Use of the Sol–Gel Method for the Preparation of Coatings of Titanium Substrates with Hydroxyapatite for Biomedical Application
Abstract
1. Introduction
2. Materials and Methods
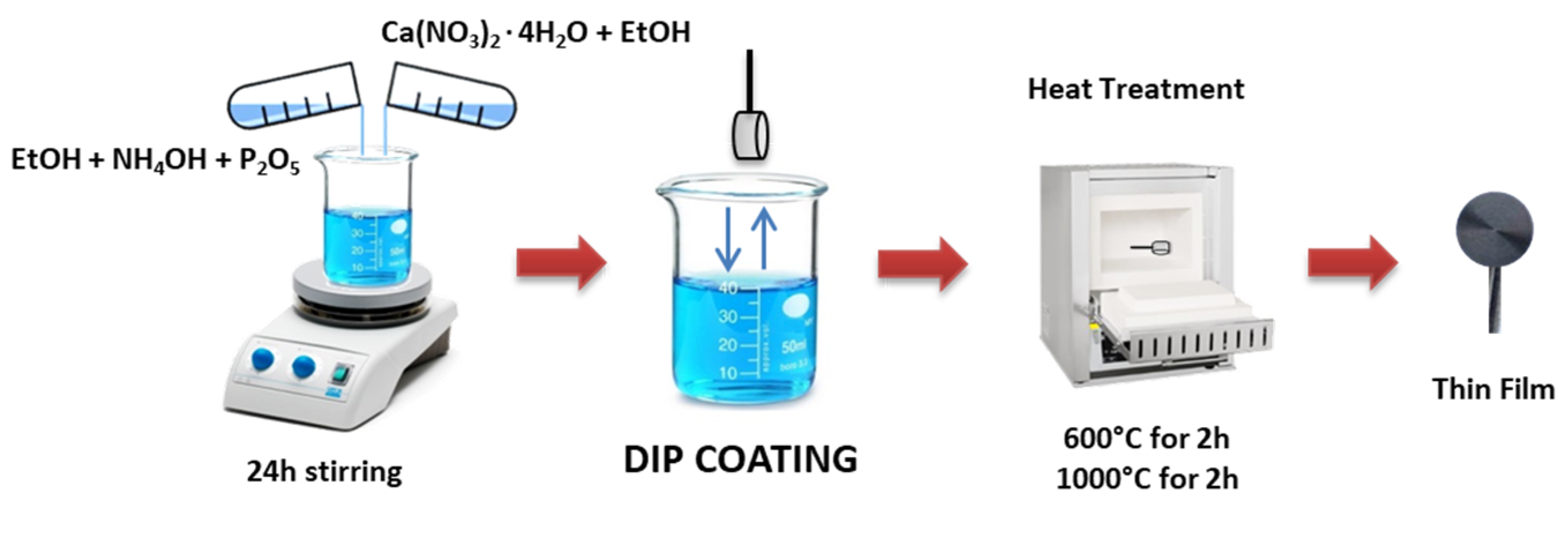
2.1. Sol–Gel Synthesis
2.2. Coating Procedure
2.3. Chemical and Morphological Characterization
2.4. In Vitro Bioactivity
2.5. WST-8 Assay
3. Results
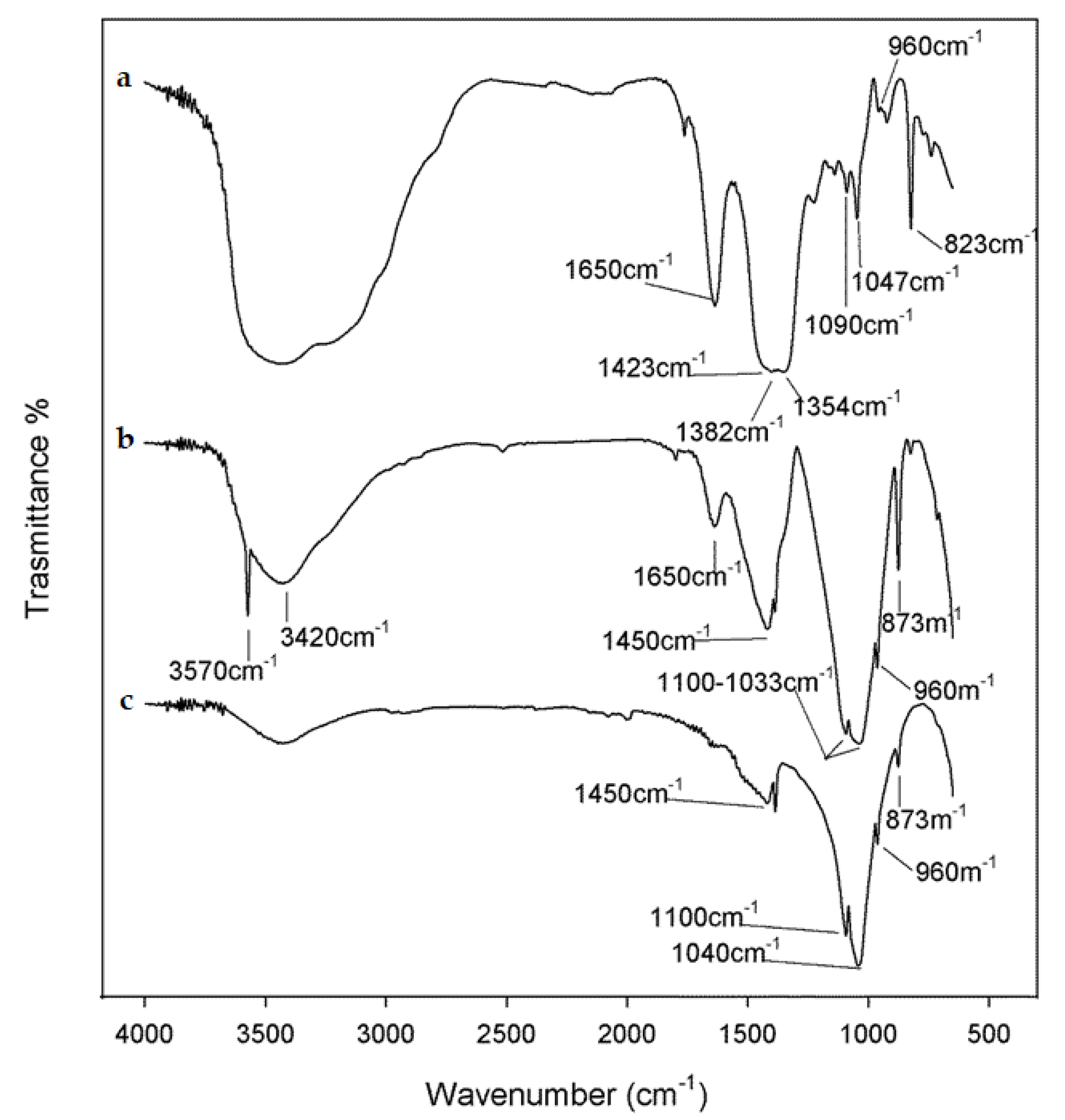
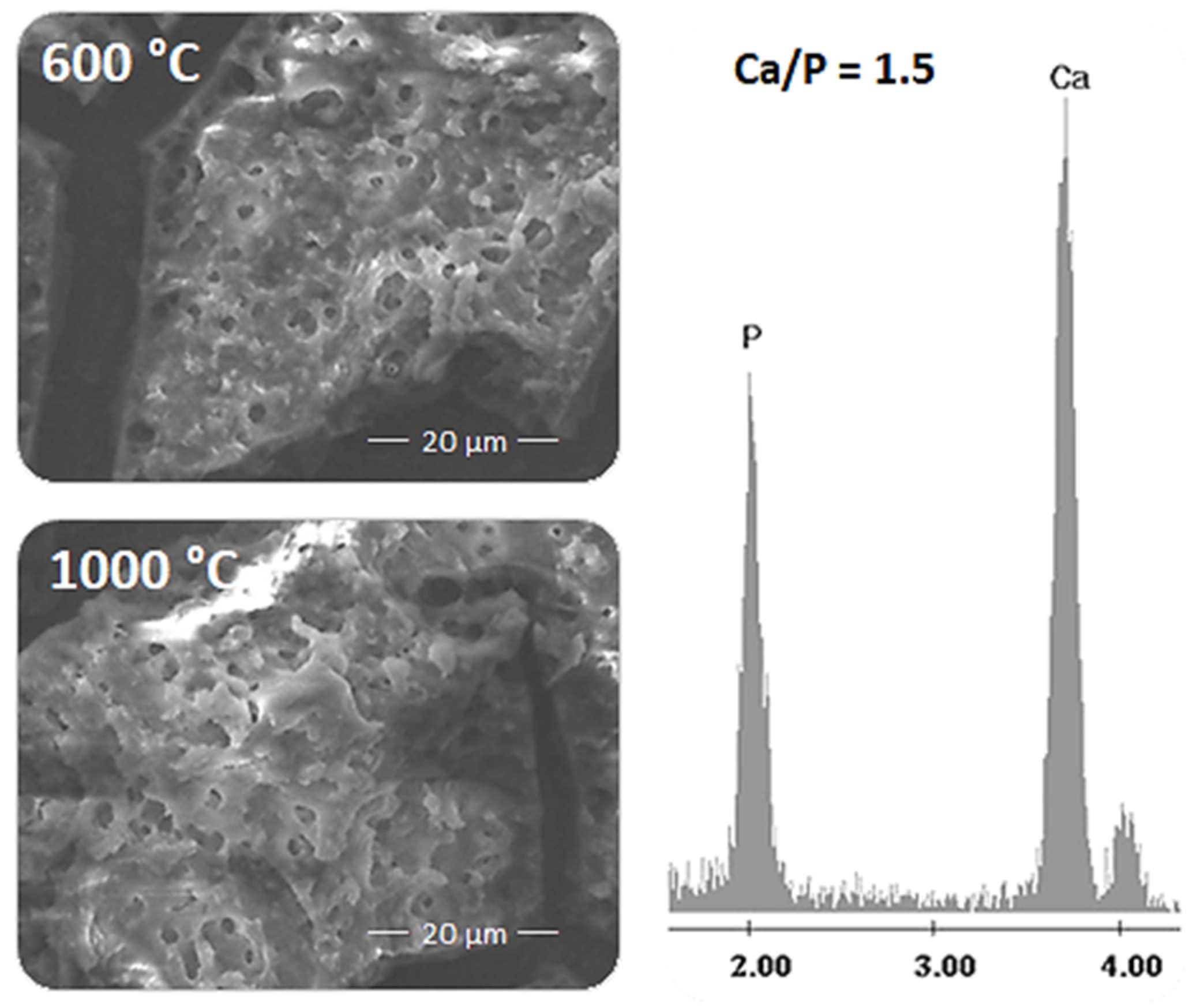
3.1. Spectroscopic and Structural Characterization
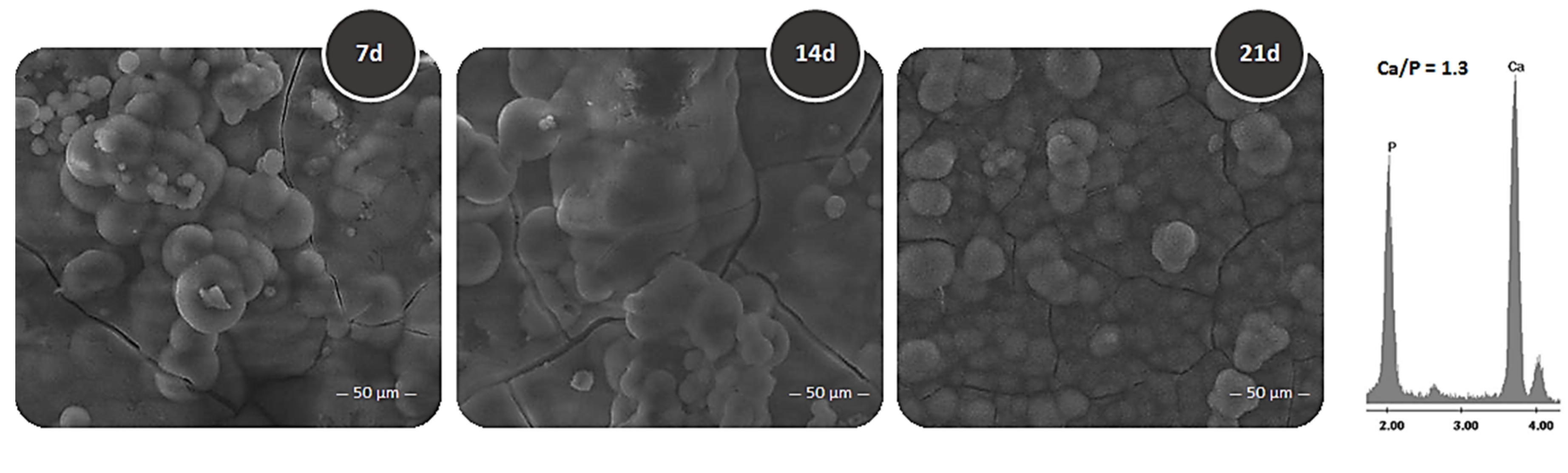
3.2. Bioactivity Test
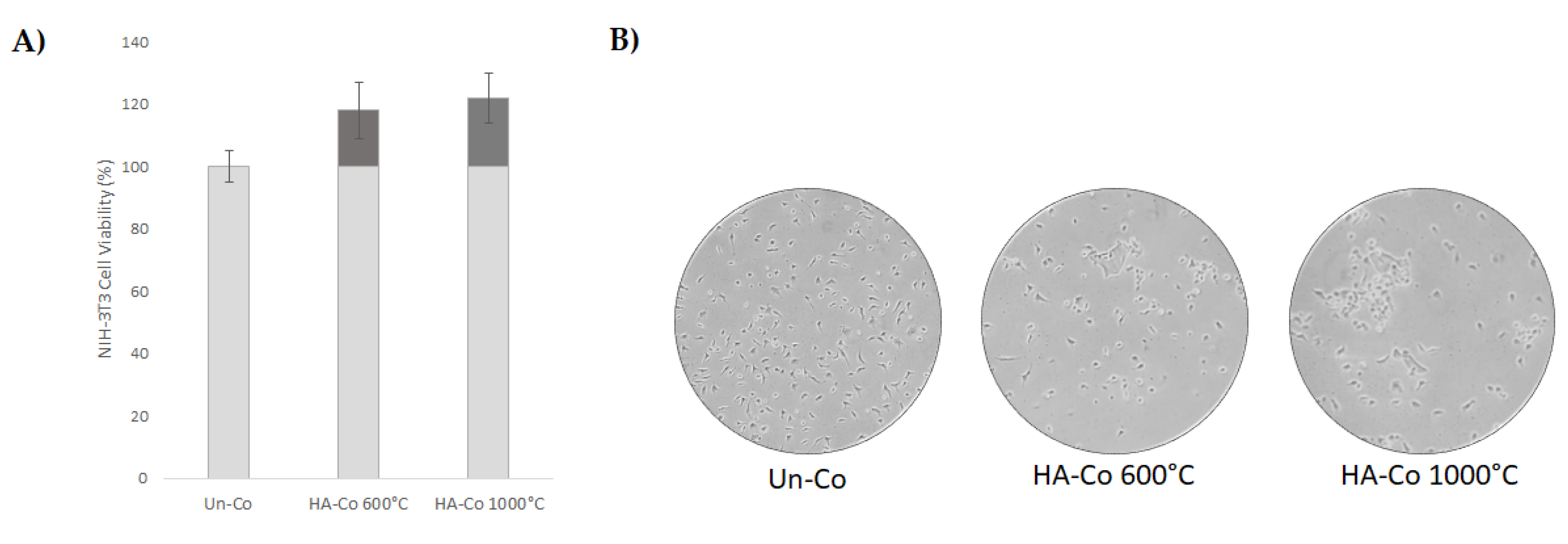
3.3. Cytotoxicity Assessment
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Langer, R.; Vacanti, J.P. Tissue engineering. Mol. Ther. 2000, 1, 12. [Google Scholar] [CrossRef] [PubMed]
- Chapekar, M.S. Tissue engineering: Challenges and opportunities. J. Biomed. Mater. Res. 2000, 53, 617–620. [Google Scholar] [CrossRef]
- Cheung, H.Y.; Lau, K.T.; Lu, T.P.; Hui, D. A critical review on polymer-based bio-engineered materials for scaffold development. Composites Part B Eng. 2007, 38, 291–300. [Google Scholar] [CrossRef]
- Catauro, M.; Bollino, F.; Papale, F.; Piccolella, S.; Pacifico, S. Sol–gel synthesis and characterization of SiO2/PCL hybrid materials containing quercetin as new materials for antioxidant implants. Mater. Sci. Eng. C 2016, 58, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Catauro, M.; Bollino, F.; Papale, F.; Mozetic, P.; Rainer, A.; Trombetta, M. Biological response of human mesenchymal stromal cells to titanium grade 4 implants coated with PCL/ZrO2 hybrid materials synthesized by sol–gel route: In vitro evaluation. Mater. Sci. Eng. C 2014, 45, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Catauro, M.; Barrino, F.; Bononi, M.; Colombini, E.; Giovanardi, R.; Veronesi, P.; Tranquillo, E. Coating of titanium substrates with ZrO2 and ZrO2-SiO2 composites by sol-gel synthesis for biomedical applications: Structural characterization, mechanic and corrosion behavior. Coatings 2019, 9, 200. [Google Scholar] [CrossRef]
- Catauro, M.; Bollino, F.; Renella, R.; Papale, F. Sol–gel synthesis of SiO2–CaO–P2O5 glasses: Influence of the heat treatment on their bioactivity and biocompatibility. Ceram. Int. 2015, 41, 12578–12588. [Google Scholar] [CrossRef]
- Cai, S.; Wang, Y.; Lv, H.; Peng, Z.; Yao, K. Synthesis of carbonated hydroxyapatite nanofibers by mechanochemical methods. Ceram. Int. 2005, 31, 135–138. [Google Scholar]
- Balazic, M.; Kopac, J.; Jackson, M.J.; Ahmed, W. Titanium and titanium alloy applications in medicine. Int. J. Nano Biomater. 2007, 1, 3–34. [Google Scholar] [CrossRef]
- Jokstad, A.; Braegger, U.; Brunski, J.B.; Carr, A.B.; Naert, I.; Wennerberg, A. Quality of dental implants. Int. Dent. J. 2003, 53, 409–443. [Google Scholar] [CrossRef]
- Catauro, M.; Bollino, F.; Tranquillo, E.; Tuffi, R.; Dell’Era, A.; Vecchio Ciprioti, S. Morphological and thermal characterization of zirconia/hydroxyapatite composites prepared via sol-gel for biomedical applications. Ceram. Int. 2019, 45, 2835–2845. [Google Scholar] [CrossRef]
- Pourhashem, S.; Afshar, A. Double layer bioglass-silica coatings on 316L stainless steel by sol–gel method. Ceram. Int. 2014, 40, 993–1000. [Google Scholar] [CrossRef]
- Yoshimura, M.; Suda, H.; Okamoto, K.; Ioku, K. Hydrothermal synthesis of biocompatible whiskers. J. Mater. Sci. 1994, 29, 3399–3402. [Google Scholar] [CrossRef]
- Silva, C.C.; Pinheiro, A.G.; Miranda, M.A.R.; Góes, J.C.; Sombra, A.S.B. Structural properties of hydroxyapatite obtained by mechanosynthesis. Solid State Sci. 2003, 5, 553–558. [Google Scholar] [CrossRef]
- Saeri, M.R.; Afshar, A.; Ghorbani, M.; Ehsani, N.; Sorrell, C.C. The wet precipitation process of hydroxyapatite. Mater. Lett. 2003, 57, 4064–4069. [Google Scholar] [CrossRef]
- Shih, W.J.; Chen, Y.F.; Wang, M.C.; Hon, M.H. Crystal growth and morphology of the nano-sized hydroxyapatite powders synthesized from CaHPO4· 2H2O and CaCO3 by hydrolysis method. J. Cryst. Growth 2004, 270, 211–218. [Google Scholar] [CrossRef]
- Kim, I.S.; Kumta, P.N. Sol–gel synthesis and characterization of nanostructured hydroxyapatite powder. Mater. Sci. Eng. B 2004, 111, 232–236. [Google Scholar] [CrossRef]
- Klein, L.C. Sol-gel Technology for Thin Films, Fibers, Preforms, Electronics, and Specialty Shapes; William Andrew Publishing: Norwick, NY, USA, 1988. [Google Scholar]
- Zhou, Z.; Chen, J.; Xiang, L.J.; Xu, Y.; Yang, P.; Li, J.A.; Wu, J.-J.; Huang, N. Fabrication of 3D TiO2 micromesh on Silicon surface and its effects on platelet adhesion. Mater. Lett. 2014, 132, 149–152. [Google Scholar] [CrossRef]
- Roest, R.; Latella, B.A.; Heness, G.; Ben-Nissan, B. Adhesion of sol–gel derived hydroxyapatite nanocoatings on anodised pure titanium and titanium (Ti6Al4V) alloy substrates. Surf. Coat. Technol. 2011, 205, 3520–3529. [Google Scholar] [CrossRef]
- Anjaneyulu, U.; Priyadarshini, B.; Arul Xavier Stango, S.; Chellappa, M.; Geetha, M.; Vijayalakshmi, U. Preparation and characterisation of sol–gel-derived hydroxyapatite nanoparticles and its coatings on medical grade Ti-6Al-4V alloy for biomedical applications Materials Technology. Adv. Perform. Mater. 2017, 32, 800. [Google Scholar] [CrossRef]
- Liu, W.; Liu, S.; Wang, L. Surface Modification of Biomedical Titanium Alloy: Micromorphology, Microstructure Evolution and Biomedical Applications. Coatings 2019, 9, 249. [Google Scholar] [CrossRef]
- Choi, A.H. Sol-gel production of bioactive nanocoatings for medical applications. Part II: Current research and development. Nanomedicine 2007, 2, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Venkateswarlu, K.; Bose, A.C.; Rameshbabu, N. X-ray peak broadening studies of nanocrystalline hydroxyapatite by Williamson–Hall analysis. Phys. B Condens. Matter 2010, 405, 4256–4261. [Google Scholar] [CrossRef]
- Irish, D.E.; Walrafen, G.E. Raman and infrared spectral studies of aqueous calcium nitrate solutions. J. Chem. Phys. 1967, 46, 378–384. [Google Scholar] [CrossRef]
- Tiwari, B.; Dixit, A.; Kothiyal, G.P.; Pandey, M.; Deb, S.K. Preparation and characterization of phosphate glasses containing titanium. Barc. Newsl. 2007, 285, 167. [Google Scholar]
- Nguyen, K.; Garcia, A.; Sani, M.; Diaz, D.; Dubey, V.; Clayton, D.; Dal Poggetto, G.; Cornelius, F.; Payne, R.J.; Separovic, F.; et al. Interaction of N-terminal peptide analogues of the Na+, K+-ATPase with membranes. BBA Biomembr. 2018, 1860, 1282–1291. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catauro, M.; Barrino, F.; Blanco, I.; Piccolella, S.; Pacifico, S. Use of the Sol–Gel Method for the Preparation of Coatings of Titanium Substrates with Hydroxyapatite for Biomedical Application. Coatings 2020, 10, 203. https://doi.org/10.3390/coatings10030203
Catauro M, Barrino F, Blanco I, Piccolella S, Pacifico S. Use of the Sol–Gel Method for the Preparation of Coatings of Titanium Substrates with Hydroxyapatite for Biomedical Application. Coatings. 2020; 10(3):203. https://doi.org/10.3390/coatings10030203
Chicago/Turabian StyleCatauro, Michelina, Federico Barrino, Ignazio Blanco, Simona Piccolella, and Severina Pacifico. 2020. "Use of the Sol–Gel Method for the Preparation of Coatings of Titanium Substrates with Hydroxyapatite for Biomedical Application" Coatings 10, no. 3: 203. https://doi.org/10.3390/coatings10030203
APA StyleCatauro, M., Barrino, F., Blanco, I., Piccolella, S., & Pacifico, S. (2020). Use of the Sol–Gel Method for the Preparation of Coatings of Titanium Substrates with Hydroxyapatite for Biomedical Application. Coatings, 10(3), 203. https://doi.org/10.3390/coatings10030203








