Preparation and Characterization of NbxOy Thin Films: A Review
Abstract
:1. Introduction
2. Properties of Niobium Oxide Thin Films
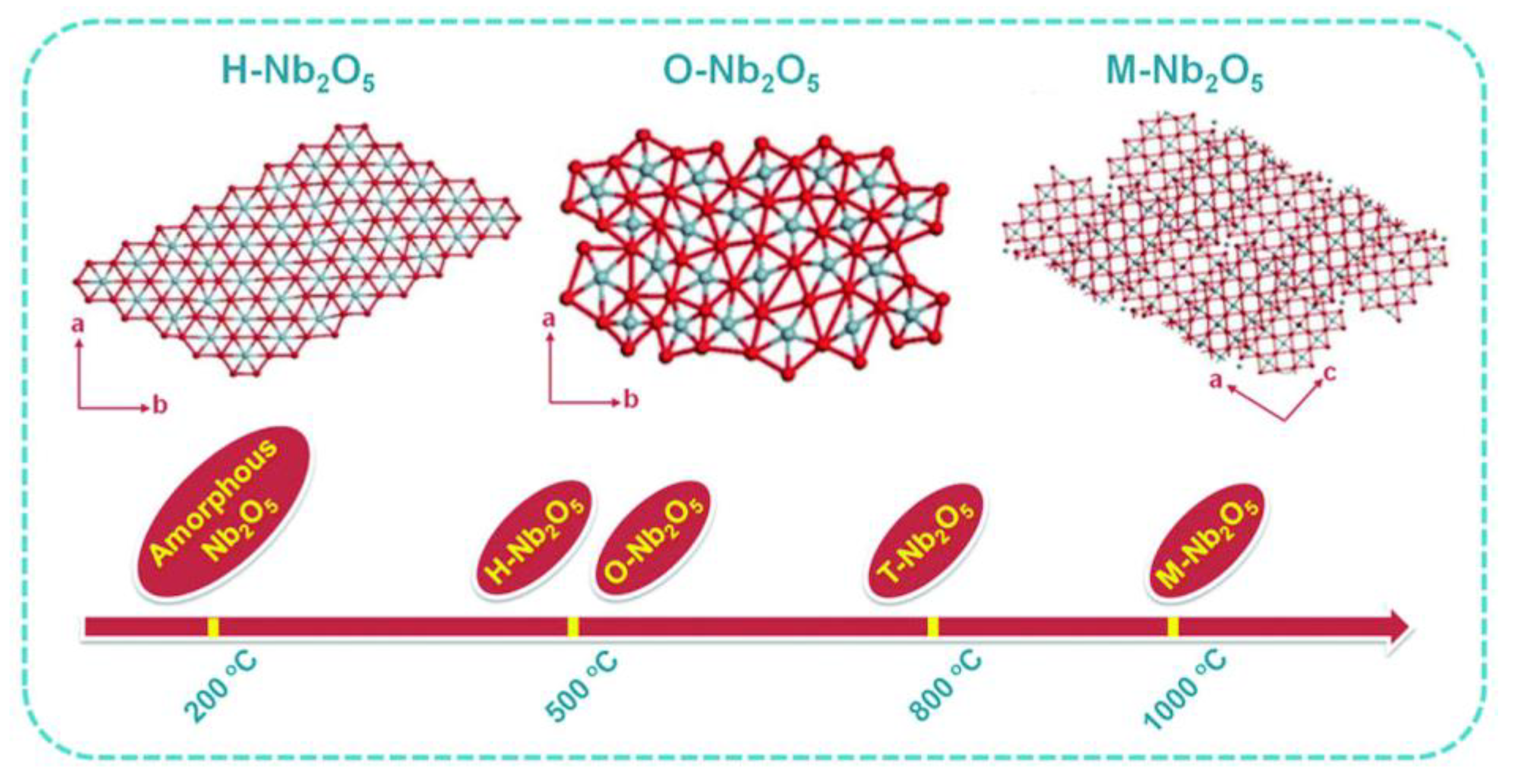
2.1. Surface Morphology
2.2. Electrical Qualities
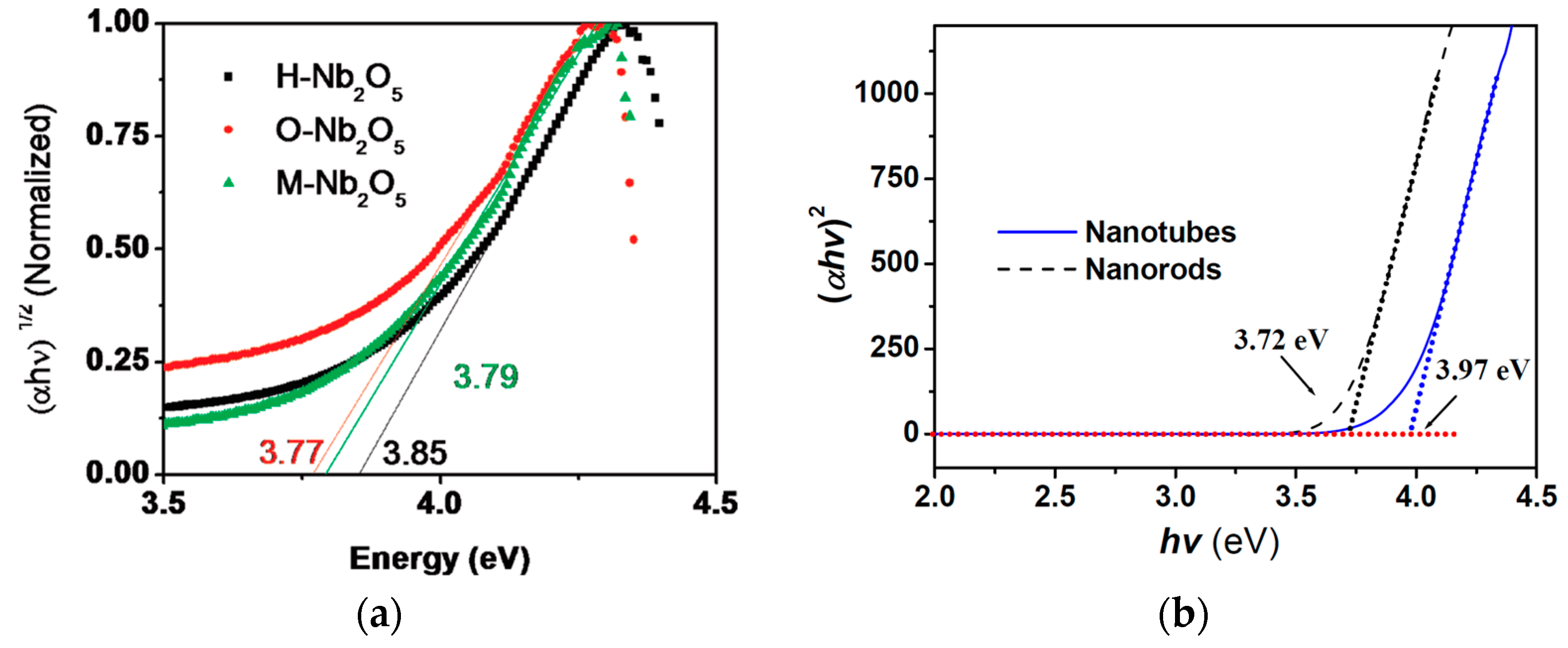
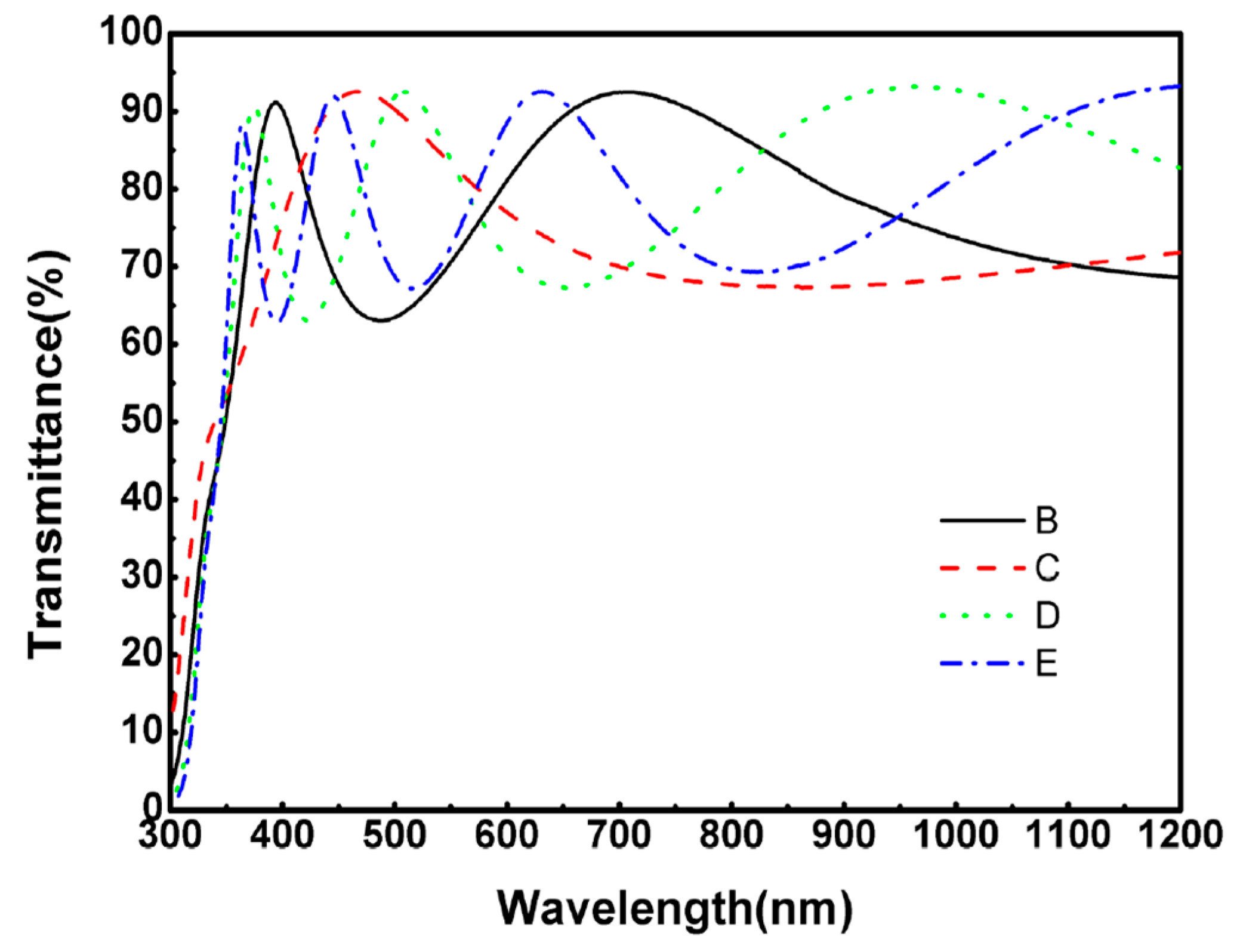
2.3. Optical Features
2.4. Mechanical Properties
3. Methods of Deposition
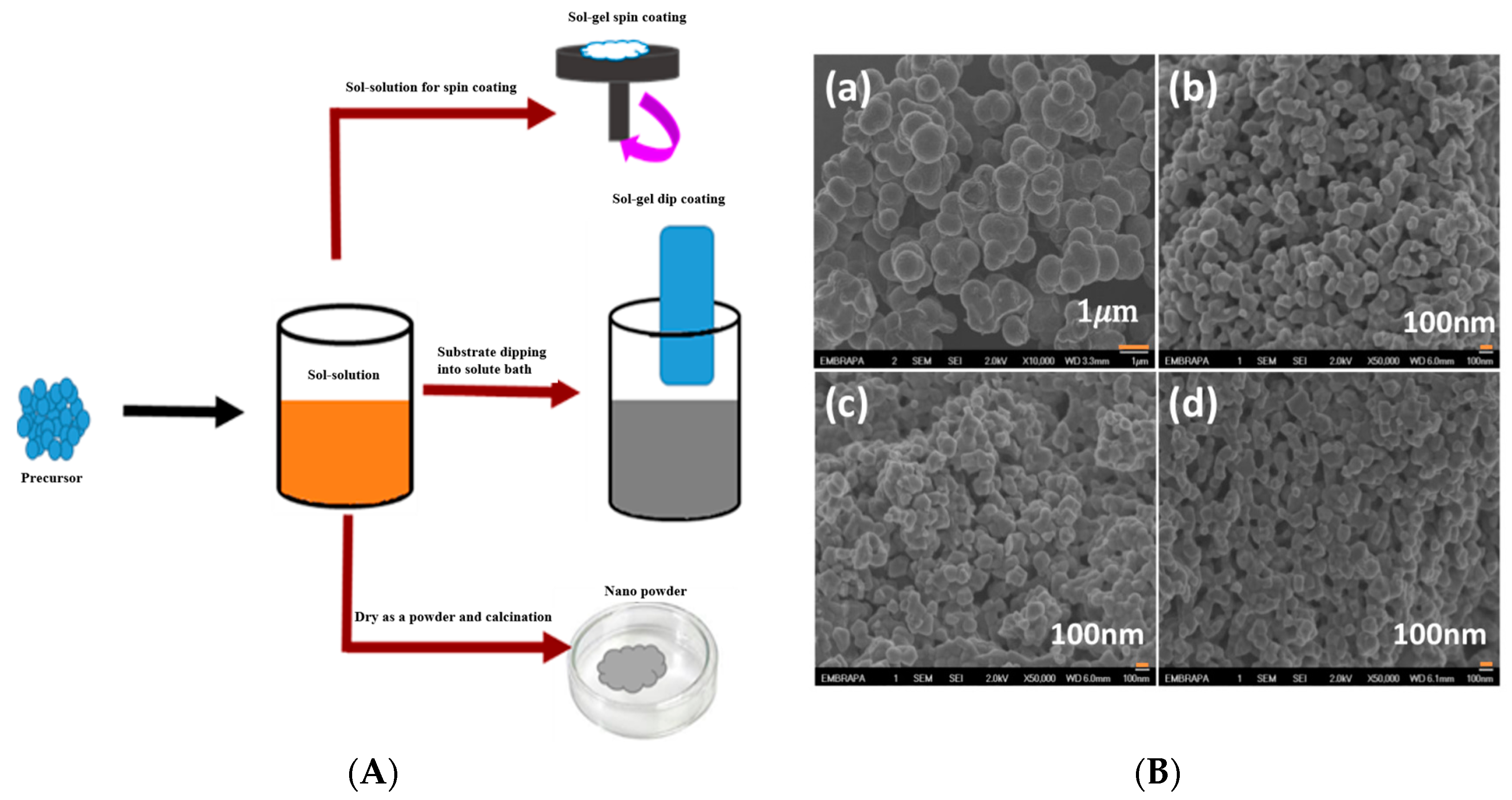
3.1. Sol Gel
3.2. Chemical Spray Pyrolysis
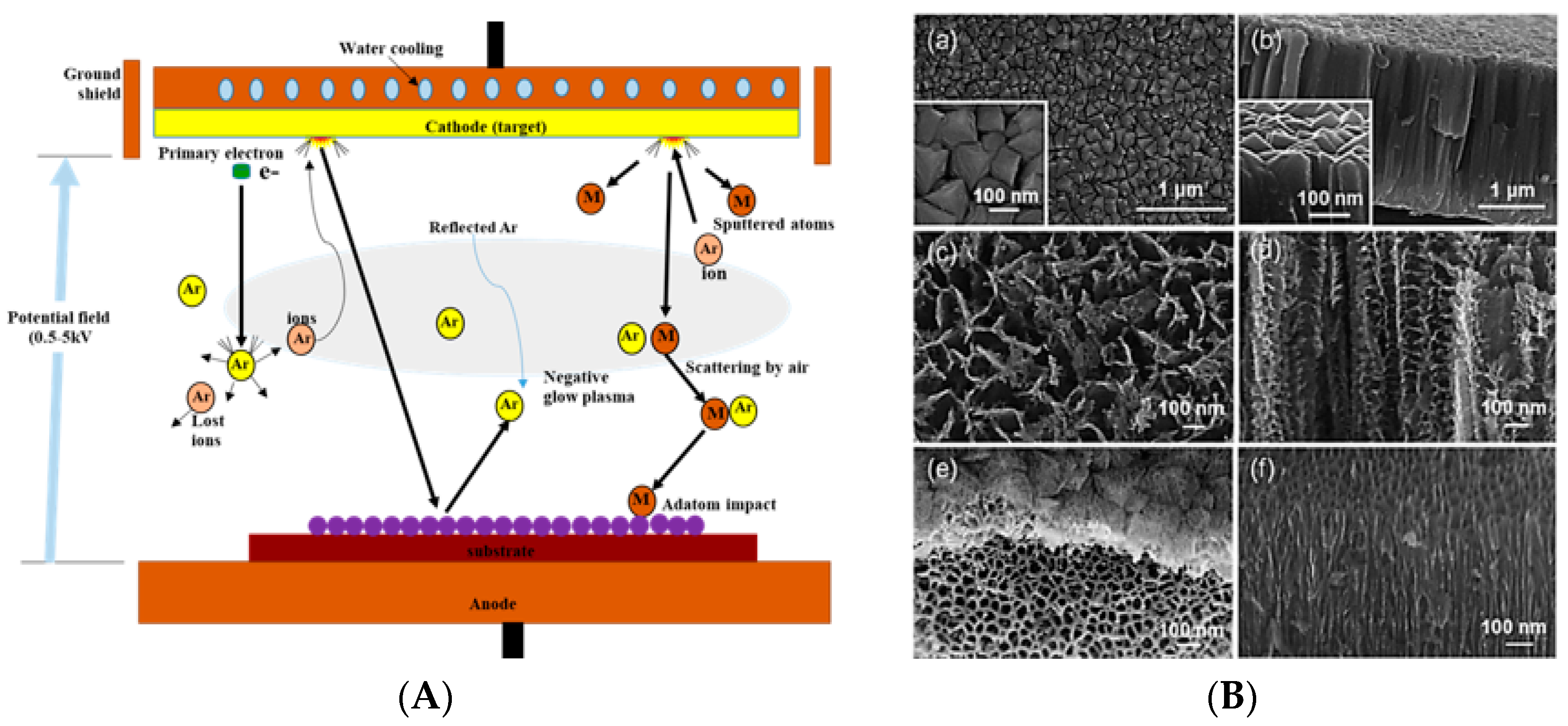
3.3. Sputtering
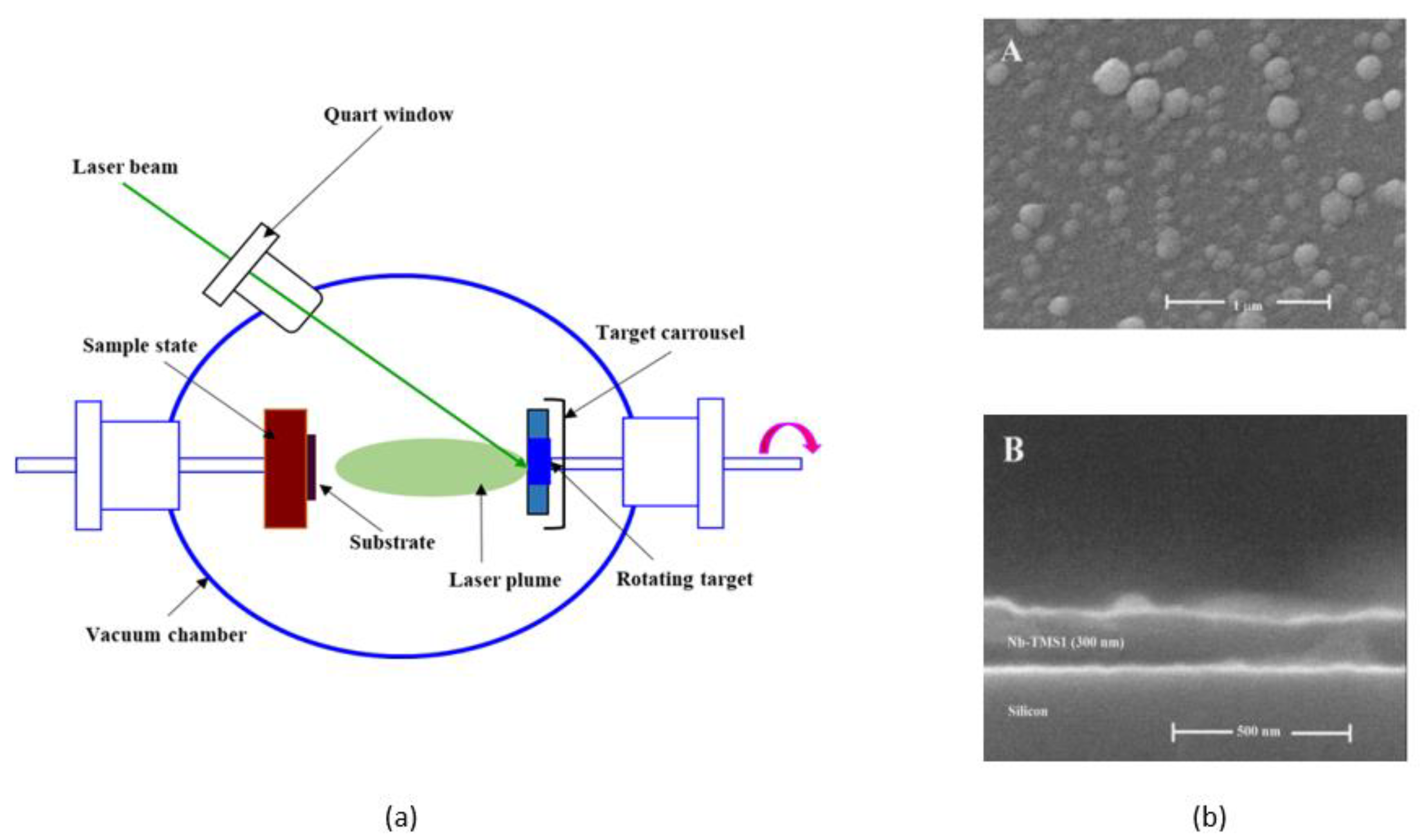
3.4. Pulsed Laser Deposition (PLD)
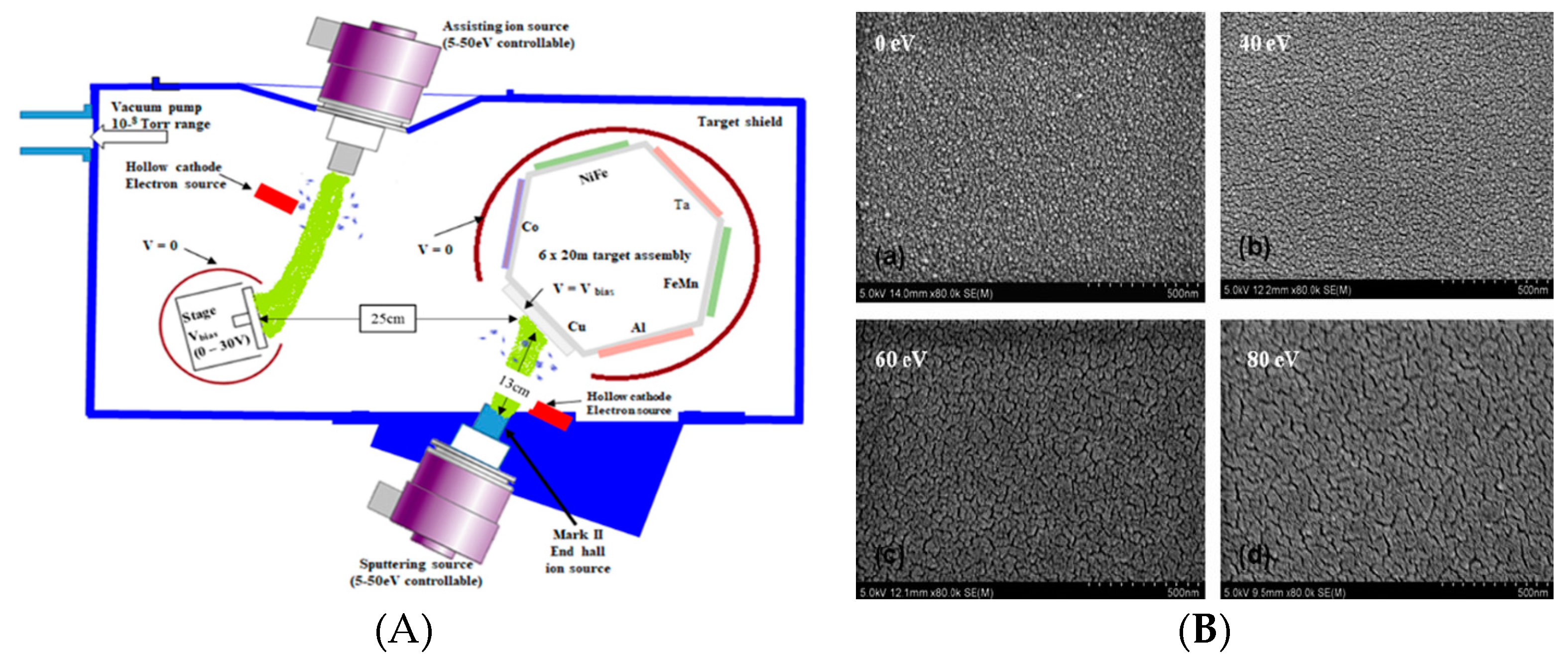
3.5. Biased Target Ion Beam Deposition (BTIBD)
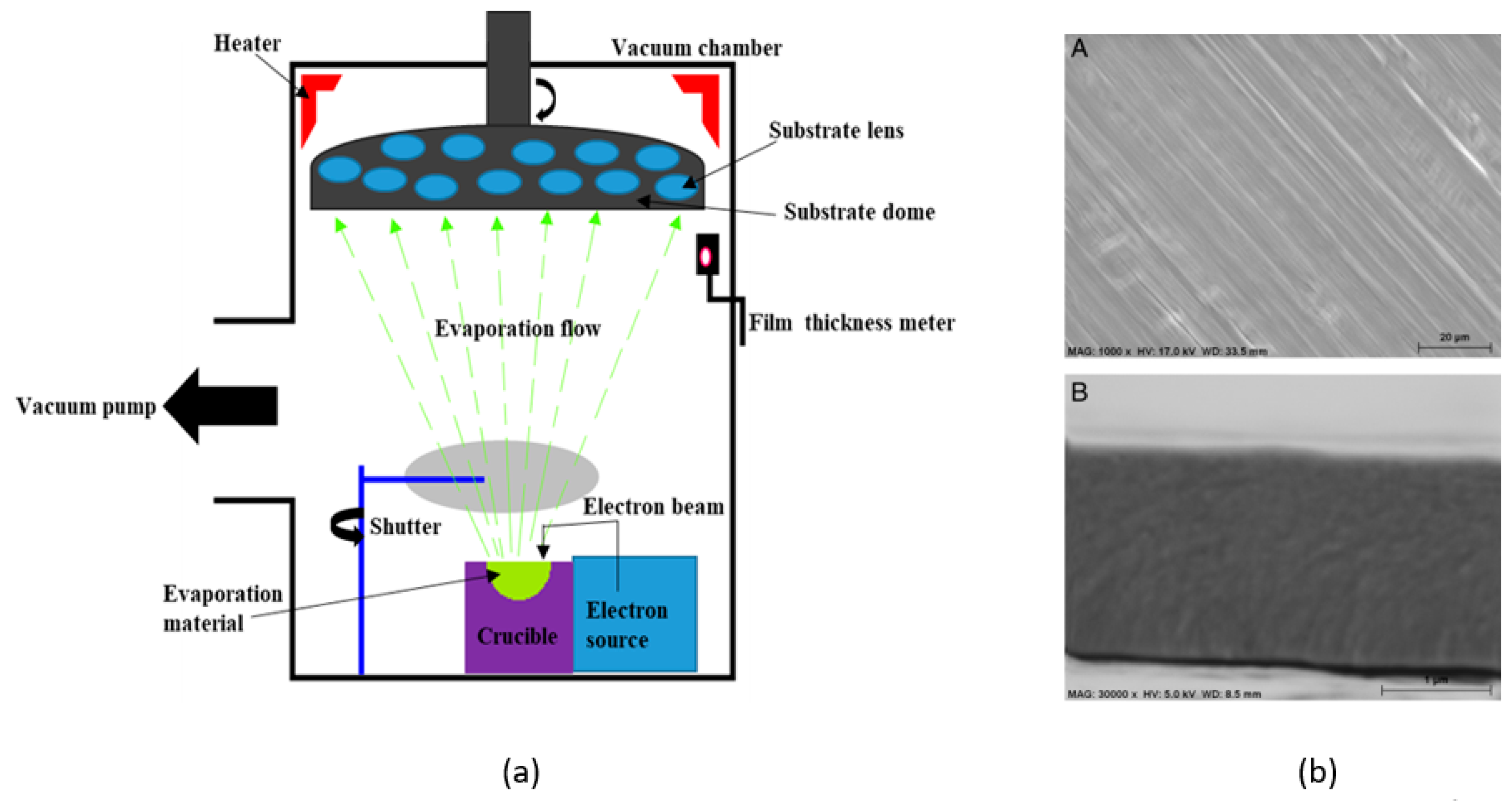
3.6. Electron Beam Evaporation (E-beam)
3.7. Electrochemical Deposition Process (ECD)
3.8. Chemical Vapour Deposition Process (CVD)
3.9. Atomic Layer Deposition (ALD)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Meija, J.; Coplen, T.B.; Berglund, M.; Brand, W.A.; De Bièvre, P.; Gröning, M.; Holden, N.E.; Irrgeher, J.; Loss, R.D.; Walczyk, T.; et al. Atomic weights of the elements 2013 (IUPAC Technical Report). Pure Appl. Chem. 2016, 88, 265–291. [Google Scholar] [CrossRef]
- Samsonov, G.V. Mechanical Properties of the Elements. In Handbook of the Physicochemical Properties of the Elements; Springer Science and Business Media: Berlin/Heidelberg, Germany, 1968; pp. 387–446. [Google Scholar]
- Knapp, B. Elements of Geographical Hydrology; Unwin Hyman Limited: London, UK, 2002. [Google Scholar]
- Zhao, Y.; Zhang, Z.; Lin, Y. Optical and dielectric properties of a nanostructured NbO2 thin film prepared by thermal oxidation. J. Phys. D: Appl. Phys. 2004, 37, 3392–3395. [Google Scholar] [CrossRef]
- Tanabe, K. Catalytic application of niobium compounds. Catal. Today 2003, 78, 65–77. [Google Scholar] [CrossRef]
- Jacob, K.T.; Shekhar, C.; Vinay, M.; Waseda, Y. Thermodynamic Properties of Niobium Oxides. J. Chem. Eng. Data 2010, 55, 4854–4863. [Google Scholar] [CrossRef]
- Venkataraj, S.; Drese, R.; Liesch, C.; Kappertz, O.; Jayavel, R.; Wuttig, M. Temperature stability of sputtered niobium–oxide films. J. Appl. Phys. 2002, 91, 4863–4871. [Google Scholar] [CrossRef]
- Bach, D.; Stormer, H.; Schneider, R.; Gerthsen, D.; Sigle, W. EELS Investigations of Reference Niobium Oxides and Anodically Grown Niobium Oxide Layers. Microsc. Microanal. 2007, 13. [Google Scholar] [CrossRef]
- Foroughi-Abari, A.; Cadien, K.C. Growth, structure and properties of sputtered niobium oxide thin films. Thin Solid Films 2011, 519, 3068–3073. [Google Scholar] [CrossRef]
- Venkataraj, S.; Drese, R.; Kappertz, O.; Jayavel, R.; Wuttig, M. Characterization of Niobium Oxide Films Prepared by Reactive DC Magnetron Sputtering. Phys. Status Solidi 2001, 188, 1047–1058. [Google Scholar] [CrossRef]
- Ou, J.Z.; Rani, R.A.; Ham, M.-H.; Field, M.R.; Zhang, Y.; Zheng, H.; Reece, P.; Zhuiykov, S.; Sriram, S.; Bhaskaran, M.; et al. Elevated Temperature Anodized Nb2O5: A Photoanode Material with Exceptionally Large Photoconversion Efficiencies. ACS Nano 2012, 6, 4045–4053. [Google Scholar] [CrossRef]
- Reichman, B.; Bard, A.J. Electrochromism at Niobium Pentoxide Electrodes in Aqueous and Acetonitrile Solutions. J. Electrochem. Soc. 1980, 127, 241–242. [Google Scholar] [CrossRef] [Green Version]
- Granqvist, C. Inorganic Non-Oxide Electrochromic Materials. In Handbook of Inorganic Electrochromic Materials; Elsevier: Amsterdam, The Netherlands, 1995; pp. 421–430. [Google Scholar]
- Cabanel, R.; Chaussy, J.; Mazuer, J.; Delabouglise, G.; Joubert, J.C.; Barral, G.; Montella, C. Electrochemichromism of Nb2O5 Thin Films Obtained by Oxidation of Magnetron-Sputtered NbNx. J. Electrochem. Soc. 1990, 137, 1444–1451. [Google Scholar] [CrossRef]
- Schmitt, M.; Aegerter, M. Electrochromic properties of pure and doped Nb2O5 coatings and devices. Electrochimica Acta 2001, 46, 2105–2111. [Google Scholar] [CrossRef] [Green Version]
- Mujawar, S.; Inamdar, A.; Patil, P.; Patil, P. Electrochromic properties of spray-deposited niobium oxide thin films. Solid State Ionics 2006, 177, 3333–3338. [Google Scholar] [CrossRef]
- Granqvist, C. Electrochromism and smart window design. Solid State Ionics 1992, 53, 479–489. [Google Scholar] [CrossRef]
- E Gimon-Kinsel, M.; Balkus, J.K.J. Pulsed laser deposition of mesoporous niobium oxide thin films and application as chemical sensors. Microporous Mesoporous Mater. 1999, 28, 113–123. [Google Scholar] [CrossRef]
- Rho, S.; Jahng, D.; Lim, J.H.; Choi, J.; Chang, J.H.; Lee, S.C.; Kim, K.J. Electrochemical DNA biosensors based on thin gold films sputtered on capacitive nanoporous niobium oxide. Biosens. Bioelectron. 2008, 23, 852–856. [Google Scholar] [CrossRef]
- Ficarro, S.B.; Parikh, J.R.; Blank, N.C.; Marto, J.A. Niobium(V) Oxide (Nb2O5): Application to Phosphoproteomics. Anal. Chem. 2008, 80, 4606–4613. [Google Scholar] [CrossRef]
- Tamai, T.; Haneda, M.; Fujitani, T.; Hamada, H. Promotive effect of Nb2O5 on the catalytic activity of Ir/SiO2 for NO reduction with CO under oxygen-rich conditions. Catal. Commun. 2007, 8, 885–888. [Google Scholar] [CrossRef]
- Cvelbar, U.; Mozetič, M. Behaviour of oxygen atoms near the surface of nanostructured Nb2O5. J. Phys. D: Appl. Phys. 2007, 40, 2300–2303. [Google Scholar] [CrossRef]
- Prado, A.G.; Bolzon, L.B.; Pedroso, C.P.; Moura, A.O.; Costa, L.L. Nb2O5 as efficient and recyclable photocatalyst for indigo carmine degradation. Appl. Catal. B Environ. 2008, 82, 219–224. [Google Scholar] [CrossRef]
- Eisenbarth, E.; Velten, D.; Breme, J. Biomimetic implant coatings. Biomol. Eng. 2007, 24, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Starikov, V.V.; Starikova, S.L.; Mamalis, A.G.; Lavrynenko, S.N.; Ramsden, J.J. The application of niobium and tantalum oxides for implant surface passivation. J. Biol. Phys. Chem. 2007, 7, 65–68. [Google Scholar] [CrossRef]
- Tanvir, M.T.; Aoki, Y.; Habazaki, H. Improved electrical properties of silicon-incorporated anodic niobium oxide formed on porous Nb–Si substrate. Appl. Surf. Sci. 2009, 255, 8383–8389. [Google Scholar] [CrossRef]
- Le Viet, A.; Reddy, M.V.; Jose, R.; Chowdari, B.V.R.; Ramakrishna, S. Nanostructured Nb2O5 Polymorphs by Electrospinning for Rechargeable Lithium Batteries. J. Phys. Chem. C 2010, 114, 664–671. [Google Scholar] [CrossRef]
- Blanquart, T.; Niinistö, J.; Heikkilä, M.; Sajavaara, T.; Kukli, K.; Puukilainen, E.; Xu, C.; Hunks, W.; Ritala, M.; Leskelä, M. Evaluation and Comparison of Novel Precursors for Atomic Layer Deposition of Nb2O5 Thin Films. Chem. Mater. 2012, 24, 975–980. [Google Scholar] [CrossRef]
- Zhou, X.; Li, Z.; Wang, Y.; Sheng, X.; Zhang, Z. Photoluminescence of amorphous niobium oxide films synthesized by solid-state reaction. Thin Solid Films 2008, 516, 4213–4216. [Google Scholar] [CrossRef]
- Lai, F.; Lin, L.; Huang, Z.; Gai, R.; Qu, Y. Effect of thickness on the structure, morphology and optical properties of sputter deposited Nb2O5 films. Appl. Surf. Sci. 2006, 253, 1801–1805. [Google Scholar] [CrossRef]
- Störmer, H.; Weber, A.; Fischer, V.; Ivers-Tiffée, E.; Gerthsen, D. Anodically formed oxide films on niobium: Microstructural and electrical properties. J. Eur. Ceram. Soc. 2009, 29, 1743–1753. [Google Scholar] [CrossRef]
- Yoon, J.S.; Cho, S.W.; Kim, Y.S.; Kim, B.I. The production of niobium powder and electric properties of niobium capacitors. Met. Mater. Int. 2009, 15, 405–408. [Google Scholar] [CrossRef]
- Choi, J.; Lim, J.H.; Lee, J.; Kim, K.J. Porous niobium oxide films prepared by anodization–annealing–anodization. Nanotechnology 2007, 18, 055603. [Google Scholar] [CrossRef]
- Matylitskaya, V.A.; Bock, W.; Kolbesen, B.O. Nitridation of niobium oxide films by rapid thermal processing. Anal. Bioanal. Chem. 2008, 390, 1507–1515. [Google Scholar] [CrossRef] [PubMed]
- Kukli, K.; Ritala, M.; Leskelä, M. Development of Dielectric Properties of Niobium Oxide, Tantalum Oxide, and Aluminum Oxide Based Nanolayered Materials. J. Electrochem. Soc. 2001, 148, F35–F41. [Google Scholar] [CrossRef]
- Bharti, D.C.; Rhee, S.-W. Dielectric properties and X-ray photoelectron spectroscopic studies of niobium oxide thin films prepared by direct liquid injection chemical vapor deposition method. Thin Solid Films 2013, 548, 195–201. [Google Scholar] [CrossRef]
- Cho, K.; Lee, J.; Lim, J.-S.; Lim, H.; Lee, J.; Park, S.; Yoo, C.-Y.; Kim, S.-T.; Chung, U.-I.; Moon, J.-T. Low temperature crystallized Ta2O5/Nb2O5 bi-layers integrated into RIR capacitor for 60 nm generation and beyond. Microelectron. Eng. 2005, 80, 317–320. [Google Scholar] [CrossRef]
- Naito, K.; Matsui, T. Review on phase equilibria and defect structures in the niobium-oxygen system. Solid State Ionics 1984, 12, 125–134. [Google Scholar] [CrossRef]
- Augustyn, V.; Come, J.; Lowe, M.A.; Kim, J.W.; Taberna, P.-L.; Tolbert, S.H.; Abruña, H.D.; Simon, P.; Dunn, B. High-rate electrochemical energy storage through Li+ intercalation pseudocapacitance. Nat. Mater. 2013, 12, 518–522. [Google Scholar] [CrossRef]
- Tamang, R.; Varghese, B.; Mhaisalkar, S.; Tok, E.S.; Sow, C.H. Probing the photoresponse of individual Nb2O5 nanowires with global and localized laser beam irradiation. Nanotechnol. 2011, 22, 115202. [Google Scholar] [CrossRef]
- Hulm, J.K.; Jones, C.K.; Hein, R.A.; Gibson, J.W. Superconductivity in the TiO and NbO systems. J. Low Temp. Phys. 1972, 7, 291–307. [Google Scholar] [CrossRef]
- Lo, W.; A Cardwell, D.; Bradley, A.D.; Doyle, R.; Shi, Y.; Lloyd, S. Development of non-weak link bulk YBCO grain boundaries for high magnetic field engineering applications. IEEE Trans. Appl. Supercond. 1999, 9, 2042–2045. [Google Scholar] [CrossRef]
- Shin, S.H.; Halpern, T.; Raccah, P.M. High-speed high-current field switching of NbO2. J. Appl. Phys. 1977, 48, 3150. [Google Scholar] [CrossRef]
- Sasaki, K.; Adzic, R.R. Monolayer-level Ru-and NbO2-supported platinum electrocatalysts for methanol oxidation. J. Electrochem. Soc. 2008, 155, B180–B186. [Google Scholar] [CrossRef]
- Bolzan, A.A.; Fong, C.; Kennedy, B.J.; Howard, C.J. A Powder Neutron Diffraction Study of Semiconducting and Metallic Niobium Dioxide. J. Solid State Chem. 1994, 113, 9–14. [Google Scholar] [CrossRef]
- Eyert, V. The metal-insulator transition of NbO2: An embedded Peierls instability. EPL Europhysics Lett. 2002, 58, 851–856. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Park, J.; Woo, J.; Cho, C.; Lee, W.; Shin, J.; Choi, G.; Park, S.; Lee, D.; Lee, B.H.; et al. Threshold-switching characteristics of a nanothin-NbO2-layer-based Pt/NbO2/Pt stack for use in cross-point-type resistive memories. Microelectron. Eng. 2013, 107, 33–36. [Google Scholar] [CrossRef]
- Pickett, M.D.; Williams, R.S. Sub-100 fJ and sub-nanosecond thermally driven threshold switching in niobium oxide crosspoint nanodevices. Nanotechnol 2012, 23, 215202. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Comes, R.B.; Kittiwatanakul, S.; Wolf, S.A.; Lu, J. Epitaxial niobium dioxide thin films by reactive-biased target ion beam deposition. J. Vac. Sci. Technol. A 2015, 33, 21516. [Google Scholar] [CrossRef]
- Lee, S.-H.; Yoon, H.-N.; Yoon, I.; Kim, B.-S. Single Crystalline NbO2 Nanowire Synthesis by Chemical Vapor Transport Method. Bull. Korean Chem. Soc. 2012, 33, 839–842. [Google Scholar] [CrossRef] [Green Version]
- Ramírez, G.; Rodil, S.; Muhl, S.; Turcio-Ortega, D.; Olaya, J.; Rivera, M.; Camps, E.; Escobar-Alarcón, L. Amorphous niobium oxide thin films. J. Non-Crystalline Solids 2010, 356, 2714–2721. [Google Scholar] [CrossRef]
- Kato, K. Structure refinement of H-Nb2O5. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1976, 32, 764–767. [Google Scholar] [CrossRef]
- Kato, K.; Tamura, S. Die Kristallstruktur von T-Nb2O5. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1975, 31, 673–677. [Google Scholar] [CrossRef]
- Holser, W.T. Compounds with the α-U3O8-type structure. Acta Crystallogr. 1956, 9, 196. [Google Scholar] [CrossRef] [Green Version]
- Ikeya, T.; Senna, M. Change in the structure of niobium pentoxide due to mechanical and thermal treatments. J. Non-Crystalline Solids 1988, 105, 243–250. [Google Scholar] [CrossRef]
- Cho, N.-H.; Kang, H.B.; Kim, Y.H. Dielectric characteristics and chemical structures of Nb2O5 thin films grown by sol-gel techniques. Ferroelectrics 1994, 152, 43–48. [Google Scholar] [CrossRef]
- Kumagai, N.; Tanno, K.; Nakajima, T.; Watanabe, N. Structural changes of Nb2O5 and V2O5 as rechargeable cathodes for lithium battery. Electrochimica Acta 1983, 28, 17–22. [Google Scholar] [CrossRef]
- Maček, M.; Orel, B. Electrochromism of sol–gel derived niobium oxide films. Sol. Energy Mater. Sol. Cells 1998, 54, 121–130. [Google Scholar] [CrossRef] [Green Version]
- Jelle, B.P. Electrochromic Smart Windows for Dynamic Daylightand Solar Energy Control in Buildings. Electrochromic Mater. Devices 2015, 419–502. [Google Scholar] [CrossRef]
- Zhitomirsky, I. Electrolytic deposition of niobium oxide films. Mater. Lett. 1998, 35, 188–193. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, Y.; Ding, S.-J.; Lu, H.-L.; Sun, Q.-Q.; Zhang, D.W.; Chen, Z. Thermal stability of atomic-layer-deposited ultra-thin niobium oxide film on Si (100). Appl. Surf. Sci. 2011, 257, 7305–7309. [Google Scholar] [CrossRef]
- Nowak, I.; Ziolek, M. Niobium Compounds: Preparation, Characterization, and Application in Heterogeneous Catalysis. Chem. Rev. 1999, 99, 3603–3624. [Google Scholar] [CrossRef]
- Rani, R.A.; Zoolfakar, A.S.; O’Mullane, A.P.; Austin, M.W.; Kalantar-Zadeh, K. Thin films and nanostructures of niobium pentoxide: fundamental properties, synthesis methods and applications. J. Mater. Chem. A 2014, 2, 15683–15703. [Google Scholar] [CrossRef] [Green Version]
- Le Viet, A.; Jose, R.; Reddy, M.V.; Chowdari, B.V.R.; Ramakrishna, S. Nb2O5 Photoelectrodes for Dye-Sensitized Solar Cells: Choice of the Polymorph. J. Phys. Chem. C 2010, 114, 21795–21800. [Google Scholar] [CrossRef]
- Reznichenko, L.A.; Akhnazarova, V.V.; Shilkina, L.A.; Razumovskaya, O.N.; Dudkina, S.I. Invar effect in n-Nb2O5, α ht-Nb2O5, and L-Nb2O5. Crystallogra. Rep. 2009, 54, 483–491. [Google Scholar] [CrossRef]
- Schäfer, H.; Gruehn, R.; Schulte, F. The Modifications of Niobium Pentoxide. Angew. Chem. Int. Ed. 2003, 5, 40–52. [Google Scholar] [CrossRef]
- Ko, E.; Weissman, J. Structures of niobium pentoxide and their implications on chemical behavior. Catal. Today 1990, 8, 27–36. [Google Scholar] [CrossRef]
- Lubimtsev, A.A.; Kent, P.R.C.; Sumpter, B.G.; Ganesh, P. Understanding the origin of high-rate intercalation pseudocapacitance in Nb2O5 crystals. J. Mater. Chem. A 2013, 1, 14951–14956. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, Y.; Wang, W.; Zhang, X.; Wang, B.; Tian, H.; Wang, Y.; Guan, J.; Gu, H. Fast and highly-sensitive hydrogen sensing of Nb2O5 nanowires at room temperature. Int. J. Hydrogen Energy 2012, 37, 4526–4532. [Google Scholar] [CrossRef]
- Palatnikov, M.N.; Shcherbina, O.B.; Sidorov, N.; Skab, I.; Bormanis, K. The structure of niobium and tantalum oxides processed by concentrated light flux. Ukr. J. Phys. Opt. 2012, 13, 207. [Google Scholar] [CrossRef]
- Frevel, L.K.; Rinn, H.W. Powder Diffraction Standards for Niobium Pentoxide and Tantalum Pentoxide. Anal. Chem. 1955, 27, 1329–1330. [Google Scholar] [CrossRef]
- Norin, R.; Magneli, A. New Niobium Oxide Phases. Naturwissenschaften 1960, 47, 354–355. [Google Scholar] [CrossRef]
- Gatehouse, B.M.; Wadsley, A.D. The crystal structure of the high temperature form of niobium pentoxide. Acta Crystallogr. 2002, 17, 1545–1554. [Google Scholar] [CrossRef]
- Kubaschewski, O.; Hopkins, B. Oxidation mechanisms of niobium, tantalum, molybdenum and tungsten. J. Less Common Met. 1960, 2, 172–180. [Google Scholar] [CrossRef]
- Yan, L.; Rui, X.; Chen, G.; Xu, W.; Zou, G.; Luo, H. Recent advances in nanostructured Nb-based oxides for electrochemical energy storage. Nanoscale 2016, 8, 8443–8465. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Song, W.; Hoertz, P.G.; Hanson, K.; Ghosh, R.; Rangan, S.; Brennaman, M.K.; Concepcion, J.J.; Binstead, R.A.; Bartynski, R.A.; et al. A Sensitized Nb2O5 Photoanode for Hydrogen Production in a Dye-Sensitized Photoelectrosynthesis Cell. Chem. Mater. 2012, 25, 122–131. [Google Scholar] [CrossRef]
- Schultze, J.; Lohrengel, M. Stability, reactivity and breakdown of passive films. Problems of recent and future research. Electrochimica Acta 2000, 45, 2499–2513. [Google Scholar] [CrossRef]
- Yoffe, A.D. Low-dimensional systems: Quantum size effects and electronic properties of semiconductor microcrystallites (zero-dimensional systems) and some quasi-two-dimensional systems. Adv. Phys. 2010, 51, 799–890. [Google Scholar] [CrossRef]
- Sattler, K. The energy gap of clusters, nanoparticles, and quantum dots. In Handbook of Thin Films; Elsevier: Amsterdam, The Netherlands, 2002; pp. 61–97. [Google Scholar]
- Liu, J.; Xue, D.; Li, K. Single-crystalline nanoporous Nb2O5 nanotubes. Nanoscale Res. Lett. 2011, 6, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greener, E.H.; Whitmore, D.H.; Fine, M.E. Electrical Conductivity of Near-Stoichiometric α-Nb2O5. J. Chem. Phys. 1961, 34, 1017. [Google Scholar] [CrossRef]
- Lee, C.-C.; Tien, C.-L.; Hsu, J.-C. Internal stress and optical properties of Nb2O5 thin films deposited by ion-beam sputtering. Appl. Opt. 2002, 41, 2043–2047. [Google Scholar] [CrossRef]
- Özer, N.; Rubin, M.D.; Lampert, C.M. Optical and electrochemical characteristics of niobium oxide films prepared by sol-gel process and magnetron sputtering A comparison. Sol. Energy Mater. Sol. Cells 1996, 40, 285–296. [Google Scholar] [CrossRef]
- Aegerter, M.A.; Schmitt, M.; Guo, Y. Sol-gel niobium pentoxide coatings: Applications to photovoltaic energy conversion and electrochromism. Int. J. Photoenergy 2002, 4, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Pawlicka, A.; Atik, M.; Aegerter, M. Synthesis of multicolor Nb2O5 coatings for electrochromic devices. Thin Solid Films 1997, 301, 236–241. [Google Scholar] [CrossRef] [Green Version]
- Xiao, X.; Dong, G.; Xu, C.; He, H.; Qi, H.; Fan, Z.; Shao, J. Structure and optical properties of Nb2O5 sculptured thin films by glancing angle deposition. Appl. Surf. Sci. 2008, 255, 2192–2195. [Google Scholar] [CrossRef]
- Çetinörgü-Goldenberg, E.; Klemberg-Sapieha, J.-E.; Martinu, L. Effect of postdeposition annealing on the structure, composition, and the mechanical and optical characteristics of niobium and tantalum oxide films. Appl. Opt. 2012, 51, 6498–6507. [Google Scholar] [CrossRef] [PubMed]
- Matias, C.R.; Nassar, E.J.; Verelst, M.; Rocha, L.A. Synthesis and Characterization of Nb2O5:La3+, Eu3+ Phosphors Obtained by the Non-Hydrolytic Sol-Gel Process. J. Braz. Chem. Soc. 2015, 26, 2558–2570. [Google Scholar] [CrossRef]
- Sajiki, G.; Benino, Y.; Nanba, T.; Okano, H. Electrochemical and Photoelectrochemical Properties of Nano-Islands of Zinc and Niobium Oxides Deposited on Aluminum Thin Film by RF Magnetron Reactive Sputtering. Mater. Sci. Appl. 2015, 6, 292–309. [Google Scholar] [CrossRef] [Green Version]
- Shao, Y.; Yi, K.; Fang, M.; Zhang, J. Properties of niobium oxide films deposited by pulsed DC reactive magnetron sputtering. In Pacific Rim Laser Damage Symposium: Optical Materials for High Power Lasers; SPIE (Society of Photo-Optical Instrumentation Engineers): Bellingham, WA, USA, 2011; Volume 8206, p. 82060M. [Google Scholar]
- Shcherbina, O.B.; Palatnikov, M.N.; Efremov, V.V. Mechanical properties of Nb2O5 and Ta2O5 prepared by different procedures. Inorg. Mater. 2012, 48, 433–438. [Google Scholar] [CrossRef]
- Özer, N.; Barreto, T.; Büyüklimanli, T.; Lampert, C.M. Characterization of sol-gel deposited niobium pentoxide films for electrochromic devices. Sol. Energy Mater. Sol. Cells 1995, 36, 433–443. [Google Scholar] [CrossRef]
- Pierre, A.C. Introduction to Sol-Gel Processing; Springer International Publishing: Cham, Switzerland, 2020; pp. 597–685. [Google Scholar]
- Mazdiyasni, K. Powder synthesis from metal-organic precursors. Ceram. Int. 1982, 8, 42–56. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Chironi, I.; Karatchevtseva, I.; Triani, G.; Sorrell, C.C. Single and mixed phase TiO2powders prepared by excess hydrolysis of titanium alkoxide. Adv. Appl. Ceram. 2012, 111, 149–158. [Google Scholar] [CrossRef] [Green Version]
- Thiagarajan, S.; Sanmugam, A.; Vikraman, D. Facile Methodology of Sol-Gel Synthesis for Metal Oxide Nanostructures. In Recent Applications in Sol-Gel Synthesis; IntechOpen: London, UK, 2017; pp. 1–17. [Google Scholar]
- Joya, M.R.; Ortega, J.J.B.; Paez, A.M.R.; Filho, J.D.S.; Freire, P.T.C. Synthesis and Characterization of Nano-Particles of Niobium Pentoxide with Orthorhombic Symmetry. Metals 2017, 7, 142. [Google Scholar] [CrossRef] [Green Version]
- Kumar, D.S.; Kumar, B.J.; Mahesh, H. Quantum Nanostructures (QDs): An Overview. In Synthesis of Inorganic Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2018; pp. 59–88. [Google Scholar]
- Romero, R.; Ramos-Barrado, J.; Martin, F.; Leinen, D. Nb2O5 thin films obtained by chemical spray pyrolysis. Surf. Interface Anal. 2004, 36, 888–891. [Google Scholar] [CrossRef]
- Romero, R.; Dalchiele, E.; Martín, F.; Leinen, D.; Ramos-Barrado, J. Electrochromic behaviour of Nb2O5 thin films with different morphologies obtained by spray pyrolysis. Sol. Energy Mater. Sol. Cells 2009, 93, 222–229. [Google Scholar] [CrossRef]
- Mwakikunga, B.W. Progress in Ultrasonic Spray Pyrolysis for Condensed Matter Sciences Developed From Ultrasonic Nebulization Theories Since Michael Faraday. Crit. Rev. Solid State Mater. Sci. 2014, 39, 46–80. [Google Scholar] [CrossRef] [Green Version]
- Alves, A.K.; Bergmann, C.P.; Berutti, F.A. Novel Synthesis and Characterization of Nanostructured Materials; Springer Science and Business Media: Berlin, Germany, 2013; pp. 11–22. [Google Scholar]
- Depla, D.; Mahieu, S.; Greene, J. Sputter deposition processes. In Handbook of Deposition Technologies for Films and Coatings; William Andrew Publishing: Norwich, NY, USA, 2010; pp. 253–296. [Google Scholar]
- Simon, A.H. Sputter processing. In Handbook of Thin Film Deposition; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 195–230. [Google Scholar]
- Baptista, A.; Silva, F.; Porteiro, J.; Míguez, J.; Pinto, G. Sputtering Physical Vapour Deposition (PVD) Coatings: A Critical Review on Process Improvement and Market Trend Demands. Coatings 2018, 8, 402. [Google Scholar] [CrossRef] [Green Version]
- Rossnagel, S. Sputtering and sputter deposition. In Handbook of Thin Film Deposition Processes and Techniques; William Andrew Publishing: Norwich, NY, USA, 2001; pp. 319–348. [Google Scholar]
- Rani, R.A.; Zoolfakar, A.S.; Ou, J.Z.; Ab Kadir, R.; Nili, H.; Latham, K.; Sriram, S.; Bhaskaran, M.; Zhuiykov, S.; Kaner, R.B.; et al. Reduced impurity-driven defect states in anodized nanoporous Nb2O5: the possibility of improving performance of photoanodes. Chem. Commun. 2013, 49, 6349–6351. [Google Scholar] [CrossRef] [PubMed]
- Pou, J.; Lusquiños, F.; Comesaña, R.; Boutinguiza, M. Production of biomaterial coatings by laser-assisted processes. In Advances in Laser Materials Processing; Woodhead Publishing: Sawston, Cambridge, UK, 2010; pp. 394–425. [Google Scholar]
- Jung, K.; Kim, Y.; Im, H.; Kim, H.; Park, B. Leakage Transport in the High-resistance State of a Resistive-switching NbOx Thin Film Prepared by Pulsed Laser Deposition. J. Korean Phys. Soc. 2011, 59, 2778–2781. [Google Scholar] [CrossRef]
- Norton, D.P. Pulsed laser deposition of complex materials: Progress towards applications. In Pulsed laser Deposition of Thin FILMS: Applications-Led Growth of Functional Materials; Eason, R., Ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2007; pp. 3–5. [Google Scholar]
- Miller, J.C. 1. Introduction to Laser Desorption and Ablation. Exp. Methods Phys. Sci. 1997, 30, 1–13. [Google Scholar] [CrossRef]
- Christen, H.M.; Eres, G. Recent advances in pulsed-laser deposition of complex oxides. J. Physics: Condens. Matter 2008, 20, 264005. [Google Scholar] [CrossRef] [Green Version]
- Kuzanyan, A.A. Pulsed Laser Deposition of Large-Area Thin Films and Coatings. In Applications of Laser Ablation-Thin Film Deposition, Nanomaterial Synthesis and Surface Modification; IntechOpen: Rijeka, Croatia, 2016. [Google Scholar]
- Kittiwatanakul, S.; Anuniwat, N.; Dao, N.; Wolf, S.A.; Lu, J. Surface morphology control of Nb thin films by biased target ion beam deposition. J. Vac. Sci. Technol. A 2018, 36, 031507. [Google Scholar] [CrossRef] [Green Version]
- Hylton, T.L.; Ciorneiu, B.; Baldwin, D.A.; Escorcia, O.; Son, J.; McClure, M.T.; Waters, G. Thin film processing by biased target ion beam deposition. IEEE Trans. Magn. 2000, 36, 2966–2971. [Google Scholar] [CrossRef]
- Heo, K.C.; Son, P.K.; Sohn, Y.; Yi, J.; Kwon, J.H.; Gwag, J.S. Characteristics of Ion Beam Assisted ITO Thin Films Deposited by RF Magnetron Sputtering. Mol. Cryst. Liq. Cryst. 2014, 601, 57–63. [Google Scholar] [CrossRef]
- Ohring, M. Thin-Film Evaporation Processes. In Materials Science of Thin Films; Elsevier: Amsterdam, The Netherlands, 2002; pp. 95–144. [Google Scholar]
- Ahmad, I.; Ashraf, M.; Khan, Y.; Khawaja, E.E.; Ali, Z.; Abbas, T.A.; Waheed, A.; Akhtar, S.M.J. Effects of preparation conditions on the optical properties of niobium oxide thin films. Inorg. Nano-Metal Chem. 2019, 50, 22–27. [Google Scholar] [CrossRef]
- Bockute, K.; Laukaitis, G.; Milčius, D. The properties of nonstoichiometric lanthanum niobium oxide thin films formed using an e-beam deposition technique. Surf. Coat. Technol. 2013, 214, 97–100. [Google Scholar] [CrossRef]
- Bishop, C.A. Electron beam (E-Beam) evaporation. Vac. Depos. Onto Webs Films Foils 2015, 289–299. [Google Scholar]
- Agarwal, D.C.; Chauhan, R.; Kumar, A.; Kabiraj, D.; Singh, F.; Khan, S.A.; Avasthi, D.K.; Pivin, J.C.; Kumar, M.; Ghatak, J.; et al. Synthesis and characterization of ZnO thin film grown by electron beam evaporation. J. Appl. Phys. 2006, 99, 123105. [Google Scholar] [CrossRef]
- Kerdcharoen, T.; Wongchoosuk, C. Carbon nanotube and metal oxide hybrid materials for gas sensing. In Semiconductor Gas Sensors; Elsevier: Amsterdam, The Netherlands, 2013; pp. 386–407. [Google Scholar]
- Shin, H.-C.; Dong, J.; Liu, M. Nanoporous Structures Prepared by an Electrochemical Deposition Process. Adv. Mater. 2003, 15, 1610–1614. [Google Scholar] [CrossRef]
- Lee, G.R.; Crayston, J.A. Studies on the electrochemical deposition of niobium oxide. J. Mater. Chem. 1996, 6, 187–192. [Google Scholar] [CrossRef]
- Cai, Y.-N.; Yang, S.-H.; Jin, S.-M.; Yang, H.-P.; Hou, G.-F.; Xia, J.-Y. Electrochemical synthesis, characterization and thermal properties of niobium ethoxide. J. Central South Univ. Technol. 2011, 18, 73–77. [Google Scholar] [CrossRef]
- Ismail, M.I. Periodic reverse current electroplating and surface finishing. J. Appl. Electrochem. 1979, 9, 407–410. [Google Scholar] [CrossRef]
- Feng, L.; Sun, X.; Yao, S.; Liu, C.; Xing, W.; Zhang, J. Electrocatalysts and Catalyst Layers for Oxygen Reduction Reaction. In Rotating Electrode Methods and Oxygen Reduction Electrocatalysts; Elsevier: Amsterdam, The Netherlands, 2014; pp. 67–132. [Google Scholar]
- Jayakrishnan, D.S. Electrodeposition: the versatile technique for nanomaterials. In Corrosion Protection and Control Using Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2012; pp. 86–125. [Google Scholar]
- Fomanyuk, S.S.; Krasnov, Y.S.; Kolbasov, G.; Zaichenko, V.N. Electrochemical deposition of electrochromic niobium oxide films from an acidic solution of niobium peroxo complexes. Russ. J. Appl. Chem. 2013, 86, 644–647. [Google Scholar] [CrossRef]
- Fiz, R.; Appel, L.; Gutiérrez-Pardo, A.; Ramírez-Rico, J.; Mathur, S. Electrochemical energy storage applications of CVD grown niobium oxide thin films. ACS Appl. Mater. Interfaces 2016, 8, 21423–21430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaur, R.; Mishra, L.; Siddiqi, M.A.; Atakan, B. Ruthenium complexes as precursors for chemical vapor-deposition (CVD). RSC Adv. 2014, 4, 33785–33805. [Google Scholar] [CrossRef] [Green Version]
- George, S.M.; Park, B.K.; Kim, C.G.; Chung, T.-M. Heteroleptic Group 2 Metal Precursors for Metal Oxide Thin Films. Eur. J. Inorg. Chem. 2014, 2014, 2002–2010. [Google Scholar] [CrossRef]
- Wang, J.-T. CVD and its Related Theories in Inorganic Synthesis and Materials Preparations. In Modern Inorganic Synthetic Chemistry; Elsevier: Amsterdam, The Netherlands, 2011; pp. 151–171. [Google Scholar]
- Eftekhari, A.; Jafarkhani, P.; Moztarzadeh, F. High-yield synthesis of carbon nanotubes using a water-soluble catalyst support in catalytic chemical vapor deposition. Carbon 2006, 44, 1343–1345. [Google Scholar] [CrossRef]
- Chen, L.; Sun, Q.-Q.; Gu, J.-J.; Xu, Y.; Ding, S.-J.; Zhang, D.W. Bipolar resistive switching characteristics of atomic layer deposited Nb2O5 thin films for nonvolatile memory application. Curr. Appl. Phys. 2011, 11, 849–852. [Google Scholar] [CrossRef]
- Kalantar-zadeh, K.; Fry, B. Nanotechnology-Enabled Sensors; Springer Science & Business Media: Berlin, Germany, 2007. [Google Scholar]
- Hashmi, M. Introduction to Sensor Materials, Technologies and Applications. Comprehensive Materials Processing 2014, 1–3. [Google Scholar] [CrossRef]
- Ritala, M.; Niinistö, J. Industrial Applications of Atomic Layer Deposition. ECS Trans. 2019, 25, 641–652. [Google Scholar] [CrossRef]
- He, W. ALD: Atomic Layer Deposition—Precise and Conformal Coating for Better Performance. In Handbook of Manufacturing Engineering and Technology; Springer: London, UK, 2015; pp. 2959–2996. [Google Scholar]
- Nivedita, L.R.; Haubert, A.; Battu, A.K.; Ramana, C.V. Correlation between Crystal Structure, Surface/Interface Microstructure, and Electrical Properties of Nanocrystalline Niobium Thin Films. Nanomaterials 2020, 10, 1287. [Google Scholar] [CrossRef]
- Marques, A.C.; Almeida, R.M.; Thiema, A.; Wang, S.; Falk, M.; Jain, H. Sol-gel-derived glass scaffold with high pore interconnectivity and enhanced bioactivity. J. Mater. Res. 2009, 24, 3495–3502. [Google Scholar] [CrossRef]
- Lee, J.-H. Technological realization of semiconducting metal oxide–based gas sensors. In Gas Sensors Based on Conducting Metal Oxides; Elsevier: Amsterdam, The Netherlands, 2019; pp. 167–216. [Google Scholar]
- Schneider, C.W.; Lippert, T. Laser Ablation and Thin Film Deposition. In Laser Processing of Materials; Springer Science & Business Media: Berlin, Germany, 2010; pp. 89–112. [Google Scholar]
- DeMeo, D.; Macnaughton, S.; Sonkusale, S.; Vandervelde, T. Electrodeposited Copper Oxide and Zinc Oxide Core-Shell Nanowire Photovoltaic Cells. Nanowires Implement. Appl. 2011, 141–156. [Google Scholar] [CrossRef] [Green Version]
- Rockett, A. The Materials Science of Semiconductors; Springer Science and Business Media: Berlin, Germany, 2008. [Google Scholar]
- Oviroh, P.O.; Akbarzadeh, R.; Pan, D.; Coetzee, R.A.M.; Jen, T.-C. New development of atomic layer deposition: processes, methods and applications. Sci. Technol. Adv. Mater. 2019, 20, 465–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nwanna, E.; Coetzee, R.A.M.; Jen, T.-C. Investigating the Purge Flow Rate in a Reactor Scale Simulation of an Atomic Layer Deposition Process. In Volume 2B: Advanced Manufacturing; ASME International: New York, NY, USA, 2019. [Google Scholar]






| Lattice Constant | ||||||
|---|---|---|---|---|---|---|
| Crystal Phase | Space Group | a (Ȃ) | b(Ȃ) | c(Ȃ) | Temperature (°C) | Refs |
| Tetragonal | I4/mmm | 20.44 | 3.83 | 3.82 | 900 | [68,69] |
| Pseudohexagonal | P6/mmm | 3.60 | 3.61 | 3.92 | 500 | [70,71] |
| Monoclinic β = 119.9° ± 0.4° | P12m1, P2, P2/m | 21.14 | 3.82 | 19.45 | ˃1000 | [64,72,73] |
| Orthorhombic | Pbam | 6.19 | 3.625 | 3.94 | - | [70,74] |
| Methods | Merits | Demerits | Applications | References |
|---|---|---|---|---|
| Sol-gel | (1) It allows the deposition of thin films on different surfaces. (2) Due to the process being a simple one, it requires no expensive equipment. (3) Energy Consumption is minimized. | (1) It is moisture sensitive. (2) Possibility of high cost of precursors. (3) Tendency of the wet gel to shrink when dried. | Piezoelectric devices, catalysts, superconductors, etc, are some of the film’s applications process. | [93,94,95,96] |
| Chemical Spray Pyrolysis | (1) Easy addition of doping materials. (2) Increased film growth rate. (3) The final product possesses chemical homogeneity. | (1) Scaling up can be quite challenging. (2) It has an excessive low yield. (3) There is the possibility of the sulphides oxidizing when being processed in the air. | It is applied in solid oxide fuel cells, sensors, and solar cells. | [98,99,101,102] |
| Sputtering | (1) Materials with high melting point can be sputtered. (2) Capable of depositing a vast range of metals, alloys, composites and insulators. (3) Zero X-ray defect. | (1) Low deposition rates of some materials. (2) Expensive sputtering targets. (3) Dielectrics require RF source. | It is applied in computer hard disk, integrated circuits, thin-film transistors, polymerization and dehydration processes, capacitors, optical systems. | [103,104,105,106] |
| Pulsed Laser Deposition | (1) High versatility for the deposition of materials of distinct film thicknesses. (2) The process is a fast and flexible one. (3) It is inexpensive. | (1) It possesses irregular material coverage. (2) There is the occurrence of splashing of liquid droplets, and solid particles. (3) Loss of evaporative materials. | The microelectronics, hybrid systems, superconductors, and optical industries are some of its film applications. | [110,111,112,113] |
| Biased target ion beam deposition | (1) The ions incident on the growing surface can be controlled. (2) Enables the variation of independent ion species as well as current density of the ion beams. (3) High quality of surface. | (1) High complexity level. (2) Cost of maintenance is high. (3) Possesses low deposition rates and scaling up difficulties. | It is used in the application of circuits, electronic devices, high reflective mirrors, RF neutralizers, quartz discharge chambers. | [48,114,115] |
| Electron Beam Evaporation | (1) Its efficiency in material utilization is quite high. (2) There is extremely high deposition rate. (3) Provision of room for morphological, as well as structural film control. | (1) Internal surfaces of complex geometries are hardly coated using this process. (2) Step coverage are not easily improved. (3) E-beam evaporation could lead to X-ray destruction. | It is made use of in fuel cells, solar energy layers, bio medics, thermal barrier coatings, wear-resistance, hard coatings, and thin-film solar applications. | [120,121,122] |
| Electrochemical Deposition Process | (1) It is quite affordable. (2) Possesses scalability. (3) Very simple to use. | (1) It has the propensity to produce non-conformal growths on irregular surfaces. (2) The procedure involves numerous metal coatings which consumes a lot of time. (3) There is the issue of waste disposal derived from the electroplating process, as it pollutes the surroundings. | It is applied in sensors, catalysis, energy storages, separations techniques as micro separators, and in template synthesizing of nanomaterials | [126,127,128] |
| Chemical Vapour Deposition | (1) The films derived from the CVD process are fairly conformal. (2) There is high deposition rate of film. (3) Concurrent materials introduction. | (1) High deposition temperatures are a necessity. (2) Not suitable for extremely thin films. (3) Lacks conformity on the surfaces of very high aspect ratios. | Employed in conductor applications, as well as in dielectrics, high-temperature, passivation layers, solar cells, composites of fibre, and particles of definite dimensions. | [131,132,133,134] |
| Atomic Layer Deposition | (1) The ALD process produces large areas and batch depositions since it permits only reactions at the interface. (2) It efficiently creates ultra-uniform thin films on surfaces of extremely high-aspect-ratios. (3) It is capable of producing film thicknesses that are precise, reproducible, and of easy control. | (1) The process acquires a lengthy deposition rate. (2) The very high cost of the equipment is also a restraint. (3) Nanoparticles emission in the course of the ALD process is as well a limiting factor. | Used in the application of nanotechnology, photonics, nanolaminates, biotechnology photovoltaics, as well as many other applications that are energy related. | [136,137,138,139] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emeka, N.C.; Imoisili, P.E.; Jen, T.-C. Preparation and Characterization of NbxOy Thin Films: A Review. Coatings 2020, 10, 1246. https://doi.org/10.3390/coatings10121246
Emeka NC, Imoisili PE, Jen T-C. Preparation and Characterization of NbxOy Thin Films: A Review. Coatings. 2020; 10(12):1246. https://doi.org/10.3390/coatings10121246
Chicago/Turabian StyleEmeka, Nwanna Charles, Patrick Ehi Imoisili, and Tien-Chien Jen. 2020. "Preparation and Characterization of NbxOy Thin Films: A Review" Coatings 10, no. 12: 1246. https://doi.org/10.3390/coatings10121246
APA StyleEmeka, N. C., Imoisili, P. E., & Jen, T.-C. (2020). Preparation and Characterization of NbxOy Thin Films: A Review. Coatings, 10(12), 1246. https://doi.org/10.3390/coatings10121246






