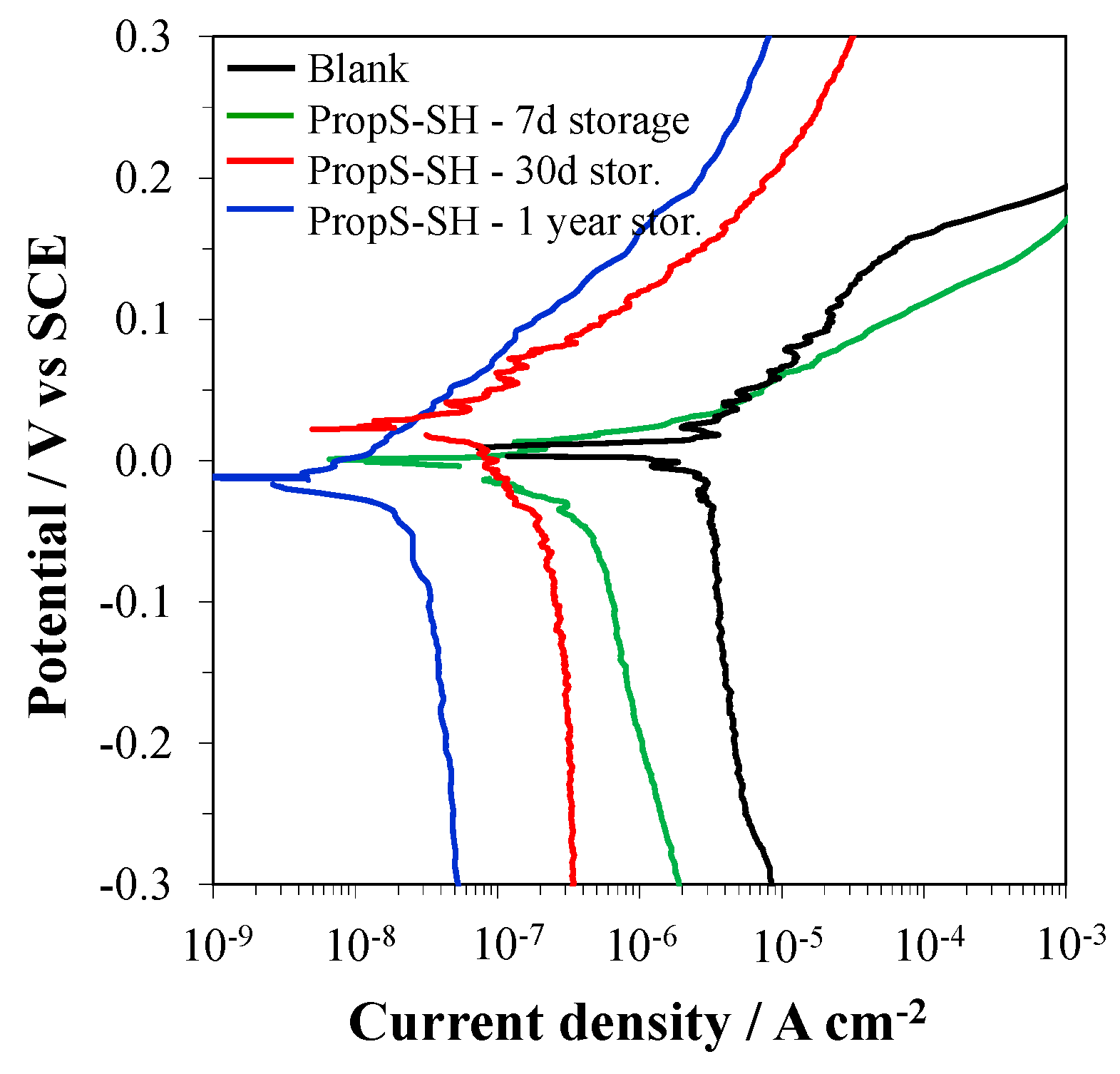
- the storage time of the silane solution at 4 °C. Values of 7 days, 30 days or 1 year were tested. The subsequent coating preparation was always performed at RT;
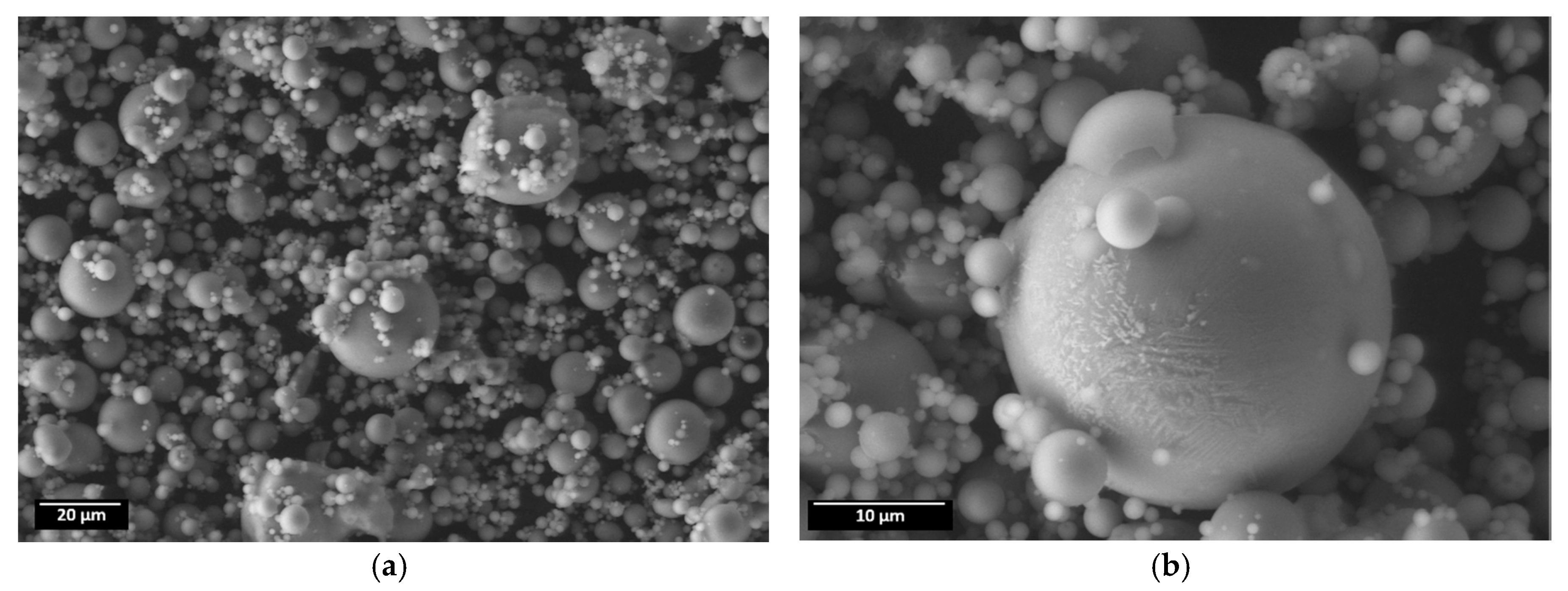
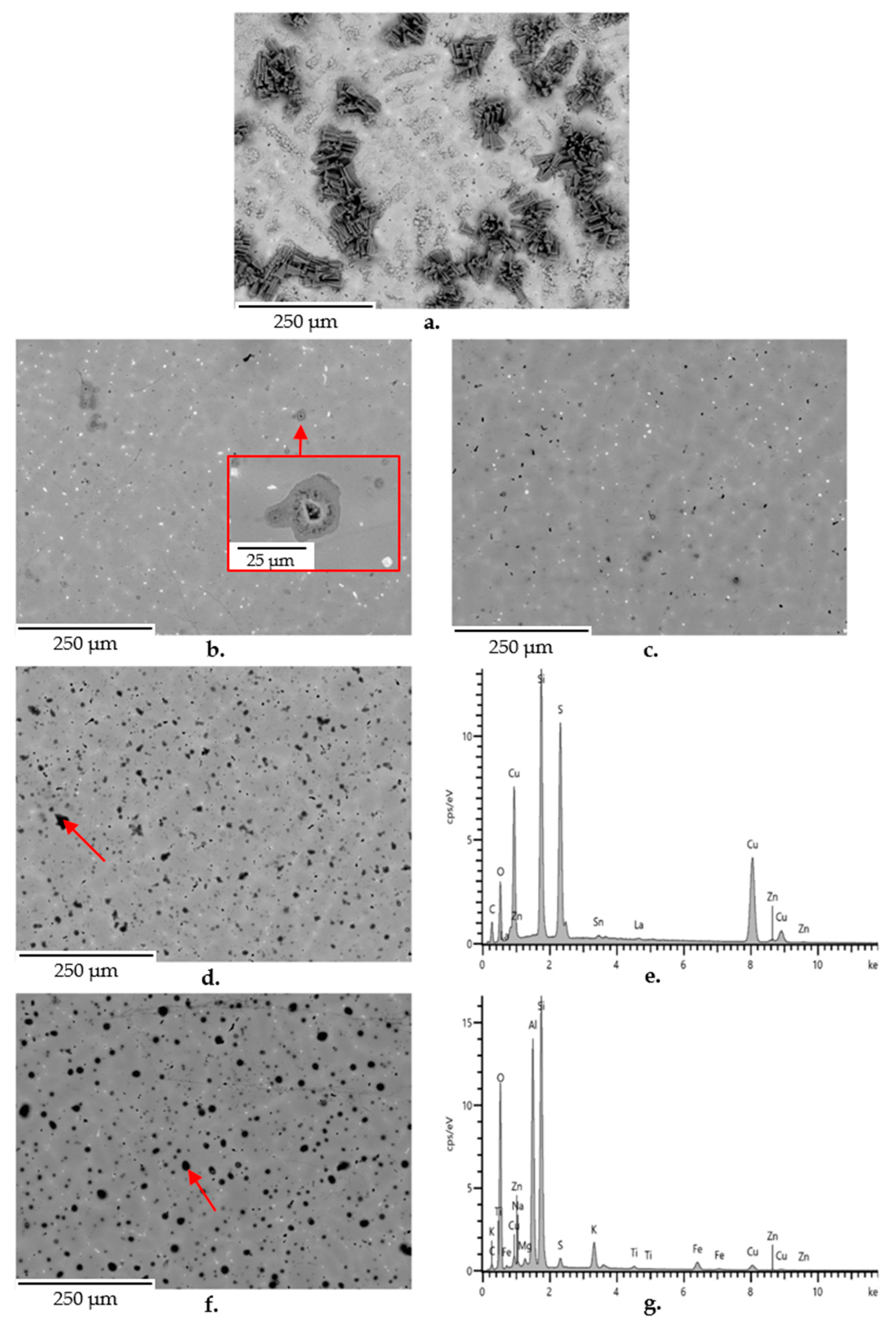
- the addition of 250 ppm nano- or microparticles. The nanoparticles (CeO2 (nano-sized, Aldrich cod. 544841), La2O3 (nano-sized, Aldrich cod. 634271), and TiO2 (nano-sized, Aldrich cod. 718467)) were dispersed into the hydrolyzed silane solution by 15 min ultrasonication. Moreover, the effect of micrometric Class F Fly Ash (FA) addition in the coatings was investigated. FA is a waste product of thermal power plant (from Aboño, Asturias, Spain), which was used after sieving to afford a maximum particle size of 30 μm (Figure 1). Its composition, reported in Table 1, reveals it is a silica- and alumina-rich powder, which, according to X-Ray Diffraction, contains crystalline mullite, quarz, and magnetite, and large amounts of amorphous phases;
- the aging time of nanoparticle suspensions in silane solution, before coating application. The coating performances were evaluated in the absence and in the presence of a 7-day aging of the nanopowder suspensions at 4 °C;
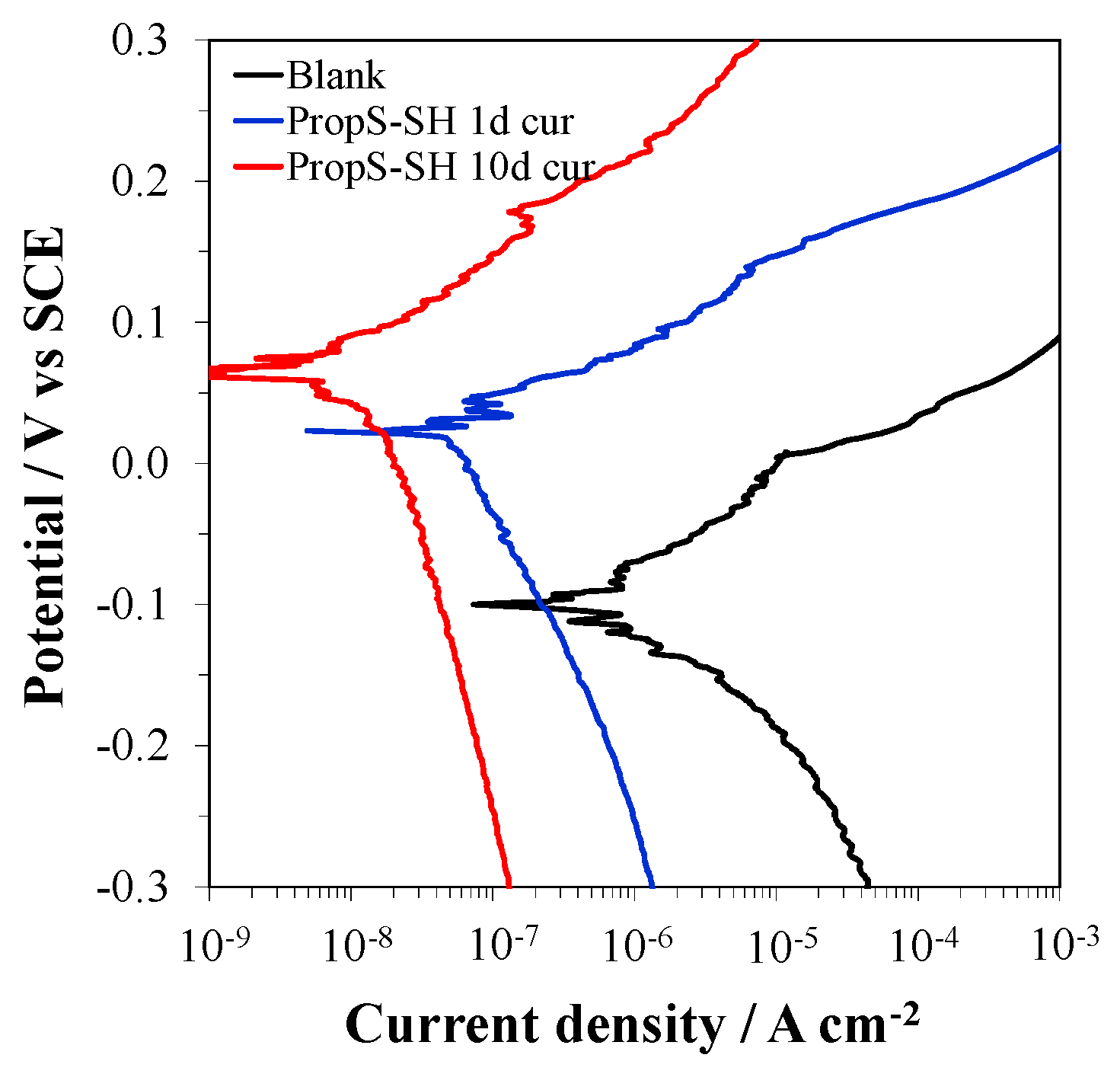
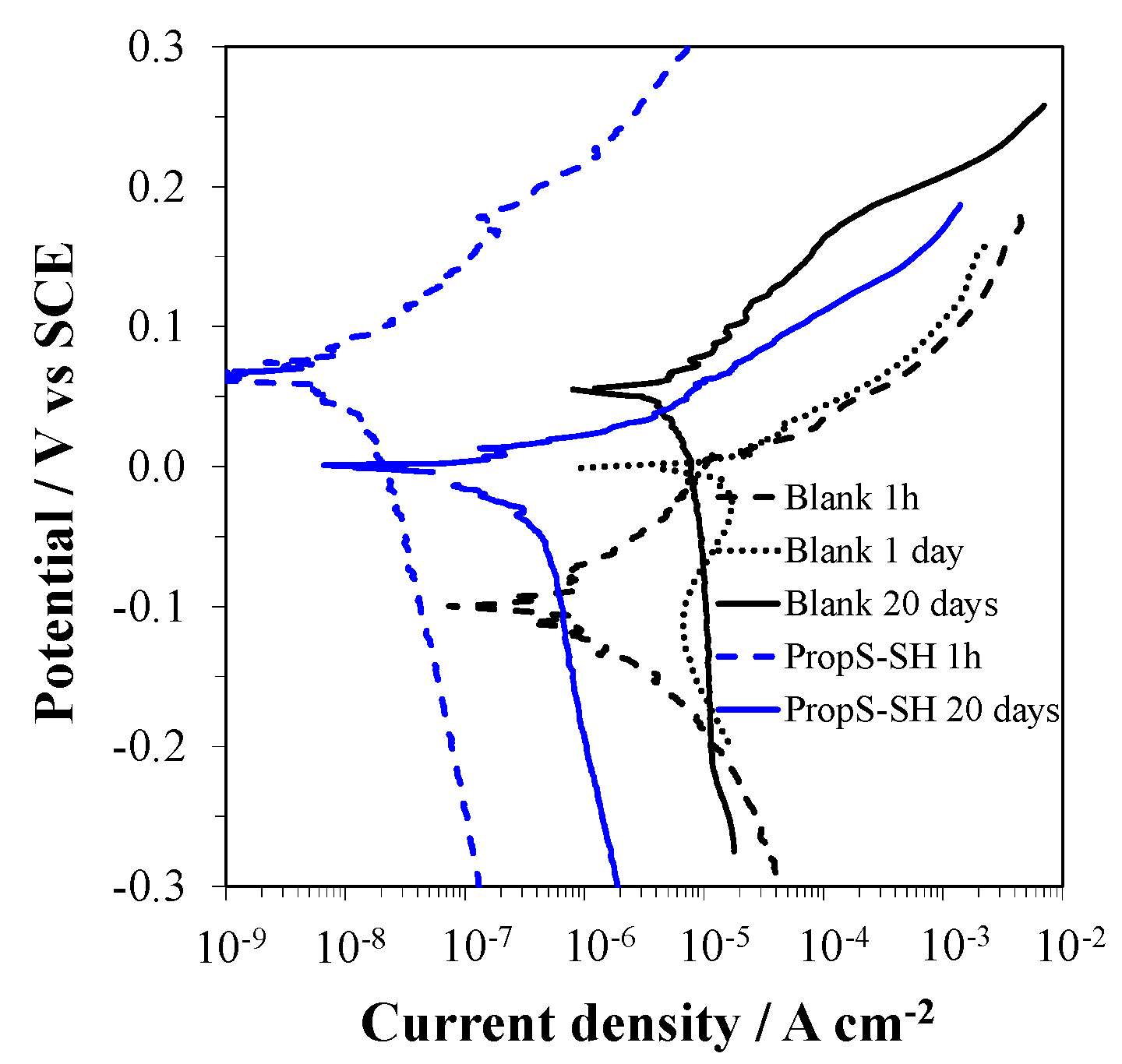
- the coating curing time at RT before immersion in the aggressive solution. Values of 1 or 10 days were tested.
2.2. Corrosion Tests and Characterization Methodology
2.3. Surface Analyses
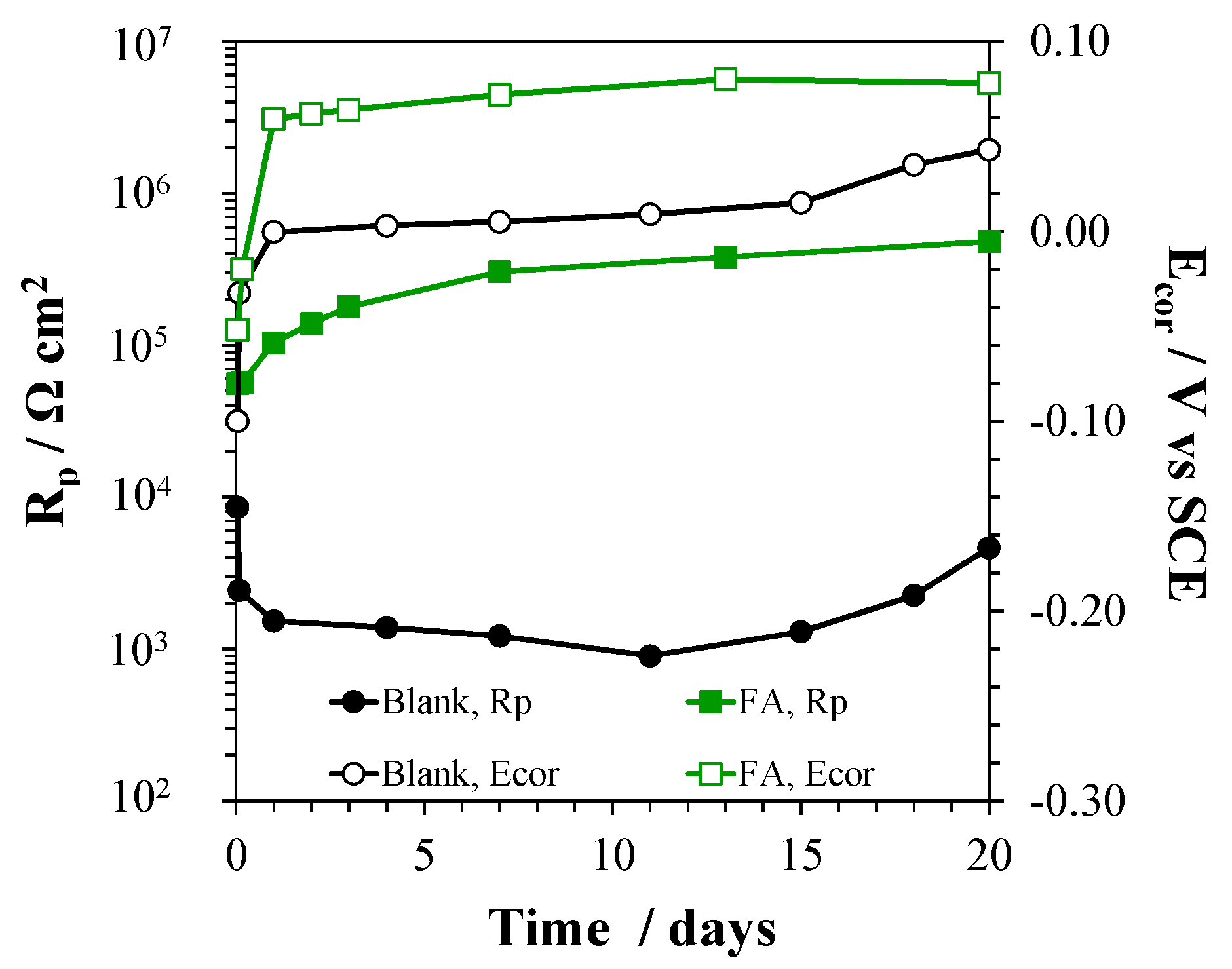
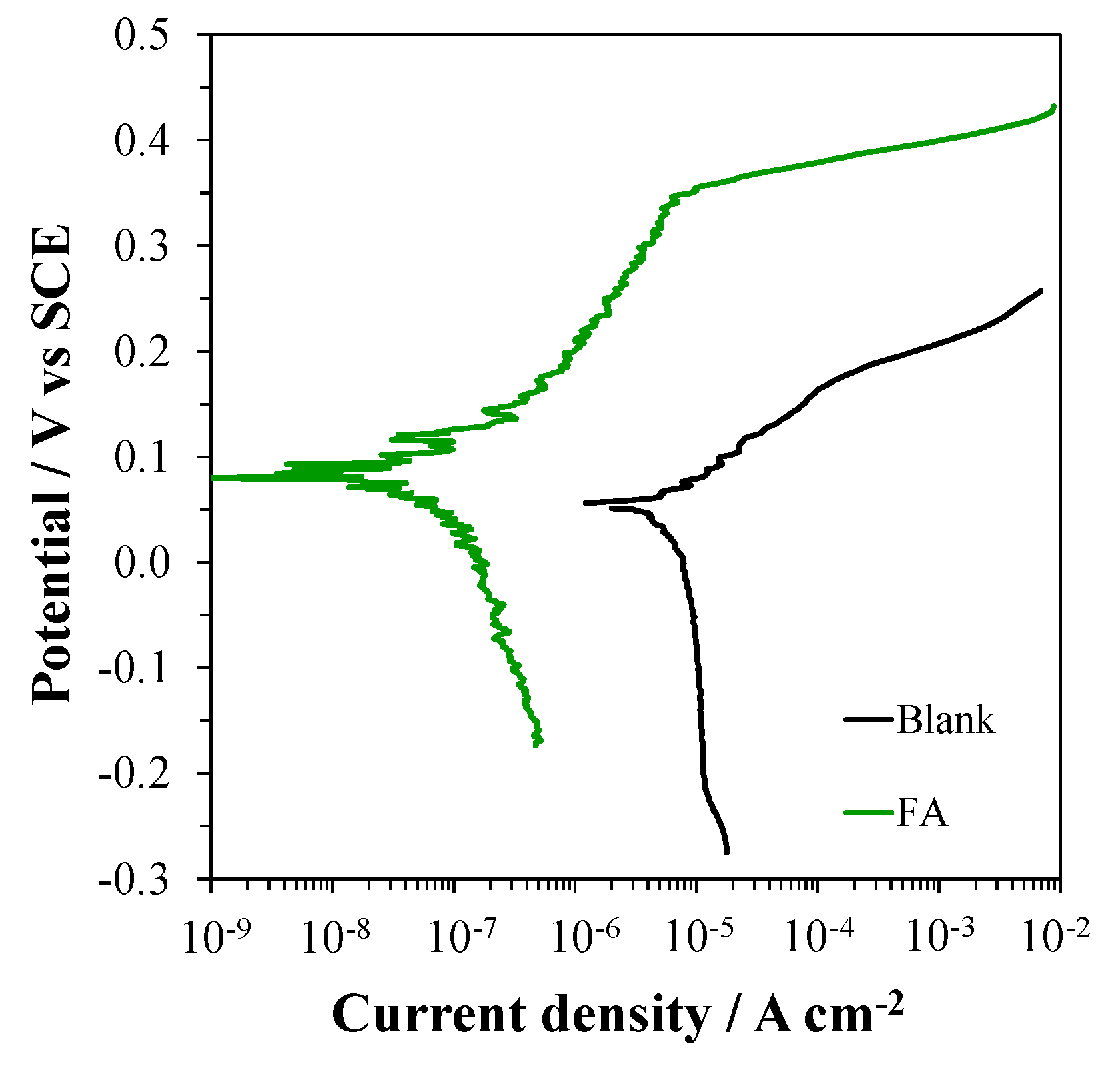
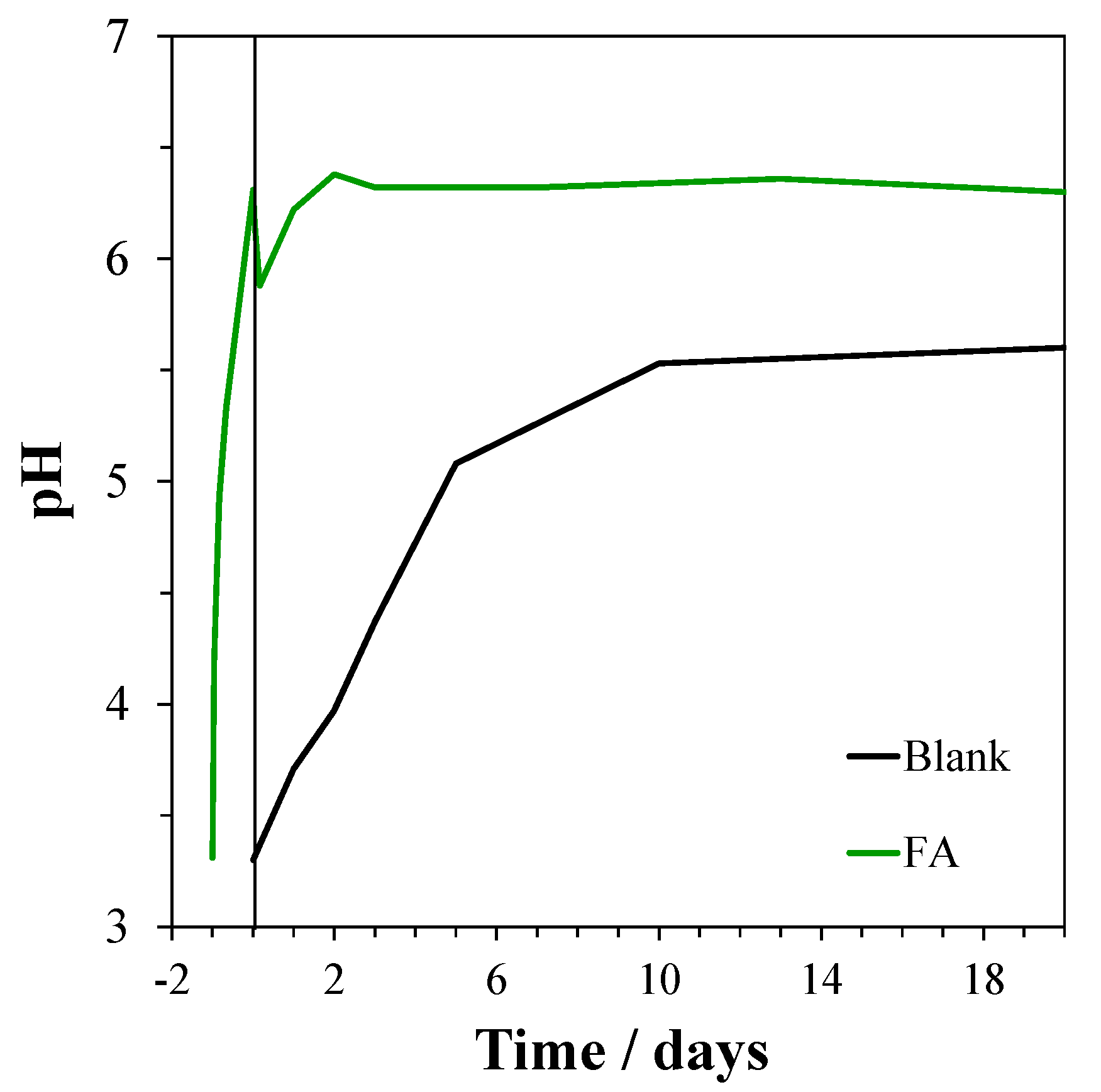
2.4. Tests in FA Suspensions
3. Results
3.1. Influence of Silane Solution Storage Time
3.2. Influence of RT Curing Time
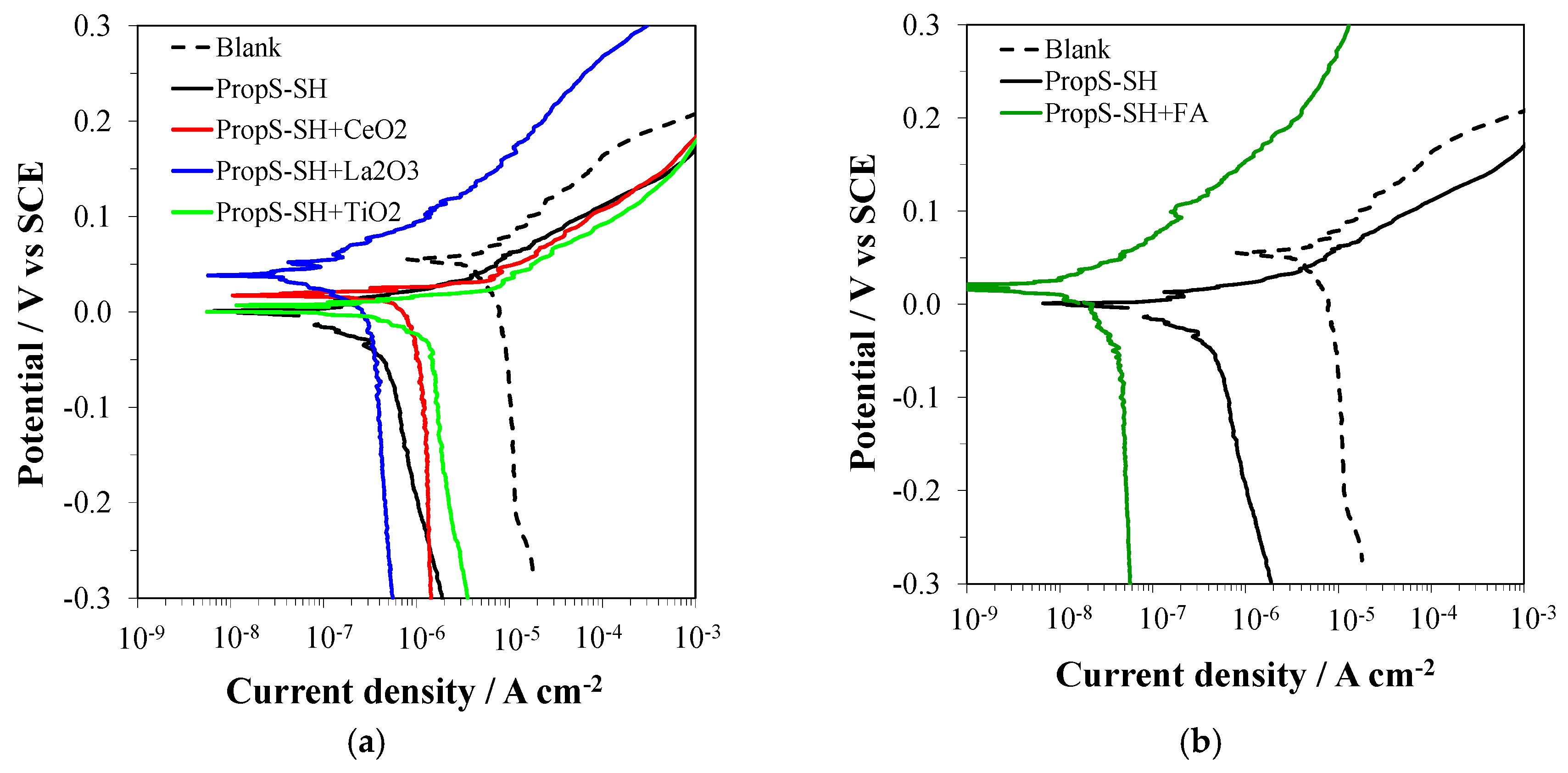
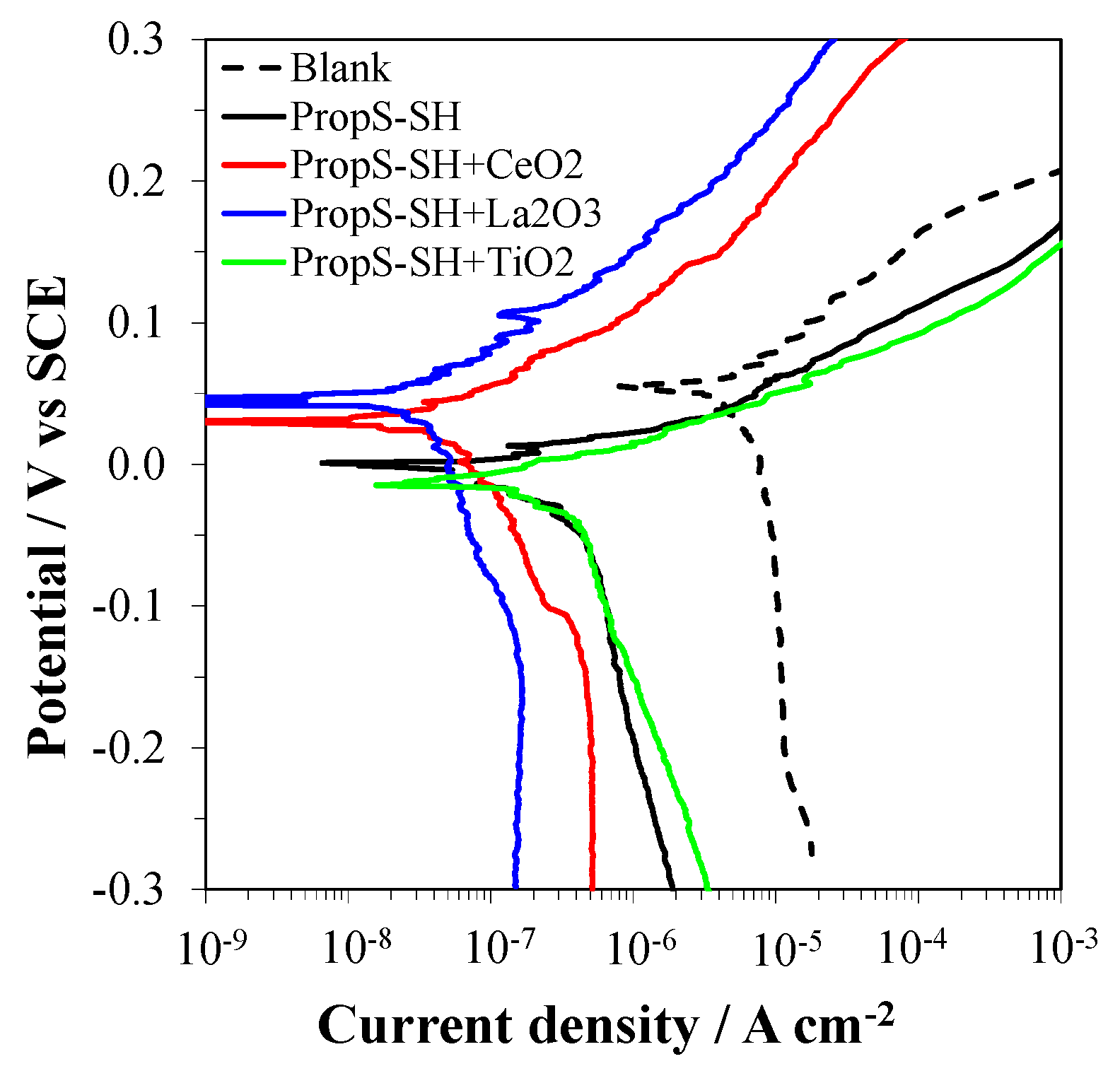
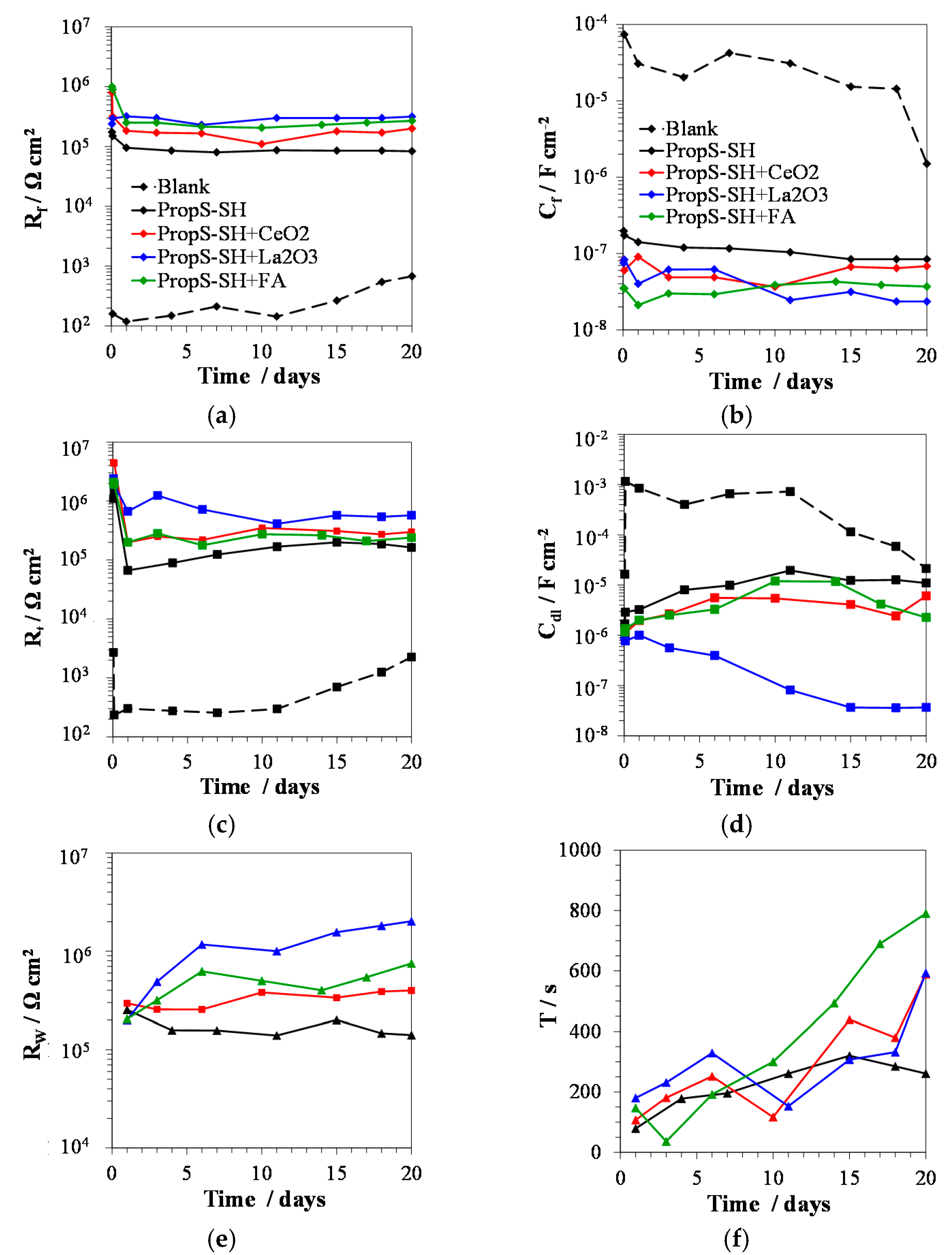
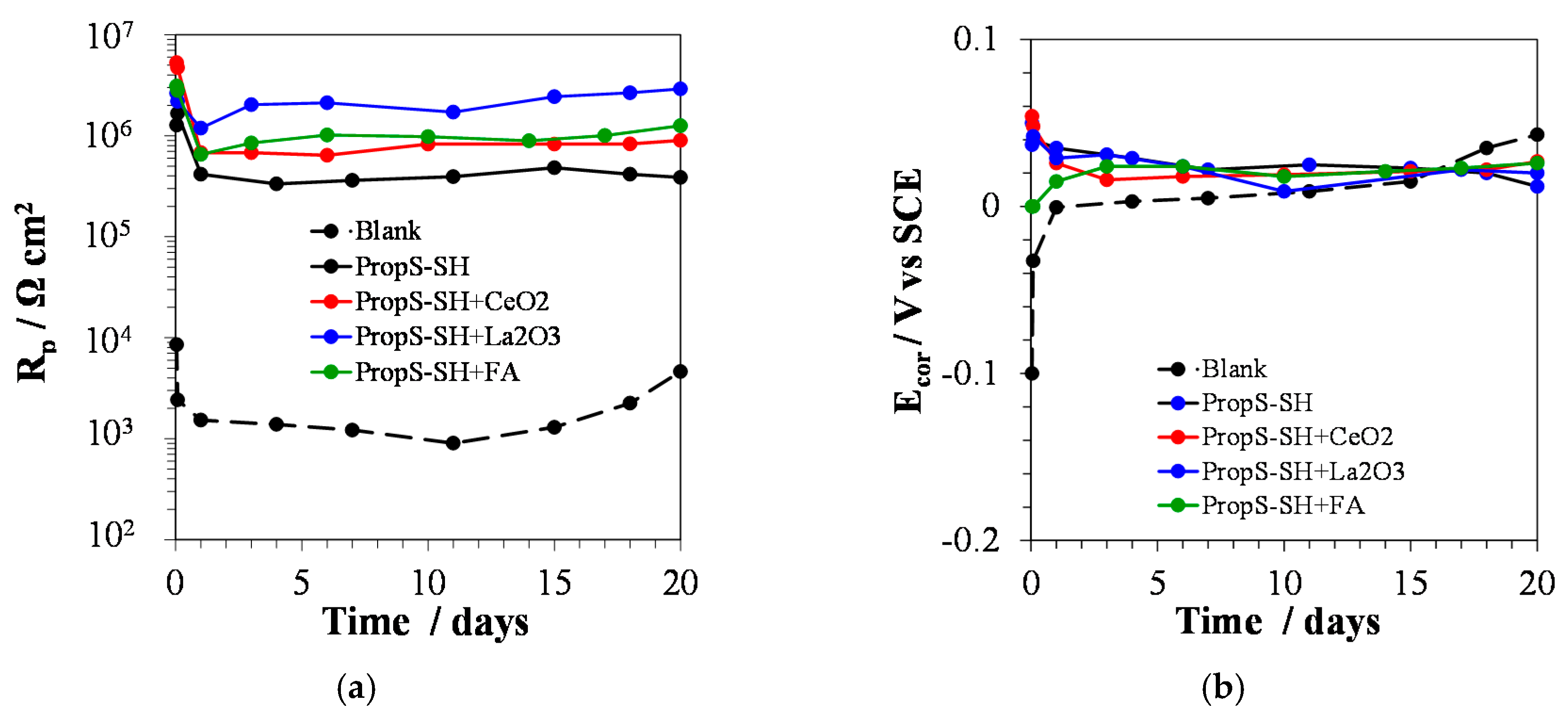
3.3. Influence of Nano and Microparticle Addition in Coating Formulations
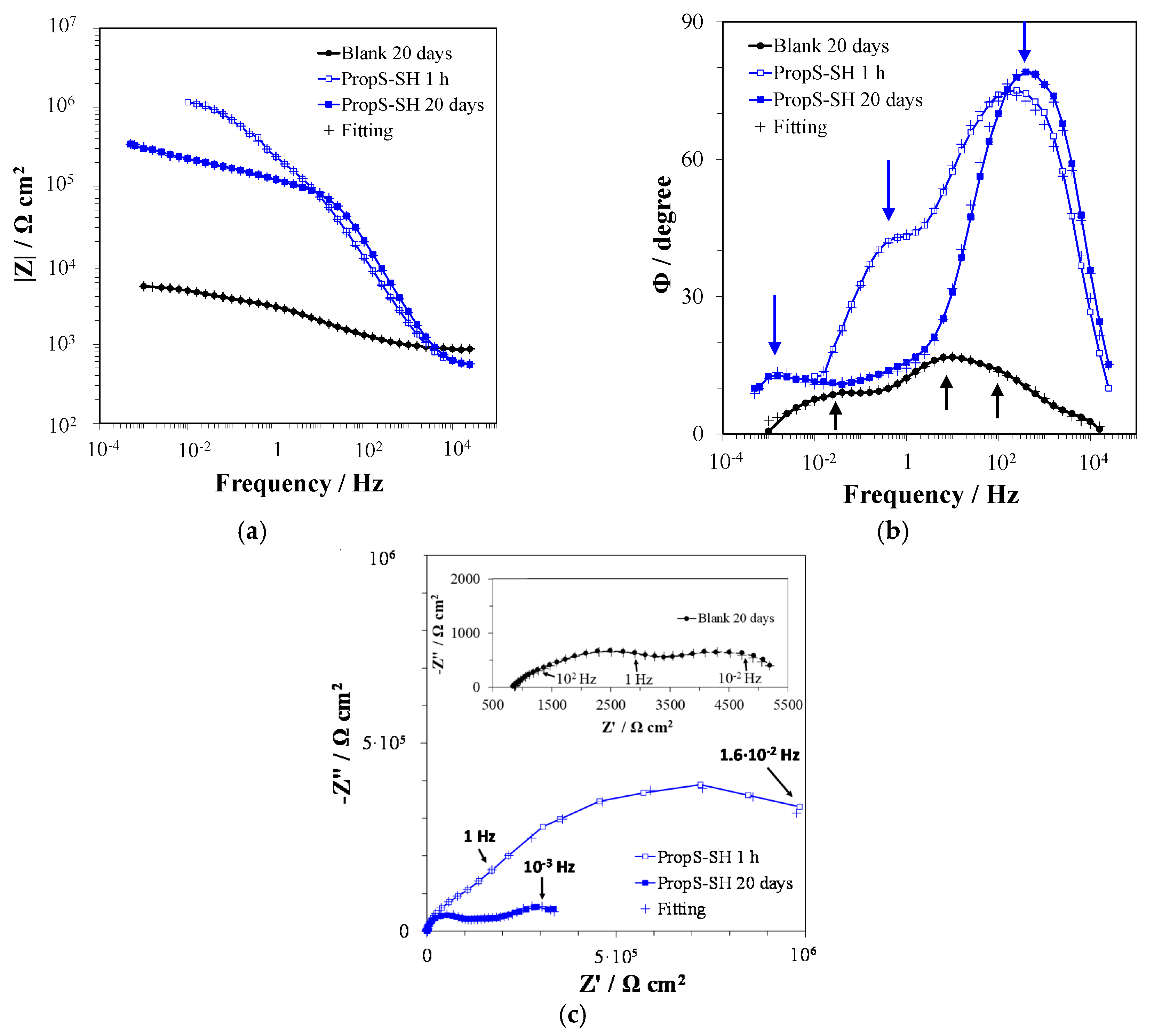
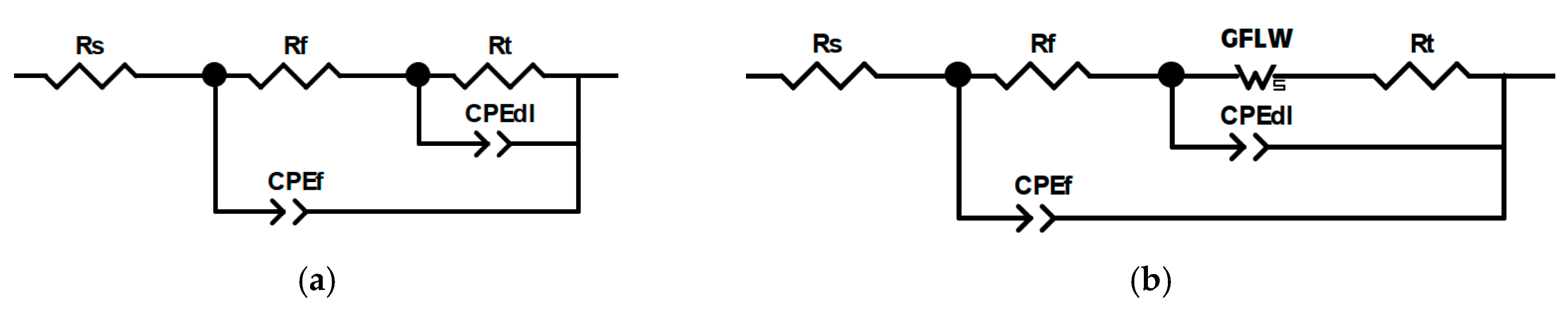
3.4. EIS Analysis of PropS-SH Coating Protectiveness in the Presence of Nano and Microparticles
- a Rf-CPEf couple, related to the dielectric properties of the surface corrosion product film,
- a Rt-CPEdl couple, correlated to the charge transfer reaction, and
- a RF-CPEF couple, due to faradaic reactions in the patina layer.
- the solution resistance (Rs),
- the resistance and CPE of the silane film (Rf and CPEf), and
- the charge transfer resistance and the CPE of the double layer (Rt and CPEdl).
3.5. SEM-EDS Observations
3.6. Tests in FA Suspensions
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Robbiola, L.; Fiaud, C.; Pennec, S. New Model of Outdoor Bronze Corrosion and its Implications for Conservation. In Proceedings of the ICOM Committee for Conservation Tenth Triennial Meeting, Washington, DC, USA, 22–27 August 1993; Volume 2, pp. 796–802, ISBN 0935868658. [Google Scholar]
- De Marco, A.; Screpanti, A.; Mircea, M.; Piersanti, A.; Fornasier, M.F. High resolution estimates of the corrosion risk for cultural heritage in Italy. Environ. Pollut. 2017, 226, 260–267. [Google Scholar] [CrossRef] [PubMed]
- ConservationSupportSystems. Available online: http://www.conservationsupportsystems.com/product/show/incralac-solvent-based/metal-coatings (accessed on 7 January 2020).
- Masi, G.; Balbo, A.; Esvan, J.; Monticelli, C.; Avila, J.; Robbiola, L.; Bernardi, E.; Bignozzi, M.C.; Asensio, M.C.; Martini, C.; et al. X-ray Photoelectron Spectroscopy as a tool to investigate silane-based coatings for the protection of outdoor bronze: the role of alloying elements. Appl. Surf. Sci. 2018, 433, 468–479. [Google Scholar] [CrossRef]
- Masi, G.; Josse, C.; Esvan, J.; Chiavari, C.; Bernardi, E.; Martini, C.; Bignozzi, M.C.; Monticelli, C.; Zanotto, F.; Balbo, A.; et al. Evaluation of the protectiveness of an organosilane coating on patinated Cu-Si-Mn bronze for contemporary art. Prog. Org. Coat. 2019, 127, 286–299. [Google Scholar] [CrossRef]
- Monticelli, C.; Fantin, G.; Di Carmine, G.; Zanotto, F.; Balbo, A. Inclusion of 5-mercapto-1-phenyl-tetrazole into β-cyclodextrin for entrapment in silane coatings: An improvement in bronze corrosion protection. Coatings 2019, 9, 508. [Google Scholar] [CrossRef]
- Aufray, M.; Balbo, A.; Benetti, F.; Bernardi, E.; Bignozzi, M.C.; Chiavari, C.; Esvan, J.; Gartner, N.; Grassi, V.; Josse, C.; et al. Protection of Outdoor Bronzes with Eco-Friendly and Non-Hazardous Coatings Based on Silane and Fluoropolymers: Results from the B-IMPACT Project. In Proceedings of the Interim Meeting of the ICOM-CC Metals Working Group (METAL 2019), Neuchâtel, Switzerland, 2–6 September 2019. [Google Scholar]
- Phanasgaonkar, A.; Raja, V.S. Influence of curing temperature, silica nanoparticles- and cerium on surface morphology and corrosion behaviour of hybrid silane coatings on mild steel. Surf. Coat. Technol. 2009, 203, 2260–2271. [Google Scholar] [CrossRef]
- Van Ooij, W.J.; Zhu, D.; Stacy, M.; Seth, A.; Mugada, T.; Gandhi, J.; Puomi, P. Corrosion protection properties of organofunctional silanes—An overview. Tsinghua Sci. Technol. 2005, 10, 639–664. [Google Scholar] [CrossRef]
- Materne, T.; de Buyl, F.; Witucki, G.L. Organosilane Technology in Coating Applications: Review and Perspectives. Available online: https://www.academia.edu/10140050/Organosilane_Technology_in_Coating_Applications_Review_and_Perspectives (accessed on 7 January 2020).
- Montemor, M.F.; Ferreira, M.G.S. Cerium salt activated nanoparticles as fillers for silane films: Evaluation of the corrosion inhibition performance on galvanised steel substrates. Electrochim. Acta 2007, 52, 6976–6987. [Google Scholar] [CrossRef]
- Montemor, M.F.; Ferreira, M.G.S. Analytical characterization of silane films modified with cerium activated nanoparticles and its relation with the corrosion protection of galvanised steel substrates. Prog. Org. Coat. 2008, 63, 330–337. [Google Scholar] [CrossRef]
- Montemor, M.F.; Pinto, R.; Ferreira, M.G.S. Chemical composition and corrosion protection of silane films modified with CeO2 nanoparticles. Electrochim. Acta 2009, 54, 5179–5189. [Google Scholar] [CrossRef]
- Zandi Zand, R.; Verbeken, K.; Flexer, V.; Adriaens, A. Effects of ceria nanoparticle concentrations on the morphology and corrosion resistance of ceriumesilane hybrid coatings on electro-galvanized steel substrates. Mat. Chem. Phys. 2014, 145, 450–460. [Google Scholar] [CrossRef]
- Brusciotti, F.; Batan, A.; De Graeve, I.; Wenkin, M.; Biessemans, M.; Willem, R.; Reniers, F.; Pireaux, J.J.; Piens, M.; Vereecken, J.; et al. Characterization of thin water-based silane pre-treatments on aluminium with the incorporation of nano-dispersed CeO2 particles. Surf. Coat. Technol. 2010, 205, 603–613. [Google Scholar] [CrossRef]
- Schem, M.; Schmidt, T.; Gerwann, J.; Wittmar, M.; Veith, M.; Thompson, G.E.; Molchan, I.S.; Hashimoto, T.; Skeldon, P.; Phani, A.R.; et al. CeO2-filled sol–gel coatings for corrosion protection of AA2024-T3 aluminium alloy. Corros. Sci. 2009, 51, 2304–2315. [Google Scholar] [CrossRef]
- Chen, C.; Dong, S.; Hou, R.; Hu, J.; Jiang, P.; Ye, C.; Du, R.; Lin, C. Insight into the anti-corrosion performance of electrodeposited silane/nano-CeO2 film on carbon steel. Surf. Coat. Technol. 2017, 326, 183–191. [Google Scholar] [CrossRef]
- Calado, L.M.; Taryba, M.G.; Carmezim, M.J.; Montemor, M.F. Self-healing ceria-modified coating for corrosion protection of AZ31 magnesium alloy. Corros. Sci. 2018, 142, 12–21. [Google Scholar] [CrossRef]
- Balan, P.; Singh Raman, R.K.; Chan, E.-S.; Harun, M.K. Effectiveness of lanthanum triflate activated silica nanoparticles as fillers in silane films for corrosion protection of low carbon steel. Prog. Org. Coat. 2016, 90, 222–234. [Google Scholar] [CrossRef]
- Palanivel, V.; Zhu, D.; van Ooij, W.J. Nanoparticle-filled silane films as chromate replacements for aluminum alloys. Prog. Org. Coat. 2003, 47, 384–392. [Google Scholar] [CrossRef]
- Suegama, P.H.; Recco, A.A.C.; Tschiptschin, A.P.; Aoki, I.V. Influence of silica nanoparticles added to an organosilane film on carbon steel electrochemical and tribological behavior. Prog. Org. Coat. 2007, 60, 90–98. [Google Scholar] [CrossRef]
- Niknahad, M.; Mannari, V. Corrosion protection of aluminum alloy substrate with nano-silica reinforced organic–inorganic hybrid coatings. J. Coat. Technol. Res. 2016, 13, 1035–1046. [Google Scholar] [CrossRef]
- Liu, L.; Hu, J.-M.; Zhang, J.-Q.; Cao, C.-N. Improving the formation and protective properties of silane films by the combined use of electrodeposition and nanoparticles incorporation. Electrochim. Acta 2006, 52, 538–545. [Google Scholar] [CrossRef]
- Fan, H.-Q.; Xia, D.-H.; Li, M.-C.; Li, Q. Self-assembled (3-mercaptopropyl)trimethoxylsilane film modified with La2O3 nanoparticles for brass corrosion protection in NaCl solution. J. Alloy. Compd. 2017, 702, 60–67. [Google Scholar] [CrossRef]
- Saravanan, P.; Jayamoorthy, K.; Kumar, S.A. Design and characterization of non-toxic nano-hybrid coatings for corrosion and fouling resistance. J. Sci. Adv. Mater. Dev. 2016, 1, 367–378. [Google Scholar] [CrossRef]
- Zhang, S.L.; Zhang, M.M.; Yao, Y.; Sun, F. Use of silane films modified with Y2O3 nanoparticles for improved corrosion resistance of AA 6061. Trans. IMF 2011, 89, 320–324. [Google Scholar] [CrossRef]
- Naderi, R.; Fedel, M.; Deflorian, F.; Poelman, M.; Olivier, M. Synergistic effect of clay nanoparticles and cerium component on the corrosion behavior of eco-friendly silane sol-gel layer applied on pure aluminium. Surf. Coat. Technol. 2013, 224, 93–100. [Google Scholar] [CrossRef]
- Santana, I.; Pepe, A.; Schreiner, W.; Pellice, S.; Ceré, S. Hybrid sol-gel coatings containing clay nanoparticles for corrosion protection of mild steel. Electrochim. Acta 2016, 203, 396–403. [Google Scholar] [CrossRef]
- Montemor, M.F.; Trabelsi, W.; Lamaka, S.V.; Yasakau, K.A.; Zheludkevich, M.L.; Bastos, A.C.; Ferreira, M.G.S. The synergistic combination of bis-silane and CeO2·ZrO2 nanoparticles on the electrochemical behaviour of galvanised steel in NaCl solutions. Electrochim. Acta 2008, 53, 5913–5922. [Google Scholar] [CrossRef]
- Zanotto, F.; Frignani, A.; Balbo, A.; Grassi, V.; Monticelli, C. Influence of CeAlO3 nanoparticles on the performances of silane coatings for AZ31 alloy. Int. J. Corros. Scale Inhib. 2019, 8, 954–973. [Google Scholar]
- Alibakhshi, E.; Ghasemi, E.; Mahdavian, M.; Ramezanzadeh, B. A comparative study on corrosion inhibitive effect of nitrate and phosphate intercalated Zn-Al- layered double hydroxides (LDHs) nanocontainers incorporated into a hybrid silane layer and their effect on cathodic delamination of epoxy topcoat. Corros. Sci. 2017, 115, 159–174. [Google Scholar] [CrossRef]
- Chiavari, C.; Balbo, A.; Bernardi, E.; Martini, C.; Zanotto, F.; Vassura, I.; Bignozzi, M.C.; Monticelli, C. Organosilane coatings applied on bronze: influence of UV radiation and thermal cycles on the protectiveness. Prog. Org. Coat. 2015, 82, 91–100. [Google Scholar] [CrossRef]
- Yasakau, K.A.; Carneiro, J.; Zheludkevich, M.L.; Ferreira, M.G.S. Influence of sol-gel process parameters on the protection properties of sol–gel coatings applied on AA2024. Surf. Coat. Technol. 2014, 246, 6–16. [Google Scholar] [CrossRef]
- Balbo, A.; Chiavari, C.; Martini, C.; Monticelli, C. Effectiveness of corrosion inhibitor films for the conservation of bronzes and gilded bronzes. Corros. Sci. 2012, 59, 204–212. [Google Scholar] [CrossRef]
- Zucchi, F.; Grassi, V.; Frignani, A.; Trabanelli, G. Inhibition of copper corrosion by silane coatings. Corros. Sci. 2004, 46, 2853–2865. [Google Scholar] [CrossRef]
- Zucchi, F.; Frignani, A.; Grassi, V.; Trabanelli, G.; Dal Colle, M. The formation of a protective layer of 3-mercapto-propyl-trimethoxy-silane on copper. Corros Sci. 2007, 49, 1570–1583. [Google Scholar] [CrossRef]
- Chiavari, C.; Bernardi, E.; Balbo, A.; Monticelli, C.; Raffo, S.; Bignozzi, M.C.; Martini, C. Atmospheric corrosion of fire-gilded bronze: corrosion and corrosion protection during accelerated ageing tests. Corros. Sci. 2015, 100, 435–447. [Google Scholar] [CrossRef]
- Chen, M.-A.; Lu, X.-B.; Guo, Z.-H.; Huang, R. Influence of hydrolysis time on the structure and corrosion protective performance of (3-mercaptopropyl)triethoxysilane film on copper. Corros. Sci. 2011, 53, 2793–2802. [Google Scholar] [CrossRef]
- Monticelli, C.; Balbo, A.; Esvan, J.; Chiavari, C.; Martini, C.; Zanotto, F.; Marvelli, L.; Robbiola, L. Evaluation of 2 (salicylideneimino) thiophenol and other Schiff bases as bronze corrosion inhibitors by electrochemical techniques and surface analysis. Corros. Sci. 2019, 148, 144–158. [Google Scholar] [CrossRef]
- Marušić, K.; Otmačić Ćurković, H.; Horvat-Kurbegović, Š.; Takenouti, H.; Stupnišek-Lisac, E. Comparative studies of chemical and electrochemical preparation of artificial bronze patinas and their protection by corrosion inhibitor. Electrochim. Acta 2009, 54, 7106–7113. [Google Scholar] [CrossRef]
- Bostan, R.; Varvara, S.; Găină, L.; Muresan, L.M. Evaluation of some phenothiazine derivatives as corrosion inhibitors for bronze in weakly acidic solution. Corros. Sci. 2012, 63, 275–286. [Google Scholar] [CrossRef]
- Macdonald, J.R. Impedance Spectroscopy; Wiley: Hoboken, NJ, USA, 1987. [Google Scholar]
- Jorcin, J.B.; Orazem, M.E.; Pébère, N.; Tribollet, B. CPE analysis by local electrochemical impedance spectroscopy. Electrochim. Acta 2006, 51, 1473–1479. [Google Scholar] [CrossRef]
- Hsu, C.H.; Mansfeld, F. Concerning the conversion of the constant phase element parameter Y0 into a capacitance. Corrosion 2001, 57, 747–748. [Google Scholar] [CrossRef]
- Wang, X.T.; Wang, J.; Wu, Y. The inhibition effect and mechanism of L -cysteine on the corrosion of bronze covered with a CuCl patina. Corros. Sci. 2015, 97, 89–99. [Google Scholar] [CrossRef]
- Zucchi, F.; Grassi, V.; Frignani, A.; Monticelli, C.; Trabanelli, G. Influence of a silane treatment on the corrosion resistance of a WE43 magnesium alloy. Surf. Coat. Technol. 2006, 200, 4136–4143. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Yan, M.; Chen, F. Reconstruction of relaxation time distribution from linear electrochemical impedance spectroscopy. J. Power Sources 2015, 283, 464–477. [Google Scholar] [CrossRef]
- Metikoš-Huković, M.; Babić, R.; Škugor Rončević, I.; Grubač, Z. Corrosion behavior of the filmed copper surface in saline water under static and jet impingement conditions. Corrosion 2012, 68, 025002-1–025002-8. [Google Scholar] [CrossRef]
- Choucri, J.; Zanotto, F.; Grassi, V.; Balbo, A.; Ebn Touhami, M.; Mansouri, I.; Monticelli, C. Corrosion behavior of different brass alloys for drinking water distribution systems. Metals 2019, 9, 649. [Google Scholar] [CrossRef]
- Deflorian, F.; Rossi, S.; Fedrizzi, L.; Fedel, M. Integrated electrochemical approach for the investigation of silane pre-treatments for painting copper. Prog. Org. Coat. 2008, 63, 338–344. [Google Scholar] [CrossRef]
- Peng, S.; Zeng, Z.; Zhao, W.; Chen, J.; Han, J.; Wu, X. Performance evaluation of mercapto functional hybrid silica sol–gel coating on copper surface. Surf. Coat. Technol. 2014, 251, 135–142. [Google Scholar] [CrossRef]
- Montemor, M.F.; Cabral, A.M.; Zheludkevich, M.L.; Ferreira, M.G.S. The corrosion resistance of hot dip galvanized steel pretreated with bis-functional silanes modified with microsilica. Surf. Coat. Technol. 2006, 200, 2875–2885. [Google Scholar] [CrossRef]
- Van Ooij, W.J.; Zhu, D. Electrochemical impedance spectroscopy of bis-[triethoxysilypropyl]tetrasulfide on Al 2024-T3 substrates. Corrosion 2001, 57, 413–427. [Google Scholar] [CrossRef]
- Scully, J.R. Polarization resistance method for determination of instantaneous corrosion rates. Corrosion 2000, 56, 199–217. [Google Scholar] [CrossRef]
- Lintereur, P.A.; Duranceau, S.J.; Taylor, J.S. Sodium silicate impacts on copper release in a potable water comprised of ground, surface and desalted sea water supplies. Desalin. Water Treat. 2011, 30, 348–360. [Google Scholar] [CrossRef]
- Bonora, P.L.; Deflorian, F.; Fedrizzi, L. Electrochemical impedance spectroscopy as a tool for investigating underpaint corrosion. Electrochim. Acta 1996, 41, 1073–1082. [Google Scholar] [CrossRef]

| Material | L.o.I.1 | IR2 | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | K2O | Na2O | MnO | TiO2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fly Ash | 3.59 | 2.23 | 51.78 | 27.80 | 6.18 | 4.59 | 1.52 | 0.71 | 2.51 | 0.59 | 0.06 | 1.35 |
| Species | Concentration/mg·L−1 |
|---|---|
| Cl− | 12.7 |
| NO3- | 46.4 |
| NH4+ | 10.6 |
| SO42− | 19.4 |
| HCOO− | 0.5 |
| CH3COO− | 2.3 |
| Na+ | 5.3 |
| Ca2+ | 3.4 |
| Electrical Conductivity /μS·cm−1 (at 25 °C) | 345 |
| pH | 3.3 |
| Coating | Immersion Time | Ecor/V(SCE) | icor/µA·cm–2 |
|---|---|---|---|
| Blank | 1 h | −0.100 | 1.6 |
| 20 days | 0.055 | 8.3 | |
| PropS-SH | 1 h | 0.066 | 0.015 |
| 20 days | 0.001 | 0.5 |
| Coating | Aging Time/days | Ecor/V(SCE) | icor/µA·cm−2 |
|---|---|---|---|
| PropS-SH + CeO2 | 0 | 0.017 | 1.1 |
| PropS-SH + La2O3 | 0 | 0.038 | 0.31 |
| PropS-SH + TiO2 | 0 | 0.004 | 1.5 |
| PropS-SH + FA | 0 | 0.018 | 0.041 |
| PropS-SH + CeO2 | 7 | 0.030 | 0.08 |
| PropS-SH + La2O3 | 7 | 0.042 | 0.045 |
| PropS-SH + TiO2 | 7 | −0.015 | 0.40 |
| Element | Concentration/mg·L–1 |
|---|---|
| Al | 0.14 |
| Si | 19.1 |
| Ca | 98.9 |
| Fe | <0.32 |
| Mg | 18.9 |
| K | 0.27 |
| Na | 5.05 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monticelli, C.; Zanotto, F.; Grassi, V.; Seyedi, M.; Balbo, A. Improving the Protectiveness of 3-Mercaptopropyl-Trimethoxysilane Coatings on Bronze by Addition of Oxidic Nano- and Microparticles. Coatings 2020, 10, 225. https://doi.org/10.3390/coatings10030225
Monticelli C, Zanotto F, Grassi V, Seyedi M, Balbo A. Improving the Protectiveness of 3-Mercaptopropyl-Trimethoxysilane Coatings on Bronze by Addition of Oxidic Nano- and Microparticles. Coatings. 2020; 10(3):225. https://doi.org/10.3390/coatings10030225
Chicago/Turabian StyleMonticelli, Cecilia, Federica Zanotto, Vincenzo Grassi, Mahla Seyedi, and Andrea Balbo. 2020. "Improving the Protectiveness of 3-Mercaptopropyl-Trimethoxysilane Coatings on Bronze by Addition of Oxidic Nano- and Microparticles" Coatings 10, no. 3: 225. https://doi.org/10.3390/coatings10030225
APA StyleMonticelli, C., Zanotto, F., Grassi, V., Seyedi, M., & Balbo, A. (2020). Improving the Protectiveness of 3-Mercaptopropyl-Trimethoxysilane Coatings on Bronze by Addition of Oxidic Nano- and Microparticles. Coatings, 10(3), 225. https://doi.org/10.3390/coatings10030225







