Surface Functionalization of Cotton and PC Fabrics Using SiO2 and ZnO Nanoparticles for Durable Flame Retardant Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
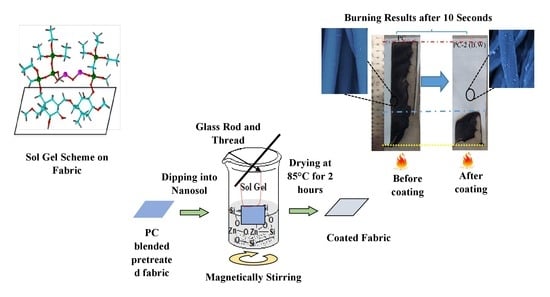
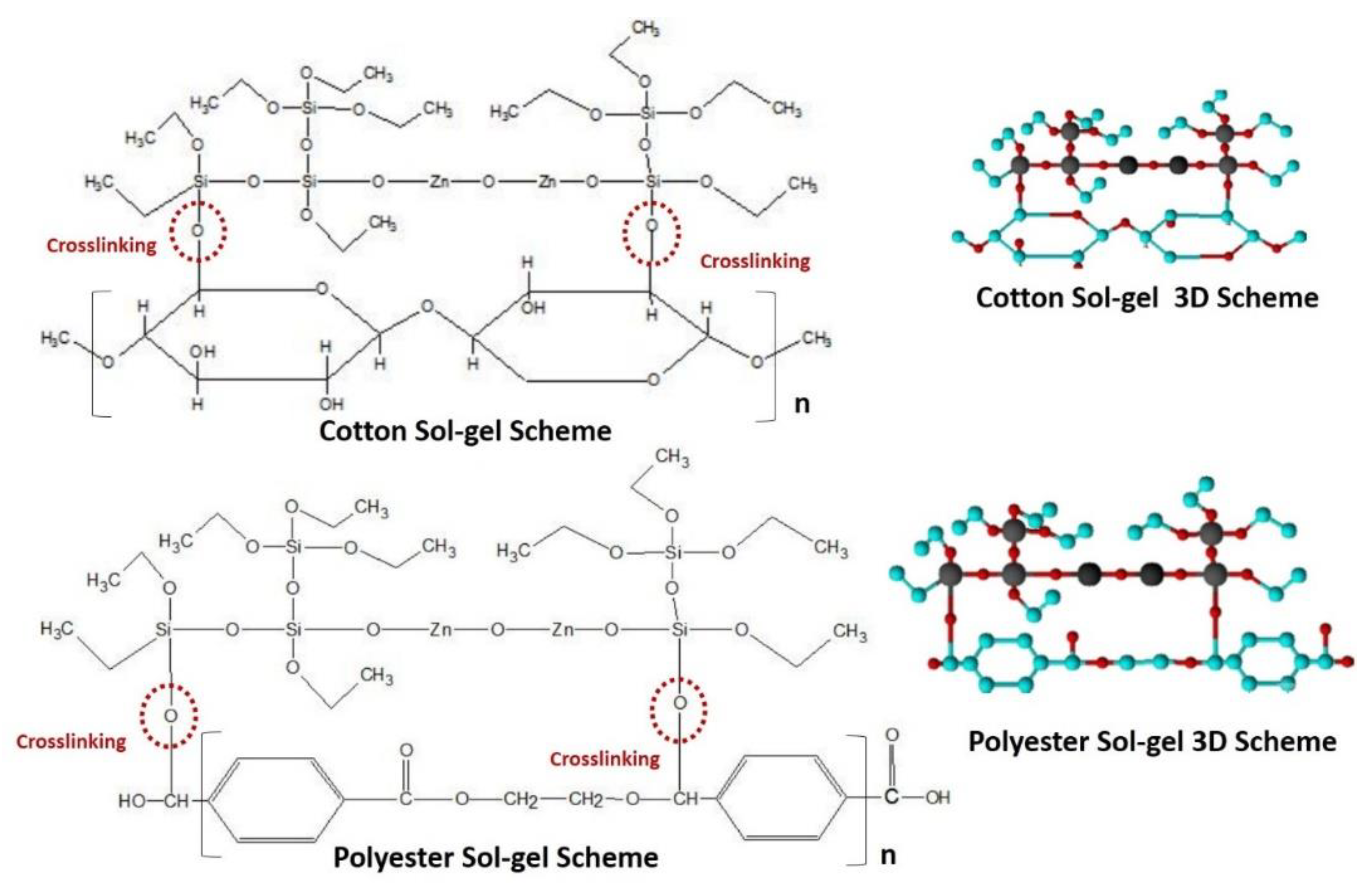
2.2. Sol-Gel Process and Preparation
2.3. Characterization
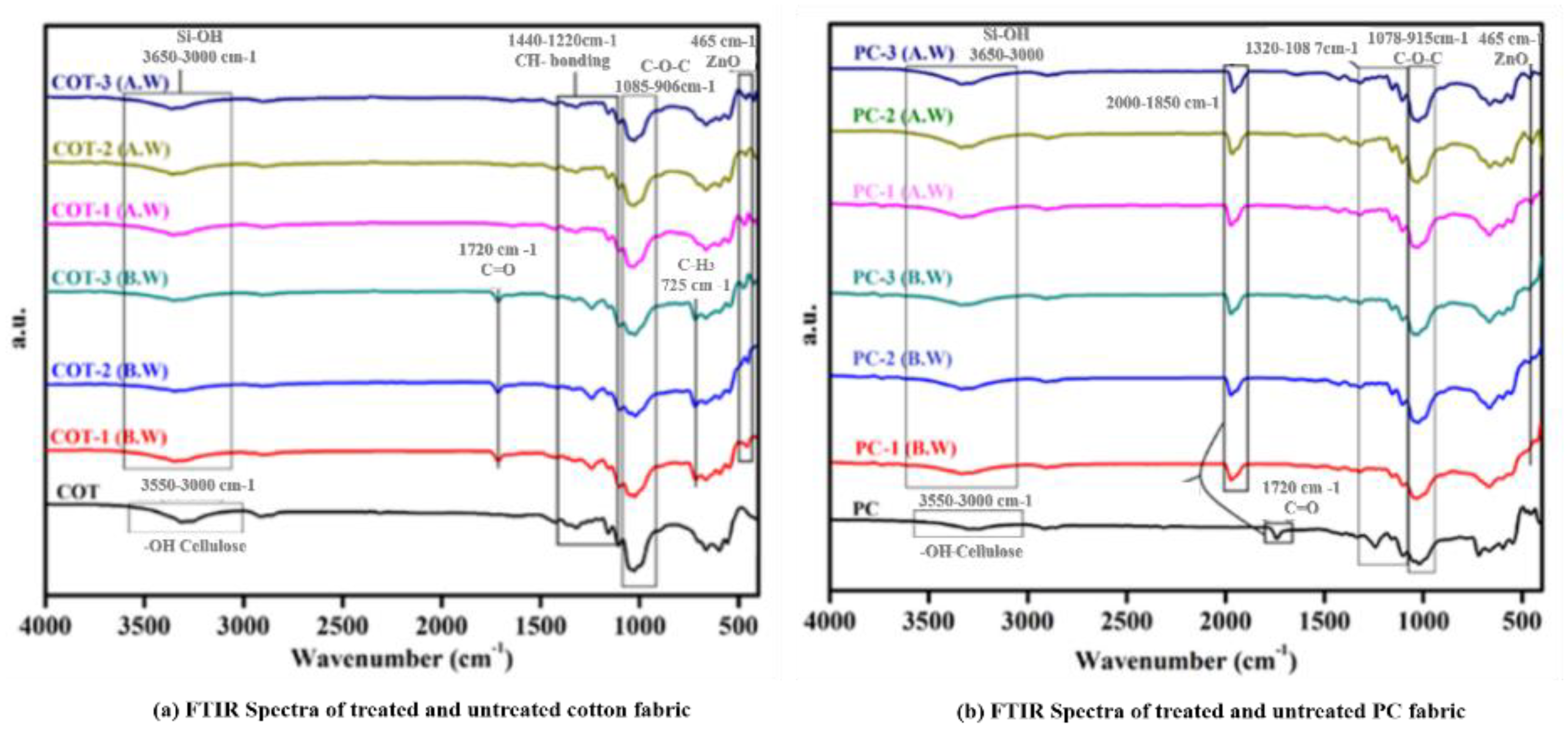
2.3.1. FTIR Analysis
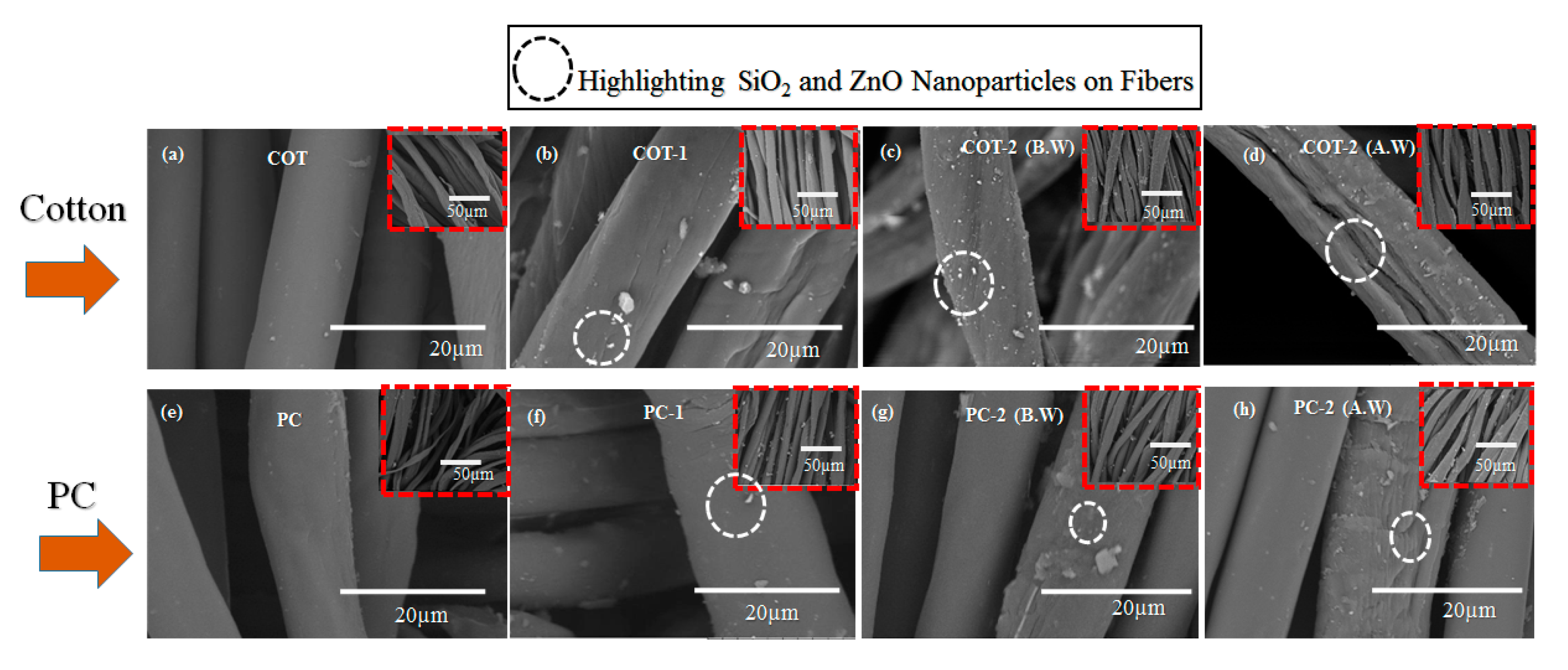
2.3.2. Morphological Structures
2.3.3. Thermal Analysis
2.3.4. Flammability Analysis
2.4. Physical Properties
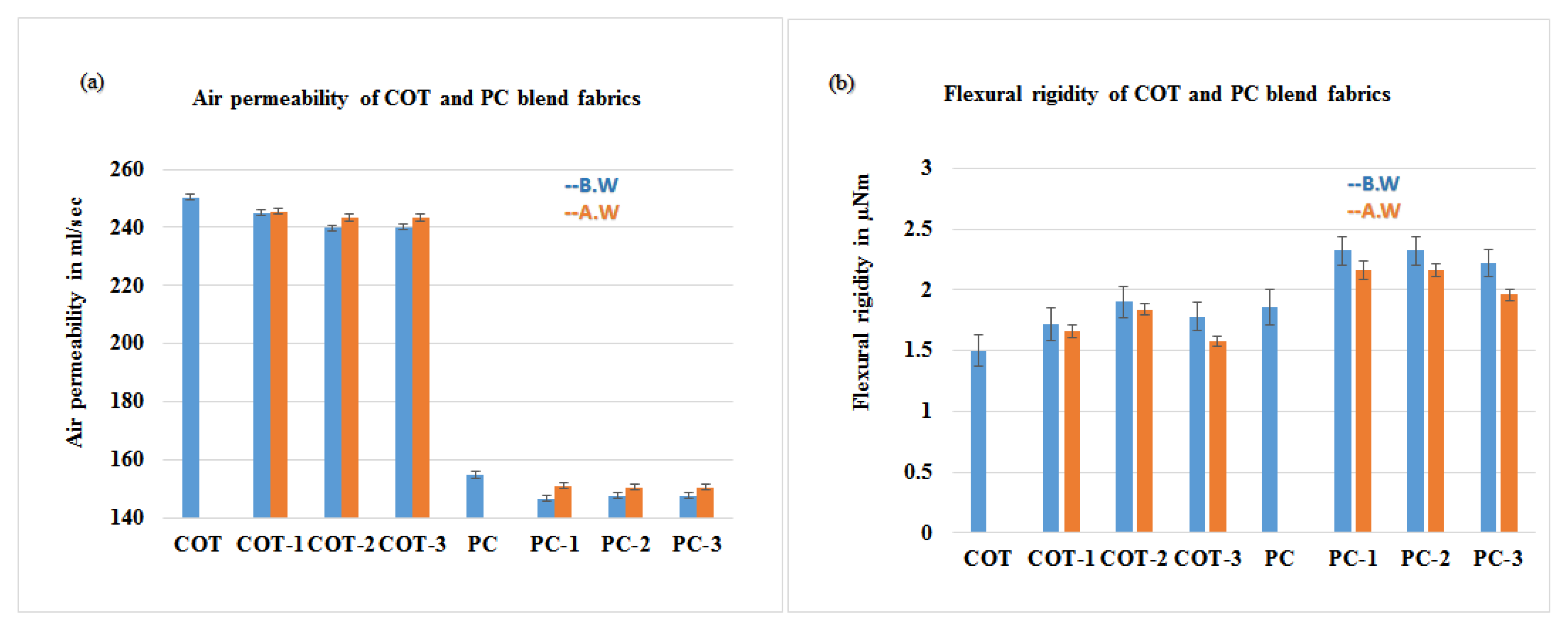
2.4.1. Air Permeability Analysis
2.4.2. Flexural Analysis
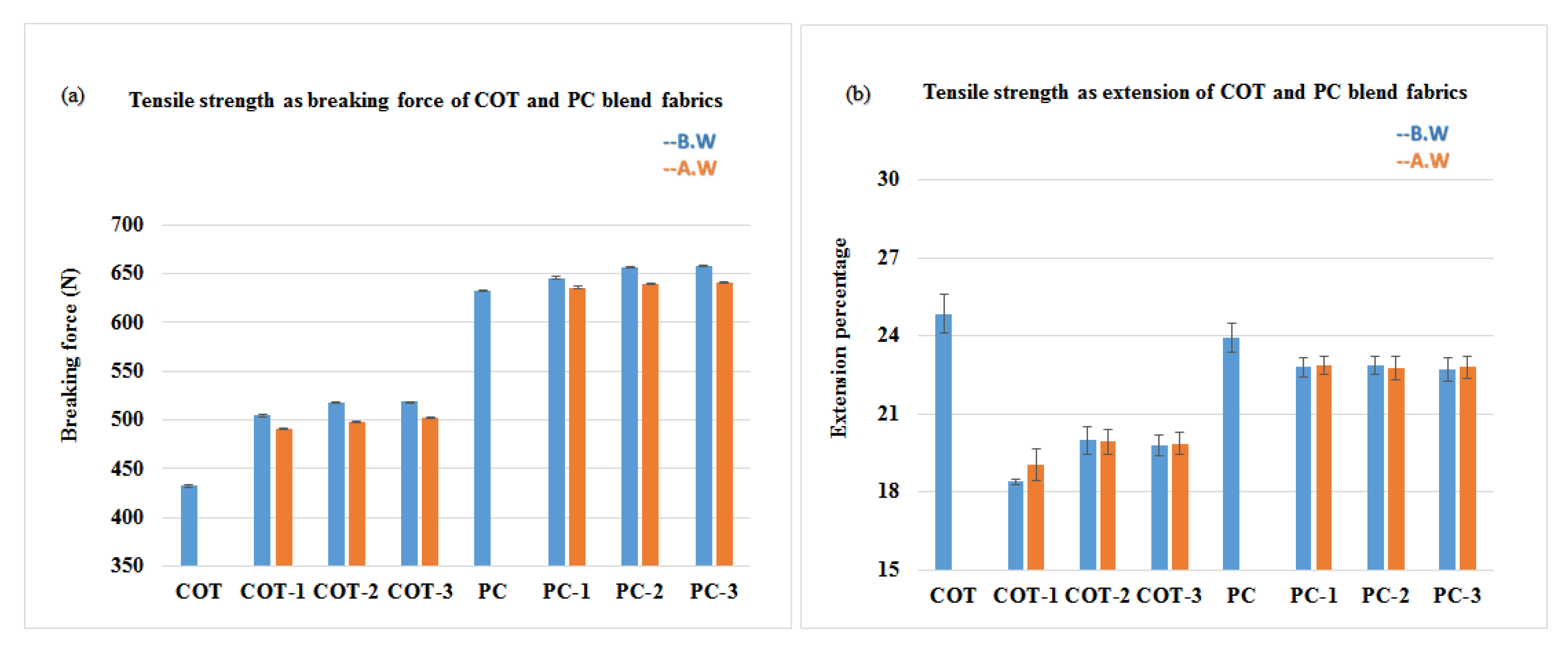
2.4.3. Tensile Strength Analysis
2.5. Durability Analysis
3. Results and Discussion
3.1. FTIR Results
3.2. Morphological Structure
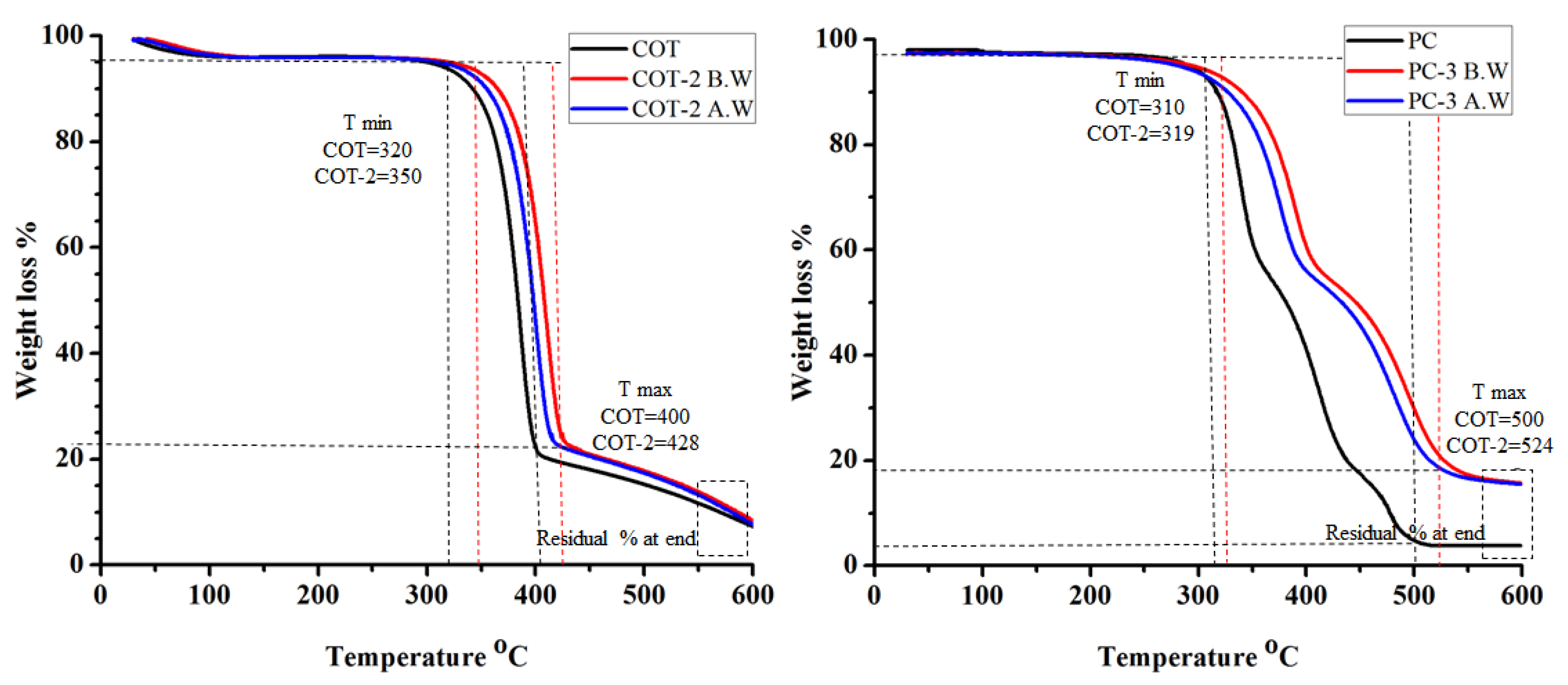
3.3. Thermal Analysis
3.3.1. Thermal Degradation
3.3.2. Flammability at 45°
3.4. Assessment of the Physical Properties
4. Conclusions
- The flame retardancy was more improved with the addition of silica nanoparticles than zinc oxide in both COT samples and PC samples.
- The blending of SiO2 with ZnO showed more impact than their separate usage on both COT and PC samples.
- The flame retardancy was more improved on PC fabric samples than the COT fabric samples. FTIR and SEM revealed that these coatings were durable and showed an insignificant decrease after five industrial washes.
- The physical properties, i.e., strength and flexural rigidity of treated fabrics, also increased due to the crosslinking of nanoparticles, which results in decreased tensile force at extension.
- The air permeability was insignificantly decreased since the nanoparticles also resist the passage of air through them.
Author Contributions
Funding
Conflicts of Interest
References
- Mahltig, B.; Haufe, H.; Böttcher, H. Functionalisation of textiles by inorganic sol–gel coatings. J. Mater. Chem. 2005, 15, 4385. [Google Scholar] [CrossRef]
- Malucelli, G. Surface-Engineered Fire Protective Coatings for Fabrics through Sol-Gel and Layer-by-Layer Methods: An Overview. Coatings 2016, 6, 33. [Google Scholar] [CrossRef]
- Miraftab, M. Comparison of air permeability and moisture management properties of jersey, interlock and pique knitted fabrics. J. Text. Inst. 2012, 2, 1–5. [Google Scholar]
- Zhu, Q.; Gao, Q.; Guo, Y.; Yang, C.Q.; Shen, L. Modified Silica Sol Coatings for Highly Hydrophobic Cotton and Polyester Fabrics Using a One-Step Procedure. Ind. Eng. Chem. Res. 2011, 50, 5881–5888. [Google Scholar] [CrossRef]
- Anantha-Iyengar, G.; Shanmugasundaram, K.; Nallal, M.; Lee, K.P.; Whitcombe, M.J.; Lakshmi, D.; Sai-Anand, G. Functionalized conjugated polymers for sensing and molecular imprinting applications. Prog. Polym. Sci. 2019, 88, 1–129. [Google Scholar] [CrossRef]
- Islam, S.R.; Yu, W.; Naveed, T. Influence of silica aerogels on fabric structural feature for thermal isolation properties of weft-knitted spacer fabrics. J. Eng. Fibers Fabr. 2019, 14, 1558925019866446. [Google Scholar] [CrossRef]
- Rehman, F.; Sanbhal, N.; Naveed, T.; Farooq, A.; Wang, Y.; Wei, W. Antibacterial performance of Tencel fabric dyed with pomegranate peel extracted via ultrasonic method. Cellulose 2018, 25, 4251–4260. [Google Scholar] [CrossRef]
- Nallal, M.; Iyengar, G.A.; Pill-Lee, K. New Titanium Dioxide-Based Heterojunction Nanohybrid for Highly Selective Photoelectrochemical–Electrochemical Dual-Mode Sensors. Acs Appl. Mater. Interfaces 2017, 9, 37166–37183. [Google Scholar] [CrossRef]
- Muthuchamy, N.; Atchudan, R.; Edison, T.N.J.I.; Perumal, S.; Lee, Y.R. High-performance glucose biosensor based on green synthesized zinc oxide nanoparticle embedded nitrogen-doped carbon sheet. J. Electroanal. Chem. 2018, 816, 195–204. [Google Scholar] [CrossRef]
- Brancatelli, G.; Colleoni, C.; Massafra, M.; Rosace, G. Effect of hybrid phosphorus-doped silica thin films produced by sol-gel method on the thermal behavior of cotton fabrics. Polym. Degrad. Stab. 2011, 96, 483–490. [Google Scholar] [CrossRef]
- Alongi, J.; Ciobanu, M.; Malucelli, G. Thermal stability, flame retardancy and mechanical properties of cotton fabrics treated with inorganic coatings synthesized through sol–gel processes. Carbohydr. Polym. 2012, 87, 2093–2099. [Google Scholar] [CrossRef]
- Mostashari, S.M.; Moafi, H.F.; Mostashari, S.Z. TG comparison between the efficiency of deposited ammonium bromide and ammonium chloride on the flame-retardancy imparted to cotton fabric. J. Therm. Anal. Calorim. 2009, 96, 535–540. [Google Scholar] [CrossRef]
- A De Wit, C. An overview of brominated flame retardants in the environment. Chemosphere 2002, 46, 583–624. [Google Scholar] [CrossRef]
- Horrocks, A. Flame-retardant finishing of textiles. Rev. Prog. Coloration Relat. Top. 1986, 16, 62–101. [Google Scholar] [CrossRef]
- Xie, K.; Gao, A.; Zhang, Y. Flame retardant finishing of cotton fabric based on synergistic compounds containing boron and nitrogen. Carbohydr. Polym. 2013, 98, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Wawro, D. Novel method of preparing flame retardant cellulose-silicate fibres. Fibres Text. East. Eur. 2010, 18, 80. [Google Scholar]
- Erdem, N.; Cireli, A.A.; Erdogan, U.H. Flame retardancy behaviors and structural properties of polypropylene/nano-SiO2 composite textile filaments. J. Appl. Polym. Sci. 2009, 111, 2085–2091. [Google Scholar] [CrossRef]
- Hribernik, S.; Smole, M.S.; Kleinschek, K.S.; Bele, M.; Jamnik, J.; Gaberscek, M. Flame retardant activity of SiO2-coated regenerated cellulose fibres. Polym. Degrad. Stab. 2007, 92, 1957–1965. [Google Scholar] [CrossRef]
- Alongi, J.; Ciobanu, M.; Malucelli, G. Sol–gel treatments for enhancing flame retardancy and thermal stability of cotton fabrics: Optimisation of the process and evaluation of the durability. Cellulose 2011, 18, 167–177. [Google Scholar] [CrossRef]
- Visakh, P.; Yoshihiko, A. Flame Retardants: Polymer Blends, Composites and Nanocomposites; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Li, F.; Xing, Y.; Ding, X. Silica xerogel coating on the surface of natural and synthetic fabrics. Surf. Coat. Technol. 2008, 202, 4721–4727. [Google Scholar] [CrossRef]
- Muthuchamy, N.; Gopalan, A.; Lee, K.-P. A new facile strategy for higher loading of silver nanoparticles onto silica for efficient catalytic reduction of 4-nitrophenol. Rsc Adv. 2015, 5, 76170–76181. [Google Scholar] [CrossRef]
- Textor, T.; Bahners, T.; Schollmeyer, E. Surface modification of textile fabrics by coatings based on the sol-gel process. Melliand Text. Int. Text. Rep. 1999, 80, E229. [Google Scholar]
- Liu, C.; Xing, T.; Wei, B.; Chen, G. Synergistic Effects and Mechanism of Modified Silica Sol Flame Retardant Systems on Silk Fabric. Materials 2018, 11, 1842. [Google Scholar] [CrossRef] [PubMed]
- Alongi, J.; Tata, J.; Carosio, F.; Rosace, G.; Frache, A.; Camino, G. A comparative analysis of nanoparticle adsorption as fire-protection approach for fabrics. Polymers 2015, 7, 47–68. [Google Scholar] [CrossRef]
- Chiang, C.; Chang, R. Synthesis, characterization and properties of novel self-extinguishing organic–inorganic nanocomposites containing nitrogen, silicon and phosphorus via sol–gel method. Compos. Sci. Technol. 2008, 68, 2849–2857. [Google Scholar] [CrossRef]
- Ren, Y.; Huo, T.; Qin, Y.; Liu, X. Preparation of Flame Retardant Polyacrylonitrile Fabric Based on Sol-Gel and Layer-by-Layer Assembly. Materials 2018, 11, 483. [Google Scholar] [CrossRef] [PubMed]
- Alongi, J.; Han, Z.; Bourbigot, S. Intumescence: Tradition versus novelty. A comprehensive review. Prog. Polym. Sci. 2015, 51, 28–73. [Google Scholar] [CrossRef]
- Saleemi, S.; Malik, S.A.; Syed, U.; Tanwari, A. Investigation of Wash Durability of Silica Nanoparticle Coated 100% Cotton Reactive Dyed Fabric Treated by Sol-Gel Technique. J. Eng. Fibers Fabr. 2014, 9, 155892501400900402. [Google Scholar] [CrossRef]
- Alongi, J.; Ciobanu, M.; Malucelli, G. Novel flame retardant finishing systems for cotton fabrics based on phosphorus-containing compounds and silica derived from sol–gel processes. Carbohydr. Polym. 2011, 85, 599–608. [Google Scholar] [CrossRef]
- Yaman, N. Preparation and flammability properties of hybrid materials containing phosphorous compounds via sol-gel process. Fibers Polym. 2009, 10, 413–418. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, W.; Huang, J.; Lai, Y.-K.; Xing, T.; Chen, G.; Jin, W.; Liu, H.; Sun, B. Flame retardance and thermal stability of wool fabric treated by boron containing silica sols. Mater. Des. 2015, 85, 796–799. [Google Scholar] [CrossRef]
- Zhang, Q.-H.; Gu, J.; Chen, G.-Q.; Xing, T.-L. Durable flame retardant finish for silk fabric using boron hybrid silica sol. Appl. Surf. Sci. 2016, 387, 446–453. [Google Scholar] [CrossRef]
- Wang, S.; Sui, X.; Li, Y.; Li, J.; Xu, H.; Zhong, Y.; Zhang, L.; Mao, Z. Durable flame retardant finishing of cotton fabrics with organosilicon functionalized cyclotriphosphazene. Polym. Degrad. Stab. 2016, 128, 22–28. [Google Scholar] [CrossRef]
- Januszewski, R.; Dutkiewicz, M.; Maciejewski, H.; Marciniec, B. Synthesis and characterization of phosphorus-containing, silicone rubber based flame retardant coatings. React. Funct. Polym. 2018, 123, 1–9. [Google Scholar] [CrossRef]
- Xu, Y.-J.; Wang, J.; Tan, Y.; Qi, M.; Chen, L.; Wang, Y.-Z. A novel and feasible approach for one-pack flame-retardant epoxy resin with long pot life and fast curing. Chem. Eng. J. 2018, 337, 30–39. [Google Scholar] [CrossRef]
- Dutkiewicz, M.; Przybylak, M.; Januszewski, R.; Maciejewski, H. Synthesis and flame retardant efficacy of hexakis(3-(triethoxysilyl)propyloxy)cyclotriphosphazene/silica coatings for cotton fabrics. Polym. Degrad. Stab. 2018, 148, 10–18. [Google Scholar] [CrossRef]
- Przybylak, M.; Maciejewski, H.; Dutkiewicz, A. Preparation of highly hydrophobic cotton fabrics by modification with bifunctional silsesquioxanes in the sol-gel process. Appl. Surf. Sci. 2016, 387, 163–174. [Google Scholar] [CrossRef]
- Przybylak, M.; Maciejewski, H.; Dutkiewicz, A.; Dąbek, I.; Nowicki, M. Fabrication of superhydrophobic cotton fabrics by a simple chemical modification. Cellulose 2016, 23, 2185–2197. [Google Scholar] [CrossRef]
- Vihodceva, S.; Kukle, S. Cotton textile surface investigation before and after deposition of the zno coating by sol-gel method. Журнал нанo-та електрoннoї фізики 2013. [Google Scholar]
- Alongi, J.; Ciobanu, M.; Tata, J.; Carosio, F.; Malucelli, G. Thermal stability and flame retardancy of polyester, cotton, and relative blend textile fabrics subjected to sol–gel treatments. J. Appl. Polym. Sci. 2011, 119, 1961–1969. [Google Scholar] [CrossRef]
- Lam, Y.L.; Kan, C.-W.; Yuen, C.W.M. Flame-retardant finishing in cotton fabrics using zinc oxide co-catalyst. J. Appl. Polym. Sci. 2011, 121, 612–621. [Google Scholar] [CrossRef]
- Guido, E.; Colleoni, C.; De Clerck, K.; Plutino, M.R.; Rosace, G. Influence of catalyst in the synthesis of a cellulose-based sensor: Kinetic study of 3-glycidoxypropyltrimethoxysilane epoxy ring opening by Lewis acid. Sens. Actuators B Chem. 2014, 203, 213–222. [Google Scholar] [CrossRef]
- ASTM D-1776. Textile Standard Atmosphere for Conditioning and Testing; ASTM International: West Conshohocken, PA, USA, 1998. [Google Scholar]
- Iqbal, M.; Sohail, M.; Aleem, A.; Ahmed, K.; Moiz, A.; Khalil, A. Textile environmental conditioning: Effect of relative humidity variation on the tensile properties of different fabrics. J. Anal. Sci. Methods Instrum. 2012, 2, 92. [Google Scholar] [CrossRef][Green Version]
- ASTM D 1388. Standard Test Method for Stiffness of Fabrics; ASTM International: West Conshohocken, PA, USA, 2008. [Google Scholar]
- ASTM D 5035. Standard Test Method for Breaking Force and Elongation of Textile Fabrics (Strip Method); ASTM International: West Conshohocken, PA, USA, 2011. [Google Scholar]
- Bentis, A.; Boukhriss, A.; Grancaric, A.M.; El Bouchti, M.; El Achaby, M.; Gmouh, S. Flammability and combustion behavior of cotton fabrics treated by the sol gel method using ionic liquids combined with different anions. Cellulose 2019, 26, 2139–2153. [Google Scholar] [CrossRef]
- Tammer, M.G. Sokrates: Infrared and Raman characteristic group frequencies: tables and charts. Colloid Polym. Sci. 2004, 283, 235. [Google Scholar] [CrossRef]
- Castellano, A.; Colleoni, C.; Iacono, G.; Mezzi, A.; Plutino, M.R.; Malucelli, G.; Rosace, G. Synthesis and characterization of a phosphorous/nitrogen based sol-gel coating as a novel halogen- and formaldehyde-free flame retardant finishing for cotton fabric. Polym. Degrad. Stab. 2019, 162, 148–159. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, J.-C.; Zhang, C.-J.; Guo, Y.; Zhu, P.; Wang, D.-Y. Effect of manganese and cobalt ions on flame retardancy and thermal degradation of bio-based alginate films. J. Mater. Sci. 2016, 51, 1052–1065. [Google Scholar] [CrossRef]

| Fabric Samples | Blend Fabric Ratio % | Warp Count (Tex) | Weft Count (Tex) | Ends/Inch | Picks/Inch | Weight g/m2 | Thickness mm | Structure | Surface Wettability |
|---|---|---|---|---|---|---|---|---|---|
| Cotton fabric (COT) | 100 | 22 | 22 | 76 | 68 | 155 | 0.20 | Plain weave | Highly absorbent |
| Polyester-cotton fabric (PC) | 50/50 | 22 | 22 | 76 | 68 | 155 | 0.20 | Plain weave | Highly absorbent |
| Samples | Silica dioxide Wt.% | Zinc oxide Wt.% | Water | TEOS | Ethanol | HCl (0.01N) | Add-On (%) |
|---|---|---|---|---|---|---|---|
| COT | Pure cotton | ||||||
| COT-1 | 0.25% | 0.25% | 20 mL | 20 mL | 45 mL | 24 mL | 1.66 |
| COT-2 | 0.5% | 0.25% | 20 mL | 20 mL | 45 mL | 24 mL | 1.91 |
| COT-3 | 0.25% | 0.5% | 20 mL | 20 mL | 45 mL | 24 mL | 1.91 |
| PC | Pure PC | ||||||
| PC-1 | 0.25% | 0.25% | 20 mL | 20 mL | 45 mL | 24 mL | 1.66 |
| PC-2 | 0.5% | 0.25% | 20 mL | 20 mL | 45 mL | 24 mL | 1.91 |
| PC-3 | 0.25% | 0.5% | 20 mL | 20 mL | 45 mL | 24 mL | 1.91 |
| Substrate Type | Tmin (°C) | Tmax (°C) | Weight Loss at Tmax (%) | Residue at End (%) | Time to Ignite (s) | Flame Spread Time (s) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B.W | A.W | B.W | A.W | B.W | A.W | B.W | A.W | B.W | A.W | B.W | A.W | |
| COT | 320 | 400 | 92.91 | 7.1 | 2 | 8 | ||||||
| COT -1 | 330 | 333 | 410 | 413 | 91.1 | 92.4 | 8.9 | 7.6 | 4 | 3 | 17 | 15 |
| COT-2 | 350 | 341 | 428 | 418 | 91.5 | 92.1 | 8.5 | 7.9 | 5 | 4 | 21 | 19 |
| COT-3 | 340 | 336 | 420 | 415 | 91.4 | 92.2 | 8.6 | 7.8 | 5 | 4 | 14 | 13 |
| PC | 310 | 500 | 96.2 | 3.8 | 2 | 8 | ||||||
| PC-1 | 315 | 311 | 514 | 511 | 86.2 | 87.3 | 13.8 | 12.7 | 4 | 3 | 17 | 17 |
| PC-2 | 319 | 314 | 524 | 520 | 85.5 | 87.9 | 14.5 | 12.1 | 6 | 5 | 22 | 20 |
| PC-3 | 317 | 313 | 521 | 519 | 86.9 | 87.7 | 13.1 | 12.3 | 6 | 5 | 18 | 17 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleemi, S.; Naveed, T.; Riaz, T.; Memon, H.; Awan, J.A.; Siyal, M.I.; Xu, F.; Bae, J. Surface Functionalization of Cotton and PC Fabrics Using SiO2 and ZnO Nanoparticles for Durable Flame Retardant Properties. Coatings 2020, 10, 124. https://doi.org/10.3390/coatings10020124
Saleemi S, Naveed T, Riaz T, Memon H, Awan JA, Siyal MI, Xu F, Bae J. Surface Functionalization of Cotton and PC Fabrics Using SiO2 and ZnO Nanoparticles for Durable Flame Retardant Properties. Coatings. 2020; 10(2):124. https://doi.org/10.3390/coatings10020124
Chicago/Turabian StyleSaleemi, Sidra, Tayab Naveed, Tabinda Riaz, Hafeezullah Memon, Javeed Ashraf Awan, M. Irfan Siyal, Fujun Xu, and Jihyun Bae. 2020. "Surface Functionalization of Cotton and PC Fabrics Using SiO2 and ZnO Nanoparticles for Durable Flame Retardant Properties" Coatings 10, no. 2: 124. https://doi.org/10.3390/coatings10020124
APA StyleSaleemi, S., Naveed, T., Riaz, T., Memon, H., Awan, J. A., Siyal, M. I., Xu, F., & Bae, J. (2020). Surface Functionalization of Cotton and PC Fabrics Using SiO2 and ZnO Nanoparticles for Durable Flame Retardant Properties. Coatings, 10(2), 124. https://doi.org/10.3390/coatings10020124