Optimizing Heat Treatment for Electroplated NiP and NiP/SiC Coatings
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Deposition Methods
2.2. Coating Characterization
3. Results and Discussion
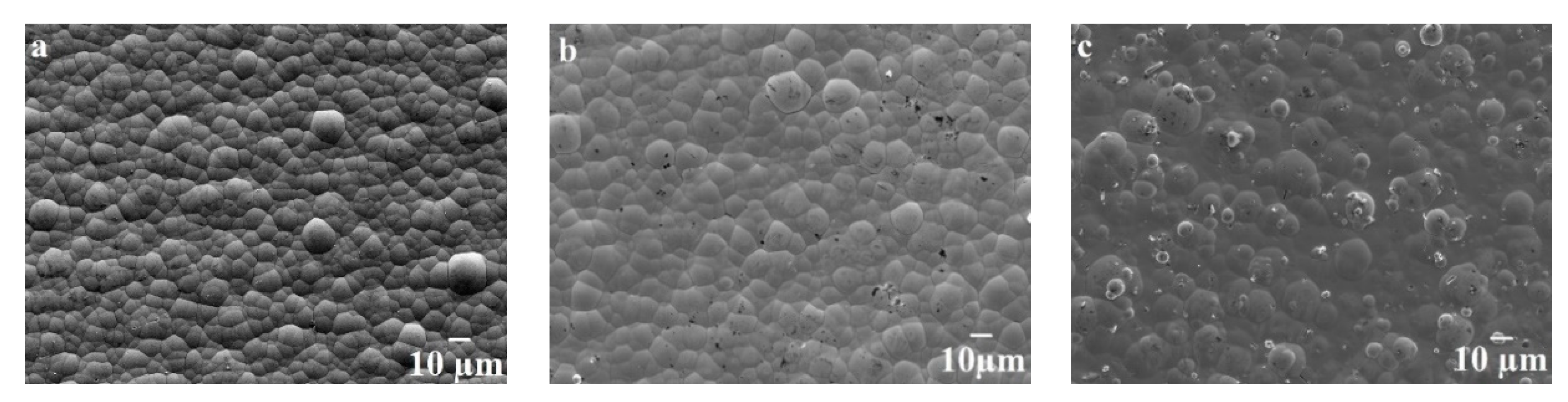
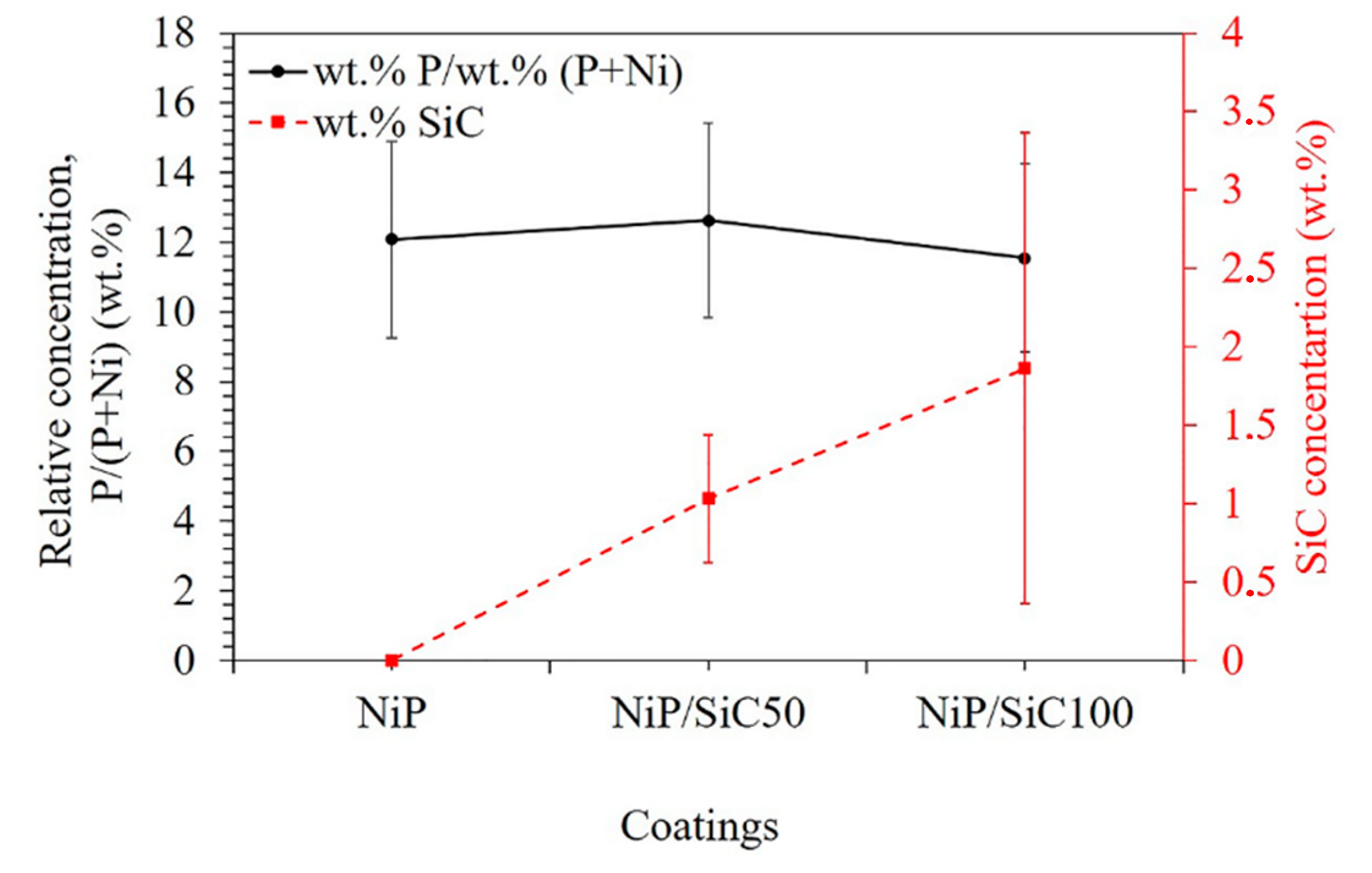
3.1. Morphology and Composition
3.2. DSC
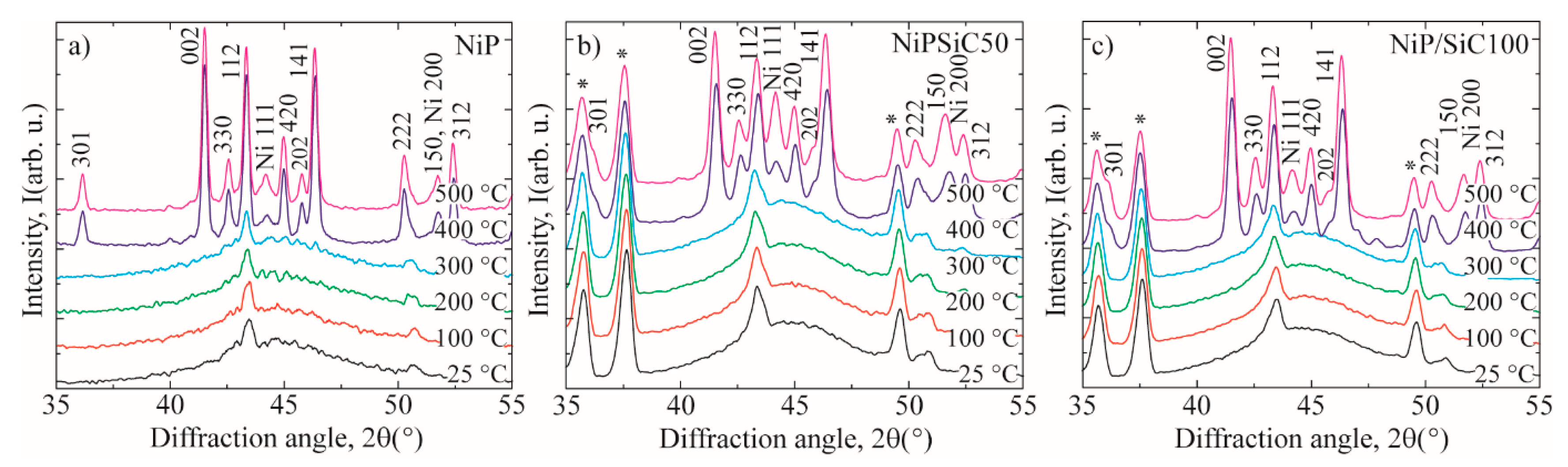
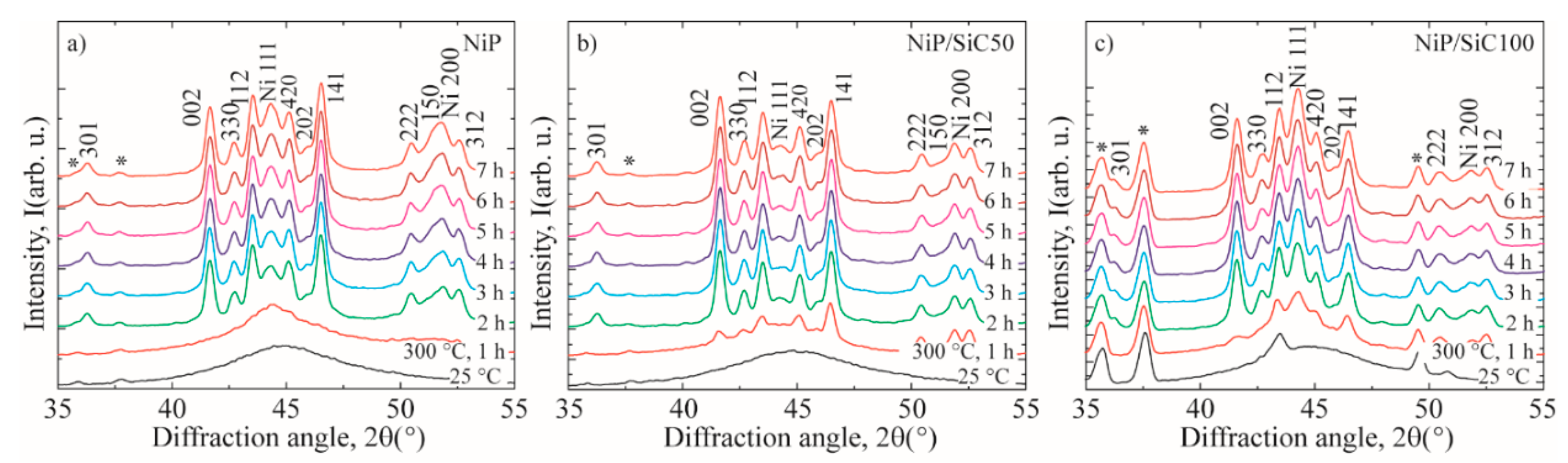
3.3. XRD
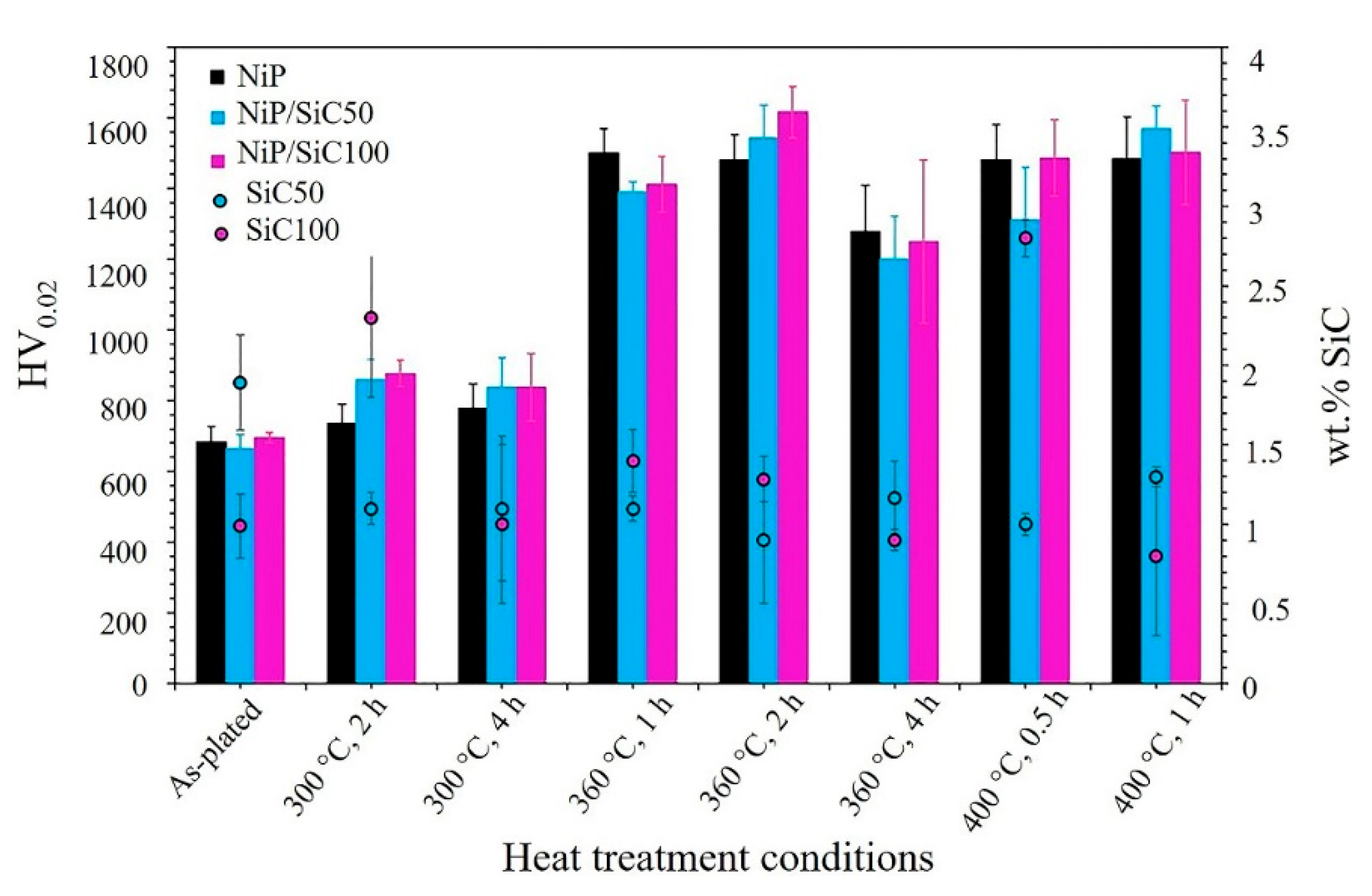
3.4. Microhardness and Nanoindentation
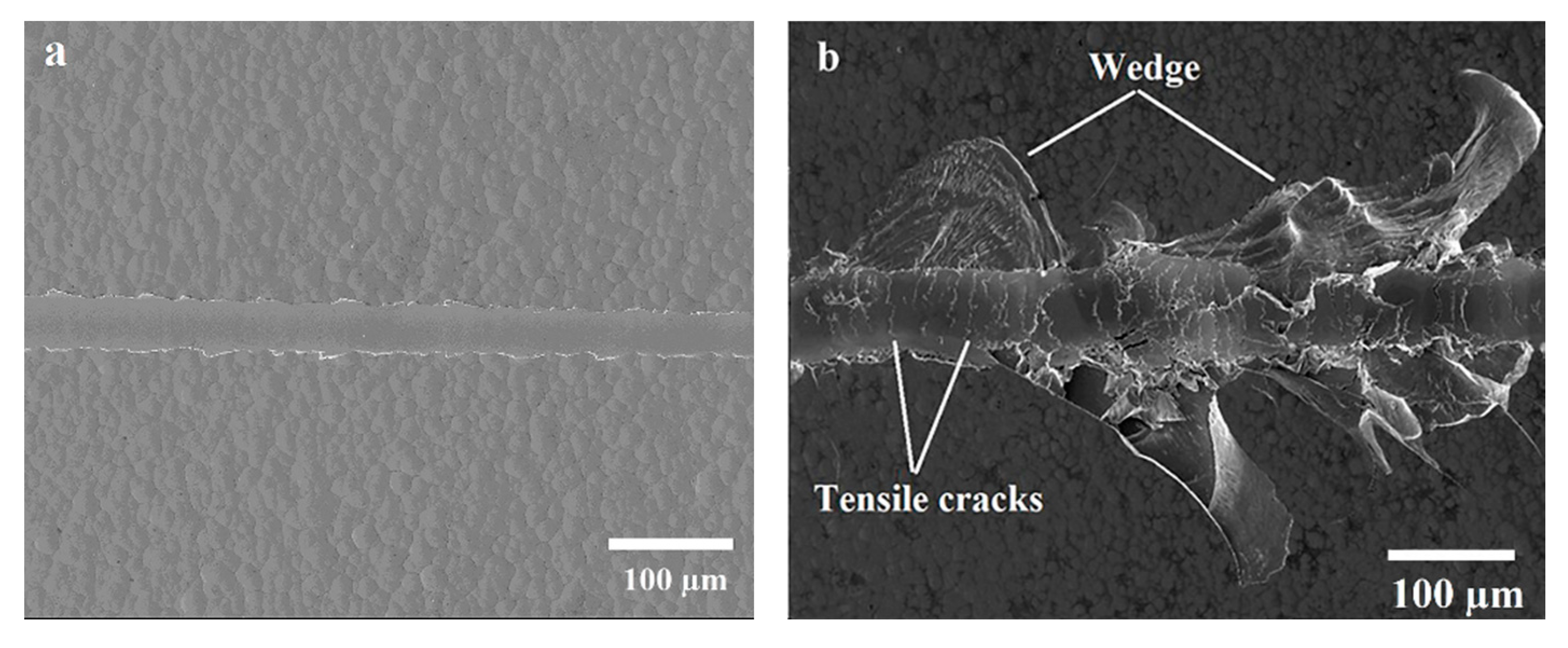
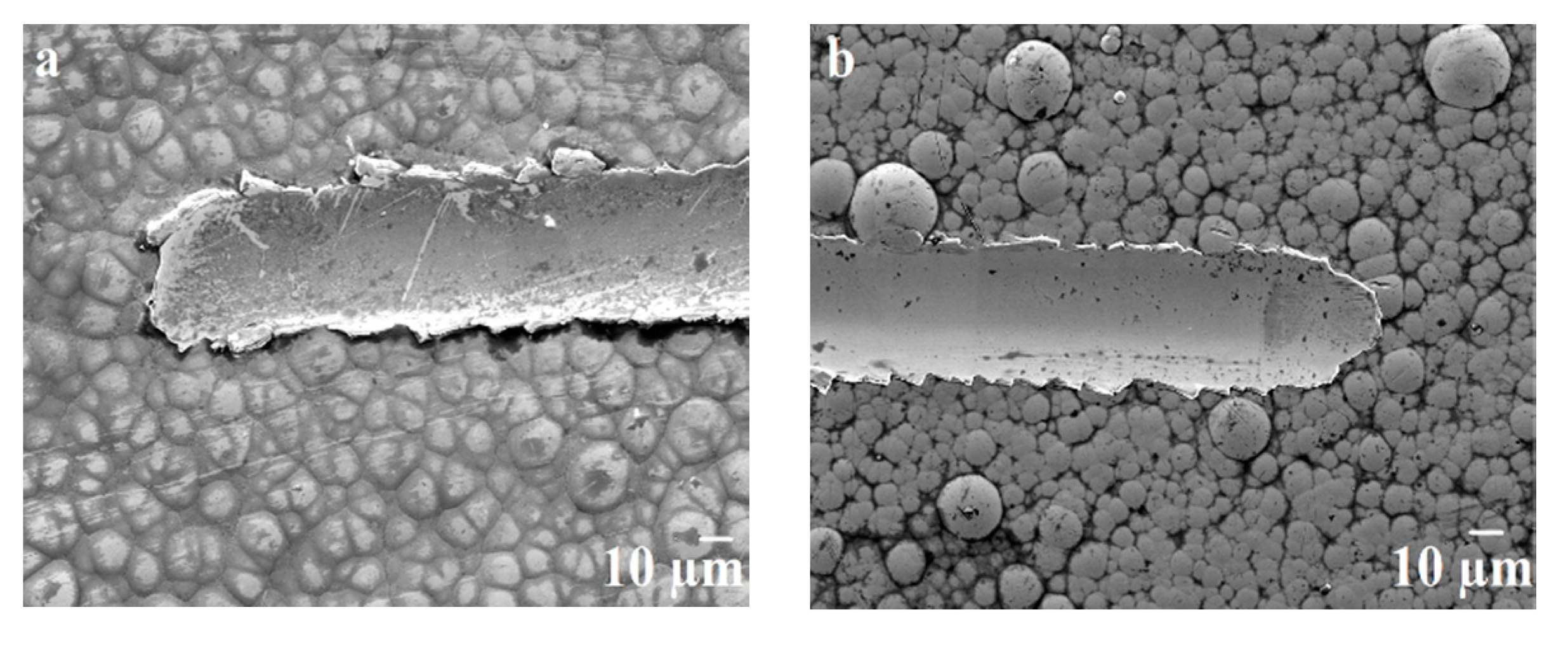
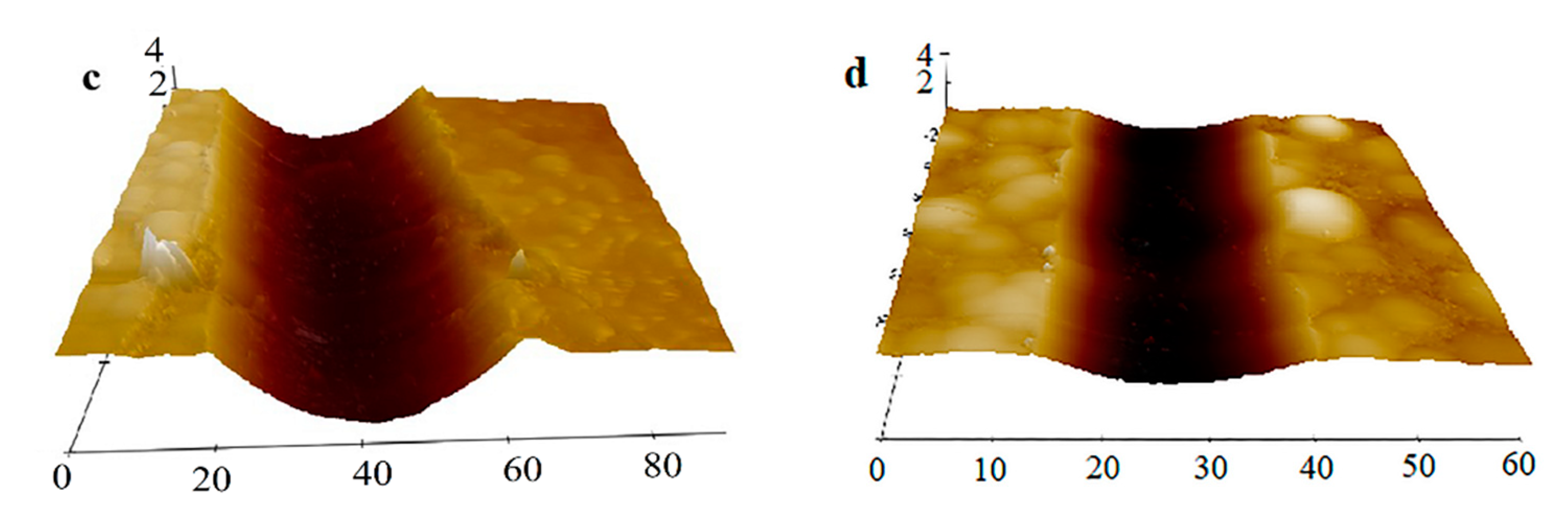
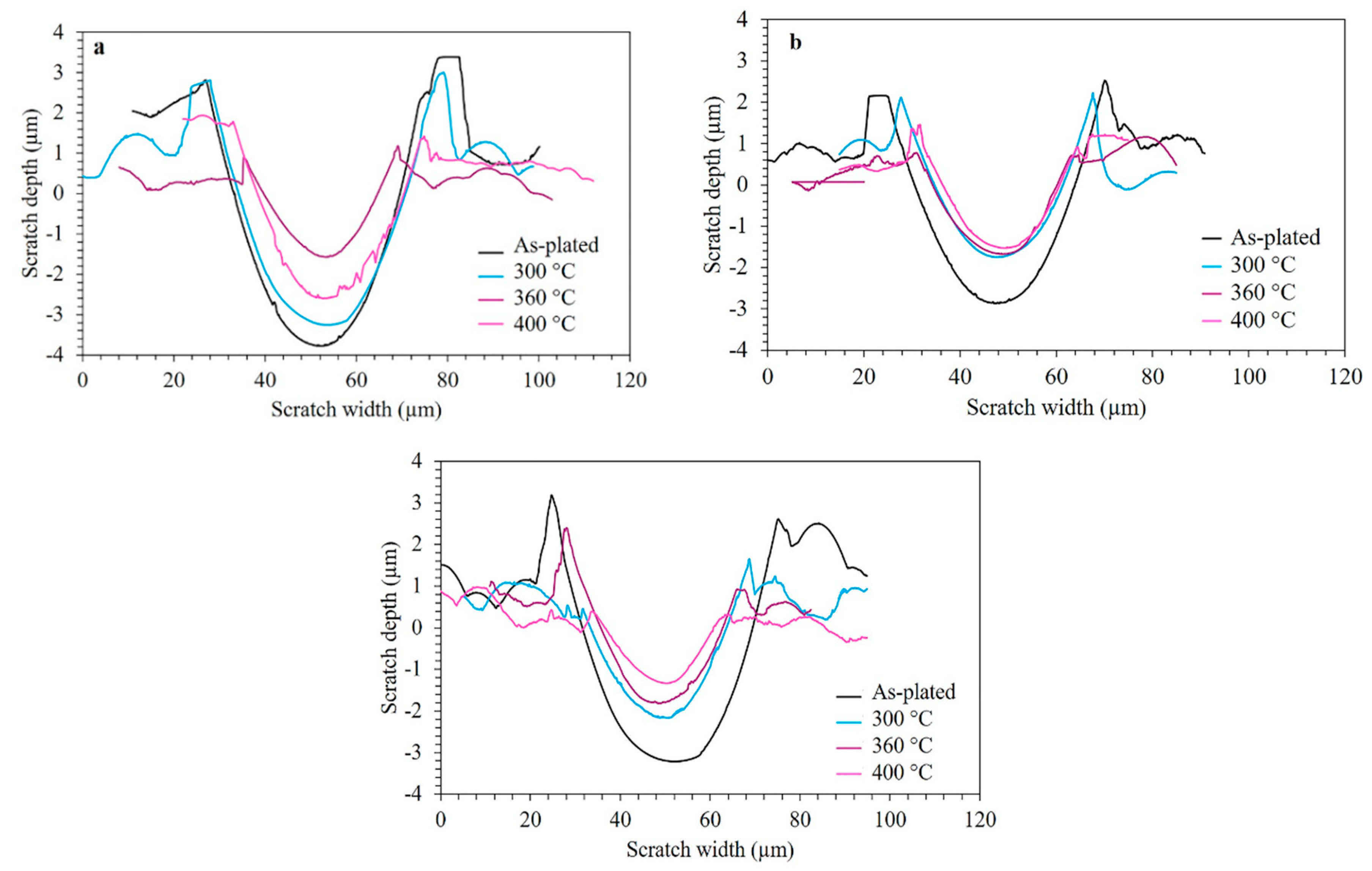
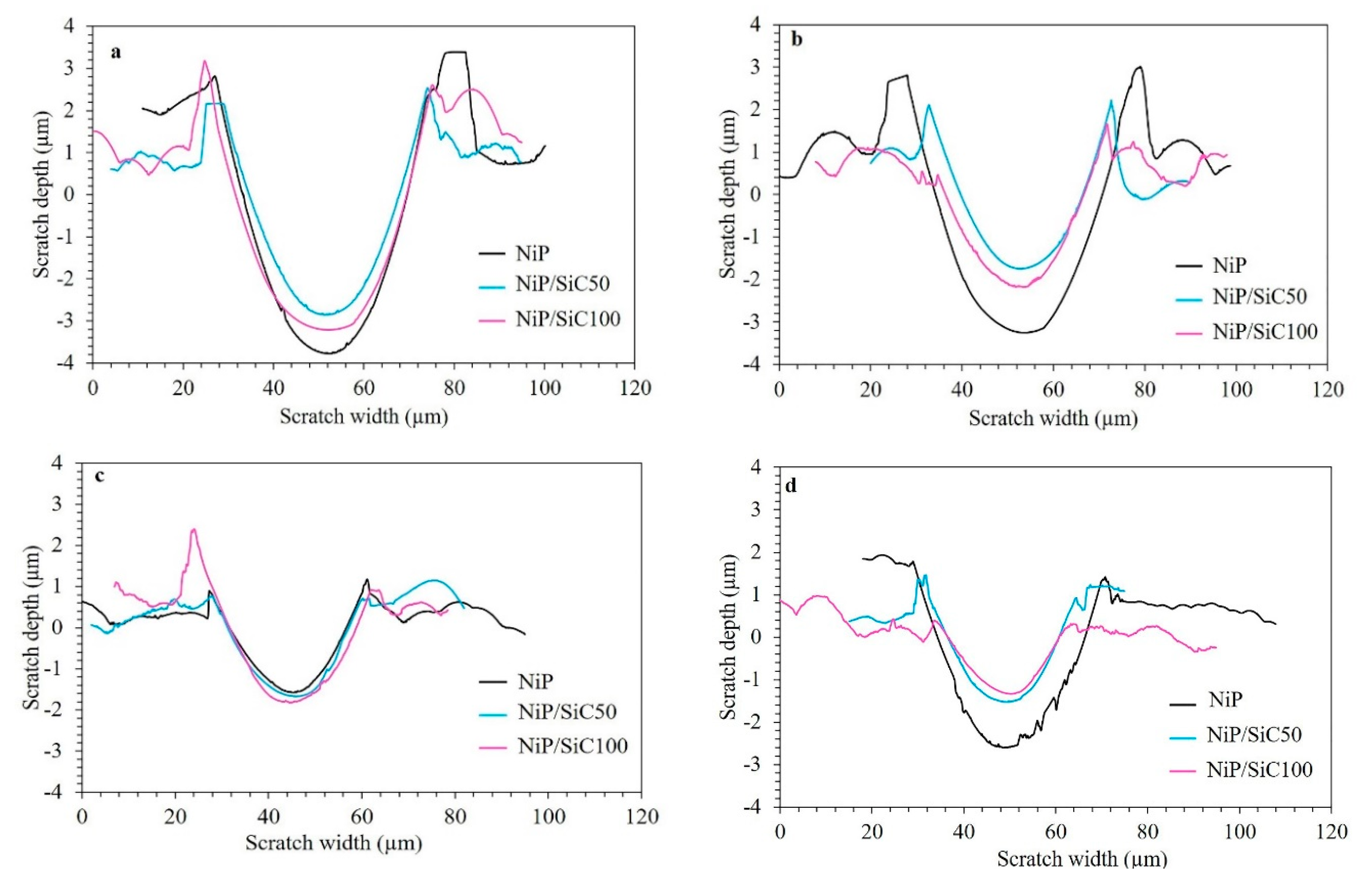
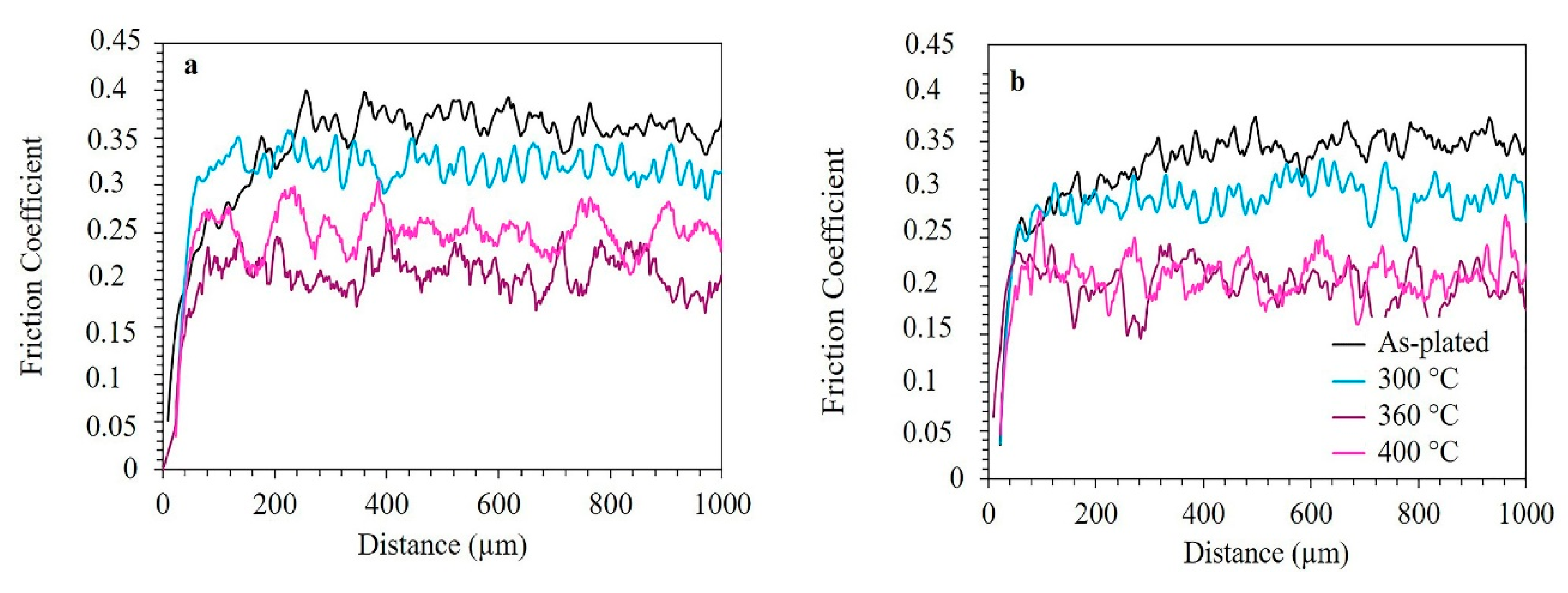
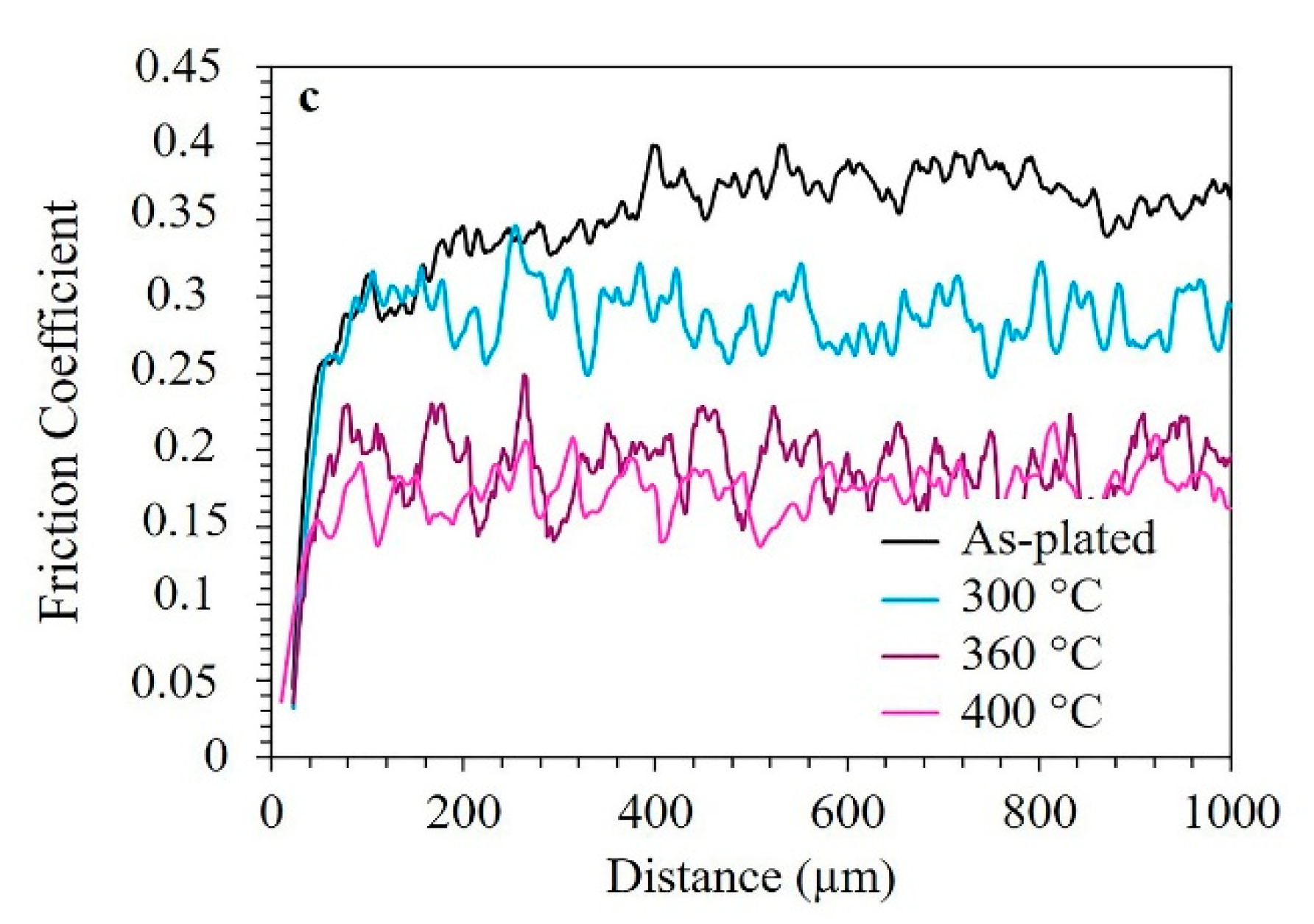
3.5. Micro-Scratch
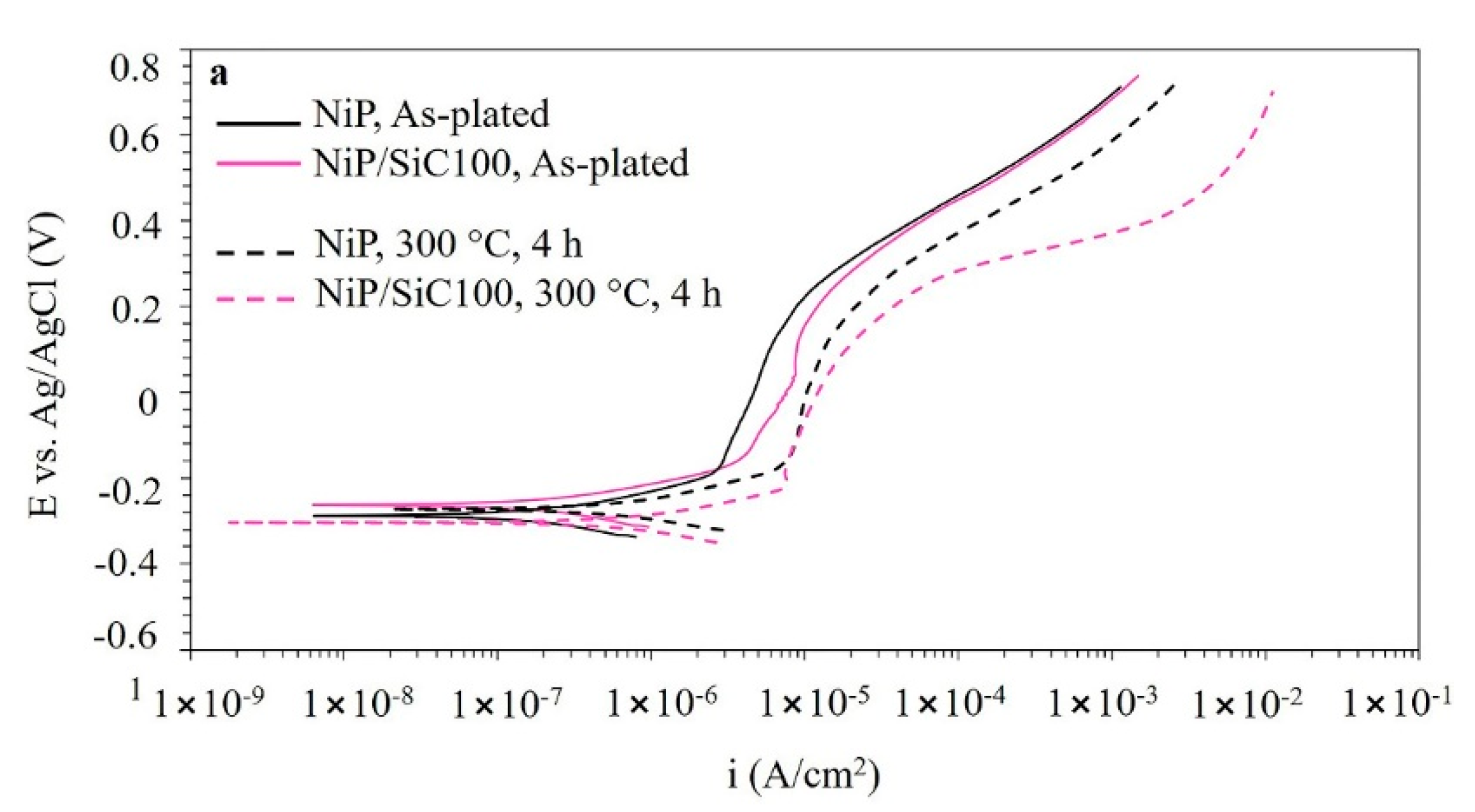
3.6. Polarization Curves
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Balaraju, J.N.; Rajam, K.S. Preparation and characterization of autocatalytic low phosphorus nickel coatings containing submicron silicon nitride particles. J. Alloys Compd. 2008, 459, 311–319. [Google Scholar] [CrossRef]
- Mazaheri, H.; Allahkaram, S.R. Deposition, characterization and electrochemical evaluation of Ni-P-nano diamond composite coatings. Appl. Surf. Sci. 2012, 258, 4574–4580. [Google Scholar] [CrossRef]
- Karrab, S.A.; Doheim, M.A.; Aboraia, M.S.; Ahmed, S.M. Effect of heat treatment and bath composition of electroless nickel-plating on cavitation erosion resistance. J. Eng. Sci. Assiut Univ. 2013, 4, 1989–2011. [Google Scholar] [CrossRef]
- Brenner, A. Electrodeposition of Alloys: Principles and Practice; Electrodeposition Alloy; Academic Press: New York, NY, USA, 1963; Volume ii. [Google Scholar] [CrossRef]
- Pillai, A.M.; Rajendra, A.; Sharma, A.K. Electrodeposited nickel-phosphorous (Ni-P) alloy coating: An in-depth study of its preparation, properties, and structural transitions. J. Coat. Technol. Res. 2012, 9, 785–797. [Google Scholar] [CrossRef]
- Harris, T.M.; Dang, Q.D. The mechanism of phosphorus incorporation during the electrodeposition of nickel-phosphorus alloys. J. Electrochem. Soc. 1993, 140, 81–83. [Google Scholar] [CrossRef]
- Lin, C.S.; Lee, C.Y.; Chen, F.J.; Chien, C.T.; Lin, P.L.; Chung, W.C. Electrodeposition of nickel-phosphorus alloy from sulfamate baths with improved current efficiency. J. Electrochem. Soc. 2006, 153, 387–392. [Google Scholar] [CrossRef]
- Bredael, E.; Celis, J.P.; Roos, J.R. NiP electrodeposition on a rotating-disc electrode and in a jet cell: Relationship between plating parameters and structural characteristics. Surf. Coat. Technol. 1993, 58, 63–71. [Google Scholar] [CrossRef]
- Hansal, W.E.G.; Sandulache, G.; Mann, R.; Leisner, P. Pulse-electrodeposited NiP–SiC composite coatings. Electrochim. Acta 2013, 114, 851–858. [Google Scholar] [CrossRef]
- Wang, L.; Gao, Y.; Xue, Q.; Liu, H.; Xu, T. A novel electrodeposited Ni-P gradient deposit for replacement of conventional hard chromium. Surf. Coat. Technol. 2006, 200, 3719–3726. [Google Scholar] [CrossRef]
- Sudagar, J.; Lian, J.; Sha, W. Electroless nickel, alloy, composite and nano coatings—A critical review. J. Alloys Compd. 2013, 571, 183–204. [Google Scholar] [CrossRef]
- Wang, Q.; Callisti, M.; Miranda, A.; McKay, B.; Deligkiozi, I.; Milickovic, T.K.; Zoikis-Karathanasis, A.; Hrissagis, K.; Magagnin, L.; Polcar, T. Evolution of structural, mechanical and tribological properties of Ni-P/MWCNT coatings as a function of annealing temperature. Surf. Coat. Technol. 2016, 302, 195–201. [Google Scholar] [CrossRef]
- Hou, K.H.; Hwu, W.H.; Ke, S.T.; der Ger, M. Ni–P–SiC composite produced by pulse and direct current plating. Mater. Chem. Phys. 2006, 100, 54–59. [Google Scholar] [CrossRef]
- Mafi, I.R.; Dehghanian, C. Studying the effects of the addition of TiN nanoparticles to Ni–P electroless coatings. Appl. Surf. Sci. 2011, 258, 1876–1880. [Google Scholar] [CrossRef]
- Shibli, S.M.A.; Dilimon, V.S. Effect of phosphorous content and TiO2-reinforcement on Ni–P electroless plates for hydrogen evolution reaction. Int. J. Hydrogen Energy 2007, 32, 1694–1700. [Google Scholar] [CrossRef]
- Islam, M.; Rizwan, M.; Fredj, N.; Burleigh, T.D.; Oloyede, O.R.; Almajid, A.A.; Shah, S.I. Influence of SiO2 nanoparticles on hardness and corrosion resistance of electroless Ni–P coatings. Surf. Coat. Technol. 2015, 261, 141–148. [Google Scholar] [CrossRef]
- Matik, U. Structural and wear properties of heat-treated electroless Ni-P alloy and Ni–P–Si3N4 composite coatings on iron based PM compacts. Surf. Coat. Technol. 2016, 302, 528–534. [Google Scholar] [CrossRef]
- Hamid, Z.A.; el Badry, S.A.; Aal, A.A. Electroless deposition and characterization of Ni–P–WC composite alloys. Surf. Coat. Technol. 2007, 201, 5948–5953. [Google Scholar] [CrossRef]
- Shakoor, R.A.; Kahraman, R.; Waware, U.; Wang, Y.; Gao, W. Properties of electrodeposited Ni–B–Al2O3 composite coatings. J. Mater. 2014, 64, 127–135. [Google Scholar] [CrossRef]
- Bernasconi, R.; Allievi, F.; Sadeghi, H.; Magagnin, L. Codeposition of nickel–phosphorus alloys reinforced with boron carbide microparticles: Direct and pulse plating. Int. J. Surf. Eng. Coat. 2017, 95, 52–59. [Google Scholar] [CrossRef]
- Sarret, M.; Müller, C.; Amell, A. Electroless NiP micro- and nano-composite coatings. Surf. Coat. Technol. 2006, 201, 389–395. [Google Scholar] [CrossRef]
- Aslanyan, I.R.; Bonino, J.P.; Celis, J.P. Effect of reinforcing submicron SiC particles on the wear of electrolytic NiP coatings. Part 2: Bi-directional sliding. Surf. Coat. Technol. 2006, 201, 581–589. [Google Scholar] [CrossRef]
- Alexis, J.; Etcheverry, B.; Beguin, J.D.; Bonino, J.P. Structure, morphology and mechanical properties of electrodeposited composite coatings Ni–P/SiC. Mater. Chem. Phys. 2010, 120, 244–250. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Vijayaraghavan, L.; Madhavan, S.; Almeida, A. Study on the mechanical properties of heat-treated electroless NiP coatings reinforced with Al2O3 nano particles. Metall. Mater. Trans. A 2016, 47, 2223–2231. [Google Scholar] [CrossRef]
- Keong, K.G.; Sha, W. Crystallisation and phase transformation behaviour of electroless nickel-phosphorus deposits and their engineering properties. Surf. Eng. 2013, 844, 329–343. [Google Scholar] [CrossRef]
- Apachitei, I.; Tichelaar, F.D.; Duszczyk, J.; Katgerman, L. The effect of heat treatment on the structure and abrasive wear resistance of autocatalytic NiP and NiP–SiC coatings. Surf. Coat. Technol. 2002, 149, 263–278. [Google Scholar] [CrossRef]
- Buchtík, M.; Kosár, P.; Wasserbauer, J.; Tkacz, J.; Doležal, P. Characterization of electroless Ni–P coating prepared on a wrought ZE10 magnesium alloy. Coatings 2018, 8, 96. [Google Scholar] [CrossRef]
- Jiaqiang, G.; Yating, W.; Lei, L.; Bin, S.; Wenbin, H. Crystallization temperature of amorphous electroless nickel—Phosphorus alloys. Mater. Lett. 2005, 59, 1665–1669. [Google Scholar] [CrossRef]
- Keong, K.G.; Sha, W.; Malinov, S. Crystallisation kinetics and phase transformation behaviour of electroless nickel-phosphorus deposits with high phosphorus content. J. Alloys Compd. 2002, 334, 192–199. [Google Scholar] [CrossRef]
- Keong, K.G.; Sha, W.; Malinov, S. Crystallization and phase transformation behaviour of electroless nickel-phosphorus deposits with low and medium phosphorus contents under continuous heating. J. Mater. Sci. 2002, 37, 4445–4450. [Google Scholar] [CrossRef]
- Hur, K.; Jeong, J.; Lee, D. Microstructures and crystallization of electroless Ni–P deposits. J. Mater. Sci. 1990, 25, 2573–2584. [Google Scholar] [CrossRef]
- Ng, P.K.; Snyder, D.D.; Lasala, J.; Clemens, B.; Fuerst, C. Structure and crystallization of nickel-phosphorus alloys prepared by high-rate electrodeposition. J. Electrochem. Soc. 1988, 135, 1376–1381. [Google Scholar] [CrossRef]
- Zanella, C.; Lekka, M.; Bonora, P.P. Influence of the particle size on the mechanical and electrochemical behaviour of micro- and nano-nickel matrix composite coatings. J. Appl. Elechtrochem. 2009, 39, 31–38. [Google Scholar] [CrossRef]
- Lekka, M.; Lanzutti, A.; Zanella, C.; Zendron, G.; Fedrizzi, L.; Bonora, P.L. Resistance to localized corrosion of pure Ni, micro- and nano-SiC composite electrodeposits. Pure Appl. Chem. 2011, 83, 295–308. [Google Scholar] [CrossRef]
- Ahmadkhaniha, D.; Eriksson, F.; Leisner, P.; Zanella, C. Effect of SiC particle size and heat-treatment on microhardness and corrosion resistance of NiP electrodeposited coatings. J. Alloys Compd. 2018, 769, 1080–1087. [Google Scholar] [CrossRef]
- Bonin, L.; Vitry, V.; Delaunois, F. Corrosion behaviour of electroless high boron-mid phosphorous nickel duplex coatings in the as-plated and heat-treated states in NaCl, H2SO4, NaOH and Na2SO4 media. Mater. Chem. Phys. 2018, 208, 77–84. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. Measurement of hardness and elastic modulus by instrumented indentation: Advances in understanding and refinements to methodology. J. Mater. Res. 2004, 19, 3–20. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Saha, R.; Nix, W.D. Effects of the substrate on the determination of thin film mechanical properties by nanoindentation. Acta Mater. 2002, 50, 23–38. [Google Scholar] [CrossRef]
- Chang, C.; Hou, K.; Ger, M.; Chung, C.; Lin, J. Effects of annealing temperature on microstructure, surface roughness, mechanical and tribological properties of Ni–P and Ni–P/SiC films. Surf. Coat. Technol. 2016, 288, 135–143. [Google Scholar] [CrossRef]
- Ahmadkhaniha, D.; Tsongas, K.; Tzetzis, D.; Zanella, C. Study of the effect of pulse plating parameters on the electrodeposition of NiP and NiP/SiC coatings and their microhardness values. Trans. IMF 2020, 98, 1–10. [Google Scholar]
- Balaraju, J.N.; Narayanan, T.S.N.S.; Seshadri, S.K. Structure and phase transformation behaviour of electroless Ni–P composite coatings. Mater. Res. Bull. 2006, 41, 847–860. [Google Scholar] [CrossRef]
- Chou, M.; Ger, M.; Ke, S.; Huang, Y.; Wu, S. The Ni–P–SiC composite produced by electro-codeposition. Mater. Chem. Phys. 2005, 92, 146–151. [Google Scholar] [CrossRef]
- Sribalaji, M.; Rahman, O.S.A.; Laha, T.; Keshri, A.K. Nanoindentation and nanoscratch behavior of electroless deposited nickel-phosphorous coating. Mater. Chem. Phys. 2016, 177, 220–228. [Google Scholar] [CrossRef]
- Lee, K.; Takai, O. Nanoindentation study on nanomechanical characteristics of a-CN film deposited by shielded arc ion plating. Diam. Relat. Mater. 2005, 14, 1444–1450. [Google Scholar] [CrossRef]
- Leyland, A.; Matthews, A. On the significance of the H/E ratio in wear control: A nanocomposite coating approach to optimised tribological behaviour. Wear 2000, 246, 1–11. [Google Scholar] [CrossRef]
- Vitry, V.; Delaunois, F.; Dumortier, C. Mechanical properties and scratch test resistance of nickel–Boron coated aluminium alloy after heat treatments. Surf. Coat. Technol. 2008, 202, 3316–3324. [Google Scholar] [CrossRef]
- Beake, B.D.; Vishnyakov, V.M.; Harris, A.J. Relationship between mechanical properties of thin nitride-based films and their behaviour in nano-scratch tests. Tribol. Int. 2011, 44, 468–475. [Google Scholar] [CrossRef]
- Bull, S.J.; Berasetegui, E.G. An overview of the potential of quantitative coating adhesion measurement by scratch testing. Tribol. Int. 2006, 9, 99–114. [Google Scholar] [CrossRef]
- Gyawali, G.; Tripathi, K.; Joshi, B.; Wohn, S. Mechanical and tribological properties of Ni–W–TiB2 composite coatings. J. Alloys Compd. 2017, 721, 757–763. [Google Scholar] [CrossRef]
- Panich, N.; Sun, Y. Mechanical characterization of nanostructured TiB2 coatings using microscratch techniques. Tribol. Int. 2006, 39, 138–145. [Google Scholar] [CrossRef]
- Zawischa, M.; Makowski, S.; Schwarzer, N.; Weihnacht, V. Scratch resistance of superhard carbon coatings—A new approach to failure and adhesion evaluation. Surf. Coat. Technol. 2016, 308, 341–348. [Google Scholar] [CrossRef]
- Liew, K.W.; Kong, H.J.; Low, K.O.; Kok, C.K.; Lee, D. The effect of heat treatment duration on mechanical and tribological characteristics of Ni–P-PTFE coating on low carbon high tensile steel. J. Mater. 2014, 62, 430–442. [Google Scholar] [CrossRef]
- Beegan, D.; Chowdhury, S.; Laugier, M.T. Comparison between nanoindentation and scratch test hardness (scratch hardness) values of copper thin films on oxidised silicon substrates. Surf. Coat. Technol. 2007, 201, 5804–5808. [Google Scholar] [CrossRef]
- Jardret, V.; Zahouani, H.; Loubet, L.; Mathia, T.G. Understanding and quantification of elastic and plastic deformation during a scratch test. Wear 2008, 218, 8–14. [Google Scholar] [CrossRef]
- Makkar, P.; Agarwala, R.C.; Agarwala, V. Wear characteristics of mechanically milled TiO2 nanoparticles incorporated in electroless Ni–P coatings. Adv. Powder Technol. 2014, 25, 1653–1660. [Google Scholar] [CrossRef]
- Ebrahimian-Hosseinabadi, M.; Azari-dorcheh, K.; Vaghefi, S.M.M. Wear behavior of electroless Ni–P–B4C composite coatings. Wear 2006, 260, 123–127. [Google Scholar] [CrossRef]
- Rabizadeh, T.; Allahkaram, S.R.; Zarebidaki, A. An investigation on effects of heat treatment on corrosion properties of Ni–P electroless nano-coatings. Mater. Des. 2010, 31, 3174–3179. [Google Scholar] [CrossRef]






| Coatings | NiP | NiP/SiC50 | NiP/SiC100 | |||
|---|---|---|---|---|---|---|
| Conditions | As-Plated | HT | As-Plated | HT | As-Plated | HT |
| Nanohardness (GPa) | 5.4 ± 0.1 | 10.1 ± 0.6 | 5.3 ± 0.3 | 10.4 ± 1.1 | 5.1 ± 0.5 | 10.4 ± 0.9 |
| Es (GPa) | 127 ± 5.8 | 178 ± 8.8 | 138 ± 5.2 | 178 ± 2.7 | 134 ± 7.8 | 182 ± 6.3 |
| H/E | 0.042 ± 0.005 | 0.056 ± 0.003 | 0.038 ± 0.008 | 0.056 ± 0.004 | 0.038 ± 0.007 | 0.057 ± 0.005 |
| WP/WT | 0.68 ± 0.02 | 0.64 ± 0.01 | 0.69 ± 0.02 | 0.63 ± 0.01 | 0.75 ± 0.01 | 0.63 ± 0.05 |
| Conditions | As-Plated | Heat Treated at 360 °C for 1 h | ||||
|---|---|---|---|---|---|---|
| Coatings | NiP | NiP/SiC50 | NiP/SiC100 | NiP | NiP/SiC50 | NiP/SiC100 |
| ΔE (V) | 0.40 | 0.43 | 0.39 | 0.28 | 0.19 | 0.22 |
| ip (A/cm2) | 1.8 × 10–6 | 6.4 × 10−6 | 3.4 × 10−6 | 9.5 × 10−6 | 1.2 × 10−5 | 1.3 × 10−5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmadkhaniha, D.; Eriksson, F.; Zanella, C. Optimizing Heat Treatment for Electroplated NiP and NiP/SiC Coatings. Coatings 2020, 10, 1179. https://doi.org/10.3390/coatings10121179
Ahmadkhaniha D, Eriksson F, Zanella C. Optimizing Heat Treatment for Electroplated NiP and NiP/SiC Coatings. Coatings. 2020; 10(12):1179. https://doi.org/10.3390/coatings10121179
Chicago/Turabian StyleAhmadkhaniha, Donya, Fredrik Eriksson, and Caterina Zanella. 2020. "Optimizing Heat Treatment for Electroplated NiP and NiP/SiC Coatings" Coatings 10, no. 12: 1179. https://doi.org/10.3390/coatings10121179
APA StyleAhmadkhaniha, D., Eriksson, F., & Zanella, C. (2020). Optimizing Heat Treatment for Electroplated NiP and NiP/SiC Coatings. Coatings, 10(12), 1179. https://doi.org/10.3390/coatings10121179







