Thermionic Vacuum Arc—A Versatile Technology for Thin Film Deposition and Its Applications
Abstract
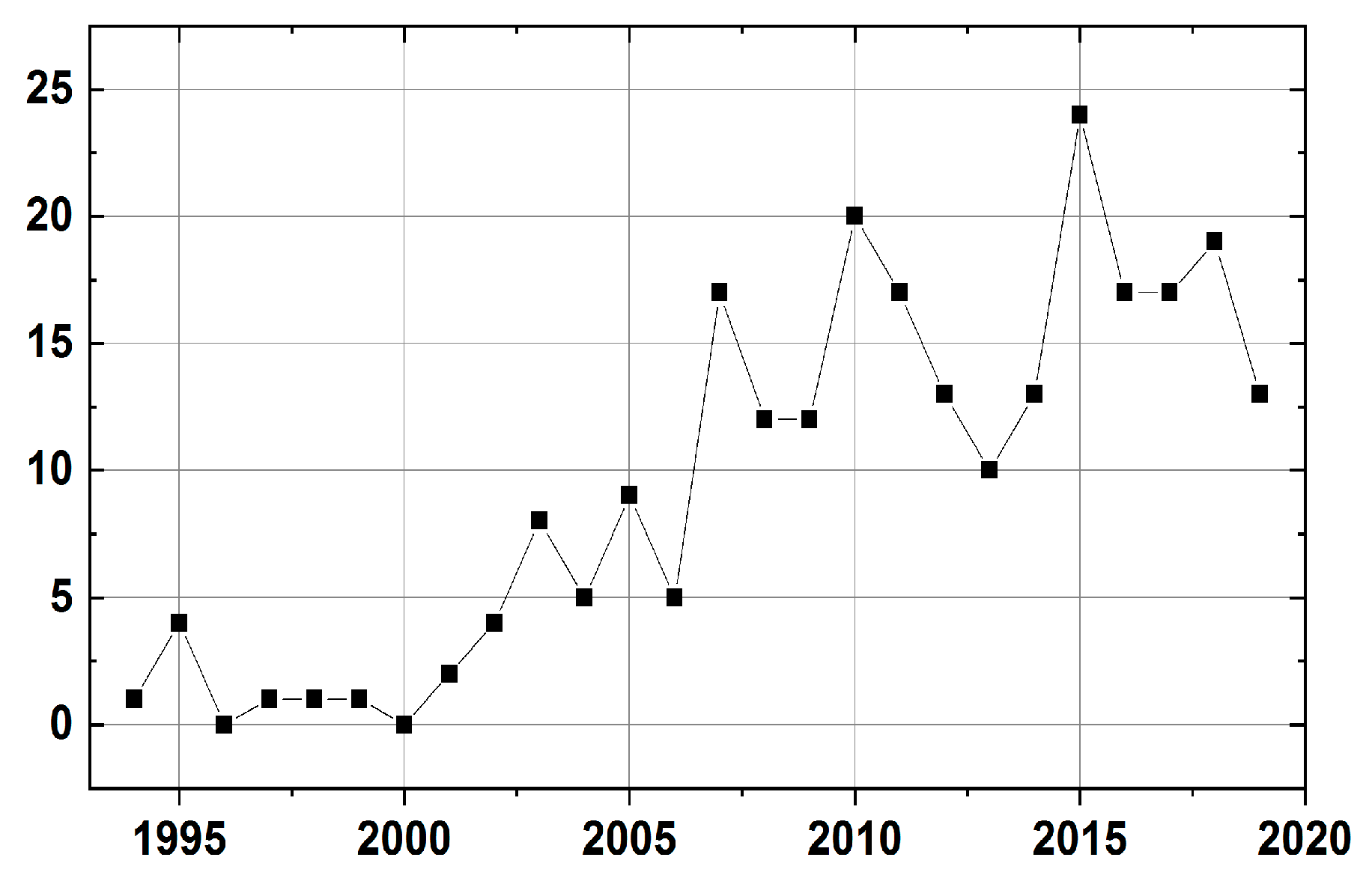
1. Introduction
2. Arrangements of the TVA Systems
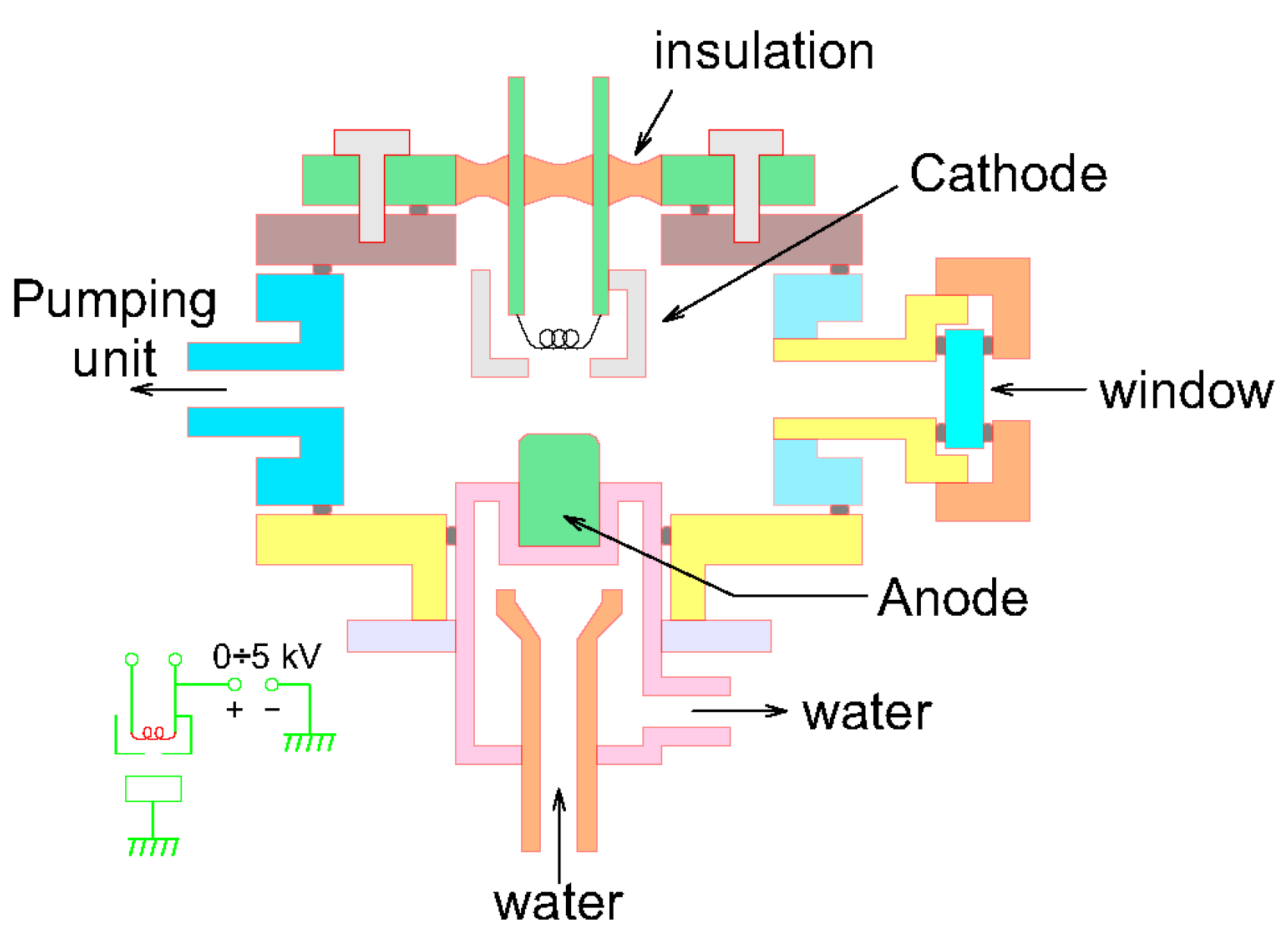
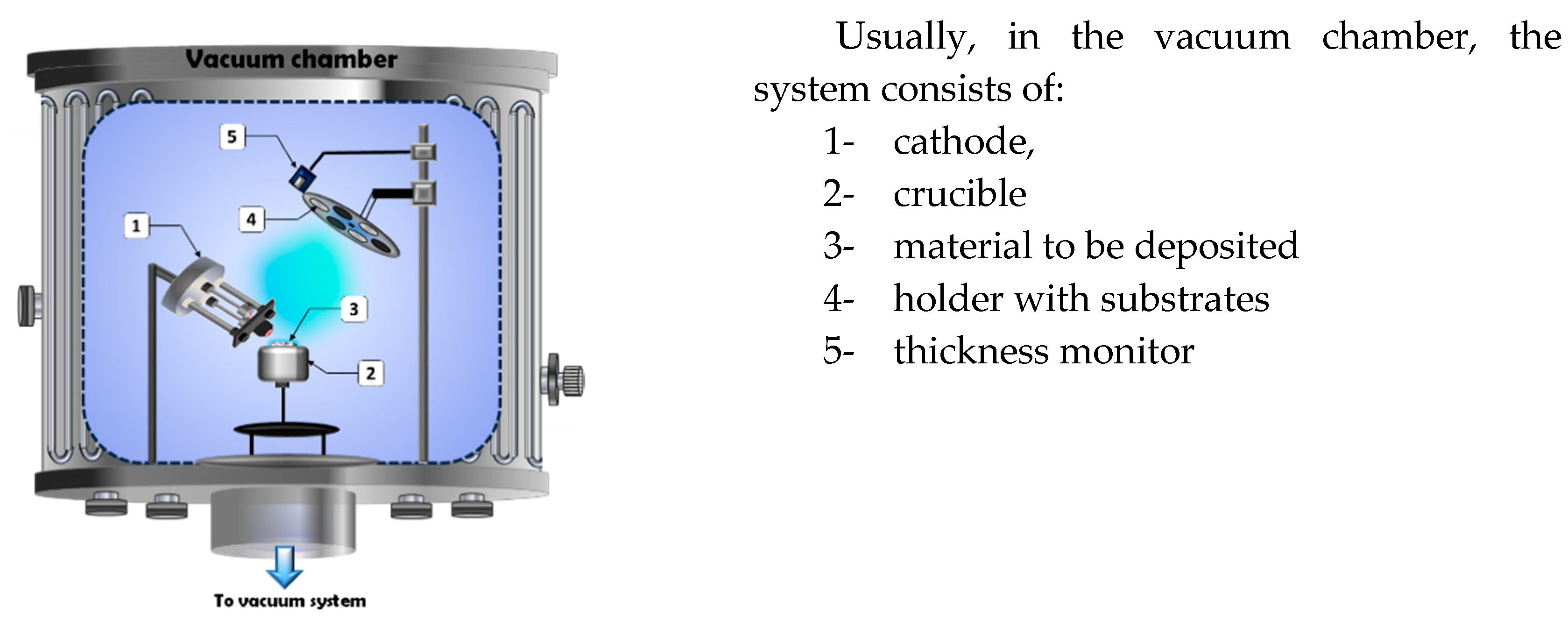
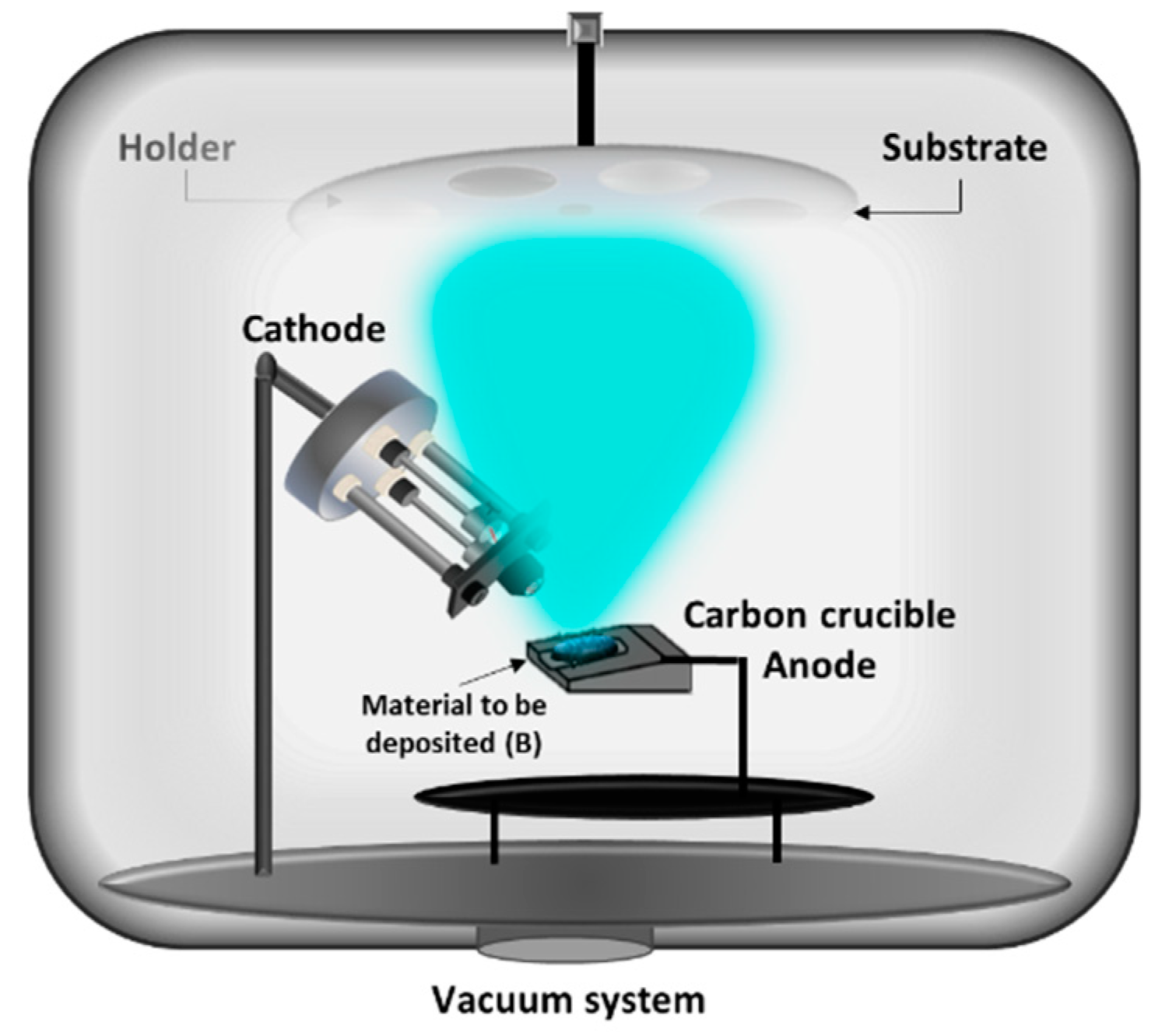
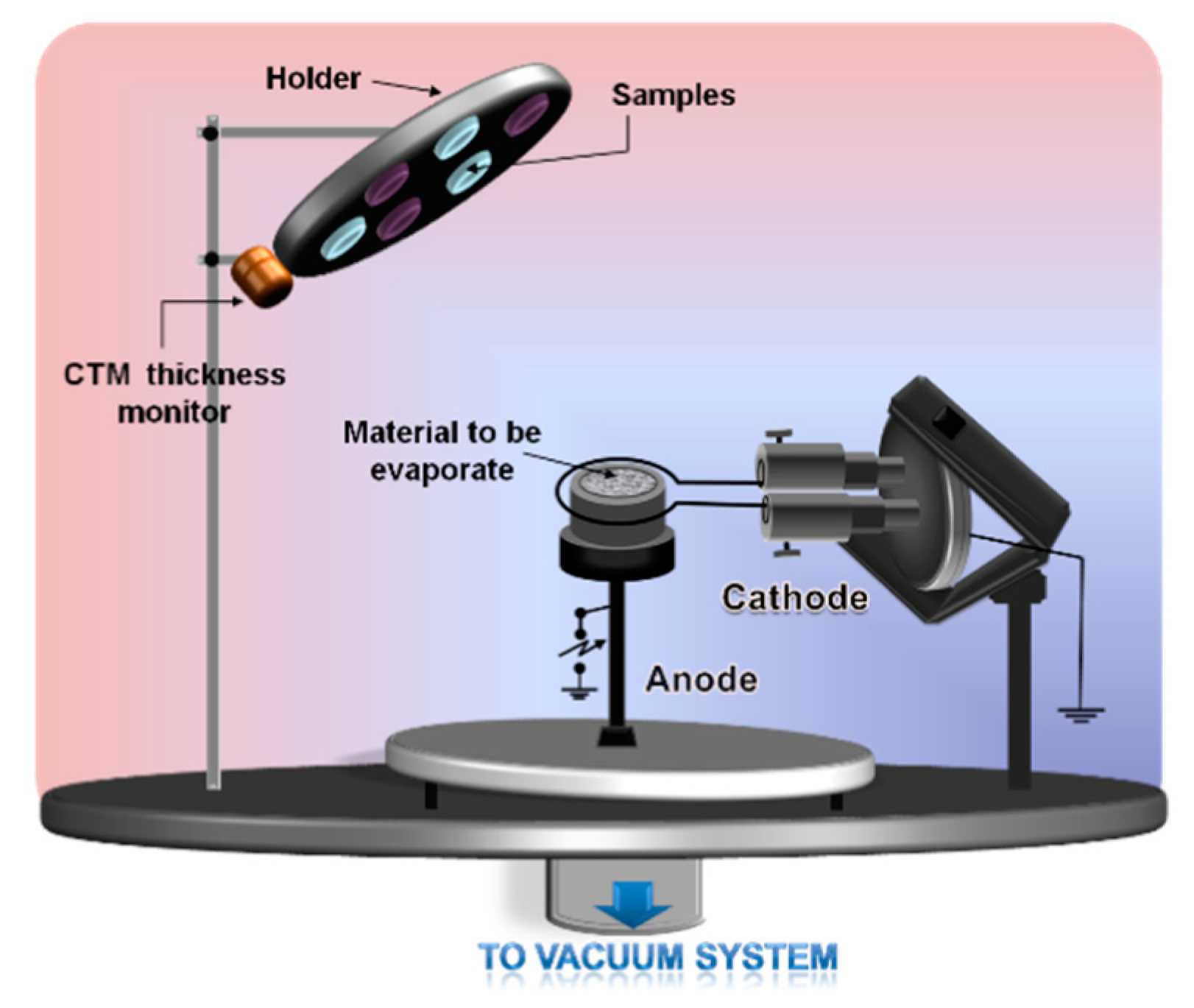
2.1. One electron Gun and One Crucible
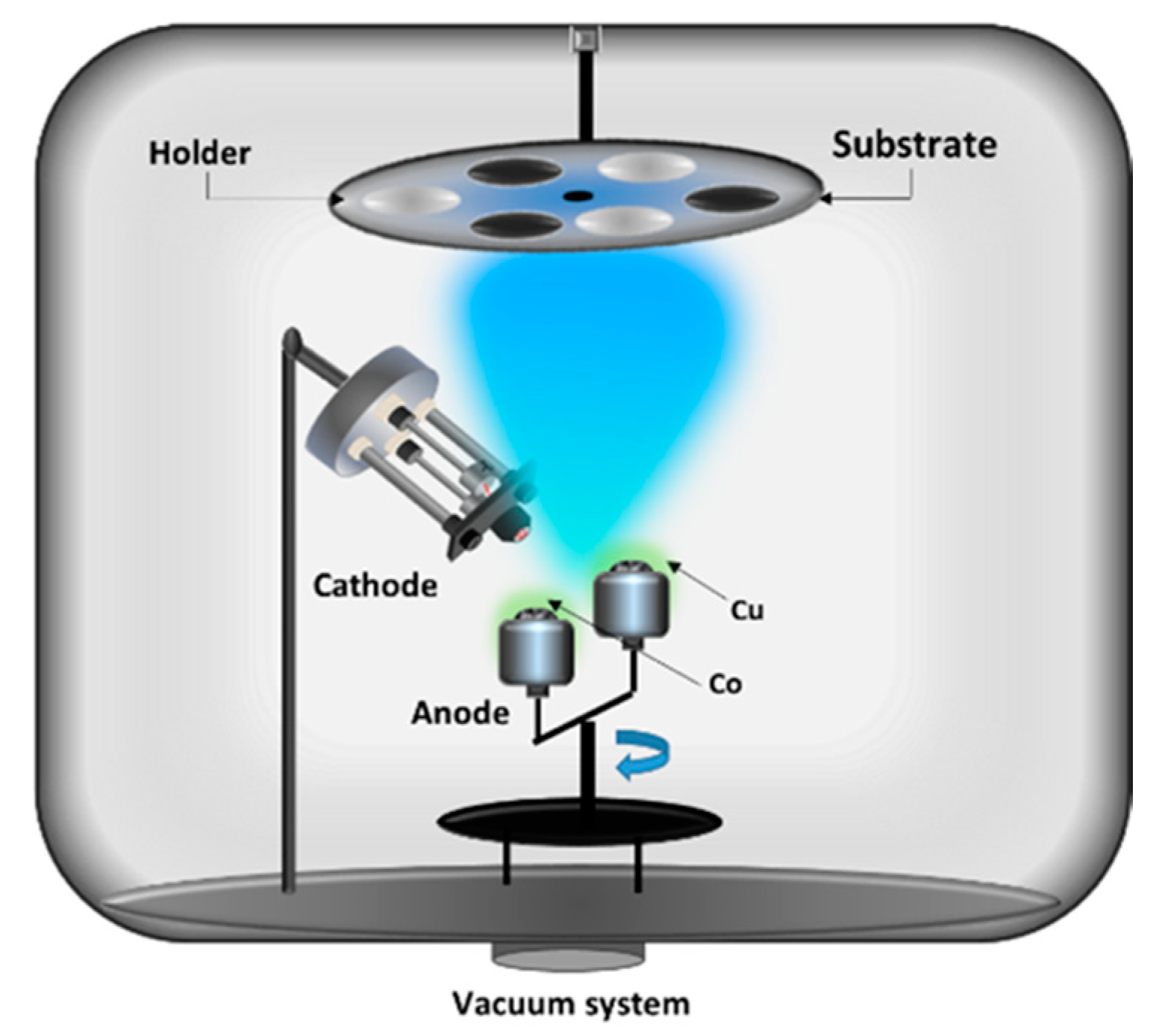
2.2. One Electron Gun and Two/Three Crucibles
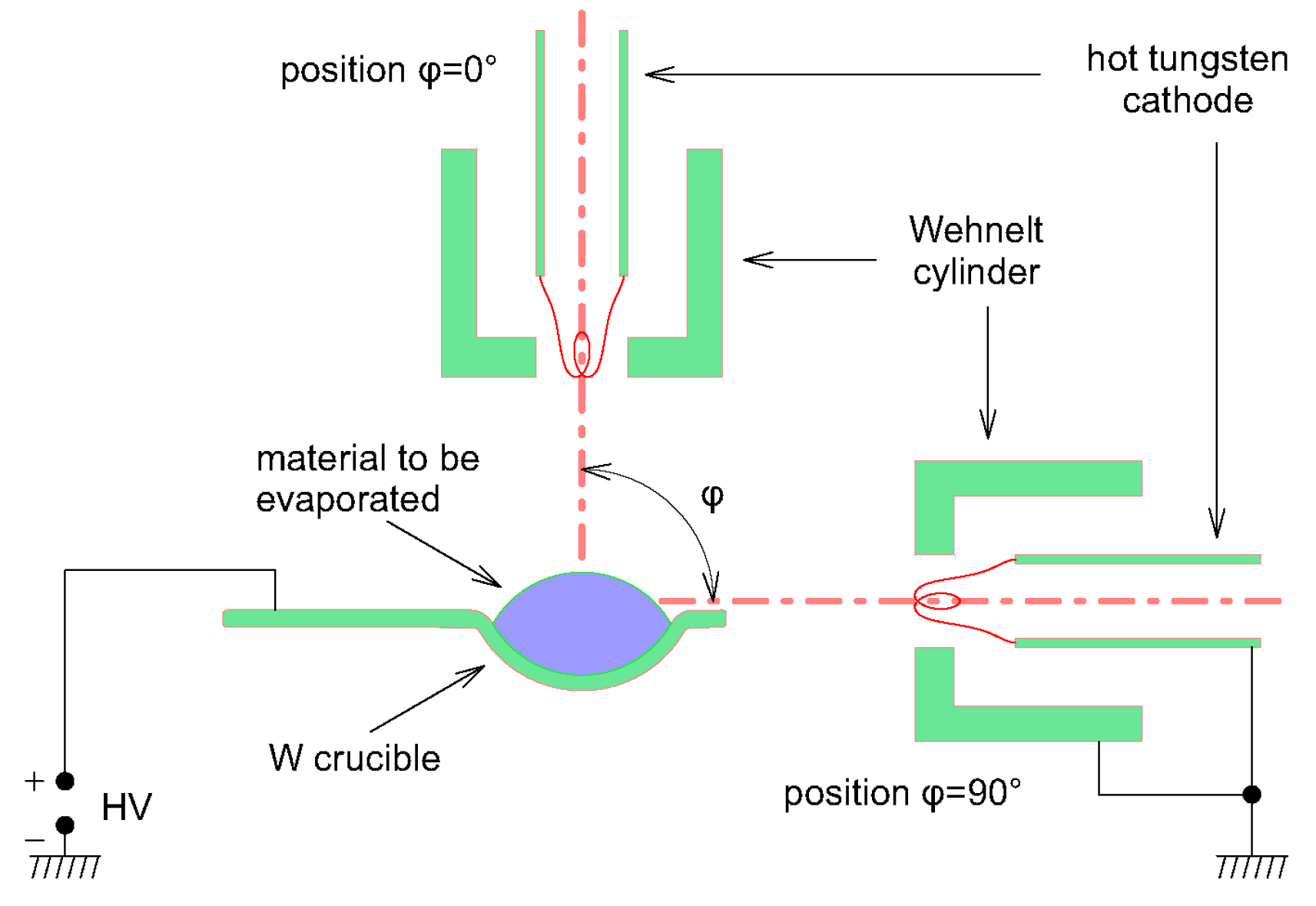
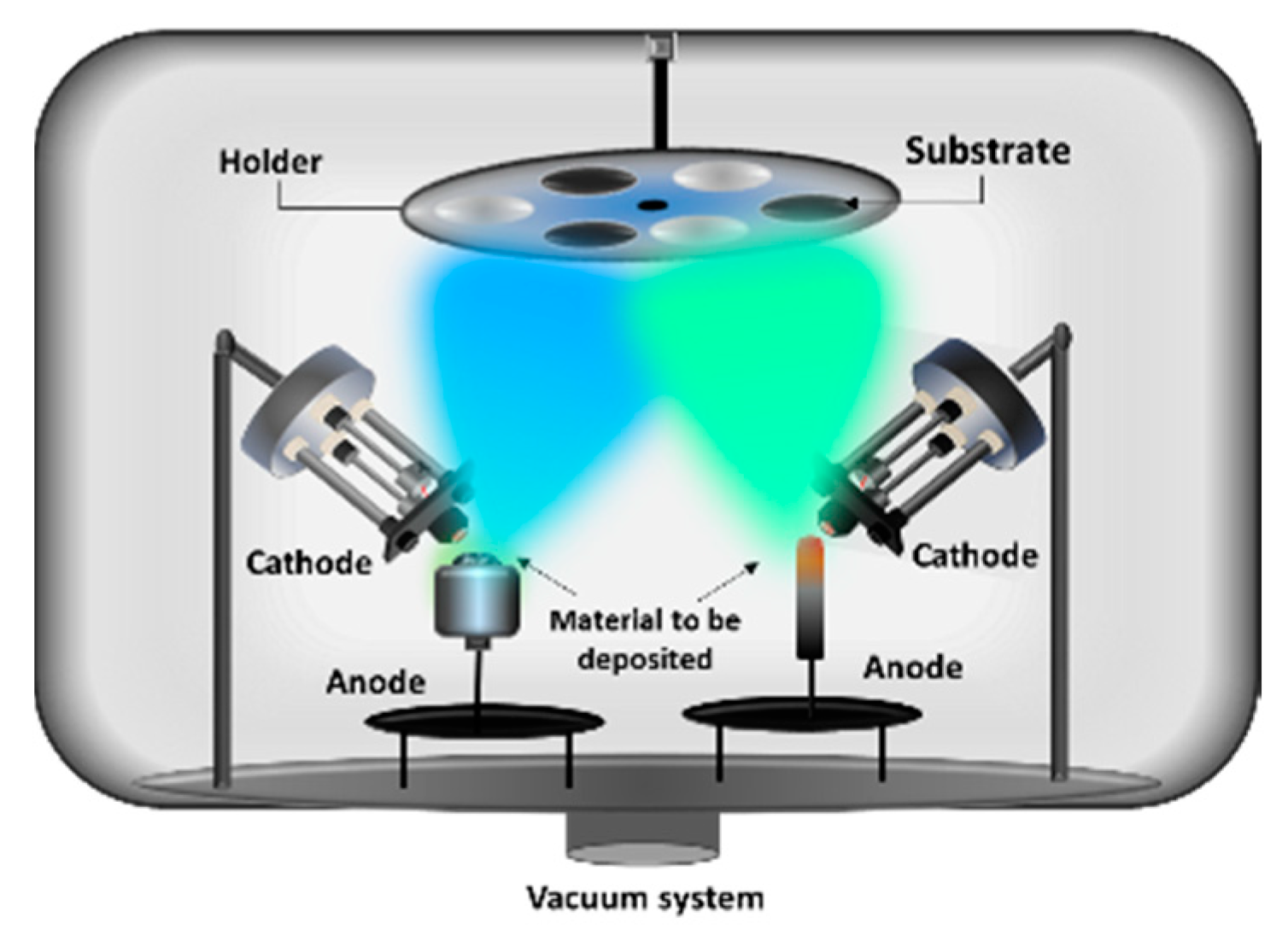
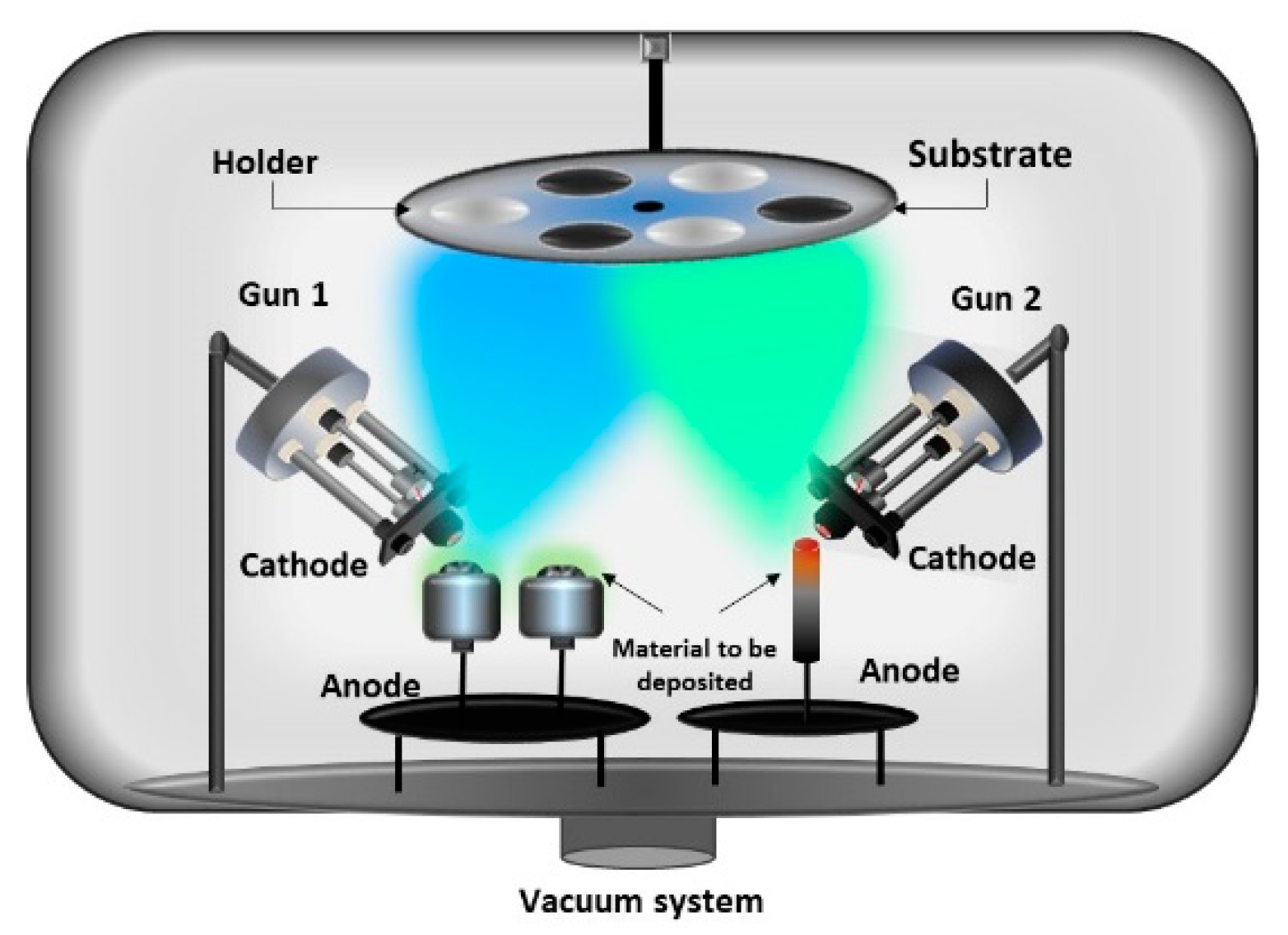
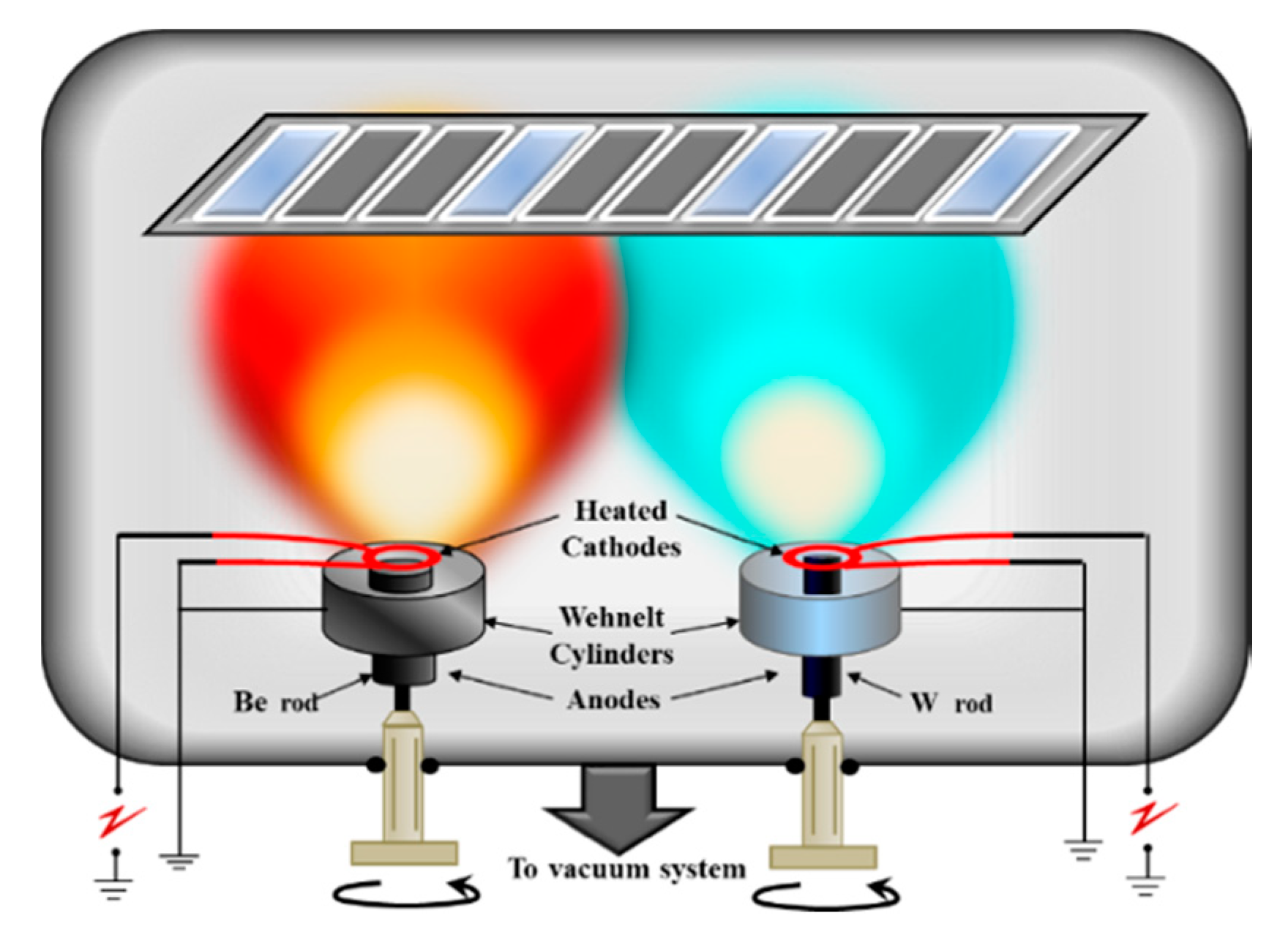
2.3. Two Electron Guns and Two Crucibles
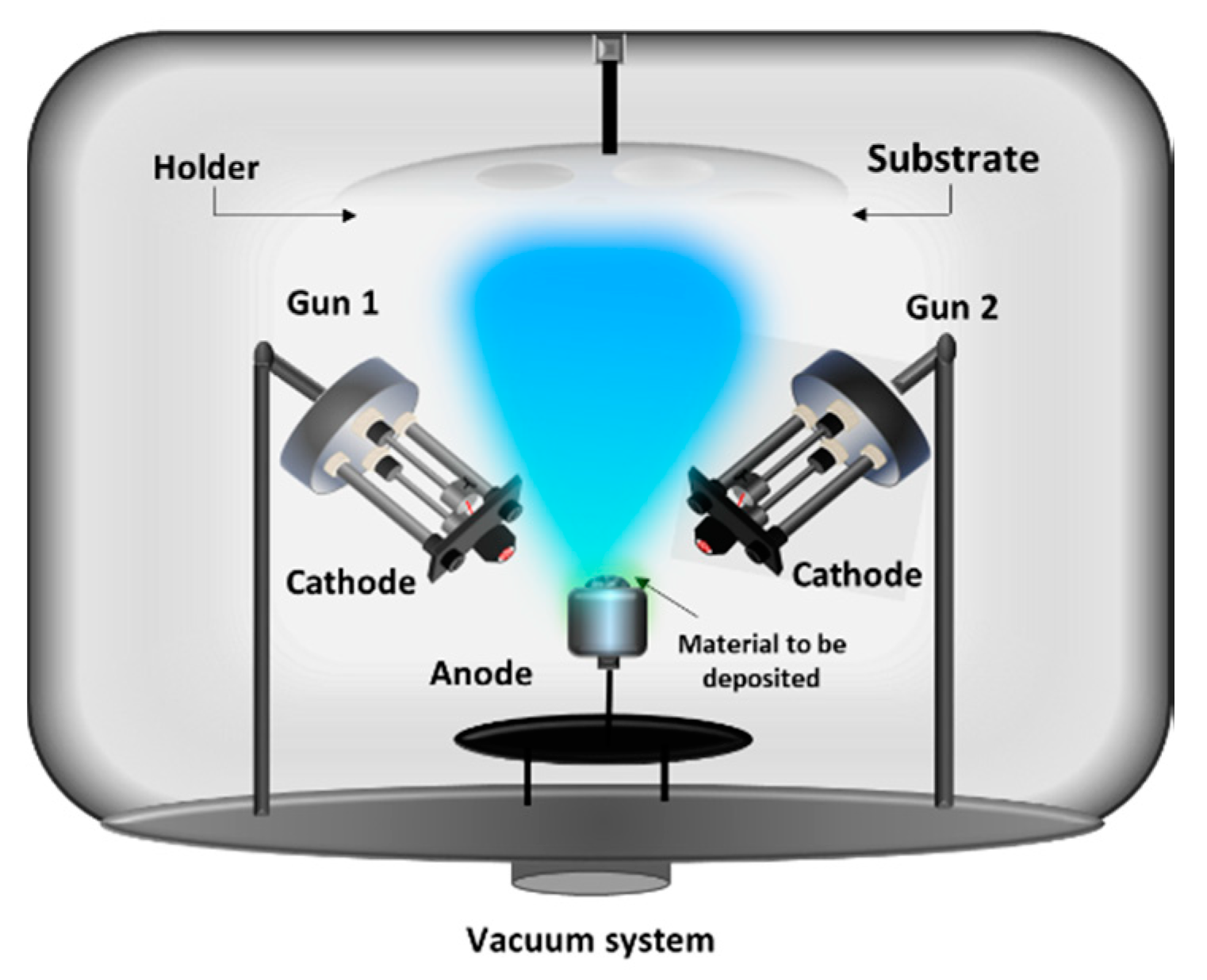
2.4. Two Electron Guns and One Crucible
2.5. Two Electron Guns and Three Crucibles
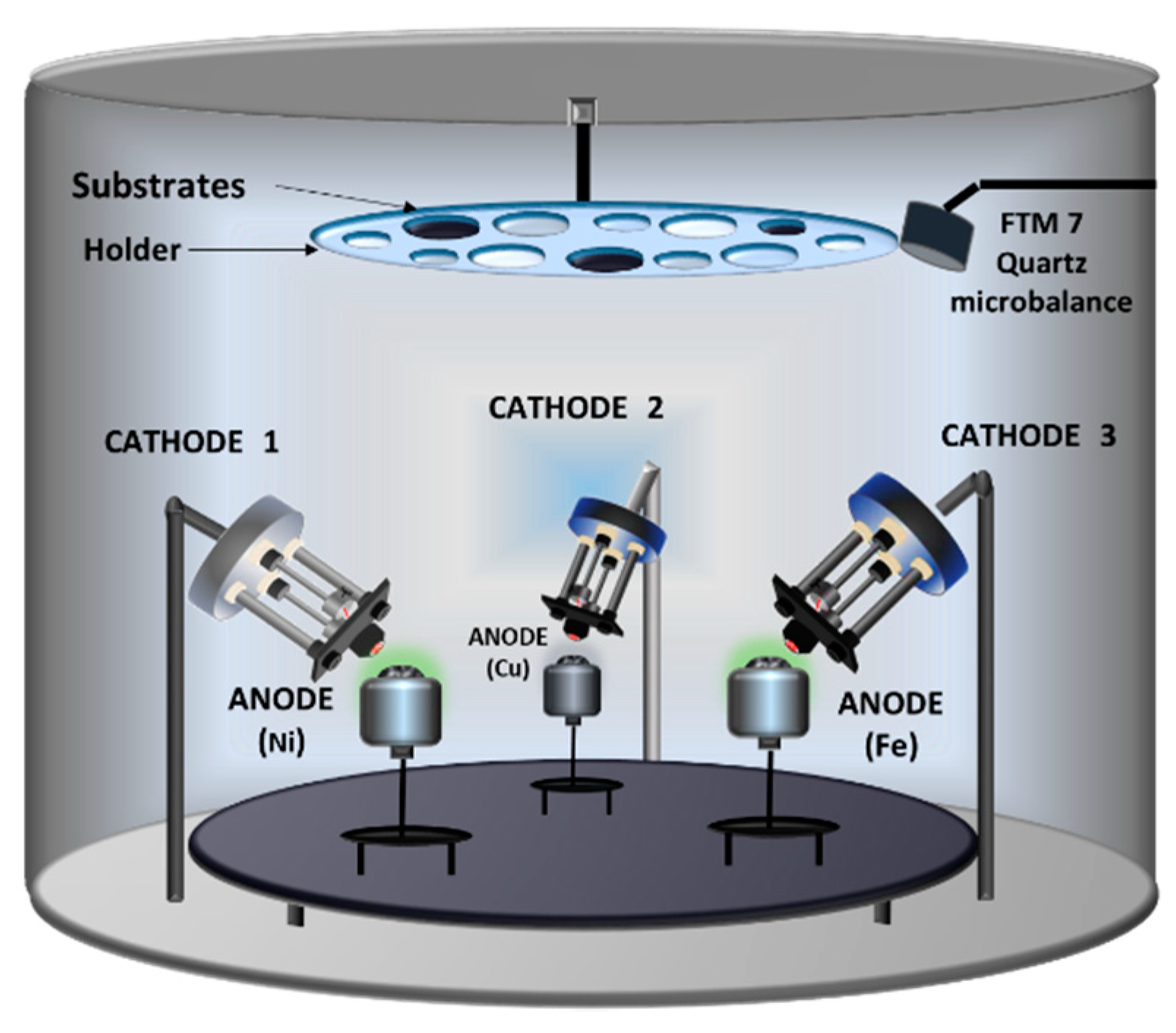
2.6. Three Electron Guns and Three Crucibles
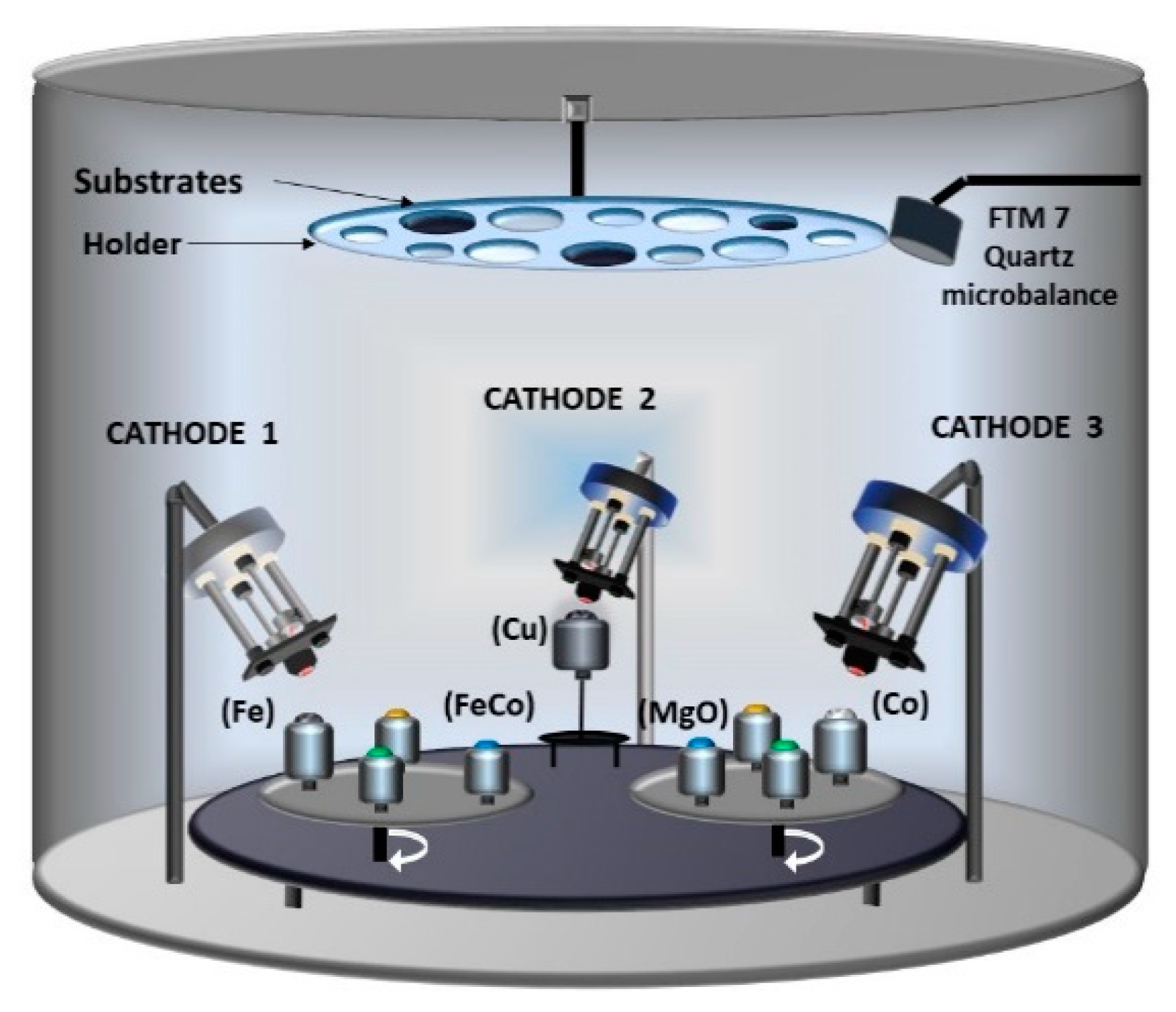
2.7. Three Electron Guns and Nine Crucibles
2.8. One Ring-Shaped Electron Gun and One Crucible
2.9. Two Ring-Shaped Electron Guns and Two Crucibles
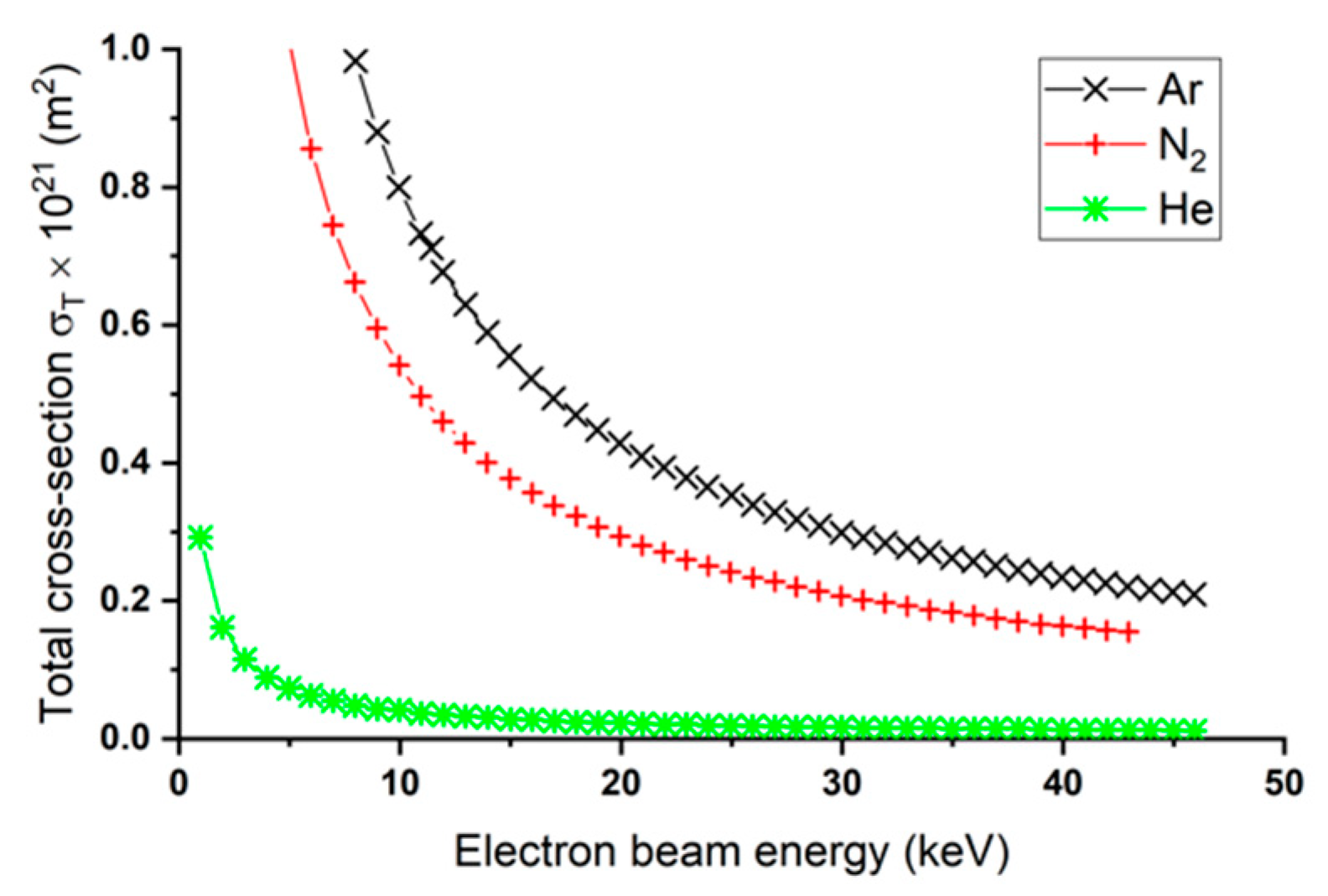
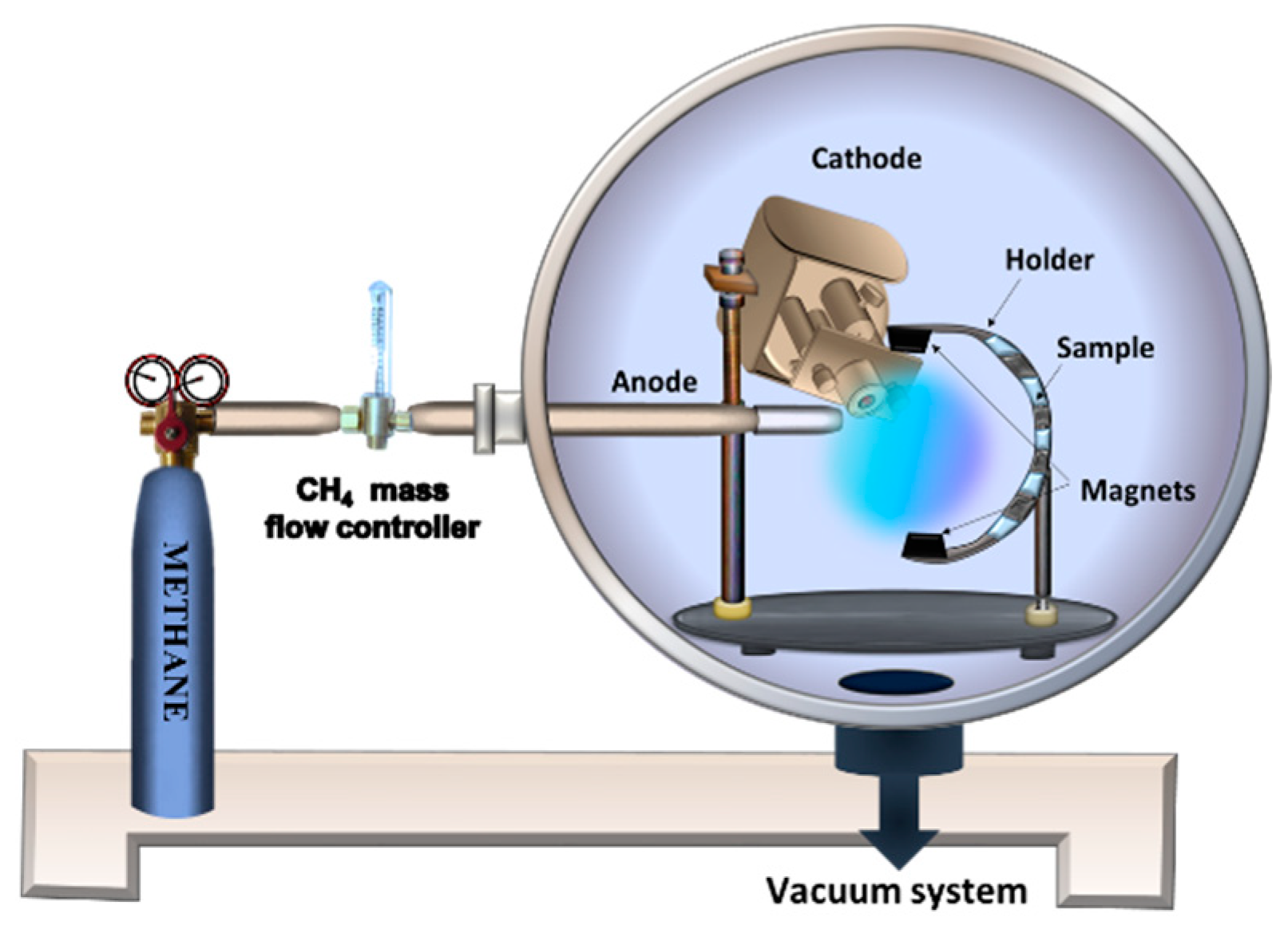
2.10. Gaseous Thermionic Vacuum Arc (G-TVA)
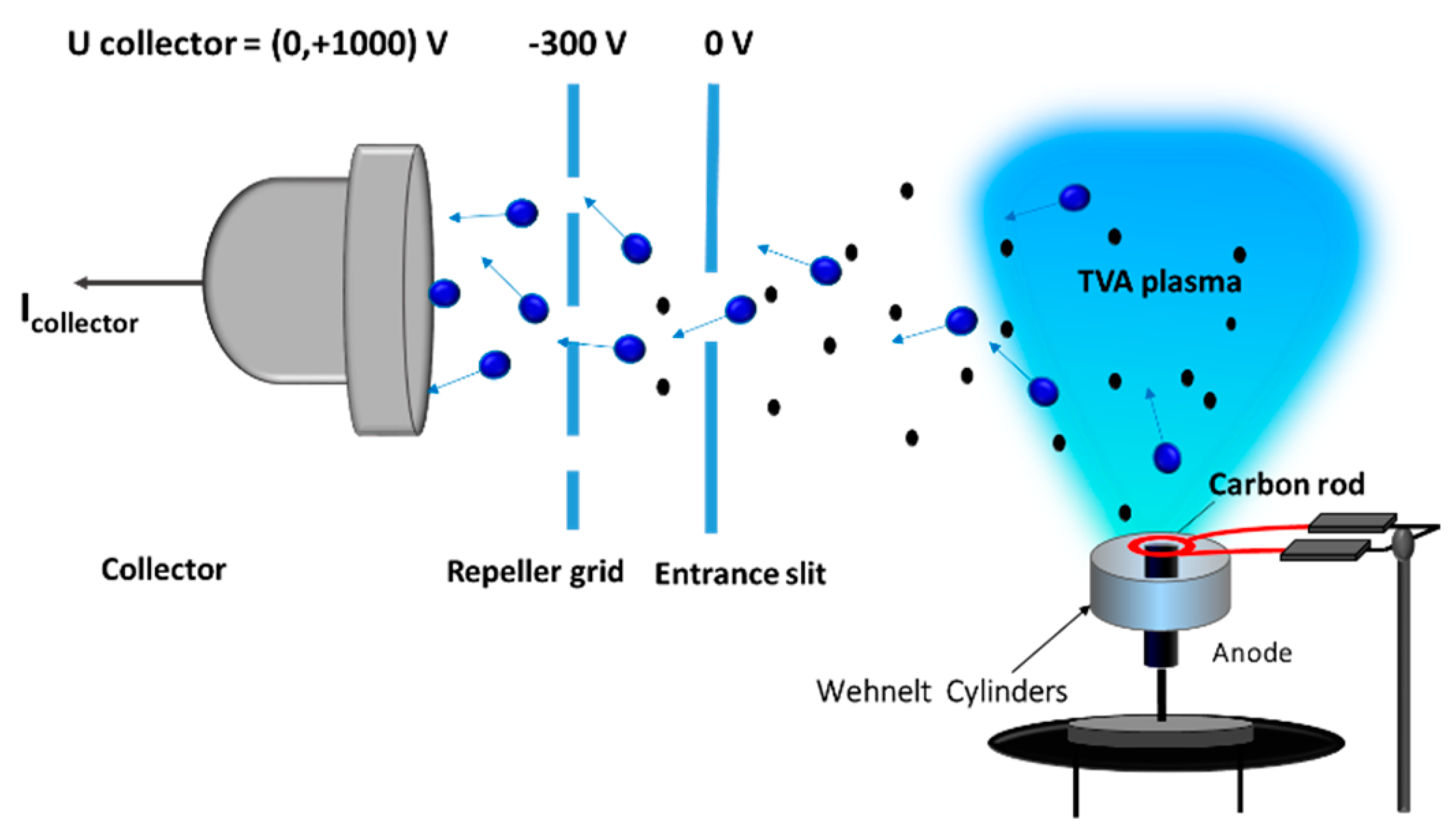
3. Diagnostic of TVA Plasma
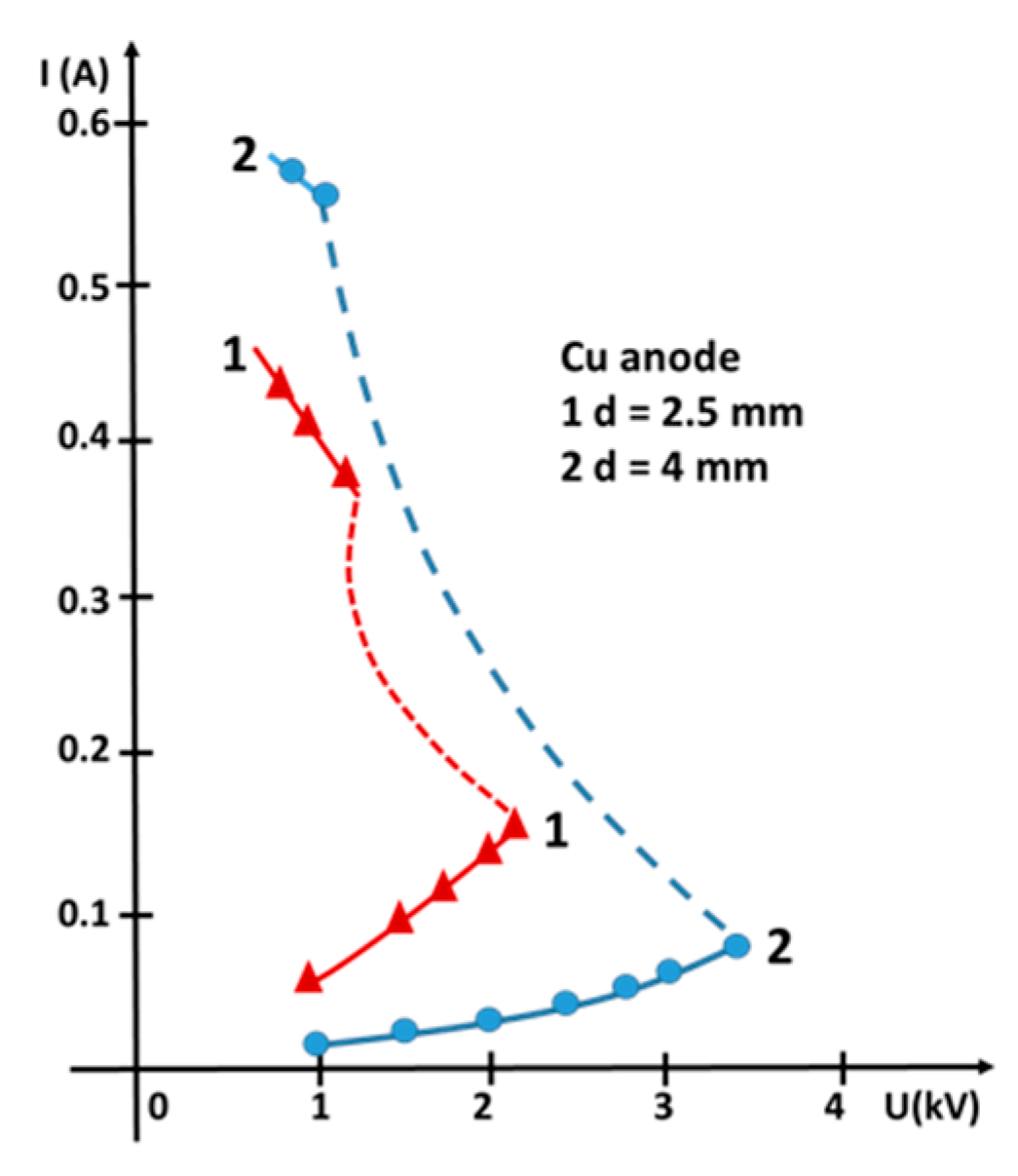
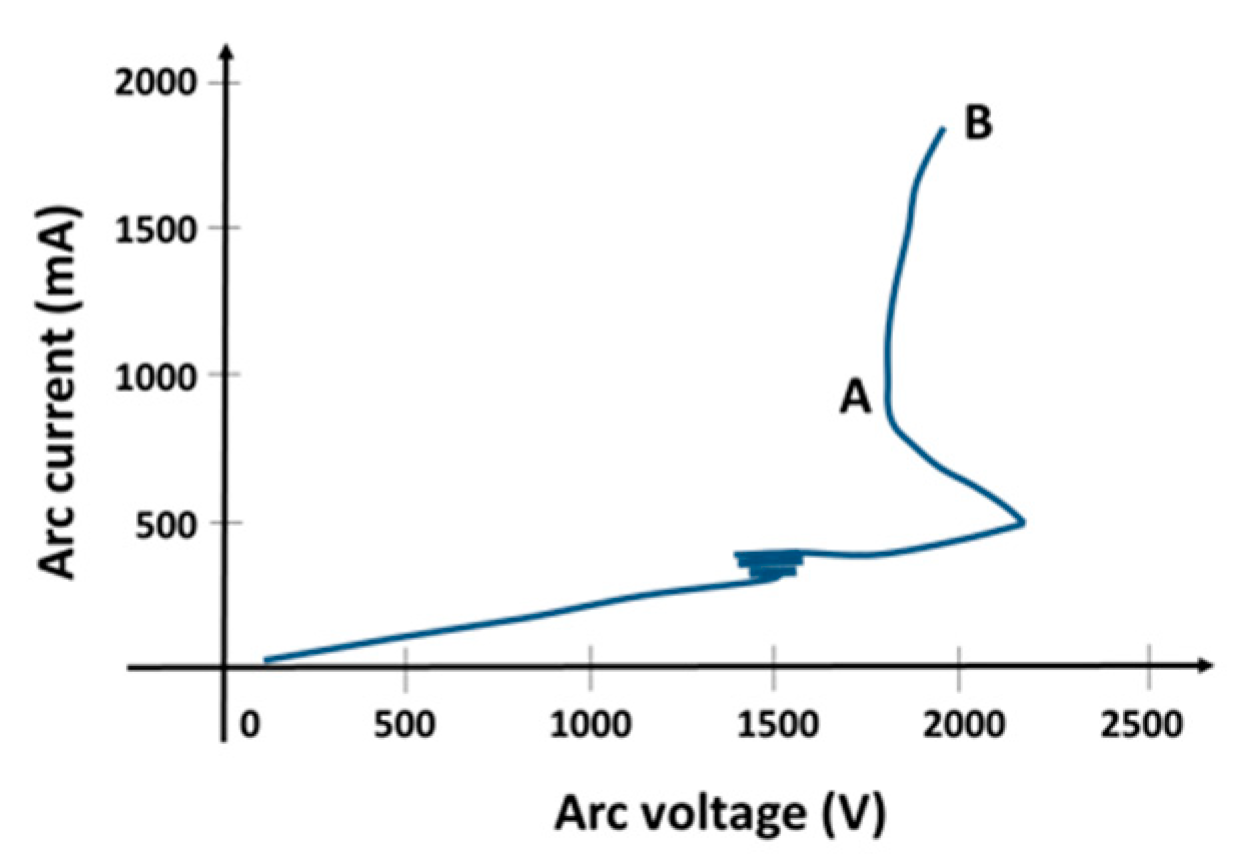
3.1. Basic Macroscopic Measurements
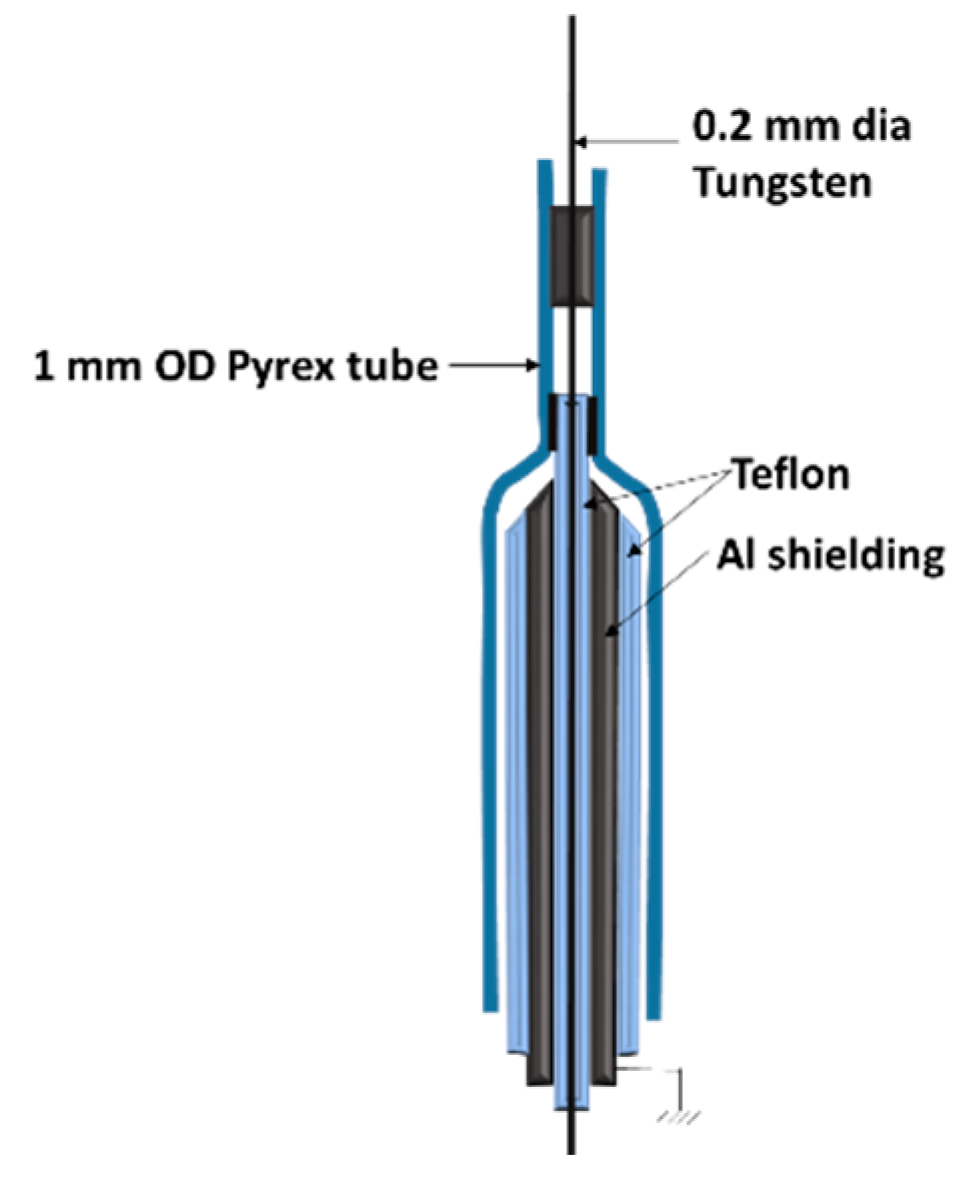
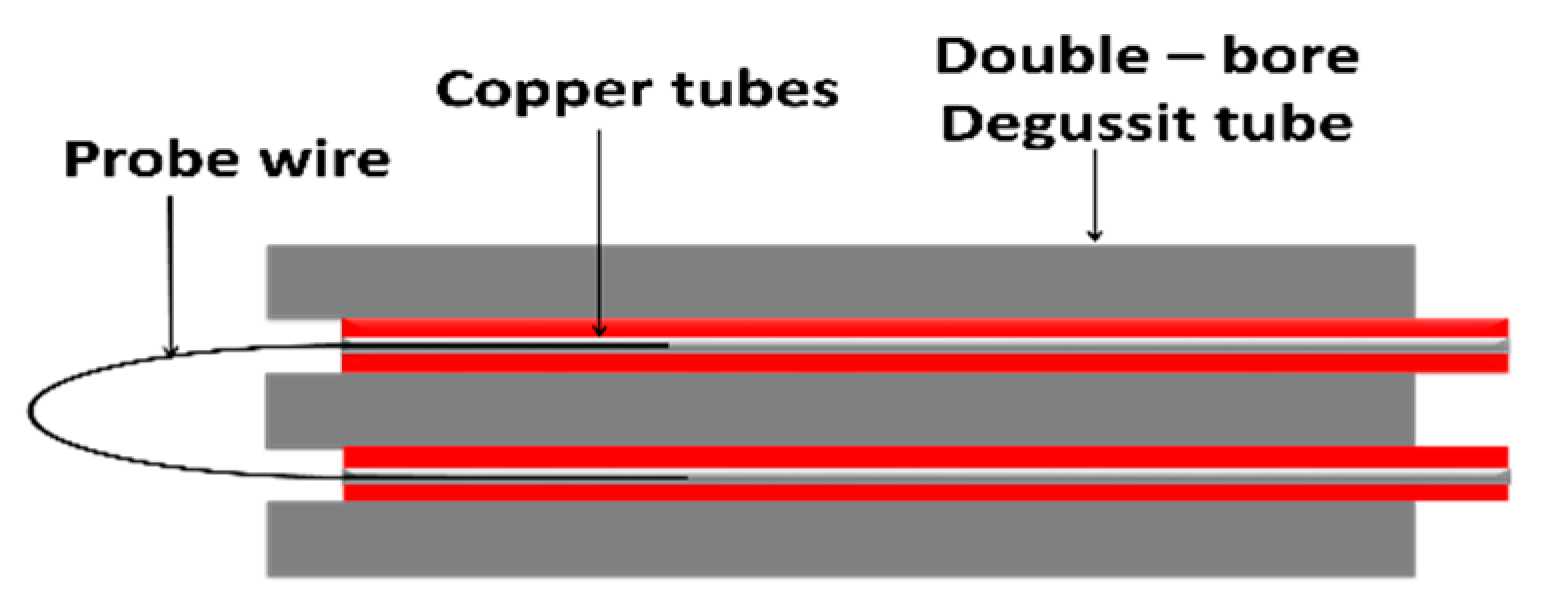
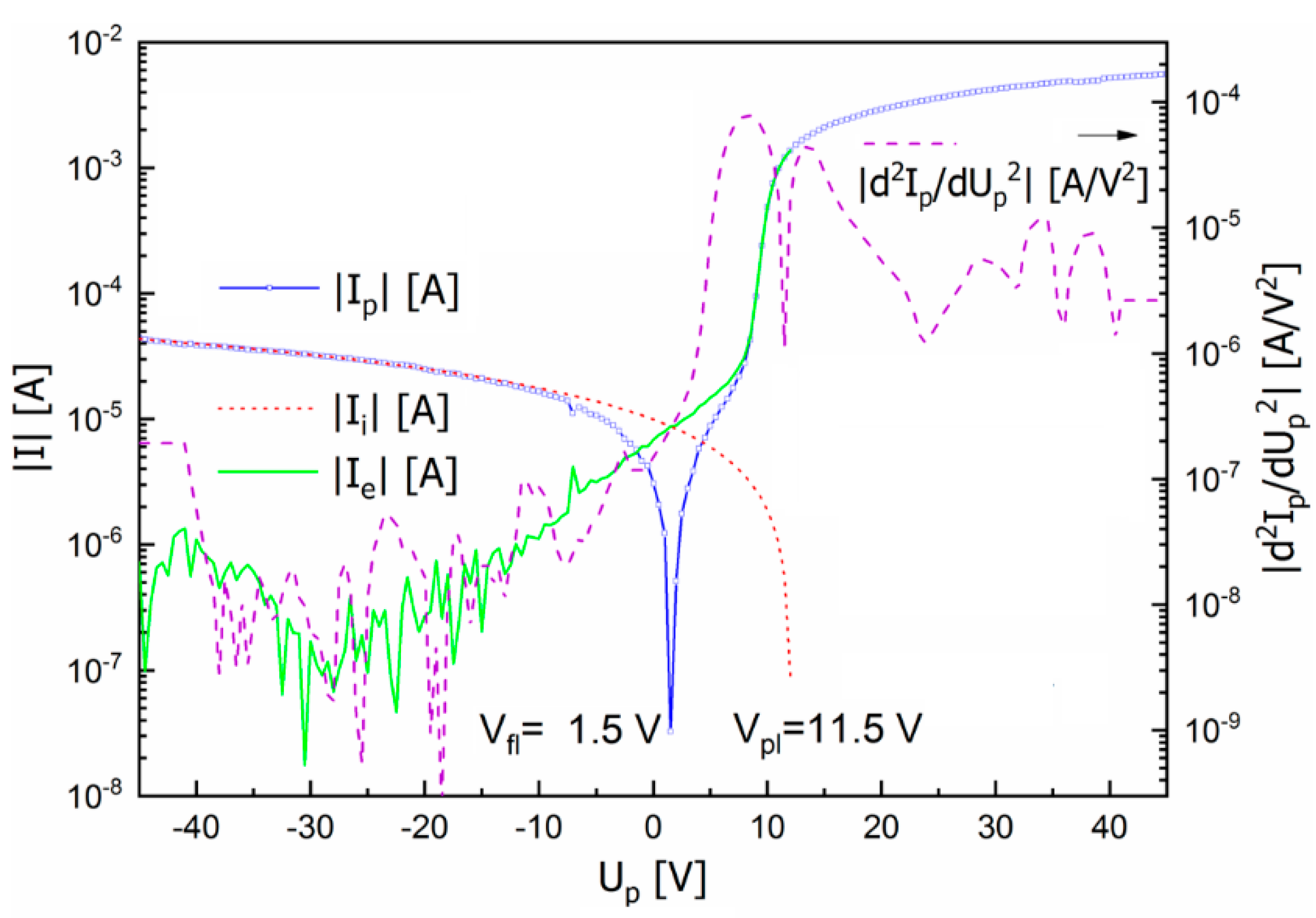
3.2. Langmuir/Emissive/Heated Probe Measurements
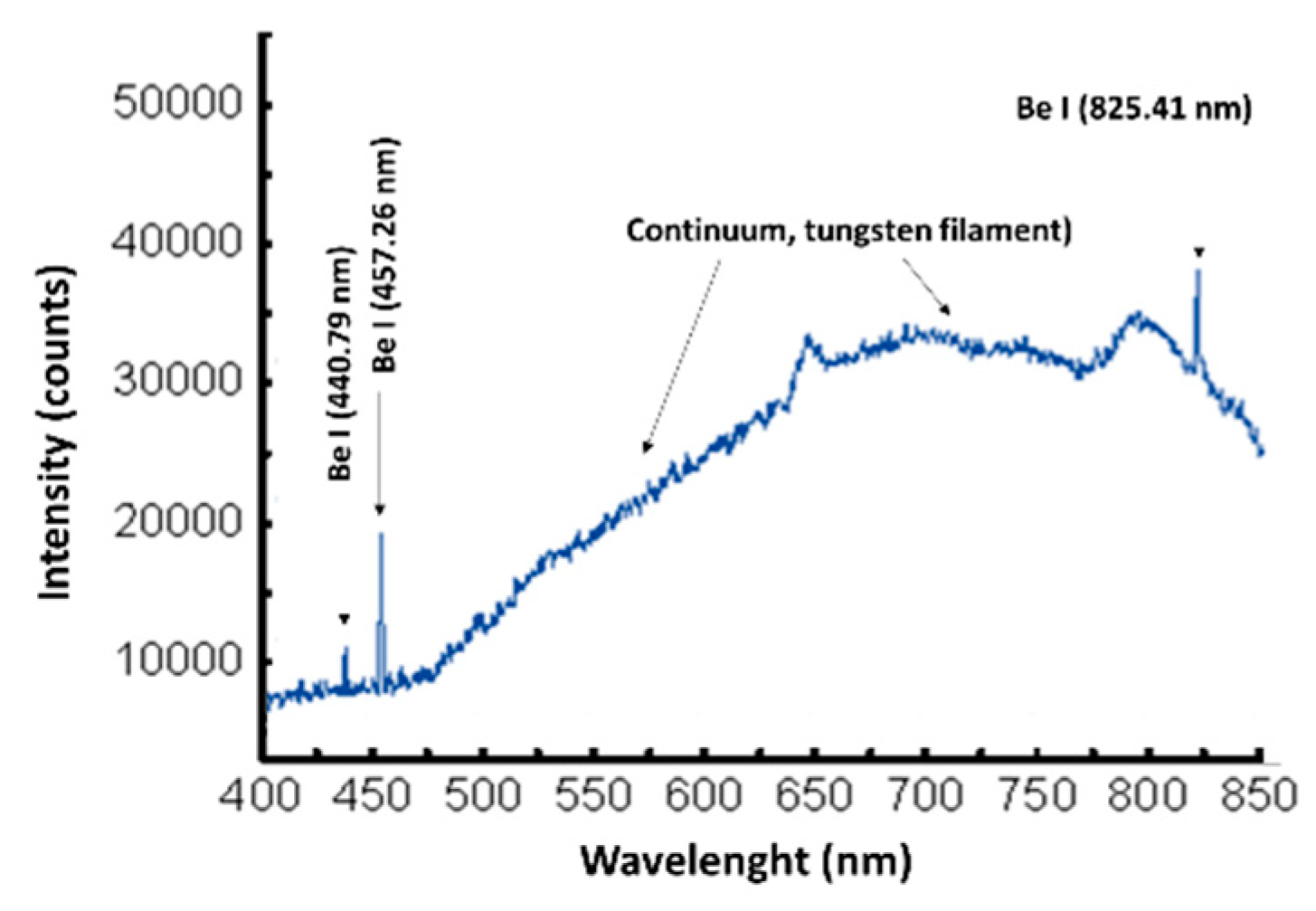
3.3. Optical Emission Spectroscopy
3.4. Mass-Spectrometry, Ion Energy Analysis
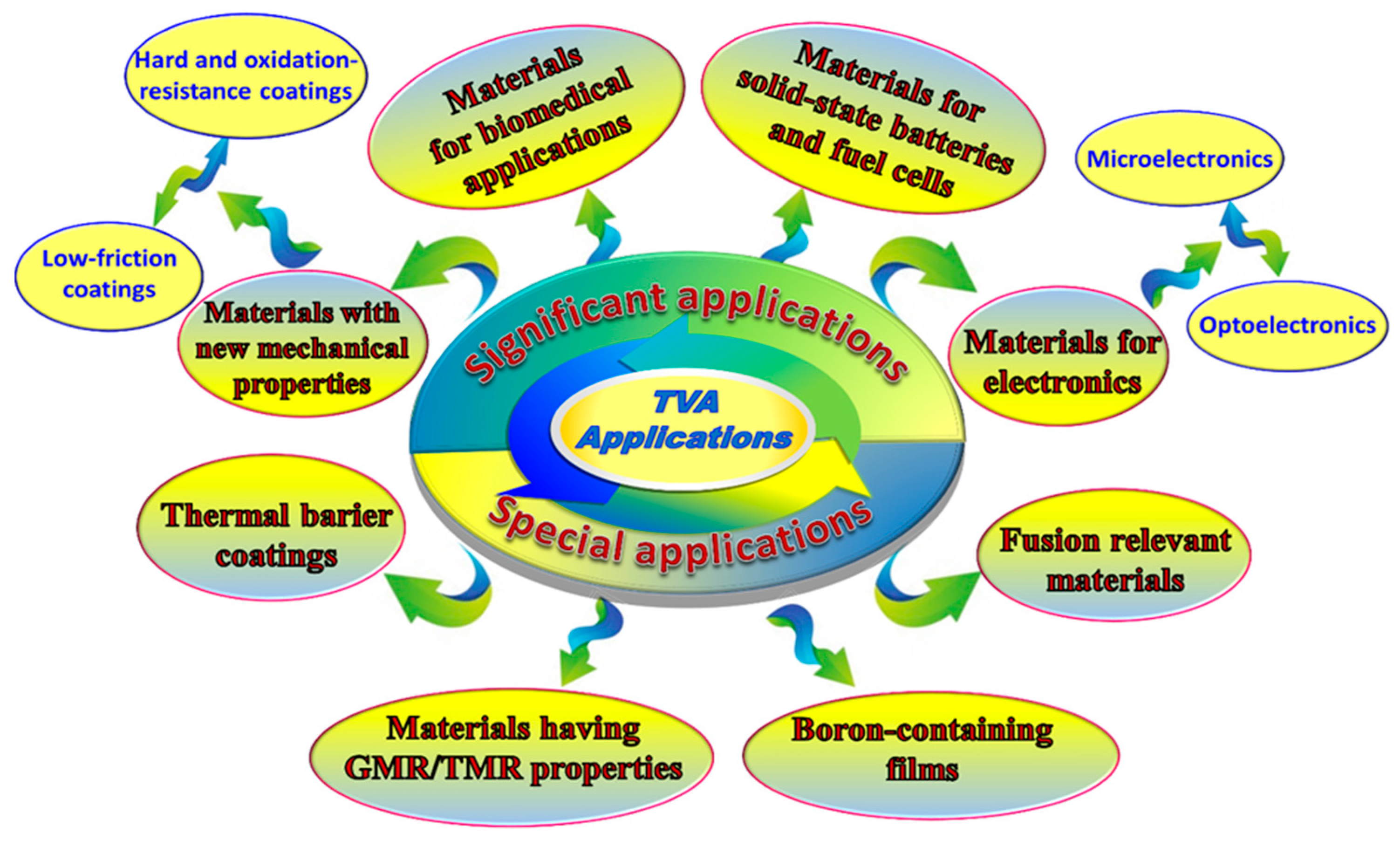
4. Applications of TVA Deposition System
4.1. Materials with New Mechanical Properties
4.2. Low-friction Coatings
4.3. Hard and Oxidation-Resistant Coatings
4.3.1. Outstanding Properties of the Deposited DLC Thin Films
4.3.2. Surface and Interface Analysis
4.3.3. Refractory Materials and Superalloys
4.3.4. Binary and Ternary Combination
4.3.5. Adhesion on the Substrate
4.3.6. Tailoring the DLC Structure
4.4. Materials for Microelectronics/Optoelectronics
4.4.1. Magnesium-Based Materials
4.4.2. Thin Films Deposited as Dielectrics
4.4.3. Deposition for Solar-Cell Technology
4.4.4. Thin Film Deposition on Special Substrates
4.4.5. Gallium-Based Materials
4.4.6. Deposition of the Semiconductors
4.4.7. Deposition of the Titanium Dioxide
4.5. Beryllium and Tungsten—Fusion-Relevant Materials
4.5.1. The Role of TVA Method in Be Coatings for Fusion Application
4.5.2. Fusion-Related Mixed Layers
4.5.3. Carbon-Metal Nanocomposites for Fusion Applications
4.6. Materials Having GMR/TMR Properties
4.7. Materials for Biomedical Applications
4.7.1. Dental Implants
4.7.2. Implantable Medical Devices
4.7.3. Biomedical Coatings
4.7.4. Antibacterial Materials
4.7.5. Sensors for Biomedical Analysis
4.8. Materials for Solid-State Batteries and Fuel Cells
4.8.1. Thin Rechargeable Batteries
4.8.2. Lithium-Based Materials
4.8.3. Platinum-Based Materials
4.9. Thermal Barrier Coatings
4.9.1. Nickel-Based Alloys
4.9.2. Carbon-Titanium Multilayer Films
4.10. Boron-Containing Films
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Boxman, R.L.; Philip, J.M.; David, M.S. Handbook of Vacuum Arc Science and Technology: Fundamentals and Applications, 1st ed.; William Andrew Publishing/Noyes Publications: Park Ridge, NJ, USA, 1995. [Google Scholar]
- Lafferty, J.W. Vacuum Arcs: Theory and Practice, 2nd ed.; John Wiley: Hoboken, NJ, USA, 1980. [Google Scholar]
- Anders, A. Available online: https://escholarship.org/uc/item/33n1m86d (accessed on 26 February 2020).
- Matthews, A. Plasma-based physical vapor deposition surface engineering processes. J. Vac. Sci. Technol. A 2003, 21, S224–S231. [Google Scholar] [CrossRef]
- Snaper, A.A. Arc Deposition Process and Apparatus. U.S. Patent 3,625,848, 7 December 1971. [Google Scholar]
- Sablev, L.P. Apparaturs for Metal Evaporating Coating. U.S. Patent 3,793,179, 19 July 1971. [Google Scholar]
- Bradley, D.E. Evaporated carbon films for use in electron microscopy. Br. J. Appl. Phys. 1954, 5, 65–66. [Google Scholar] [CrossRef]
- Kesaev, I.G. Cathode Processes of an Electric Arc; Nauka: Moscow, Russia, 1968. (In Russian) [Google Scholar]
- Rakhovskii, V.I. Physical Foundations of Switching Electrical Current in Vacuum; Nauka: Moscow, Russia, 1970. (In Russian) [Google Scholar]
- Aksenov, I.I.; Belous, V.A.; Padalka, V.G. Apparatus to rid the plasma of a vacuum arc of macroparticles. Instrum. Exp. Tech. 1978, 21, 1416–1418. [Google Scholar]
- Karpov, D.; Efremov, D.V. Arc sources of metallic plasma for coatings in vacuum and for high speed vacuum pumping. Vacuum 1995, 46, 825–826. [Google Scholar] [CrossRef]
- Bunshah, R.F. Deposition Technologies for Films and Coatings: Developments and Applications, 1st ed.; Noyes: Park Ridge, NJ, USA, 1 June 1982. [Google Scholar]
- Mattox, D.M. The Foundations of Vacuum Coating Technology; Noyes Publications: Norwich, NY, USA, 2017. [Google Scholar]
- Boxman, R.L.; Zhitomirski, V.N. Vacuum arc deposition devices. Rev. Sci. Instrum. 2006, 77, 021101. [Google Scholar] [CrossRef]
- Krutenac, R.C.; Gesick, W.R. Vapor Deposition by Liquid Phase Sputtering. J. Vac. Sci. Technol. 1970, 7, S40–S43. [Google Scholar] [CrossRef]
- Dorodnov, A.M.; Kuznetsov, A.N.; Petrosov, V.A. New anode-vapor vacuum arc with a permanent hollow cathode. Sov. Tech. Phys. Lett. 1979, 5, 418–419. [Google Scholar]
- Bunshah, R.F.; Raghuram, A.C. Activated Reactive Evaporation Process for High Rate Deposition of Compounds. J. Vac. Sci. Technol. 1972, 9, 1385–1388. [Google Scholar] [CrossRef]
- Bunshah, R.F.; Juntz, R.S. High-rate evaporation/deposition processes of metals, alloys, and ceramics for vacuum metallurgical applications. In Transactions of the Vacuum Metallurgy Conference; American Vacuum Society: New York, NY, USA, 1965; p. 200. [Google Scholar]
- Bunshah, R.F. High-rate evaporation/deposition processes of metals, alloys, and ceramics for vacuum metallurgical applications. J. Vac. Sci. Technol. 1974, 11, 814–819. [Google Scholar] [CrossRef]
- Bunshah, R.F. High Rate Deposition of Carbides by Activated Reactive Evaporation. U.S. Patent 3,791,852, 12 February 1974. [Google Scholar]
- Ehrich, H. The anodic vacuum arc. I. Basic construction and phenomenology. J. Vac. Sci. Technol. A 1988, 6, 134–138. [Google Scholar] [CrossRef]
- Ehrich, H.; Hasse, B.; Mausbach, M.; Müller, K.G. The anodic vacuum arc. II. Experimental study of arc plasma. J. Vac. Sci. Technol. A 1988, 6, 2499–2503. [Google Scholar] [CrossRef]
- Ehrich, H.; Hasse, B.; Mausbach, M.; Müller, K.G. The anodic vacuum arc and its application to coating. J. Vac. Sci. Technol. A 1990, 8, 2160–2164. [Google Scholar] [CrossRef]
- Ehrich, H.; Hasse, B.; Mausbach, M.; Muller, K.G. Plasma Deposition of Thin Films Utilizing the Anodic Vacuum Arc. IEEE Trans. Plasma Sci. 1990, 18, 895–903. [Google Scholar] [CrossRef]
- Ehrich, H. Method and Apparatus for Evaporating Material in Vacuum. U.S. Patent 4,917,786, 16 February 1990. [Google Scholar]
- Popescu, I.; Brandus, L.; Moldovan, C.; Musa, G. On the diffusion theory of the low-voltage arc plasma. Br. J. Appl. Phys. 1966, 17, 129–131. [Google Scholar] [CrossRef]
- Musa, G.; Betiu, N.; Mustata, I.; Baltog, A.; Popescu, A. Low Voltage Arc Welding in Vacuum. Rev. Roum. Phys. 1983, 28, 907. [Google Scholar]
- Musa, G.; Baltog, A.; Popescu, A.; Betiu, N.; Mustata, I. Electrical and Spectral Characteristics of a Heated Cathode Discharge in Metal Vapors. Beitr. Plasmaphys. 1986, 26, 171. [Google Scholar] [CrossRef]
- Musa, G.; Baltog, A.; Popescu, A.; Betiu, N.; Mustata, I. Possible High Power Laser Construction Using a New Discharge Type-Heated Cathode Discharge in the Copper vapours Continuously Evaporated from the Anode. Beitr. Plasmaphys. 1987, 27, 431. [Google Scholar] [CrossRef]
- Musa, G.; Popescu, A.; BaItog, A.; Betiu, N.; Mustata, I. Hot Cathode Pulsed Discharge in Copper Vapors from a Melted Anode. Rev. Roum. Phys. 1987, 32, 869. [Google Scholar]
- Musa, G.; Mustata, I.; Dinescu, G.; Bajeu, G.; Raiciu, E. Evaporation Source for Deposition of Protective Layers inside Tubes. Jpn. J. Appl. Phys. 1992, 31, 2869–2871. [Google Scholar] [CrossRef]
- Musa, G.; Ehrich, H.; Mausbach, M. Studies on thermionic cathode anodic vacuum arcs. J. Vac. Sci. Technol. A 1994, 12, 2887–2895. [Google Scholar] [CrossRef]
- Musa, G.S.; Ehrich, H.; Schuhmann, J. Pure Metal Vapor Plasma Source with Controlled Energy of Ions. IEEE Trans. Plasma Sci. 1997, 25, 386–391. [Google Scholar] [CrossRef]
- Vladoiu, R.; Musa, G.; Mustata, I. Thermoionic vacuum arc—A new method of thin film deposition. J. Optoelectron. Adv. Mater. 2003, 5, 325–330. [Google Scholar]
- Kadoun, A.; Belkorissat, R.; Khelifa, B.; Mathieu, C. Comparative study of electron beam-gas interaction in an SEM operating at pressures up to 300 Pa. Vacuum 2003, 69, 537–543. [Google Scholar] [CrossRef]
- Ciupina, V.; Vladoiu, R.; Mandes, A.; Musa, G.; Lungu, C.P. TEM investigation of the C-Me multilayer nanocomposites deposited by Thermionic Vacuum Arc (TVA) method. J. Optoelectron. Adv. Mater. 2008, 10, 2958–2962. [Google Scholar]
- Ciupina, V.; Vladoiu, R.; Lungu, C.P.; Dinca, V.; Contulov, M.; Mandes, A.; Popov, P.; Prodan, G. Investigation of the SiC thin films synthetized by Thermionic Vacuum Arc method (TVA). Eur. Phys. J. D 2012, 66, 99. [Google Scholar] [CrossRef]
- Ciupina, V.; Lungu, C.P.; Vladoiu, R.; Epure, T.D.; Prodan, G.; Rosca, C.; Porosnicu, C.; Jepu, I.; Belc, M.; Prodan, M. SiC multi-layer protective coating on carbon obtained by thermionic vacuum arc method. Proc. SPIE 2013, 8818, UNSP 881807. [Google Scholar] [CrossRef]
- Ciupina, V.; Lungu, C.P.; Vladoiu, R.; Prodan, G.; Porosnicu, C.; Belc, M.; Stanescu, I.M.; Vasile, E.; Rughinis, R. Silicon carbide multilayer protective coating on carbon obtained by thermionic vacuum arc method. J. Nanophotonics 2014, 8, 083996. [Google Scholar] [CrossRef]
- Ciupina, V.; Lungu, C.P.; Vladoiu, R.; Prodan, G.C.; Antohe, S.; Porosnicu, C.; Stanescu, I.; Jepu, I.; Iftimie, S.; Prodan, M. The effect of the substrate temperature and the acceleration potential drop on the structural and physical properties of SiC thin films deposed by TVA method. Proc. SPIE 2014, 9172, 91720Y. [Google Scholar] [CrossRef]
- Özen, S.; Senay, V.; Pat, S.; Korkmaz, S. Morphological and optical comparison of the Si doped GaN thin film deposited onto the transparent substrates. Mater. Res. Express 2016, 3, 045012. [Google Scholar] [CrossRef]
- Akan, T.; Ekem, N.; Pat, S.; Issever, U.G.; Balbag, M.Z.; Cenik, M.I.; Vladoiu, R.; Musa, G. Boron thin film deposition by using Thermionic Vacuum Arc (TVA) technology. Mater. Lett. 2007, 61, 23–26. [Google Scholar] [CrossRef]
- Akan, T.; Karakas, E. Boron processes using thermionic vacuum arc. Optoelectron. Adv. Mater. Rapid Commun. 2014, 8, 480–482. [Google Scholar]
- Ciupina, V.; Ilie, D.; Manu, R.; Prioteasa, I.; Jepu, I.; Petrasescu, L.; Dinca, P.; Vasile, E. Investigation of the Cu-Co-Fe Nanostructurated Films Deposited by Thermionic Vacuum Arc Technology. Dig. J. Nanomater. Biostructures 2017, 12, 805–813. [Google Scholar]
- Ciupină, V.; Prioteasa, I.; Ilie, D.; Manu, R.; Petrăşescu, L.; Tutun, Ş.G.; Dincă, P.; Mustaţă, I.; Lungu, C.P.; Jepu, I.; et al. Synthesis and characterization of Copper/Cobalt/Copper/Iron nanostructurated films with magnetoresistive properties. AIP Conf. Proc. 2017, 1815, 040001. [Google Scholar] [CrossRef]
- Răsleanu, D.; Ciupină, V.; Prodan, G.; Lungu, C.P.; Jepu, I.; Eugeniu, V. Obtaining and stuying of thermionic vacuum arc deposited Cu and Co nanostructured multilayers on ceramic substrate. J. Optoelectron. Adv. Mater. 2013, 15, 46–49. [Google Scholar]
- Lungu, M.; Tiseanu, I.; Porosnicu, C.; Dobrea, C.; Jepu, I.; Dinca, P.; Marcu, A.; Lungu, C.P. Tribological Investigations on Laser Irradiated Composite Thin Films Prepared by TVA Technique. Dig. J. Nanomater. Biostruct. 2016, 11, 401–410. [Google Scholar]
- Lungu, C.P.; Mustata, I.; Musa, G.; Lungu, A.M.; Brinza, O.; Moldovan, C.; Rotaru, C.; Iosub, R.; Sava, F.; Popescu, M.; et al. Unstressed carbon-metal films deposited by thermionic vacuum arc method. J. Optoelectron. Adv. Mater. 2006, 8, 74–77. [Google Scholar]
- Vladoiu, R.; Ciupina, V.; Lungu, C.P.; Pompilian, O.I.; Chiru, P.; Lungu, A.M.; Prodan, G.; Mandes, A.; Musa, G. Characterization of Nanostructured Carbon-Metal Bilayers Deposited by Thermionic Vacuum Arc (TVA) Technology. Chem. Listy 2008, 102, s1482–s1485. [Google Scholar]
- Ehrich, H.; Musa, G.; Popescu, A.; Mustata, I.; Salabas, A.; Cretu, M.; Leu, G.F. MgO thin film deposition using TVA (thermoionic vacuum arc). Solid Films 1999, 343, 63–66. [Google Scholar] [CrossRef]
- Prioteasa, I.; Porosnicu, C.; Lungu, C.P.; Jepu, I.; Schinteie, G.; Ciupina, V.; Prodan, G.; Vasile, E.; Dinca, P. GMR on CuNiCo Thin Layers Deposited Using TVA Method. Dig. J. Nanomater. Biostruct. 2015, 10, 429–436. [Google Scholar]
- Jepu, I.; Porosnicu, C.; Mustata, I.; Lungu, C.P.; Kunkser, V.; Osiac, M.; Iacobescu, G.; Ionescu, V.; Tudor, T. Simultaneously Thermionic Vacuum Arc Discharges in Obtaining Ferromagnetic Thin Films. Rom. Rep. Phys. 2011, 63, 804–816. [Google Scholar]
- Jepu, I.; Porosnicu, C.; Lungu, C.P.; Mustata, I.; Luculescu, C.; Kuncser, V.; Iacobescu, G.; Marin, A.; Ciupina, V. Combinatorial Fe–Co thin film magnetic structures obtained by thermionic vacuum arc method. Surf. Coat. Technol. 2014, 240, 344–352. [Google Scholar] [CrossRef][Green Version]
- Surdu-Bob, C.C.; Vladoiu, R.; Badulescu, M.; Musa, G. Control over the sp2/sp3 ratio by tuning plasma parameters of the Thermoionic Vacuum Arc. Diam. Relat. Mater. 2008, 17, 1625–1628. [Google Scholar] [CrossRef]
- Jepu, I.; Doerner, R.P.; Baldwin, M.J.; Porosnicu, C.; Lungu, C.P. Temperature influence on deuterium retention for Be–W mixed thin films prepared by Thermionic Vacuum Arc method exposed to PISCES B plasma. J. Nucl. Mater. 2015, 463, 983–988. [Google Scholar] [CrossRef]
- Musa, G.; Surdu Bob, C.; Lungu, C.P.; Ciupina, V.; Vladoiu, R. Gaseous Thermionic Vacuum Arc (G-TVA)—An extension of TVA (Thermionic Vacuum Arc) input materials from solid samples to gases and liquids for carbon thin film deposition. J. Optoelectron. Adv. Mater. 2007, 9, 867–870. [Google Scholar]
- Surdu-Bob, C.; Musa, G.; Vladoiu, R.; Lungu, C.P. The synthesis of DLC using a novel cathodic arc technique: Gas-TVA. J. Optoelectron. Adv. Mater. 2007, 9, 2660–2662. [Google Scholar]
- Stoica, A.; Vlădoiu, R.; Musa, G.; Ciupină, V.; Contulov, M.; Buršíková, V.; Bláhová, O. Mechanical Properties of Thin Films Deposited by TVA and G-TVA Methods. Chem. Listy 2011, 105, S132–S135. [Google Scholar]
- Vladoiu, R.; Ciupina, V.; Contulov, M.; Mandes, A.; Dinca, V.; Prodan, M. HRTEM Images of a-C:H Thin Films Deposited by G-TVA Technique. IEEE Trans. Plasma Sci. 2011, 39, 2802–2803. [Google Scholar] [CrossRef]
- Vladoiu, R.; Ciupina, V.; Contulov, M.; Dinca, V.; Mandes, A.; Bursikova, V. Synthesis and Characterization of Nanostructured a-C:H (Hydrogenated Amorphous Carbon) Thin Films by Gaseous Thermionic Vacuum Arc (G-TVA) Deposition Technique. Plasma Chem. Plasma Process. 2012, 32, 219–229. [Google Scholar] [CrossRef]
- Vladoiu, R.; Ciupina, V.; Contulov, M.; Dinca, V.; Mandes, A.; Prodan, M. DLC thin films growth in thermionic vacuum arc technologies: TVA and GTVA. In Diamond-Like Carbon Films, Chapter: DLC Thin Films Growth in Thermionic Vacuum Arc Technologies: TVA and GTVA; Nova Science Publishers: New York, NY, USA, 2012; pp. 141–150. ISBN 10 1613247915. [Google Scholar]
- Surdu-Bob, C.C.; Badulescu, M. The C-TVA plasma source—A chemical vacuum thin film deposition tool. Tech. Proc. 2013 NSTI Nanotechnol. Conf. Expo Nanotech. 2013, 2, 446–449. [Google Scholar]
- Surdu-Bob, C.C.; Badulescu, M.; Anghel, A.; Sporea, D. Compact-TVA plasma—New opportunity for thin film deposition for energy applications. In Proceedings of the 5th International Symposium on Energy Challenges & Mechanics—Working on small scales, Inverness, UK, 10–14 July 2016. [Google Scholar]
- Akan, T. Operation Parameters of the Thermionic Vacuum Arc Discharge. Turk. J. Phys. 2003, 27, 69–75. [Google Scholar]
- Akan, T.; Ekem, N.; Pat, S.; Vladoiu, R.; Musa, G. Studies on the thermionic vacuum arc discharges in the vapors of Cu-Ag and Cu-Sn alloys. J. Optoelectron. Adv. Mater. 2005, 7, 2489–2494. [Google Scholar]
- Surdu-Bob, C.; Mustata, I.; Iacob, C. General characteristics of the Thermoionic Vacuum Arc plasma. J. Optoelectron. Adv. Mater. 2007, 9, 2932–2934. [Google Scholar]
- Jepu, I.; Porosnicu, C.; Mustata, I.; Lungu, C.P.; Kuncser, V.; Miculescu, F. Optimization of Thermionic Vacuum Arc Plasma Used for Multilayer GMR/TMR Films Preparation. Rom. Rep. Phys. 2010, 62, 771–779. [Google Scholar]
- Tiron, V.; Mihaescu, L.; Lungu, C.P.; Popa, G. Strong Double Layer Structure in Thermionic Vacuum Arc Plasma. Rom. Rep. Phys. 2011, 56, 41–46. [Google Scholar]
- Tiron, V.; Dobromir, M.; Pohoata, V.; Popa, G. Ion Energy Distribution in Thermionic Vacuum Arc Plasma. IEEE Trans. Plasma Sci. 2011, 39, 1403–1407. [Google Scholar] [CrossRef]
- Akan, T.; Demirkol, S.; Ekem, N.; Pat, S.; Musa, G. Study of Metal and Ceramic Thermionic Vacuum Arc Discharges. Plasma Sci. Technol. 2007, 9, 280–283. [Google Scholar] [CrossRef]
- Ehrich, H.; Schuhmann, J.; Musa, G.; Popescu, A.; Mustata, I. Adhesive metal films obtained by thermionic vacuum arc (TVA) deposition. Thin Solid Films 1998, 333, 95–102. [Google Scholar] [CrossRef]
- Fernández Palop, J.I.; Ballesteros, J.; Colomer, V.; Hernández, M.A. A new smoothing method for obtaining the electron energy distribution function in plasmas by the numerical differentiation of the I-V probe characteristic. Rev. Sci. Instrum. 1995, 66, 4625–4636. [Google Scholar] [CrossRef]
- Sheehan, J.P.; Hershkowitz, N. Emissive probes. Plasma Sources Sci. Technol. 2011, 20, 063001. [Google Scholar] [CrossRef]
- Takamura, S.; Ohno, N.; Ye, M.Y.; Kuwabara, T. Space-Charge Limited Current from Plasma-Facing Material Surface. Contrib. Plasma Phys. 2004, 44, 126–137. [Google Scholar] [CrossRef]
- Bousselin, G.; Lemoine, N.; Cavalier, J.; Heuraux, S.; Bonhomme, G. Note: On the measurement of plasma potential fluctuations using emissive probes. Rev. Sci. Instr. 2014, 85, 056102. [Google Scholar] [CrossRef] [PubMed]
- Bousselin, G.; Plihon, N.; Lemoine, N.; Cavalier, J.; Heuraux, S. How plasma parameters fluctuations influence emissive probe measurements. Phys. Plasmas 2015, 22, 053511. [Google Scholar] [CrossRef]
- Tiron, V.; Porosnicu, C.; Dinca, P.; Velicuc, I.-L.; Cristea, D.; Munteanu, D.; Révész, Á.; Stoian, G.; Lungu, C.P. Beryllium thin films deposited by thermionic vacuum arc for nuclear applications. Appl. Surf. Sci. 2019, 481, 327–336. [Google Scholar] [CrossRef]
- Vladoiu, R.; Mandes, A.; Dinca Balan, V.; Prodan, G.; Kudrna, P.; Tichý, M. Magnesium plasma diagnostics by heated probe and characterization of the Mg thin films deposited by thermionic vacuum arc technology. Plasma Sources Sci. Technol. 2015, 24, 035008. [Google Scholar] [CrossRef]
- Vladoiu, R.; Mandes, A.; Dinca, V.; Prodan, G.; Kudrna, P.; Tichý, M. Plasma diagnostics and characterization of the Mg and Mg–Zn thin films deposited by thermionic vacuum arc (TVA) method. Vacuum 2019, 167, 129–135. [Google Scholar] [CrossRef]
- Biloiu, C.; Ehrich, H.; Musa, G. Spectral plasma temperature determination of thermionic vacuum arc in the titanium vapors. J. Vac. Sci. Technol. A 2001, 19, 757–761. [Google Scholar] [CrossRef]
- Pat, S.; Ekem, N.; Akan, T.; Küsmüs, Ö.; Demirkol, S.; Vladoiu, R.; Lungu, C.P.; Musa, G. Study on Termionic Vacuum Arc—A Novel and Advanced Technology for Surface Coating. J. Optoelectron. Adv. Mater. 2005, 7, 2495–2499. [Google Scholar]
- Lungu, C.P.; Mustata, I.; Zaroschi, V.; Lungu, A.M.; Chiru, P.; Anghel, A.; Burcea, G.; Bailescu, V.; Dinuta, G.; Din, F. Spectroscopic study of beryllium plasma produced by thermionic vacuum arc. J. Optoelectron. Adv. Mater. 2007, 9, 884–886. [Google Scholar]
- Osiac, M.; Surdu-Bob, C.C.; Badulescu, M.; Lungu, C.P. Optical emission spectroscopy diagnostics of a Ni Thermionic Vacuum Arc (TVA) plasma. J. Optoelectron. Adv. Mater. 2008, 10, 2007–2010. [Google Scholar]
- Lungu, C.P.; Marcu, A.; Porosnicu, C.; Jepu, I.; Lungu, A.M.; Chiru, P.; Luculescu, C.; Banici, R.; Ursescu, D.; Dabu, R.; et al. Terawatt laser system irradiation of carbon/tungsten bilayers. Phys. Status Solidi A 2012, 209, 1732–1737. [Google Scholar] [CrossRef]
- Surdu-Bob, C.; Musa, G.; Buck, V.; Mustata, I.; Filipov, O.; Poukhovoi, A. Mass spectrometry and ion energy analysis of the carbon TVA plasma for the synthesis of DLC films. J. Optoelectron. Adv. Mater. 2007, 9, 2657–2659. [Google Scholar]
- Badulescu, M.; Gruia, I.; Micheli, V.; Calliari, L.; Surdu-Bob, C. Diamond film nano-abrasives obtained by anodic arc. Optoelectron. Adv. Mater. Rapid Commun. 2009, 3, 1207–1209. [Google Scholar]
- Badulescu, M.; Gruia, I.; Surdu-Bob, C.; Iacob, C. Arc plasma tailoring for the synthesis of compact tungsten films. Optoelectron. Adv. Mater. Rapid Commun. 2009, 11, 1231–1234. [Google Scholar]
- Surdu-Bob, C.C.; Badulescu, M.; Iacob, C.; Porosnicu, C.; Lungu, C.P. Ion energy distribution analysis of the TVA plasma ignited in carbon vapours using RFA. J. Phys. Conf. Ser. 2010, 207, 012018. [Google Scholar] [CrossRef]
- Ehrich, H.; Musa, G.; Mustata, I. Thermoionic Vacuum Arc (TVA)—One of the Best Suitable Method for High Purity Compact Smooth Thin Films Deposition. Bonpocы Amoмнoй Hayкu u Texнuкu 2002, 12, 169–171. [Google Scholar]
- Musa, G.; Mustata, I.; Blideran, M.; Ciupina, V.; Vladoiua, R.; Prodan, G.; Vasile, E. Nanostructured Carbon Thin Films Deposition Using Thermionic Vacuum Arc (TVA) Technology. J. Optoelectron. Adv. Mater. 2003, 5, 667–673. [Google Scholar]
- Musa, G.; Mustata, I.; Ciupina, V.; Vladoiu, R.; Prodan, G.; Vasile, E.; Ehrich, H. Diamond-like nanostructured carbon film deposition using thermionic vacuum arc. Diam. Relat. Mater. 2004, 13, 1398–1401. [Google Scholar] [CrossRef]
- Robertson, J. Diamond-like amorphous carbon. Mater. Sci. Eng. R 2002, 37, 129–281. [Google Scholar] [CrossRef]
- Mustata, I.; Musa, G.; Blideran, M.; Zaroschi, V.; Lungu, A.M.; Lungu, C.P.; Iwasaki, K. Ag-DLC tribological film deposition by double thermionic vacuum arc. In Proceedings of the 26th International Conference on Phenomena in Ionized Gases, Greifswald, Germany, 15–20 July 2003; Volume 4, pp. 87–88. [Google Scholar]
- Lungu, C.P.; Mustata, I.; Musa, G.; Zaroschi, V.; Lungu, A.M.; Iwasaki, K. Low friction silver-DLC coatings prepared by thermionic vacuum arc method. Vacuum 2004, 76, 127–130. [Google Scholar] [CrossRef]
- Mandes, A.; Vladoiu, R.; Dinca, V.; Prodan, G. Binary C–Ag Plasma Breakdown and Structural Characterization of the Deposited Thin Films by Thermionic Vacuum Arc Method. IEEE Trans. Plasma Sci. 2014, 42, 2806–2807. [Google Scholar] [CrossRef]
- Lungu, C.P.; Tudor, A.; Mustata, I.; Zaroschi, V.; Lungu, A.M.; Pompilian, O.; Porosnicu, C.; Chiru, P.; Vlase, M. Low Friction C-Cu, C-Sn Films Prepared by Thermionic Vacuum Arc Method. In Proceedings of the 7th International Conference “THE Coatings in Manufacturing Engineering”, Chalkidiki, Greece, 1–3 October 2008; pp. 439–448. [Google Scholar]
- Manole, D.; Casapu, C.; Pompilian, O.; Lungu, C.P.; Prodan, G.; Ciupina, V. Carbon-metal thin films deposited by thermionic vacuum arc method (TVA). J. Optoelectron. Adv. Mater. 2008, 10, 2954–2957. [Google Scholar]
- Ionescu, V.; Lungu, C.; Osiac, M.; Cotarlan, C.; Pompilian, O.; Lungu, A.M.; Ciupina, V. Carbon-copper amorphous composite coatings grown by thermionic vacuum arc method. Ovidius Univ. Ann. Chem. 2009, 20, 193–198. [Google Scholar]
- Ionescu, V.; Lungu, C.P.; Jepu, I.; Osia, M.; Iacobescu, G.E. Characterization of Thermionic Vacuum Arc Deposited Co-MgF2 Granular Thin Films Using X-Ray Diffraction and Microscopy Techniques. Rom. Rep. Phys. 2013, 65, 1390–1397. [Google Scholar]
- Lungu, C.P.; Ionescu, V.; Osiac, M.; Cotarlan, C.; Pompilian, O.; Lungu, A.M.; Ciupina, V. Thermionic Vacuum Arc Deposited Al—Doped Amorphous Carbon Nanocomposite Coatings. J. Non-Oxide Glasses 2009, 1, 175–182. [Google Scholar]
- Lungu, C.P.; Lungu, A.M.; Chiru, P.; Pompilian, O.G.; Tudor, A.; Brescia, R. Low friction properties of nano-structured C-Ni films prepared by thermionic vacuum arc method. Int. J. Surf. Sci. Eng. 2010, 4, 191–200. [Google Scholar] [CrossRef]
- Vladoiu, R.; Ciupina, V.; Mandes, A.; Contulov, M.; Dinca, V.; Popov, P.; Lungu, C.P. Tribological Properties of Carbon-Tungsten Nanocomposites Synthetized by Thermionic Vacuum Arc (TVA) Method. Rom. Rep. Phys. 2011, 63, 1053–1060. [Google Scholar]
- Vladoiu, R.; Mandes, A.; Dinca-Balan, V.; Bursikova, V. Structural and Mechanical Properties of Nanostructured C-Ag Thin Films Synthesized by Thermionic Vacuum Arc Method. Hindawi J. Nanomater. 2018, 2018, 9632041. [Google Scholar] [CrossRef]
- Ciupina, V.; Lungu, C.P.; Vladoiu, R.; Prodan, G.C.; Porosnicu, C.; Vasile, E.; Prodan, M.; Nicolescu, V.; Mandes, A.; Dinca, V.; et al. Nanostructured Carbon-Titanium multilayer films obtained by Thermionic Vacuum Arc method. Proc. SPIE 2018, 10731, 1073107. [Google Scholar] [CrossRef]
- Ciupină, V.; Lungu, C.P.; Vasile, E.; Prodan, G.C.; Porosnicu, C.; Vladoiu, R.; Mandes, A.; Dinca, V.; Nicolescu, V.; Prodan, M.; et al. Carbon-titanium multilayer films: Synthesis and characterization. AIP Conf. Proc. 2018, 2042, 020034. [Google Scholar] [CrossRef]
- Musaoğlu, C.; Pat, S.; Özen, S.; Mohammadigharehbagh, R.; Korkmaz, Ş. Investigation of the microstructural, surface and optical properties of nano-layer MoxSy thin film deposited by thermionic vacuum arc. Mater. Res. Express 2019, 6, 036411. [Google Scholar] [CrossRef]
- Vladoiu, R.; Ciupina, V.; Mustata, I.; Lungu, C.P.; Musa, G. Characterization of Carbon Thin Film Deposited by Thermionic Vacuum Arc (TVA) Method. Rom. J. Phys. 2004, 51, 215–218. [Google Scholar]
- Musa, G.; Mustata, I.; Blideran, M.; Ciupina, V.; Vladoiu, R.; Prodan, G.; Vasile, E.; Ehrich, H. Thermionic Vacuum Arc—New Technique for High Purity Carbon Thin Film Deposition. Acta Phys. Slovaca 2005, 55, 417–421. [Google Scholar]
- Musa, G.; Mustata, I.; Ciupina, V.; Vladoiu, R.; Prodan, G.; Lungu, C.P.; Ehrich, H. Thermionic Vacuum Arc (TVA)—Carbon Thin Film Deposition. J. Optoelectron. Adv. Mater. 2005, 7, 2485–2487. [Google Scholar]
- Musa, G.; Vladoiu, R.; Ciupina, V.; Janik, J. Raman spectra of carbon thin films. J. Optoelectron. Adv. Mater. 2006, 8, 617–1620. [Google Scholar]
- Vladoiu, R.; Ciupina, V.; Mandes, A.; Dinca, V.; Contulov, M.; Prodan, G.; Musa, G. Preliminary results on comparative study of three methods for nanocarbon films deposition: Thermionic vacuum arc, magnetron sputtering and cathodic arc. J. Optoelectron. Adv. Mater. 2008, 10, 723–726. [Google Scholar]
- Ciupina, V.; Morjan, I.G.; Alexandrescu, R.; Dumitrache, F.V.; Prodan, G.; Lungu, C.; Vladoiu, R.; Mustata, I.; Zarovschi, V.; Sullivan, J.; et al. Synthesis and Characterization of Some Carbon Based Nanostructures. Proc. SPIE 2010, 7764, 77640O. [Google Scholar] [CrossRef]
- Vladoiu, R.; Dinca, V.; Musa, G. Surface energy evaluation of unhydrogenated DLC thin film deposited by thermionic vacuum arc (TVA) method. Eur. Phys. J. D 2009, 54, 433–437. [Google Scholar] [CrossRef]
- Oancea-Stanescu, I.M.; Ciupina, V.; Prodan, G.; Prodan, M.; Caraiane, A.; Dulgheru, N.; Jepu, I.; Lungu, C.P. Transmission electron microscopy analysis and electrical measurements of carbon thin films. J. Optoelectron. Adv. Mater. 2010, 12, 824–828. [Google Scholar]
- Vladoiu, R.; Ciupina, V.; Lungu, C.P.; Bursikova, V.; Musa, G. Thermoionic vacuum arc (TVA) deposited tungsten thin film characterization. J. Optoelectron. Adv. Mater. 2005, 8, 71–73. [Google Scholar]
- Lungu, C.P.; Mustata, I.; Lungu, A.M.; Zaroschi, V.; Musa, G.; Iwanaga, I.; Tanaka, R.; Matsumura, Y.; Tanaka, H.; Oi, T.; et al. Influence of Re on the Thermo-Electron Emission From Thoriated W Cathode During Re Deposition by Thermionic Vacuum Arc (TVA) Method. J. Optoelectron. Adv. Mater. 2005, 7, 2513–2519. [Google Scholar]
- Lungu, C.P.; Mustata, I.; Musa, G.; Lungu, A.M.; Zaroschi, V.; Iwasaki, K.; Tanaka, R.; Matsumura, Y.; Iwanaga, I.; Tanaka, H.; et al. Formation of nanostructured Re–Cr–Ni diffusion barrier coatings on Nb superalloys by TVA method. Surf. Coat. Technol. 2005, 200, 399–402. [Google Scholar] [CrossRef]
- Surdu-Bob, C.C.; Lungu, C.P.; Mustata, I.; Frunza, L. Re–Cr–Ni high-temperature resistant coatings on Cu substrates prepared by thermionic vacuum arc (TVA) method. J. Phys. D Appl. Phys. 2008, 41, 132001. [Google Scholar] [CrossRef]
- Pompilian, O.G.; Osiac, M.; Iacobescu, G.E.; Lungu, C.P. Layer coatings of Re and Re-NiCr obtained by thermoionic vacuum arc technique. J. Optoelectron. Adv. Mater. 2009, 11, 1779–1782. [Google Scholar]
- Vladoiu, R.; Mandes, A.; Dinca, V.; Prodan, G. Titanium-based thin films for protective coatings prepared by TVA (Thermionic Vacuum Arc) technology. MATEC Web Conf. 2018, 249, 0100. [Google Scholar] [CrossRef]
- Panjan, P.; Cekada, M.; Panjan, M.; Kek-Merl, D. Growth defects in PVD hard coatings. Vacuum 2010, 84, 209–214. [Google Scholar] [CrossRef]
- Porosnicu, C.; Lungu, C.P.; Jepu, I.; Pompilian, O.G.; Dinca, P.; Luculescu, C.; Prodan, G.; Marin, A.; Vladescu, A.; Vladoiu, R. Characterization of Ternary C-Si-Al Nanocomposite Thin Films Obtained by TVA Method. Dig. J. Nanomater. Biostruct. 2014, 9, 765–775. [Google Scholar]
- Ciupina, V.; Lungu, C.P.; Vladoiu, R.; Prodan, G.C.; Antohe, S.; Porosnicu, C.; Stanescu, I.; Jepu, I.; Iftimie, S.; Belc, M. Structural and electrical properties of N doped SiC nanostructures obtained by TVA method. Proc. SPIE 2015, 9558, 955808. [Google Scholar] [CrossRef]
- Ciupina, V.; Vasile, E.; Porosnicu, C.; Prodan, G.C.; Lungu, C.P.; Vladoiu, R.; Jepu, I.; Mandes, A.; Dinca, V.; Caraiane, A. Characterization of nitrogen doped silicon–carbon multi-layer nanostructures obtained by TVA method. Proc. SPIE 2016, 9929, 992910. [Google Scholar] [CrossRef]
- Ciupina, V.; Vasile, E.; Porosnicu, C.; Vladoiu, R.; Mandes, A.; Dinca, V.; Nicolescu, V.; Manu, R.; Dinca, P.; Zaharia, A. Nitrogen Doped Silicon-Carbon Multilayer Protective Coatings on Carbon Obtained By Thermionic Vacuum Arc (TVA) Method. AIP Conf. Proc. 2018, 1935, UNSP 050001. [Google Scholar] [CrossRef]
- Dinca-Balan, V.; Vladoiu, R.; Mandes, A.; Prodan, G. Correlation study of nanocrystalline carbon doped thin films prepared by a thermionic vacuum arc deposition technique. J. Phys. D Appl. Phys. 2017, 50, 435305. [Google Scholar] [CrossRef]
- Mandes, A.; Vladoiu, R.; Prodan, G.; Dinca, V.; Porosnicu, C.; Dinca, P. The Properties of Binary and Ternary Ti Based Coatings Produced by Thermionic Vacuum Arc (TVA) Technology. Coatings 2018, 8, 114. [Google Scholar] [CrossRef]
- Vladoiu, R.; Ciupina, V.; Surdu-Bob, C.; Lungu, C.P.; Janik, J.; Skalny, J.D.; Bursikova, V.; Bursik, J.; Musa, G. Properties of the carbon thin films deposited by thermionic vacuum arc. J. Optoelectron. Adv. Mater. 2007, 9, 862–866. [Google Scholar]
- Ekem, N.; Musa, G.; Pat, S.; Balbag, Z.; Cenik, I.; Vladoiu, R. Carbon thin film deposition by Thermionic Vacuum Arc (TVA). J. Optoelectron. Adv. Mater. 2008, 10, 672–674. [Google Scholar]
- Lungu, C.P.; Grigorescu, C.E.A.; Rusu, M.I.; Jepu, I.; Porosnicu, C.; Lungu, A.M.; Feraru, I.D.; Savastru, D. Nanodiamond crystallites embedded in carbon films prepared by thermionic vacuum arc method. Diam. Relat. Mater. 2011, 20, 1061–1064. [Google Scholar] [CrossRef]
- Ciupina, V.; Sullivan, J.; Saied, S.; Vladoiu, R.; Prodan, M.; Oancea-Stanescu, I.; Mandes, A.; Contulov, M.; Dinca, V.; Prodan, M.; et al. Synthesis and Characterization of Some Carbon Based Nanostructures. Contrib. Plasma Phys. 2011, 51, 546–553. [Google Scholar] [CrossRef]
- Balbag, M.Z.; Pat, S.; Ozkan, M.; Ekem, N.; Musa, G. Thermionic vacuum arc (TVA) technique for magnesium thin film deposition. Physica B 2010, 405, 3276–3278. [Google Scholar] [CrossRef]
- Marin, I.; Ciupina, V.; Oancea-Stanescu, I.M.; Vasile, E.; Ungureanu, G.D.; Prodan, G. Structural characterization of MgO-Co multilayers prepared by thermionic vacuum arc method. Optoelectron. Adv. Mater. Rapid Commun. 2010, 4, 2048–2051. [Google Scholar]
- Vladoiu, R.; Mandes, A.; Dinca, V.; Prodan, G.; Ciupina, V. Synthesis of Reinforced Magnesium Embedded in Carbon Matrix by using Thermionic Vacuum Arc (TVA) Technology. Rom. Rep. Phys. 2016, 68, 1076–1084. [Google Scholar]
- Pat, S.; Ozen, S.; Senay, V.; Korkmaz, S. Optical and Surface Characteristics of Mg-Doped GaAs Nanocrystalline Thin Film Deposited by Thermionic Vacuum Arc Technique. J. Electron. Mater. 2017, 46, 1–5. [Google Scholar] [CrossRef]
- Akan, T.; Ekem, N.; Demirkol, S.; Pat, S.; Balbag, M.Z.; Cenik, M.I.; Deligoz, H.; Musa, G. Studies on Ag-Al2O3 Nano-layer Composite Produced by the Thermionic Vacuum Arc Method (TVA). AIP Conf. Proc. 2007, 899, 695. [Google Scholar]
- Rasleanu, D.; Ionescu, V.; Prodan, G.; Ciupina, V.; Lungu, C.P.; Surdu-Bob, C.; Osiac, M.; Pompilian, O.; Badulescu, M.; Lungu, A.M.; et al. Nanostructured PZT type thin films prepared by thermionic vacuum arc method. J. Optoelectron. Adv. Mater. 2008, 10, 3041–3047. [Google Scholar]
- Savastru, D.; Tenciu, D.; Lungu, C.P.; Viespe, C.; Grigoriu, C.; Iordanescu, R.; Feraru, I.D.; Ionescu, V.; Monnereau, O.; Tortet, L. PZT Films Prepared by TVA and PLD From PbO2:TiO2:ZrO2 (1:1:1) Nanoceramic Targets. Dig. J. Nanomater. Biostruct. 2011, 6, 207–2011. [Google Scholar]
- Burada, M.; Soare, V.; Mitrica, D.; Lungu, C.P.; Ghenescu, V.; Ion, L. Growth of CIS Thin Films using one Step Electrodeposition Process. Metal. Int. 2009, 14, 193–196. [Google Scholar]
- Vladoiu, R.; Ciupina, V.; Mandes, A.; Dinca, V.; Prodan, M.; Musa, G. Growth and characteristics of tantalum oxide thin films deposited using thermionic vacuum arc technology. J. Appl. Phys. 2010, 108, 093301. [Google Scholar] [CrossRef]
- Korkmaz, Ş.; Elmas, S.; Ekem, N.; Pat, S.; Balbağ, M.Z. Deposition of MgF2 thin films for antireflection coating by using thermionic vacuum arc (TVA). Opt. Commun. 2012, 285, 2373–2376. [Google Scholar] [CrossRef]
- Emre Cetin, N.; Korkmaz, S.; Elmas, S.; Ekem, N.; Pat, S.; Balbag, M.Z.; Tarhan, E.; Temel, S.; Ozmumcu, M. The structural, optical and morphological properties of CaF2 thin films. Mater. Lett. 2013, 91, 175–178. [Google Scholar] [CrossRef]
- Pat, S.; Cetin, N.E.; Korkmaz, S.; Balbag, M.Z.; Ekem, N. Characterization of BaF2 Thin Film Deposited by Thermionic Vacuum Arc. J. Nanoelectron. Optoelectron. 2015, 10, 301–303. [Google Scholar] [CrossRef]
- Pat, S.; Balbag, M.Z.; Korkmaz, S. Ultra Thin Carbon Films Deposited on SrTiO3 Substrates by Thermionic Vacuum Arc. Nano 2013, 8, 1350028. [Google Scholar] [CrossRef]
- Korkmaz, S.; Pat, S.; Ekem, N.; Balbag, M.Z.; Temel, S. Thermal treatment effect on the optical properties of ZrO2 thin films deposited by thermionic vacuum arc. Vacuum 2012, 86, 1930–1933. [Google Scholar] [CrossRef]
- Demirkol, U.; Pat, S.; Mohammadigharehbagh, R.; Musaoğlu, C.; Özgür, M.; Elmas, S.; Özen, S.; Korkmaz, Ş. Determination of the structural, morphological and optical properties of graphene doped SnO thin films deposited by using thermionic vacuum arc technique. Phys. B Condens. Matter 2019, 569, 14–19. [Google Scholar] [CrossRef]
- Pat, S.; Ozmumcu, M.; Ekem, N.; Ozkan, M.; Korkmaz, S.; Balbag, M.Z. Antireflective Coating on Polyethylene Terephthalate by Thermionic Vacuum Arc. J. Plast. Film Sheeting 2010, 26, 259–270. [Google Scholar] [CrossRef]
- Balbag, M.Z.; Pat, S. Electrically conductive and optically transparent polyethylene terephthalate films coated with gold and silver by thermionic vacuum arc. J. Plast. Film Sheeting 2011, 27, 209–222. [Google Scholar] [CrossRef]
- Elmas, S.; Korkmaz, S.; Pat, S. Optical characterization of deposited ITO thin films on glass and PET substrates. Appl. Surf. Sci. 2013, 276, 641–645. [Google Scholar] [CrossRef]
- Elmas, S.; Korkmaz, Ş.; Pat, S. Investigation of physical properties and surface free energy of produced ITO thin films by TVA technique. J. Mater. Sci. Mater. Electron. 2019, 30, 8876–8882. [Google Scholar] [CrossRef]
- Özen, S.; Pat, S.; Senay, V.; Korkmaz, S.; Gecici, B. Physical Properties of the SiGe Thin Film Coatings by Thermionic Vacuum Arc (TVA). J. Nanoelectron. Optoelectron. 2015, 10, 56–60. [Google Scholar] [CrossRef]
- Pat, S.; Senay, V.; Ozen, S.; Korkmaz, S. Direct and fast growth of GaAs thin films on glass and polyethylene terephthalate substrates using a thermionic vacuum arc. J. Mater. Sci. Mater. Electron. 2015, 26, 2210–2214. [Google Scholar] [CrossRef]
- Özen, S.; Senay, V.; Pat, S.; Korkmaz, S. Deposition of a Mo doped GaN thin film on glass substrate by thermionic vacuum arc (TVA). J. Mater. Sci. Mater. Electron. 2015, 26, 5060–5064. [Google Scholar] [CrossRef]
- Özen, S.; Senay, V.; Pat, S.; Korkmaz, S. Characterization of a fast grown GaAs:Sn thin film by thermionic vacuum arc. J. Mater. Sci. Mater. Electron. 2015, 26, 8983–8987. [Google Scholar] [CrossRef]
- Senay, V.; Ozen, S.; Pat, S.; Korkmaz, S. Optical, Structural and Morphological Characterization of a Zn-Doped GaAs Semiconducting Thin Film Produced by Thermionic Vacuum Arc. Mater. Focus 2015, 4, 397–402. [Google Scholar] [CrossRef]
- Senay, V.; Ozen, S.; Pat, S.; Korkmaz, S. Direct and Fast Growth of a Si:GaAs Thin Film by means of Thermionic Vacuum Arc (TVA). In Proceedings of the 42nd IEEE International Conference on Plasma Sciences (ICOPS), Belek, Turkey, 24–28 May 2015. [Google Scholar]
- Senay, V.; Ozen, S.; Pat, S.; Korkmaz, S. Some physical properties of a Si-doped nano-crystalline GaAs thin film grown by thermionic vacuum arc. Vacuum 2015, 119, 228–232. [Google Scholar] [CrossRef]
- Pat, S.; Korkmaz, S.; Ozen, S.; Senay, V. Heavily carbon doped GaAs nanocrystalline thin film deposited by thermionic vacuum arc method. J. Alloys Compd. 2016, 657, 711–716. [Google Scholar] [CrossRef]
- Pat, S.; Korkmaz, S.; Ozen, S.; Senay, V. The Effects of Boron Alloying on the Structural and Optical Properties of GaAs Deposited by a Thermionic Vacuum Arc Method. Mater. Focus 2016, 5, 1–4. [Google Scholar] [CrossRef]
- Pat, S.; Ozen, S.; Senay, V.; Korkmaz, S.; Simsek, V. Optical and Surface Properties of the In Doped GaAs Layer Deposition. Scanning 2016, 38, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Pat, S.; Senay, V.; Ozen, S.; Korkmaz, S. Surface, Nanomechanical, and Optical Properties of Mo-Doped GeGaAs Thin Film Deposited by Thermionic Vacuum Arc. J. Electron. Mater. 2016, 45, 255–261. [Google Scholar] [CrossRef]
- Senay, V.; Ozen, S.; Pat, S.; Korkmaz, S.; Mohammadigharehbagh, R. Some physical properties of Co-doped GaAs thin films grown by thermionic vacuum arc. AIP Conf. Proc. 2016, 1722, 290016. [Google Scholar]
- Senay, V.; Ozen, S.; Pat, S.; Korkmaz, S. Optical, structural, morphological and compositional characterization of a Co-doped GaAs semiconducting thin film produced by thermionic vacuum arc. J. Alloys Compd. 2016, 663, 829–833. [Google Scholar] [CrossRef]
- Senay, V.; Ozen, S.; Pat, S.; Korkmaz, S. A study on some physical properties of a Pb-doped GaAs thin film produced by thermionic vacuum arc. J. Alloys Compd. 2017, 720, 383–387. [Google Scholar] [CrossRef]
- Özen, S.; Senay, V.; Pat, S.; Korkmaz, S. Investigation on the morphology and surface free energy of the AlGaN thin film. J. Alloys Compd. 2015, 653, 162–167. [Google Scholar] [CrossRef]
- Özen, S.; Bilgiç, E.; Gülmez, G.; Şenay, V.; Pat, S.; Korkmaz, Ş.; Mohammadigharehbagh, R. Investigation of the thickness effect to impedance analysis results AlGaN acoustic sensor. AIP Conf. Proc. 2016, 1722, 240004. [Google Scholar]
- Özen, S.; Bilgiç, E.; Gülmez, G.; Şenay, V.; Pat, S.; Korkmaz, Ş.; Mohammadigharehbagh, R. Impedance analysis of nano thickness layered AlGaN acoustic sensor deposited by thermionic vacuum arc. AIP Conf. Proc. 2016, 1722, 240005. [Google Scholar]
- Özen, S.; Senay, V.; Pat, S.; Korkmaz, S. AlGaAs film growth using thermionic vacuum arc (TVA) and determination of its physical properties. Eur. Phys. J. Plus 2015, 130, 108. [Google Scholar] [CrossRef]
- Erdoğan, E.; Kundakçı, M. Investigation of GaN/InGaN thin film growth on ITO substrate by thermionic vacuum arc (TVA). SN Appl. Sci. 2019, 1, 9. [Google Scholar] [CrossRef]
- Pat, S.; Ozen, S.; Korkmaz, S.; Senay, V. GaN thin film deposition on glass and PET substrates by thermionic vacuum arc (TVA). Mater. Chem. Phys. 2015, 159, 1–5. [Google Scholar] [CrossRef]
- Kundakçı, M.; Mantarcı, A.; Erdoğan, E. Growth and characterization of GaN thin film on Si substrate by thermionic vacuum arc (TVA). Mater. Res. Express 2017, 4, 016410. [Google Scholar] [CrossRef]
- Özen, S.; Senay, V.; Pat, S.; Korkmaz, S. The Influence of Voltage Applied Between the Electrodes on Optical and Morphological Properties of the InGaN Thin Films Grown by Thermionic Vacuum Arc. Scanning 2016, 38, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Özen, S.; Pat, S.; Korkmaz, S.; Senay, V. Mo Doped GaN Thin Film Growth Using Thermionic Vacuum Arc (TVA). In Proceedings of the 42nd IEEE International Conference on Plasma Sciences (ICOPS), Belek, Turkey, 24–28 May 2015. [Google Scholar]
- Pat, S.; Ozen, S.; Senay, V.; Korkmaz, S. Comparisons of surface and optical properties of the heavily carbon carbon doped GaN nanocrystalline films deposited by thermionic vacuum arc method. Vacuum 2016, 133, 38–42. [Google Scholar] [CrossRef]
- Özen, S.; Korkmaz, Ş.; Şenay, V.; Pat, S. The substrate effect on Ge doped GaN thin films coated by thermionic vacuum arc. J. Mater. Sci. Mater. Electron. 2017, 28, 1288–1293. [Google Scholar] [CrossRef]
- Pat, S.; Ozen, S.; Korkmaz, S. A Rapid Method for Deposition of Sn-Doped GaN Thin Films on Glass and Polyethylene Terephthalate Substrates. J. Electron. Mater. 2018, 47, 167–172. [Google Scholar] [CrossRef]
- Pat, S.; Korkmaz, S.; Ozen, S.; Senay, V. Optical, surface and magnetic properties of the Ti-doped GaN nanosheets on glass and PET substrates by thermionic vacuum arc (TVA) method. Part. Sci. Technol. 2018, 37, 333–338. [Google Scholar] [CrossRef]
- Özen, S.; Pat, S.; Senay, V.; Korkmaz, S. The surface morphology research of the BGaN thin films deposited by thermionic vacuum arc. Vacuum 2017, 135, 50–54. [Google Scholar] [CrossRef]
- Özen, S.; Pat, S.; Korkmaz, S. Characterization of Pb-Doped GaN Thin Films Grown by Thermionic Vacuum Arc. J. Electron. Mater. 2018, 47, 3727–3732. [Google Scholar] [CrossRef]
- Özen, S. Zr-doped GaN thin films grown onto glass and PET substrates. Mater. Res. Express 2019, 6, 046401. [Google Scholar] [CrossRef]
- Özkan, M.; Ekem, N.; Pat, S.; Balbag, M.Z. ZnS thin film deposition on Silicon and glass substrates by Thermionic vacuum Arc. Mater. Sci. Semicond. Process. 2012, 15, 113–119. [Google Scholar] [CrossRef]
- Özkan, M.; Ekem, N.; Balbag, M.Z.; Pat, S. ZnSe nanocrystalline thin films deposition on Si substrate by thermionic vacuum arc. Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 2012, 226, 103–108. [Google Scholar] [CrossRef]
- Sürdem, S. Determination of some properties of ZnSB thin films deposited by a thermionic vacuum arc technique. J. Mater. Sci. Mater. Electron. 2019, 30, 19060–19068. [Google Scholar] [CrossRef]
- Mohammadigharehbagh, R.; Özen, S.; Yudar, H.H.; Pat, S. Investigation of the some physical properties of Ge-doped ZnO thin films deposited by thermionic vacuum arc technique. J. Mater. Sci. Mater. Electron. 2017, 28, 14131–14137. [Google Scholar] [CrossRef]
- Mohammadigharehbagh, R.; Özen, S.; Yudar, H.H.; Pat, S.; Korkmaz, Ş. The electrical, elemental, optical, and surface properties of Si-doped ZnO thin films prepared by thermionic vacuum arc. Mater. Res. Express 2017, 4, 096404. [Google Scholar] [CrossRef]
- Demirkol, U.; Pat, S.; Mohammadigharehbagh, R.; Musaoğlu, C.; Özgür, M.; Elmas, S.; Özen, S.; Korkmaz, Ş. Investigation of the substrate effect for Zr doped ZnO thin film deposition by thermionic vacuum arc technique. J. Mater. Sci. Mater. Electron. 2018, 29, 18098–18104. [Google Scholar] [CrossRef]
- Mohammadigharehbagh, R.; Pat, S.; Musaoglu, C.; Korkmaz, Ş.; Özen, S. The investigation of the Cr doped ZnO thin films deposited by thermionic vacuum arc technique. Mater. Res. Express 2018, 5, 026403. [Google Scholar] [CrossRef]
- Mohammadigharehbagh, R.; Özen, S.; Yudar, H.H.; Senay, V.; Pat, S.; Korkmaz, S. Investigation on the physical properties of C-doped ZnO thin films deposited by the thermionic vacuum arc. Eur. Phys. J. Plus 2017, 132, 28. [Google Scholar] [CrossRef]
- Pat, S.; Mohammadigharehbagh, R.; Musaoglu, C.; Özen, S.; Korkmaz, Ş. Investigation of the surface, morphological and optical properties of boron–doped ZnO thin films deposited by thermionic vacuum arc technique. Mater. Res. Express 2018, 5, 066419. [Google Scholar] [CrossRef]
- Elmas, S.; Pat, S.; Mohammadigharehbagh, R.; Musaoğlu, C.; Özgür, M.; Demirkol, U.; Özen, S.; Korkmaz, Ş. Determination of physical properties of graphene doped ZnO (ZnO:Gr) nanocomposite thin films deposited by a thermionic vacuum arc technique. Phys. B Condens. Matter 2019, 557, 27–33. [Google Scholar] [CrossRef]
- Özgür, M.; Pat, S.; Mohammadigharehbagh, R.; Musaoğlu, C.; Demirkol, U.; Elmas, S.; Özen, S.; Korkmaz, Ş. Al doped ZnO thin film deposition by thermionic vacuum arc. J. Mater. Sci. Mater. Electron. 2019, 30, 624–630. [Google Scholar] [CrossRef]
- Kaplan, H.K.; Sarsıcı, S.; Akay, S.K.; Ahmetoglu, M. The characteristics of ZnS/Si heterojunction diode fabricated by thermionic vacuum arc. J. Alloys Compd. 2017, 724, 543–548. [Google Scholar] [CrossRef]
- Kaplan, H.K.; Akay, S.K.; Ahmetoglu, M. Photoelectrical properties of fabricated ZnS/Si heterojunction device using thermionic vacuum arc method. Superlattices Microstruct. 2018, 120, 402–409. [Google Scholar] [CrossRef]
- Musaoğlu, C.; Pat, S.; Özen, S.; Korkmaz, Ş.; Mohammadigharehbagh, R. Investigation of the structural, surface, optical and electrical properties of the Indium doped CuxO thin films deposited by a thermionic vacuum arc. Mater. Res. Express 2018, 5, 035909. [Google Scholar] [CrossRef]
- Musaoğlu, C.; Pat, S.; Mohammadigharehbagh, R.; Özen, S.; Korkmaz, S. The Thermionic Vacuum Arc Method for Rapid Deposition of Cu/CuO/Cu2O Thin Film. J. Electron. Mater. 2019, 48, 2272–2277. [Google Scholar] [CrossRef]
- Pat, S.; Mohammadigharehbagh, R.; Musaoglu, C.; Özen, S.; Korkmaz, Ş. Investigation of the optical properties of the Cr doped CuxO thin film deposited by thermionic vacuum arc plasma. Opt. Int. J. Light Electron Opt. 2019, 180, 350–354. [Google Scholar] [CrossRef]
- Silik, E.; Pat, S.; Özen, S.; Mohammadigharehbagh, R.; Yudar, H.H.; Musaoğlu, C.; Korkmaz, Ş. Electrochromic properties of TiO2 thin films grown by thermionic vacuum arc method. Thin Solid Films 2017, 640, 27–32. [Google Scholar] [CrossRef]
- Senay, V.; Ozen, S.; Pat, S.; Gecici, B.; Korkmaz, S. A new method for titania thin film production: Thermionic vacuum arc method. J. Thermoplast. Compos. Mater. 2017, 30, 808–815. [Google Scholar] [CrossRef]
- Lungu, C.P.; Mustata, I.; Zaroschi, V.; Lungu, A.M.; Anghel, A.; Chiru, P.; Rubel, M.; Coad, P.; Matthews, G.F.; JET-EFDA Contributors. Beryllium coatings on metals for marker tiles at JET: Development of process and characterization of layers. Phys. Scr. T 2007, 128, 157–161. [Google Scholar] [CrossRef]
- Gavrila, C.; Lungu, C.P.; Gruia, I. A Numerical Simulation of the Phenomena in Be Plasma. Proc. SPIE 2011, 8001, 80013D. [Google Scholar] [CrossRef]
- Anghel, A.; Porosnicu, C.; Badulescu, M.; Mustata, I.; Lungu, C.P.; Sugiyama, K.; Lindig, S.; Kriegeb, K.; Roth, J.; Nastuta, A.; et al. Surface morphology influence on deuterium retention in beryllium films prepared by thermionic vacuum arc method. Nucl. Instrum. Methods Phys. Res. B 2009, 267, 426–429. [Google Scholar] [CrossRef]
- Anghel, A.; Mustata, I.; Porosnicu, C.; Lungu, C.P. Influence of the bias voltage on the formation of beryllium films by a thermionic vacuum arc method. J. Nucl. Mater. 2009, 385, 242–245. [Google Scholar] [CrossRef]
- Nishijima, D.; Doerner, R.P.; Baldwin, M.J.; De Temmerman, G. Erosion yields of deposited beryllium layers. J. Nucl. Mater. 2009, 390, 132–135. [Google Scholar] [CrossRef]
- Nemanič, V.; Zajec, B.; Zumer, M.; Porosnicu, C.; Lungu, C.P. Hydrogen permeability of beryllium films prepared by the thermionic vacuum arc method. Fusion Eng. Des. 2011, 86, 2421–2424. [Google Scholar] [CrossRef]
- Zajec, B.; Nemanic, V.; Zumer, M.; Porosnicu, C.; Lungu, C.P. Hydrogen permeability through beryllium films and the impact of surface oxides. J. Nucl. Mater. 2013, 443, 185–194. [Google Scholar] [CrossRef]
- Nemanič, V.; Kovač, J.; Lungu, C.; Porosnicu, C.; Zajec, B. Characterization of tungsten films and their hydrogen permeability. J. Vac. Sci. Technol. A 2014, 32, 061511. [Google Scholar] [CrossRef]
- Burducea, I.; Straticiuc, M.; Racolta, P.M.; Surdu-Bob, C.C.; Badulescu, M.; Anghel, A.; Jipa, A.; Luculescu, C. Hydrogen depth profiling in DLC films using RNRA. Optoelectron. Adv. Mater. Rapid Commun. 2012, 6, 832–835. [Google Scholar]
- Zaloznik, A.; Markelj, S.; Cadez, I.; Pelicon, P.; Vavpetic, P.; Porosnicu, C.; Lungu, C.P. The influence of nitrogen co-deposition in mixed layers on deuterium retention and thermal desorption. J. Nucl. Mater. 2015, 467, 472–479. [Google Scholar] [CrossRef]
- Ciupina, V.; Morjan, I.; Lungu, C.P.; Vladoiu, R.; Prodan, G.; Prodan, M.; Zarovschi, V.; Porosnicu, C.; Stanescu, I.M.; Contulov, M. Electron Microscopy Characterization of Some Carbon Based Nanostructures with Application in Divertors Coatings From Fusion Reactor. Proc. SPIE 2011, 8104, UNSP 810411. [Google Scholar] [CrossRef]
- Ciupina, V.; Morjan, I.; Vladoiu, R.; Lungu, C.P.; Porosnicu, C.; Jepu, I.; Prodan, G.; Stanescu, I.M.; Mandes, A.; Contulov, M.; et al. Application of carbon-tungsten, carbon-beryllium and carbon-aluminium nanostructures in divertors coatings from fusion reactor. J. Optoelectron. Adv. Mater. 2013, 15, 1450–1456. [Google Scholar]
- Marcu, A.; Ticoş, C.M.; Grigoriu, C.; Jepu, I.; Porosnicu, C.; Lungu, A.M.; Lungu, C.P. Simultaneous carbon and tungsten thin film deposition using two thermionic vacuum arcs. Thin Solid Films 2011, 519, 4074–4077. [Google Scholar] [CrossRef]
- Surdu-Bob, C.C.; Racolta, P.M.; Badulescu, M.; Chiojdeanu, C.; Arsene, N.; Logofatu, C.; Ionescu, C.; Sporea, D.; Gruia, I. Pure, smooth and dense W films obtained by an anodic arc plasma. Optoelectron. Adv. Mater. Rapid Commun. 2011, 5, 1336–1340. [Google Scholar]
- Lungu, M.; Tiseanu, I.; Dobrea, C.; Porosnicu, C.; Jepu, I.; JET-EURO Fusion Contributors. Preparation and Analysis of Functional Fusion Technology Related Materials. Rom. J. Phys. 2015, 60, 560–572. [Google Scholar]
- Ciupina, V.; Lungu, C.P.; Vladoiu, R.; Epure, T.D.; Prodan, G.; Porosnicu, C.; Prodan, M.; Stanescu, I.M.; Contulov, M.; Mandes, A. Application of Carbon-Aluminum Nanostructures in Divertors Coatings from Fusion Reactor. Proc. SPIE 2012, 8465, 846508. [Google Scholar] [CrossRef]
- Lungu, C.P.; Porosnicu, C.; Jepu, I.; Lungu, M.; Marcu, A.; Luculescu, C.; Ticos, C.; Marin, A.; Grigorescu, C.E.A. The behavior of W, Be and C layers in interaction with plasma produced by terawatt laser beam pulses. Vacuum 2014, 110, 207–212. [Google Scholar] [CrossRef]
- Lungu, C.P.; Marcu, A.; Porosnicu, C.; Jepu, I.; Kovac, J.; Nemanic, V. Carbon–Tungsten Thin-Film Deposition by a Dual Thermionic Vacuum Arc. IEEE Trans. Plasma Sci. 2012, 40, 3546–3551. [Google Scholar] [CrossRef]
- Kuncser, V.; Mustata, I.; Lungu, C.P.; Lungu, A.M.; Zaroschi, V.; Keune, W.; Sahoo, B.; Stromberg, F.; Walterfang, M.; Ion, L.; et al. Fe–Cu granular thin films with giant magnetoresistance by thermionic vacuum arc method: Preparation and structural characterization. Surf. Coat. Technol. 2005, 200, 980–983. [Google Scholar] [CrossRef]
- Lungu, C.P.; Mustata, I.; Lungu, A.M.; Brinza, O.; Zaroschi, V.; Kuncser, V.; Filoti, G.; Ion, L. Giant Magnetoresistance Effects in Correlation with Local Magnetic Interactions in Fe-Cu and Co-Cu Granular Thin Films Prepared by Thermionic Vacuum Arc Method. J. Optoelectron. Adv. Mater. 2005, 7, 2507–2512. [Google Scholar]
- Anghel, A.; Lungu, C.P.; Mustata, I.; Zaroschi, V.; Lungu, A.M.; Barbu, I.; Badulescu, M.; Pompilian, O.; Schinteie, G.; Predoi, D.; et al. Giant magnetoresistive coatings using thermionic vacuum arc technology. Czechoslov. J. Phys. 2006, 5, B16–B20. [Google Scholar] [CrossRef]
- Mustata, I.; Lungu, C.P.; Lungu, A.M.; Zaroski, V.; Blideran, M.; Ciupina, V. Giant magneto-resistive granular layers deposited by TVA method. Vacuum 2004, 76, 131–134. [Google Scholar] [CrossRef]
- Mustata, I.; Anghel, A.; Lungu, C.P.; Pompilian, O.; Kuncser, V.; Schinteie, G. Tunneling magneto-resistance granular thin films deposited by thermo-ionic vacuum arc technique. J. Optoelectron. Adv. Mater. 2007, 9, 3816–3820. [Google Scholar] [CrossRef]
- Perincek, F.; Erturk, K.; Aykol, M.; Kucuk, I.; Akdeniz, M.V. Effect of Thickness on Magnetic Properties of Fe36Co36B19:2Si4:8Mo2W2 Thin Film Prepared by Thermionic Vacuum Arc. Acta Phys. Pol. A 2012, 121, 147–148. [Google Scholar] [CrossRef]
- Marin, I.; Ciupina, V.; Prodan, G.; Oancea-Stanescu, I.M. Analysis of grain size in MgO-Co multilayers using TEM investigations. Optoelectron. Adv. Mater. Rapid Commun. 2012, 6, 606–609. [Google Scholar]
- Ionescu, V.; Osiac, M.; Lungu, C.P.; Pompilian, O.G.; Jepu, I.; Mustata, I.; Iacobescu, G.E. Morphological and structural investigations of Co–MgF2 granular thin films grown by thermionic vacuum arc. Thin Solid Films 2010, 518, 3945–3948. [Google Scholar] [CrossRef]
- Bounour-Bouzamouche, W.; Chérif, S.M.; Farhat, S.; Roussigné, Y.; Lungu, C.P.; Mazaleyrat, F.; Guerioune, M. Lithography-free synthesis of nanostructured cobalt on Si (111) surfaces: Structural and magnetic properties. EPJ Web Conf. 2014, 75, 05012. [Google Scholar] [CrossRef]
- Bounour-Bouzamouche, W.; Chérif, S.M.; Farhat, S.; Roussigné, Y.; Tallaire, A.; Gicquel, A.; Lungu, C.P.; Guerioun, M. Structural and magnetic properties of cobalt nanostructures on SiO2/Si(1 1 1) substrates. Appl. Surf. Sci. 2014, 320, 858–862. [Google Scholar] [CrossRef]
- Vladoiu, R.; Lungu, C.P.; Mustata, I.; Bursikova, V.; Bursik, J. Characterization by nanoindentation and Scanning Electron Microscopy of the spin valves structures prepared by Thermionic Vacuum Arc (TVA) method. J. Optoelectron. Adv. Mater. 2007, 9, 1087–1090. [Google Scholar]
- Kuncser, V.; Valeanu, M.; Schinteie, G.; Filoti, G.; Mustata, I.; Lungu, C.P.; Anghel, A.; Chiriac, H.; Vladoiu, R.; Bartolome, J. Exchange bias and spin valve systems with Fe–Mn antiferromagnetic pinning layers, obtained by the thermo-ionic vacuum arc method. J. Magn. Magn. Mater. 2008, 320, e226–e230. [Google Scholar] [CrossRef]
- Ilie, D.; Răsleanu, D.; Ionescu, V.; Mocanu, V.; Mureşan, M.G.; Oancea-Stănescu, I.M.; Ciupină, V.; Prodan, G.; Vasile, E.; Mustaţă, I.; et al. Preparation and characterization of Copper/Nikel nanostructurated multilayers using thermionic vacuum arc method. J. Optoelectron. Adv. Mater. 2010, 35, 839–843. [Google Scholar]
- Răsleanu, D.; Ilie, D.; Ionescu, V.; Mocanu, V.; Mureşan, M.G.; Oancea-Stănescu, I.M.; Ciupină, V.; Prodan, G.; Vasile, E.; Mustaţă, I.; et al. Thermionic vacuum Arc deposited Cu and Co nanostructured multilayers: Synthesis and characterization. J. Optoelectron. Adv. Mater. 2010, 12, 834–838. [Google Scholar]
- Mustata, I.; Porosnicu, C.; Jepu, I.; Lungu, C.P. Simultaneous and Alternate Thin Film Depositions by Thermionic Vacuum Arc (TVA) Method. Rom. J. Phys. 2015, 60, 1525–1535. [Google Scholar]
- Comşa, S.; Pacioga, A.; Gheorghiu, D. Determination of Mechanical Properties of Nanostructured Materials Used for Dental Implants. In Proceedings of the 1st International Conference on Innovations, Recent Trends and Challenges in Mechatronics, Mechanical Engineering and New High-Tech Products Development, MECAHITECH’09, Bucharest, Romania, 8–9 October 2009; pp. 126–135. [Google Scholar]
- Prodan, M.; Stanescu, I.; Ciupina, V.; Gheorghiu, D.; Stanca, C.; Eugeniu, V.; Prodan, G. Nanostructured thin films for prosthetic dentistry applications. Rom. Biotechnol. Lett. 2010, 15, 109–116. [Google Scholar]
- Ionescu, V.; Lungu, C.P.; Osiac, M.; Ciupină, V. Silver Containing Carbon Amorphous Nanocomposite Films Deposited by Termionic Vacuum Arc Technique. Rom. J. Phys. 2010, 55, 119–126. [Google Scholar]
- Pat, Z.; Sanci, Ö.; Yüksel, H.; Pat, S. Antibacterial Properties of Nano-Layered Au, Ag and Al Film Coatings on Flexible Organic Substrates. Asian J. Chem. 2014, 26, 6015–6017. [Google Scholar] [CrossRef]
- Mazare, A.; Anghel, A.; Surdu-Bob, C.; Totea, G.; Demetrescu, I.; Ionita, D. Silver doped diamond-like carbon antibacterial and corrosion resistance coatings on titanium. Thin Solid Films 2018, 657, 16–23. [Google Scholar] [CrossRef]
- Calenic, B.; Greabu, M.; Caruntu, C.; Nicolescu, M.I.; Moraru, L.; Surdu-Bob, C.C.; Badulescu, M.; Anghel, A.; Logofatu, C.; Boda, D. Oral keratinocyte stem cells behavior on diamond like carbon films. Rom. Biotechnol. Lett. 2016, 21, 11914–11922. [Google Scholar]
- Stefan-van Staden, R.I.; Gugoaşă, L.A.; Badulescu, M.; Surdu-Bob, C.C. Novel textile material based disposable sensors for biomedical analysis. RSC Adv. 2015, 5, 45545. [Google Scholar] [CrossRef]
- Stefan-van Staden, R.I.; Moldoveanua, I.; Surdu-Bob, C.C.; Badulescu, M.; Frederick van Stadena, J. Carbon Modified Paper Based Sensors. J. Electrochem. Soc. 2015, 162, B360–B362. [Google Scholar] [CrossRef]
- Kuwata, N.; Iwagami, N.; Yoshinari, T.; Matsuda, Y.; Kawamura, J. Characterization of Thin-Film Lithium Batteries with Stable Thin-Film Li3PO4 Solid Electrolytes Fabricated by ArF Excimer Laser Deposition. J. Electrochem. Soc. 2010, 157, A521–A527. [Google Scholar] [CrossRef]
- Pat, S.; Yudar, H.H.; Korkmaz, Ş.; Özen, S.; Mohammadigharehbagh, R.; Pat, Z. Transparent nano layered Li3PO4 coatings on bare and ITO coated glass by thermionic vacuum arc method. J. Mater. Sci. Mater. Electron. 2017, 28, 19010–19016. [Google Scholar] [CrossRef]
- Pat, S.; Ozen, S.; Senay, V.; Korkmaz, S.; Pat, Z. Solid State Battery Manufacturing with Thermionic Vacuum Arc and RF Sputtering. In Proceedings of the 42nd IEEE International Conference on Plasma Sciences (ICOPS), Belek, Turkey, 24–28 May 2015. [Google Scholar]
- Pat, S.; Özen, S.; Senay, V.; Korkmaz, S. Optical and Surface Properties of Optically Transparent Li3PO4 Solid Electrolyte Layer for Transparent Solid Batteries. Scanning 2016, 38, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Özen, S.; Korkmaz, Ş.; Pat, S.; Yudar, H.H. A new technique for transparent solid state Li3PO4 electrolyte layer growth: Thermionic vacuum arc technique. J. Mater. Sci. Mater. Electron. 2017, 28, 11557–11561. [Google Scholar] [CrossRef]
- Özen, S.; Pat, S.; Yudar, H.H.; Korkmaz, Ş.; Pat, Z. An investigation on the half-cell production for transparent secondary type solid-state batteries. Vacuum 2018, 153, 112–116. [Google Scholar] [CrossRef]
- Petrăşescu, L.; Ciupină, V.; Tutun, Ş.G.; Vlădoiu, R.; Prodan, G.; Poroşnicu, C.; Vasile, E.; Prioteasa, I.; Manu, R. Carbon—Platinum nanostructured catalysts for hydrogen fuel cells. J. Nanoelectron. Optoelectron. 2015, 17, 1464–1470. [Google Scholar]
- Petrăşescu, L.; Ciupină, V.; Prodan, G.; Poroşnicu, C.; Vasile, E.; Prioteasa, I.; Manu, R. PtNiPd thin films obtained by Thermionic Vacuum Arc Method: Synthesis and characterization. J. Optoelectron. Adv. Mater. 2018, 20, 196–200. [Google Scholar]
- Wang, Y.; Ohnuki, S.; Shigenari, H.; Narita, T. Submicron Structure of Rhenium-Base Diffusion Barrier Coating Layer on a Nickel-Base Superalloy. Mater. Trans. 2007, 48, 526–530. [Google Scholar] [CrossRef][Green Version]
- Lungu, M.; Dobrea, C.; Craciunescu, T.; Tiseanu, I.; Porosnicu, C.; Jepu, I.; Mustata, I. Mixed Film Coatings Analyzed by Micro X-Ray Fluorescence Method. Dig. J. Nanomater. Biostruct. 2014, 9, 899–906. [Google Scholar]
- Musa, G.; Vladoiu, R.; Ciupina, V.; Lungu, C.P.; Mustata, I.; Pat, S.; Akan, T.; Ekem, N. Characteristics of boron thin films obtained by TVA technology. J. Optoelectron. Adv. Mater. 2006, 8, 617–620. [Google Scholar]
- Balbag, M.Z.; Pat, S.; Cenik, M.I.; Akan, T.; Ekem, N.; Musa, G. Boron evaporation and related difficulties. J. Optoelectron. Adv. Mater. 2007, 9, 858–861. [Google Scholar]
- Ekem, N.; Akan, T.; Pat, S.; Balbag, M.Z.; Cenik, M.I.; Karakas, E.; Vladoiu, R.; Musa, G. Investigation of Properties of Boron Thin Film Deposited By Thermionic Vacuum Arc Technology. AIP Conf. Proc. 2007, 899, 699. [Google Scholar] [CrossRef]
- Okur, S.; Kalkanci, M.; Pat, S.; Ekem, N.; Akan, T.; Balbag, Z.; Musa, G.; Tanoglu, M. MgB2 superconducting thin films sequentially fabricated using DC magnetron sputtering and thermionic vacuum arc method. Phys. C 2007, 466, 205–208. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vladoiu, R.; Tichý, M.; Mandes, A.; Dinca, V.; Kudrna, P. Thermionic Vacuum Arc—A Versatile Technology for Thin Film Deposition and Its Applications. Coatings 2020, 10, 211. https://doi.org/10.3390/coatings10030211
Vladoiu R, Tichý M, Mandes A, Dinca V, Kudrna P. Thermionic Vacuum Arc—A Versatile Technology for Thin Film Deposition and Its Applications. Coatings. 2020; 10(3):211. https://doi.org/10.3390/coatings10030211
Chicago/Turabian StyleVladoiu, Rodica, Milan Tichý, Aurelia Mandes, Virginia Dinca, and Pavel Kudrna. 2020. "Thermionic Vacuum Arc—A Versatile Technology for Thin Film Deposition and Its Applications" Coatings 10, no. 3: 211. https://doi.org/10.3390/coatings10030211
APA StyleVladoiu, R., Tichý, M., Mandes, A., Dinca, V., & Kudrna, P. (2020). Thermionic Vacuum Arc—A Versatile Technology for Thin Film Deposition and Its Applications. Coatings, 10(3), 211. https://doi.org/10.3390/coatings10030211





