Effect of Exposure Conditions on the Interfacial Bond Properties of SS400 Plate Coated with Various Epoxy Resins
Abstract
1. Introduction
2. Experimental Investigation
2.1. Epoxy Resins
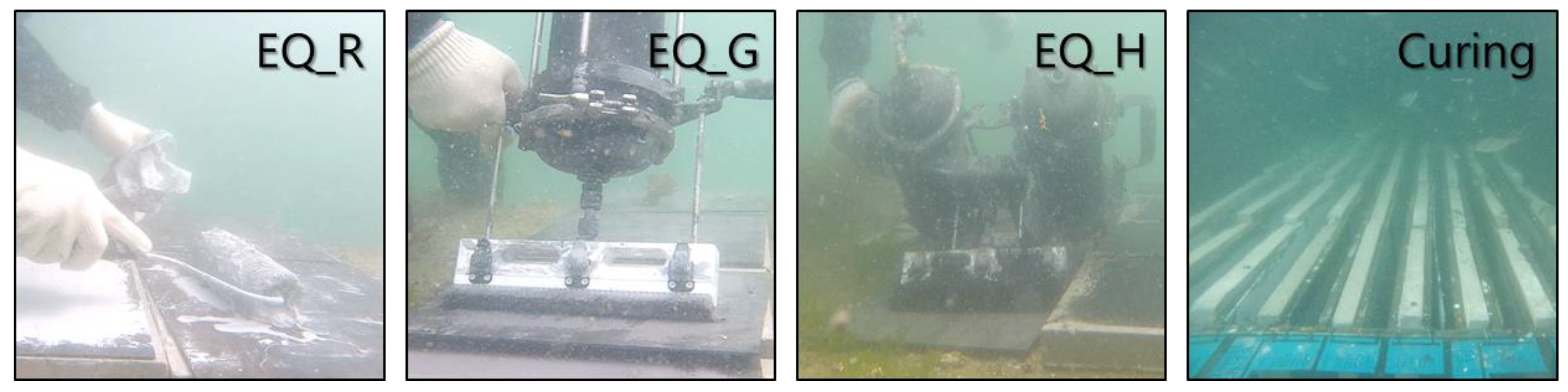
2.2. Exposure Conditions and Periods
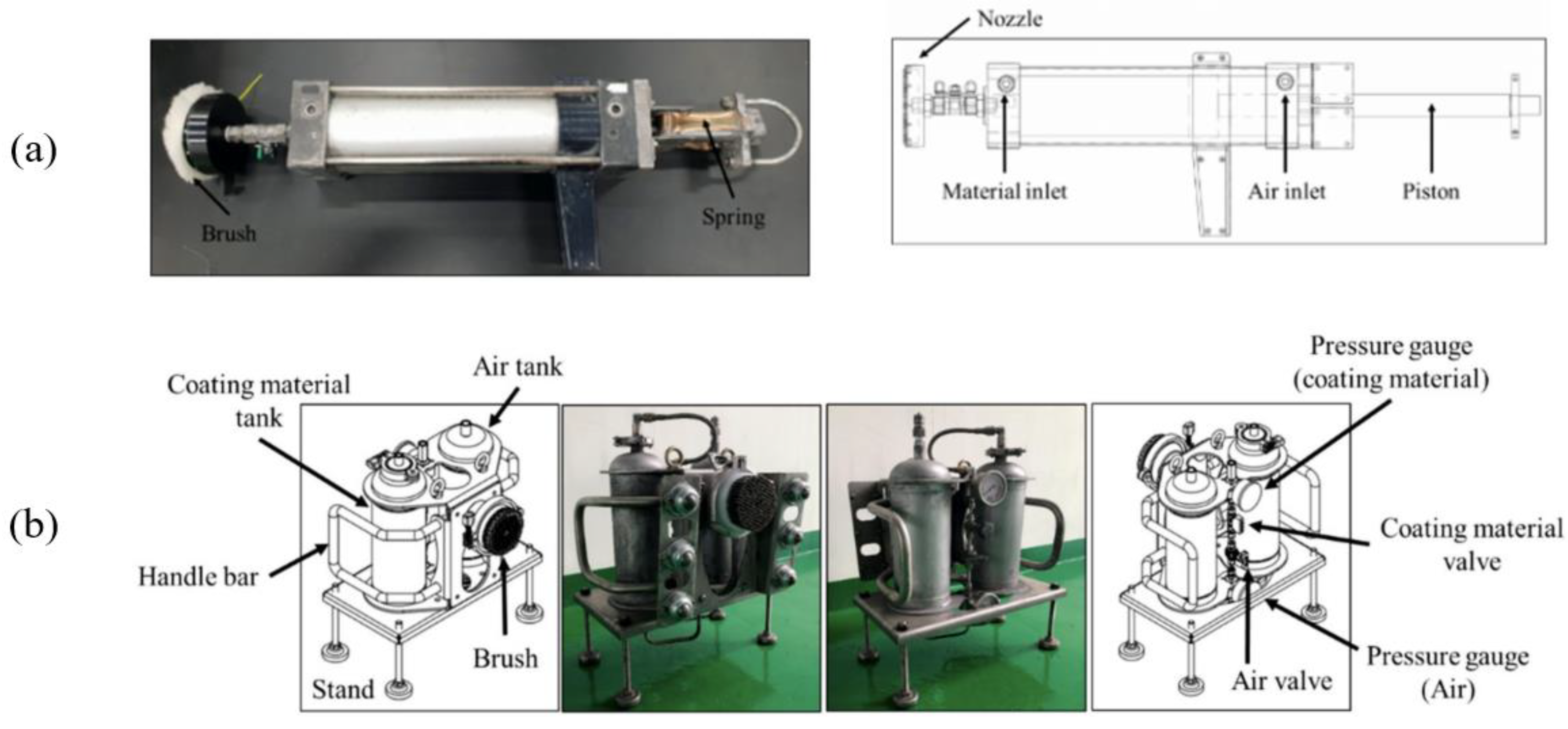
2.3. Coating Equipment
2.3.1. EQ_G
2.3.2. EQ_H
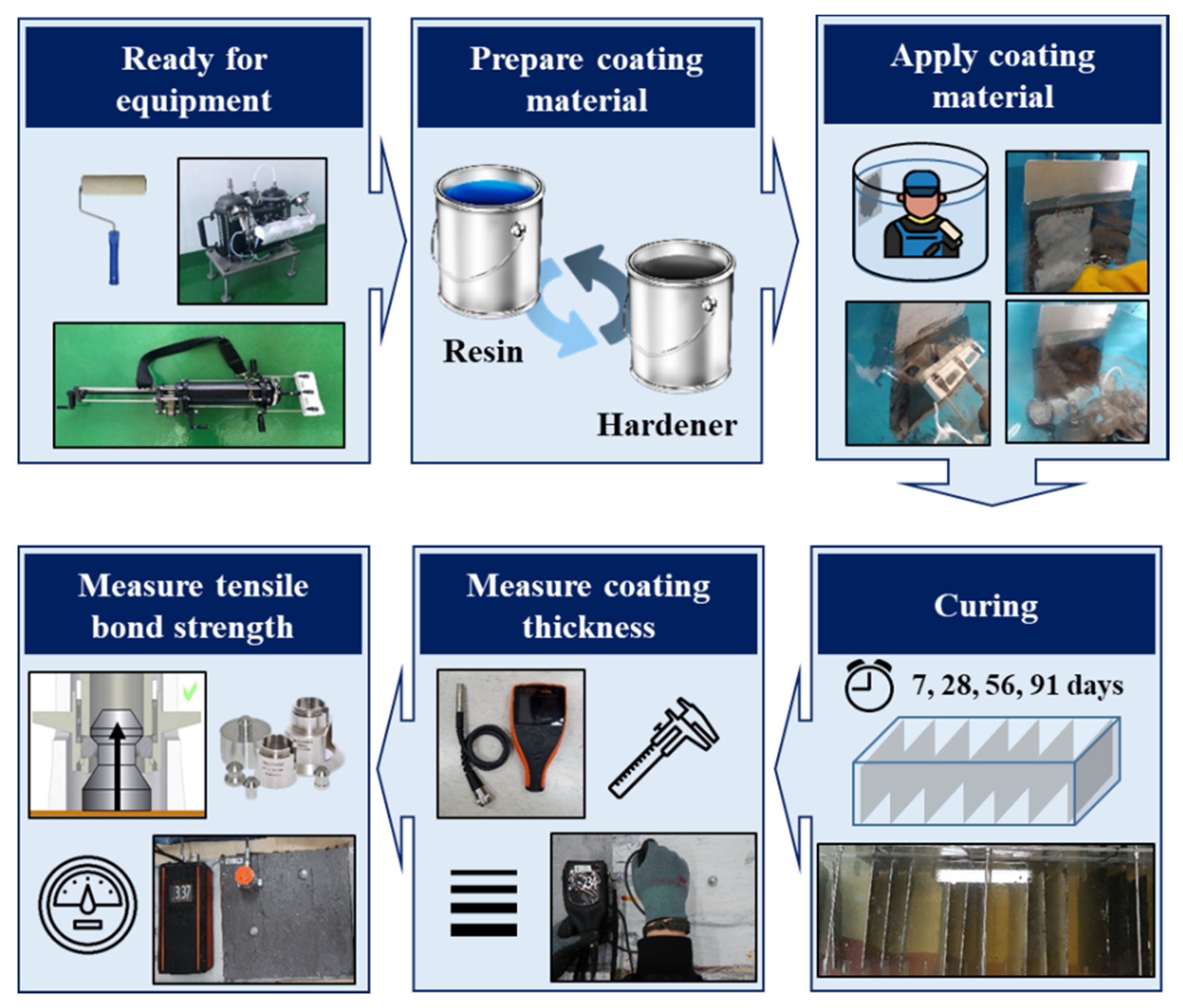
2.4. Test Procedure and Measurement
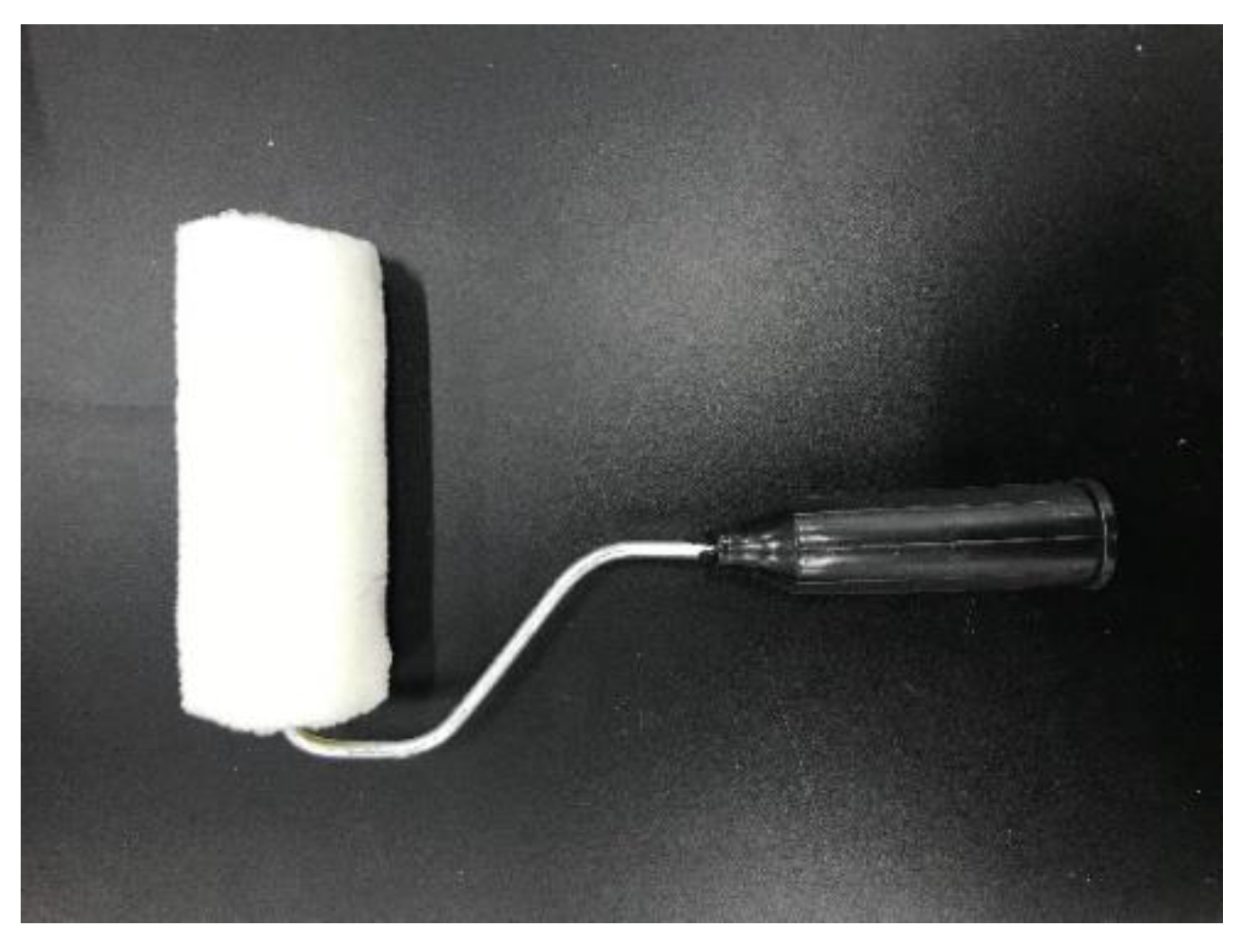
2.4.1. Coating Procedure
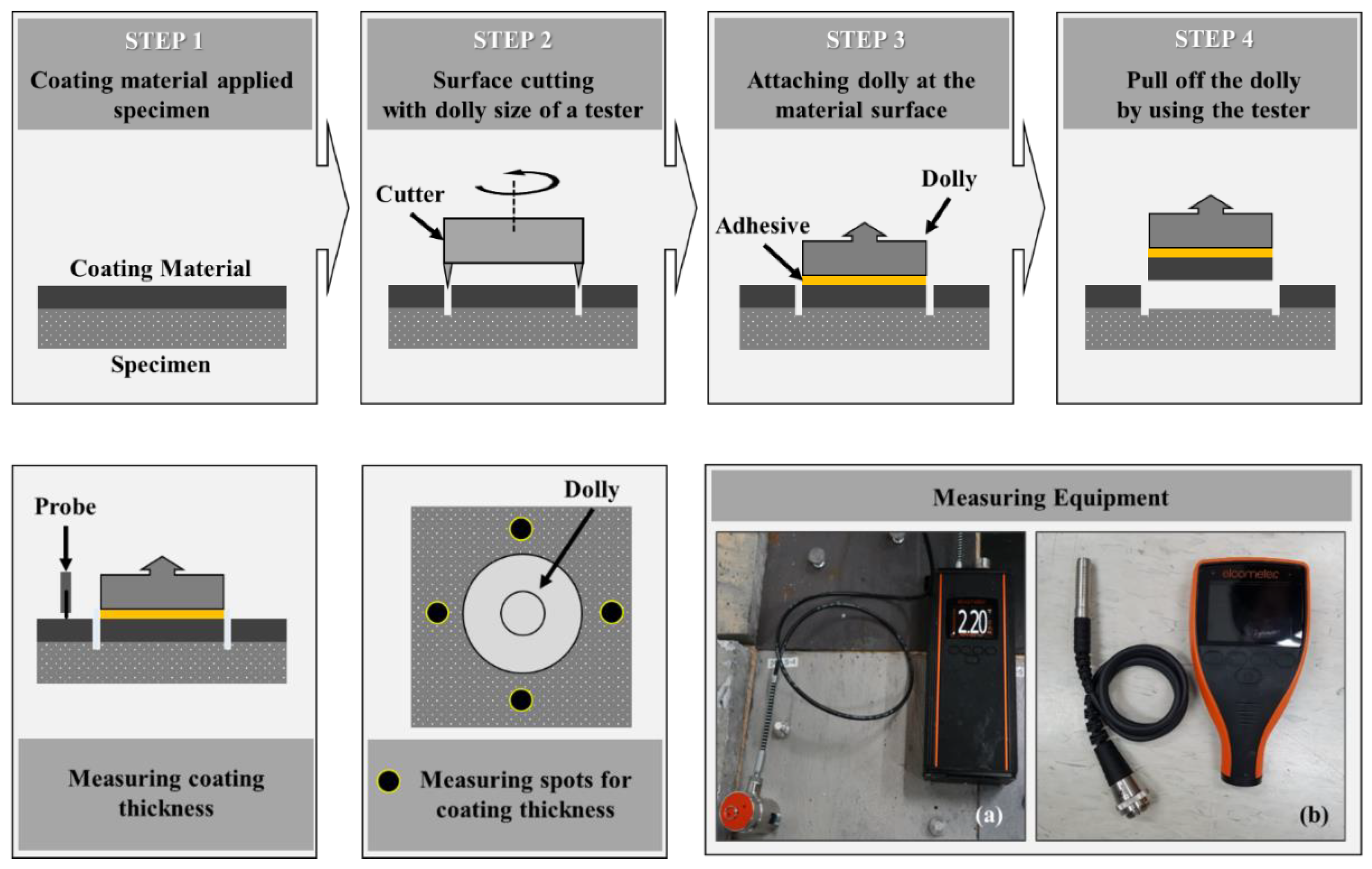
2.4.2. Measurement
3. Results and Discussion
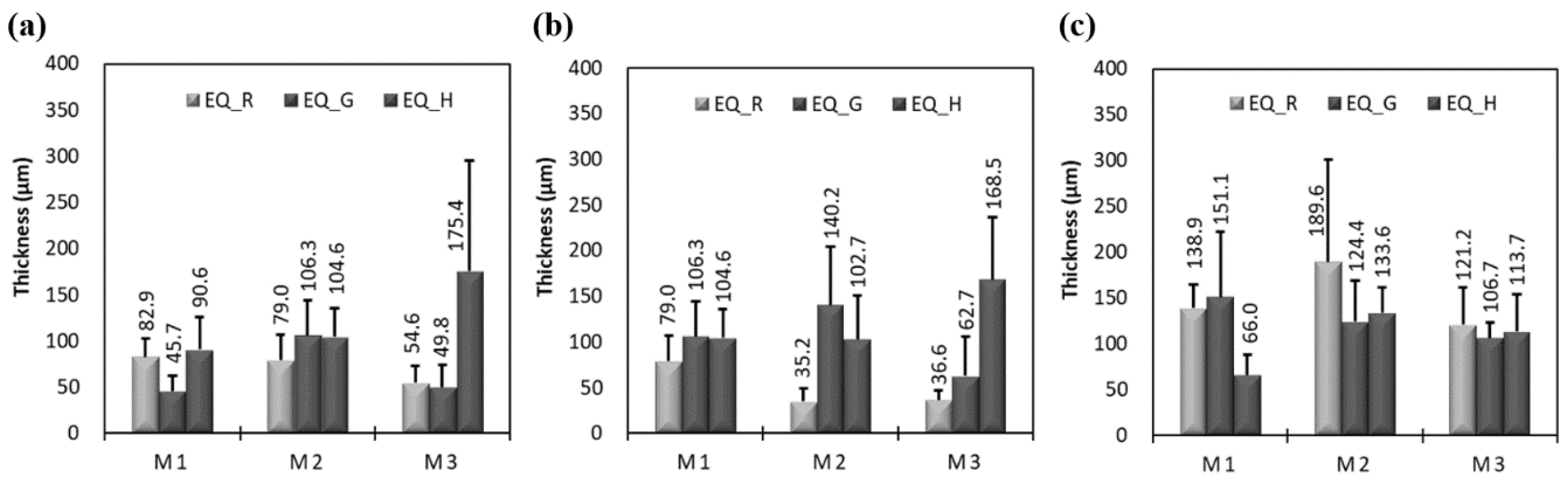
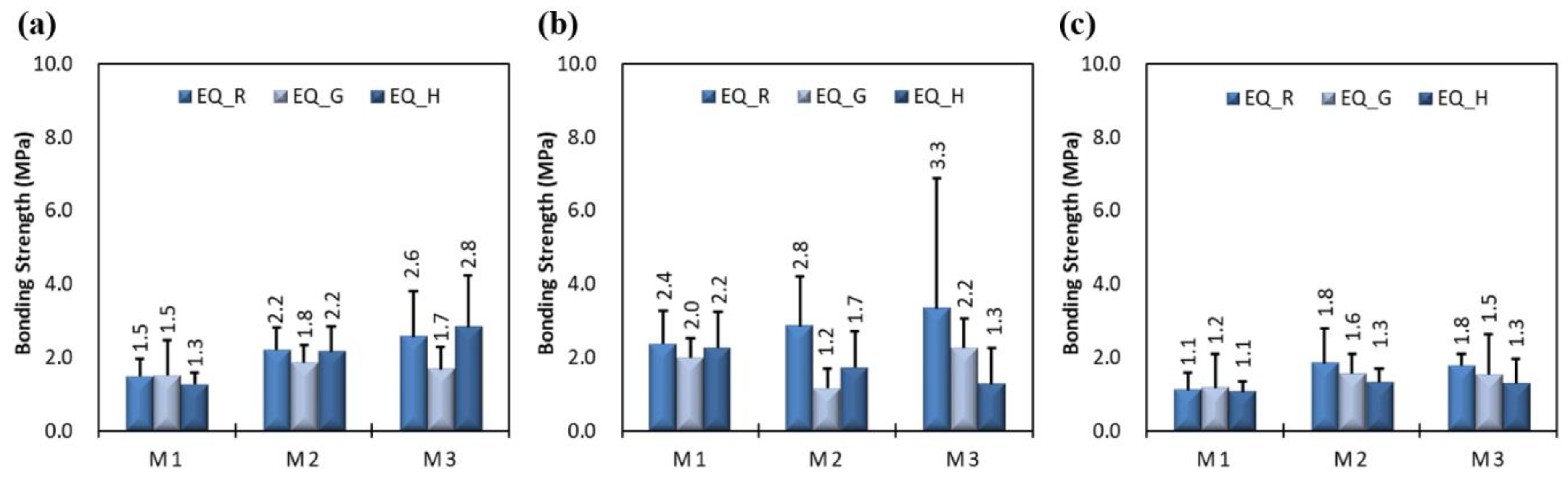
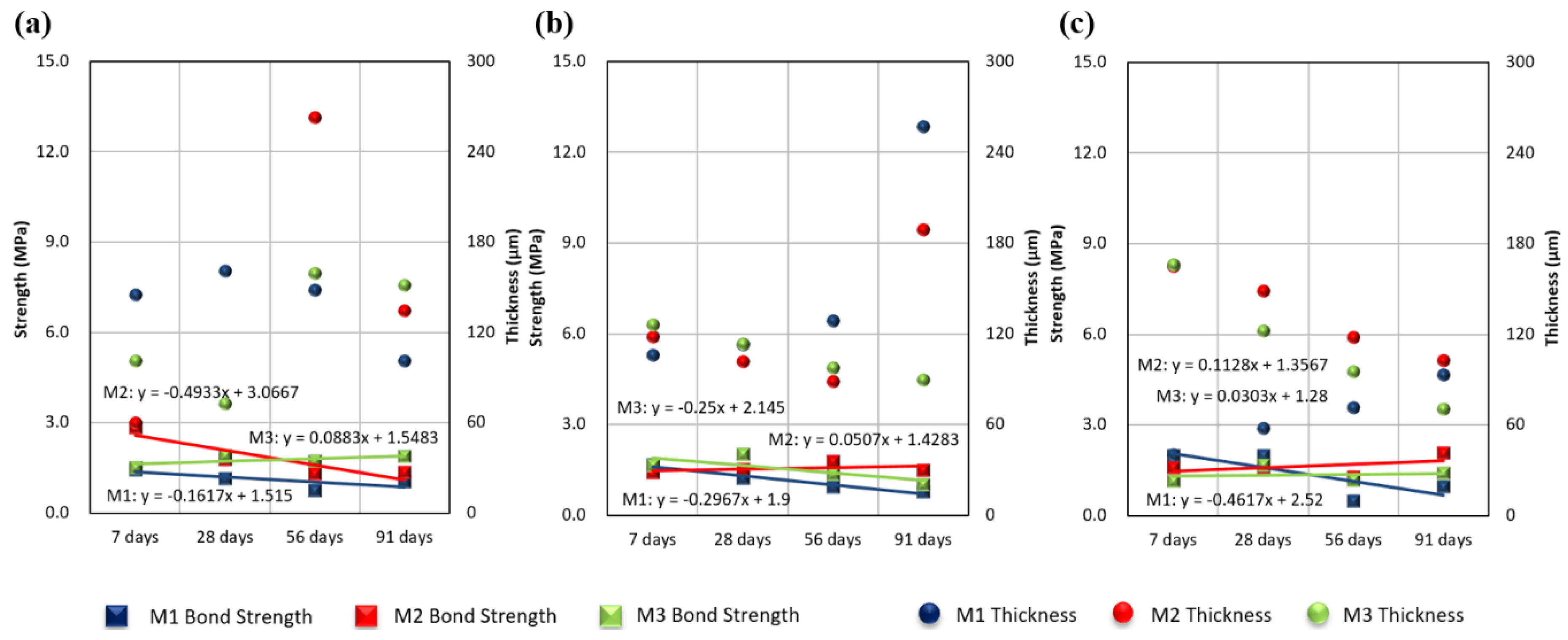
3.1. Effects of Epoxy Resin on Measured Thickness and Bond Strength
3.2. Effects of Exposure Condition on Measured Thickness and Bond Strength
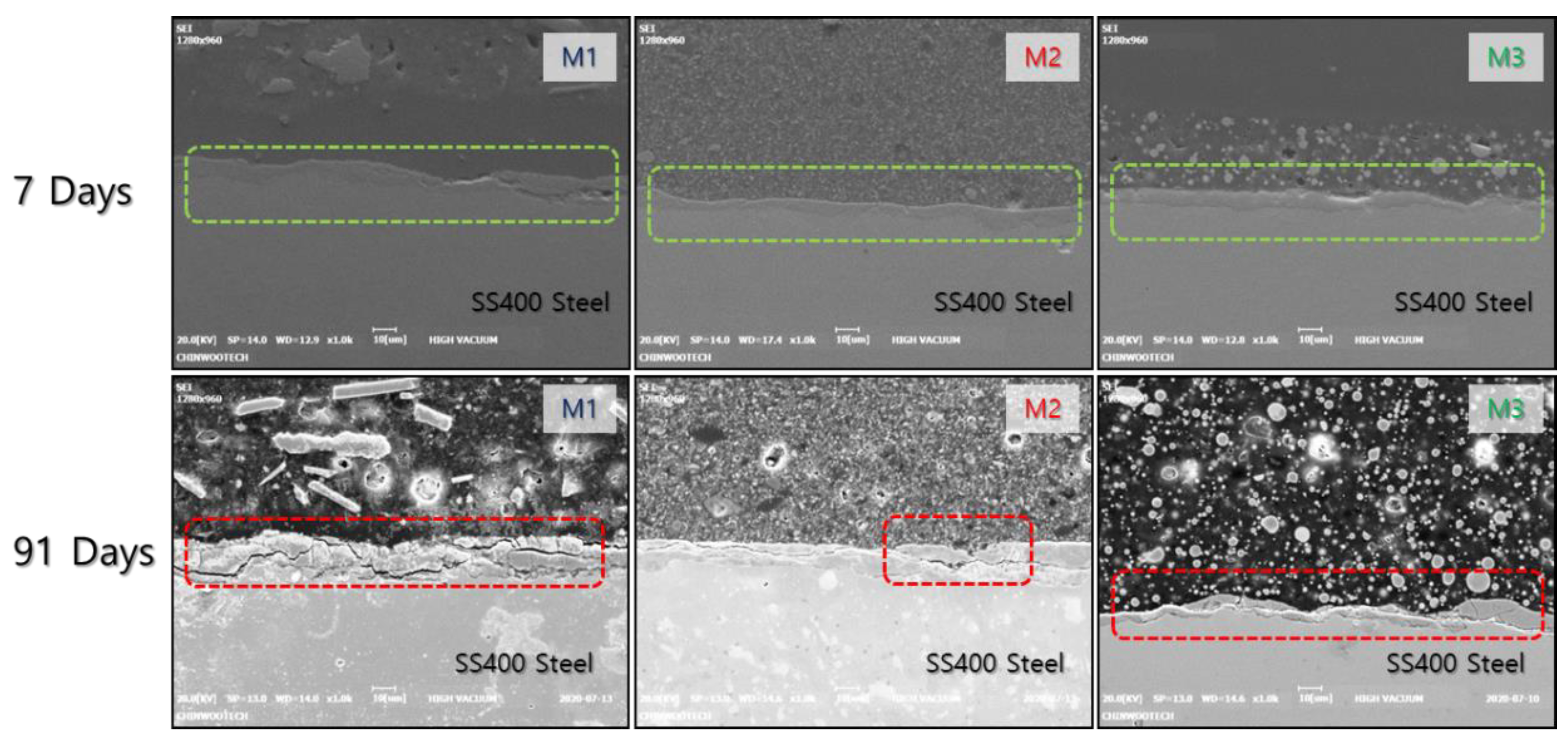
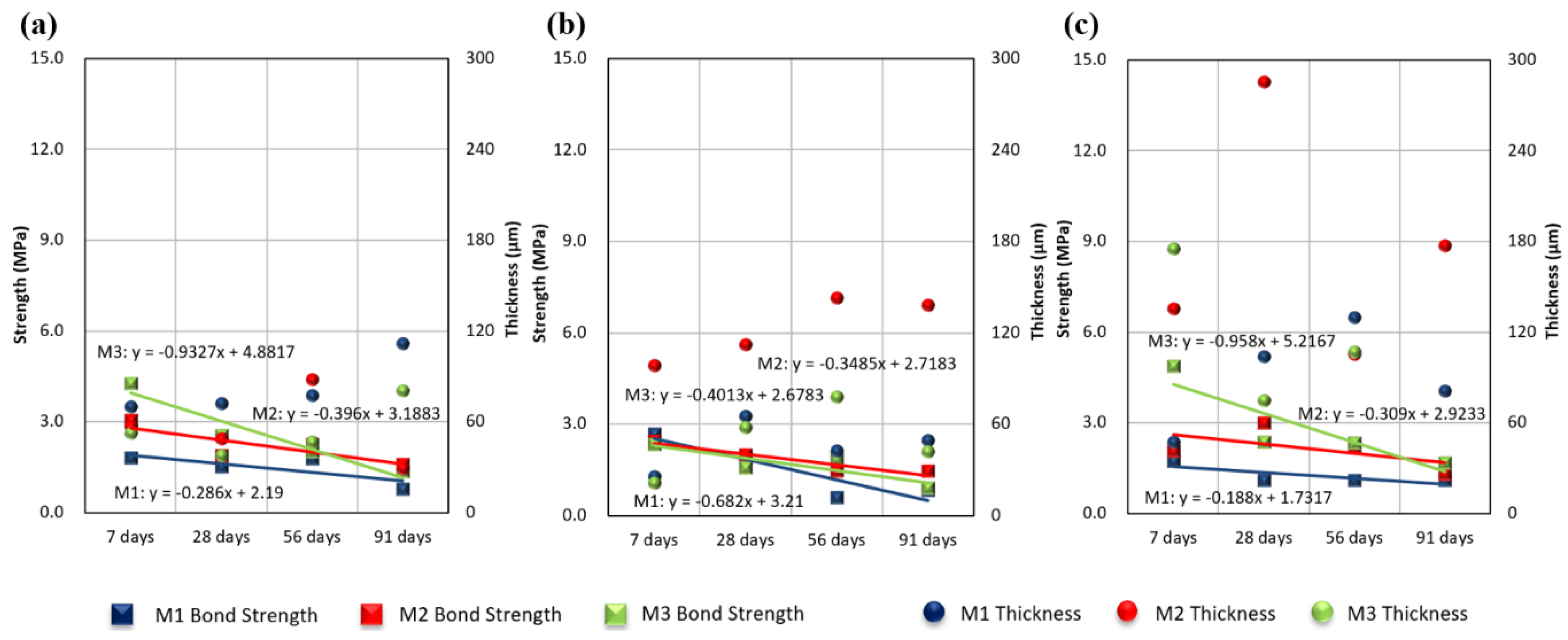
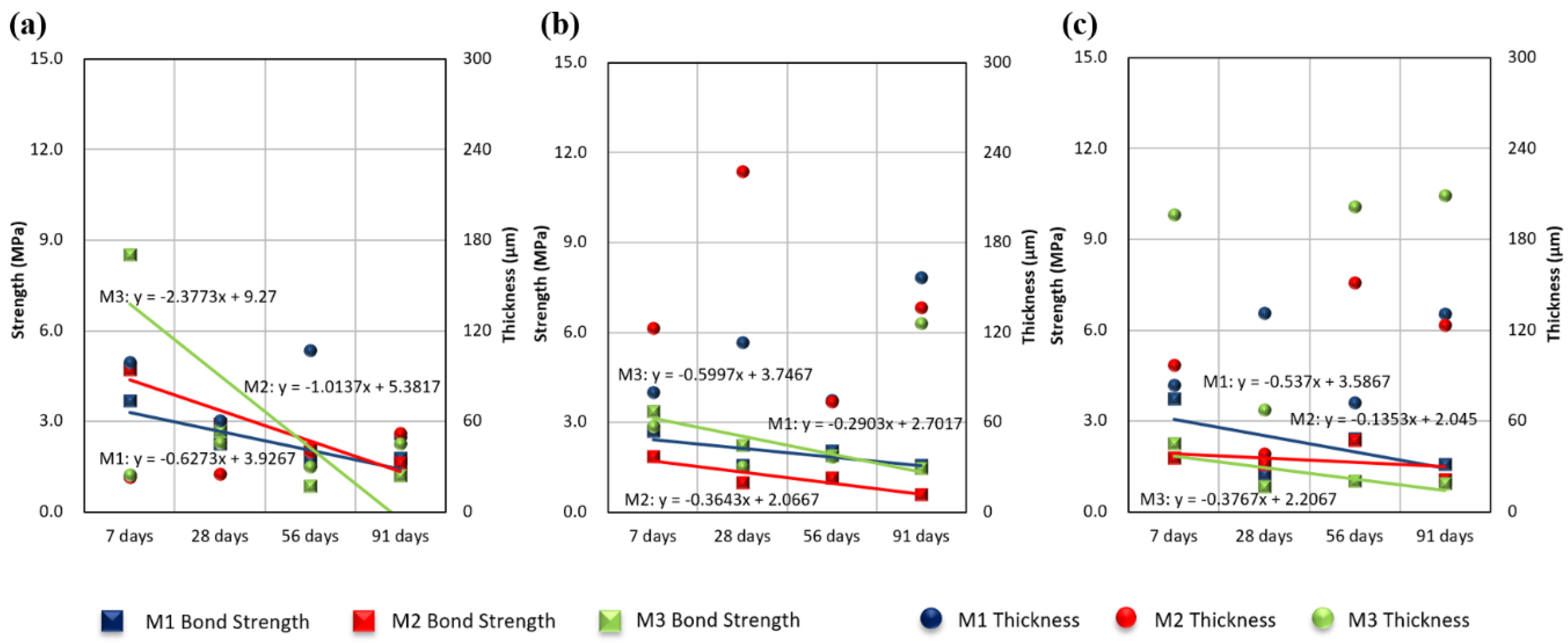
3.3. Effects of Exposure Period on Measured Thickness and Bond Strength
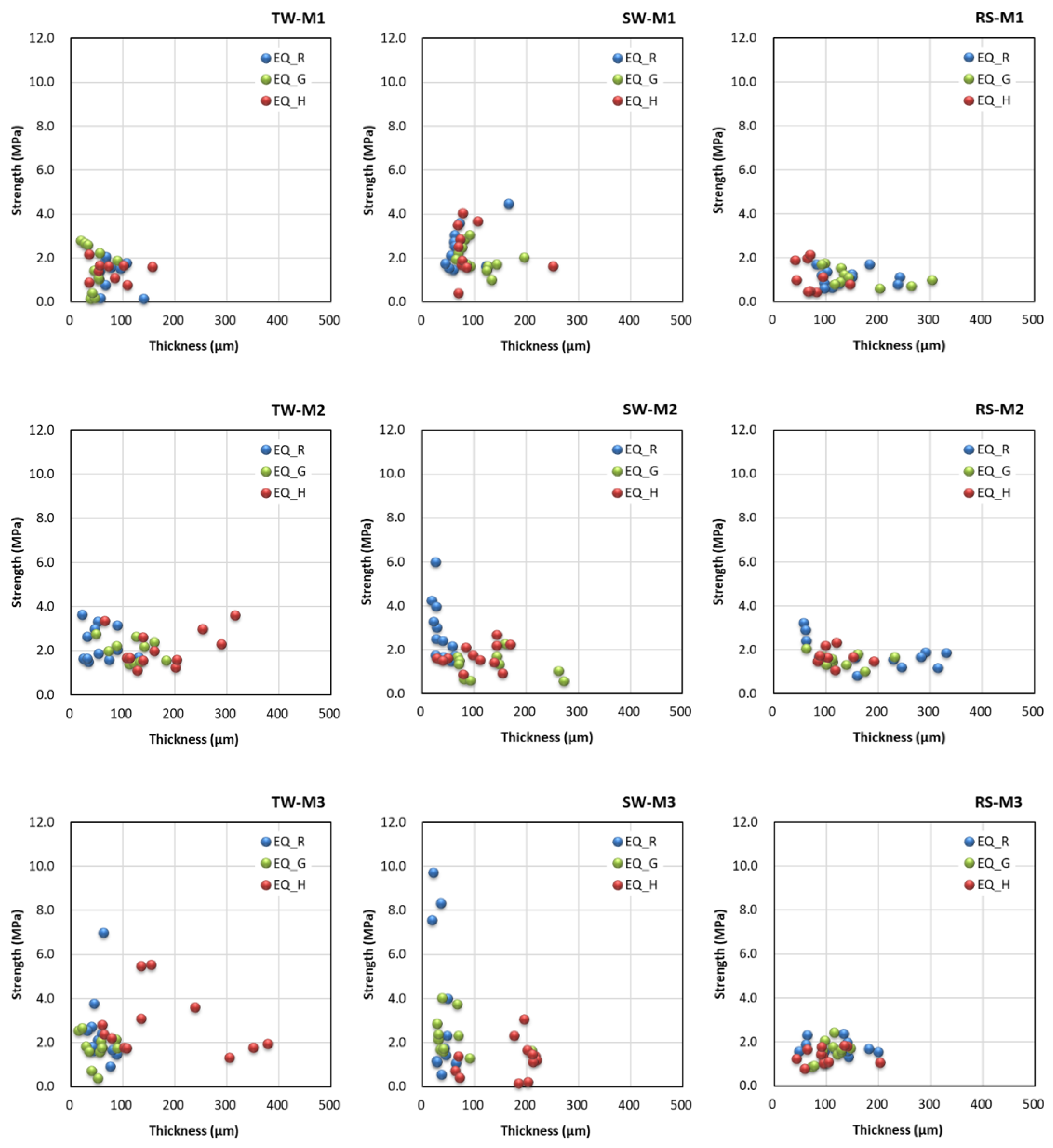
3.4. Effects of Coating Equipment on Measured Thickness and Bond Strength
3.5. Discussion
4. Conclusions
- Even when the initial bond strength was high, the strength was confirmed to weaken with increased time. This trend was the same under all environmental conditions. The initial bond strength was higher in the indoor tests than in the real sea test, but all bond strengths were similar after 91 days. In addition, the bond strength decreased more over time in the indoor tests than in the real sea test.
- No significant correlation between the measured coating thickness and bond strength was observed.
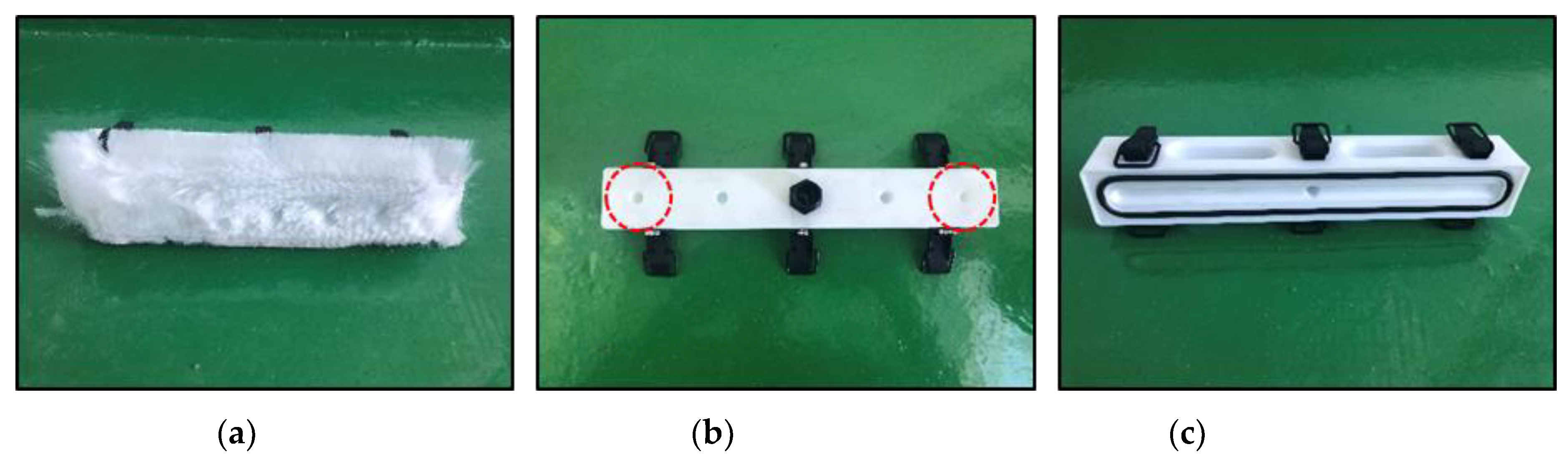
- Overall, the improved equipment types reduced the standard deviation of the coating thickness compared to the results of the previous study. The functionality of the equipment improved because of the reduced self-weight, and the coating thickness was more uniform owing to the improved brush.
- The functionality of the equipment types differed according to the environmental conditions. EQ_R had excellent functionality indoors, but the developed equipment types were more convenient to use in the real sea test. This was attributed to the latter supplying the coating material to the brush directly.
Author Contributions
Funding
Conflicts of Interest
References
- Jiang, D.; Tan, K.H.; Wang, C.M.; Ong, K.C.G.; Bra, H.; Jin, J.; Kim, M.O. Analysis and design of floating prestressed concrete structures in shallow waters. Mar. Struct. 2018, 59, 301–320. [Google Scholar] [CrossRef]
- Justnes, H.; Kim, M.O.; Ng, S.; Qian, X. Methodology of calculating required chloride diffusion coefficient for intended service life as function of concrete cover in reinforced marine structures. Cem. Concr. Compos. 2016, 73, 316–323. [Google Scholar] [CrossRef]
- Kim, M.O.; Bordelon, A.; Lee, M.K.; Oh, B.H. Cracking and failure of patch repairs in RC members subjected to bar corrosion. Constr. Build. Mater. 2016, 107, 255–263. [Google Scholar] [CrossRef]
- Andrade, C. Calculation of chloride diffusion coefficients in concrete from ionic migration measurements. Cem. Concr. Res. 1993, 23, 724–742. [Google Scholar] [CrossRef]
- Kim, M.O.; Qian, X.; Lee, M.K.; Park, W.S.; Jeong, S.T.; Oh, N.S. Determination of structural lightweight concrete mix proportion for floating concrete structures. J. Korean Soc. Coast. Ocean Eng. 2017, 29, 315–325. [Google Scholar] [CrossRef]
- Pan, X.; Shi, Z.; Shi, C.; Ling, T.; Li, N. A review on concrete surface treatment Part I: Types and mechanisms. Constr. Build. Mater. 2017, 132, 578–590. [Google Scholar] [CrossRef]
- Basheer, L.; Cleland, D.; Long, A. Protection provided by surface treatments against chloride induced corrosion. Mater. Struct. 1998, 31, 459–464. [Google Scholar] [CrossRef]
- Christodoulou, C.; Goodier, C.I.; Austin, S.A.; Webb, J.; Glass, G.K. Long-term performance of surface impregnation of reinforced concrete structures with silane. Constr. Build. Mater. 2013, 48, 708–716. [Google Scholar] [CrossRef]
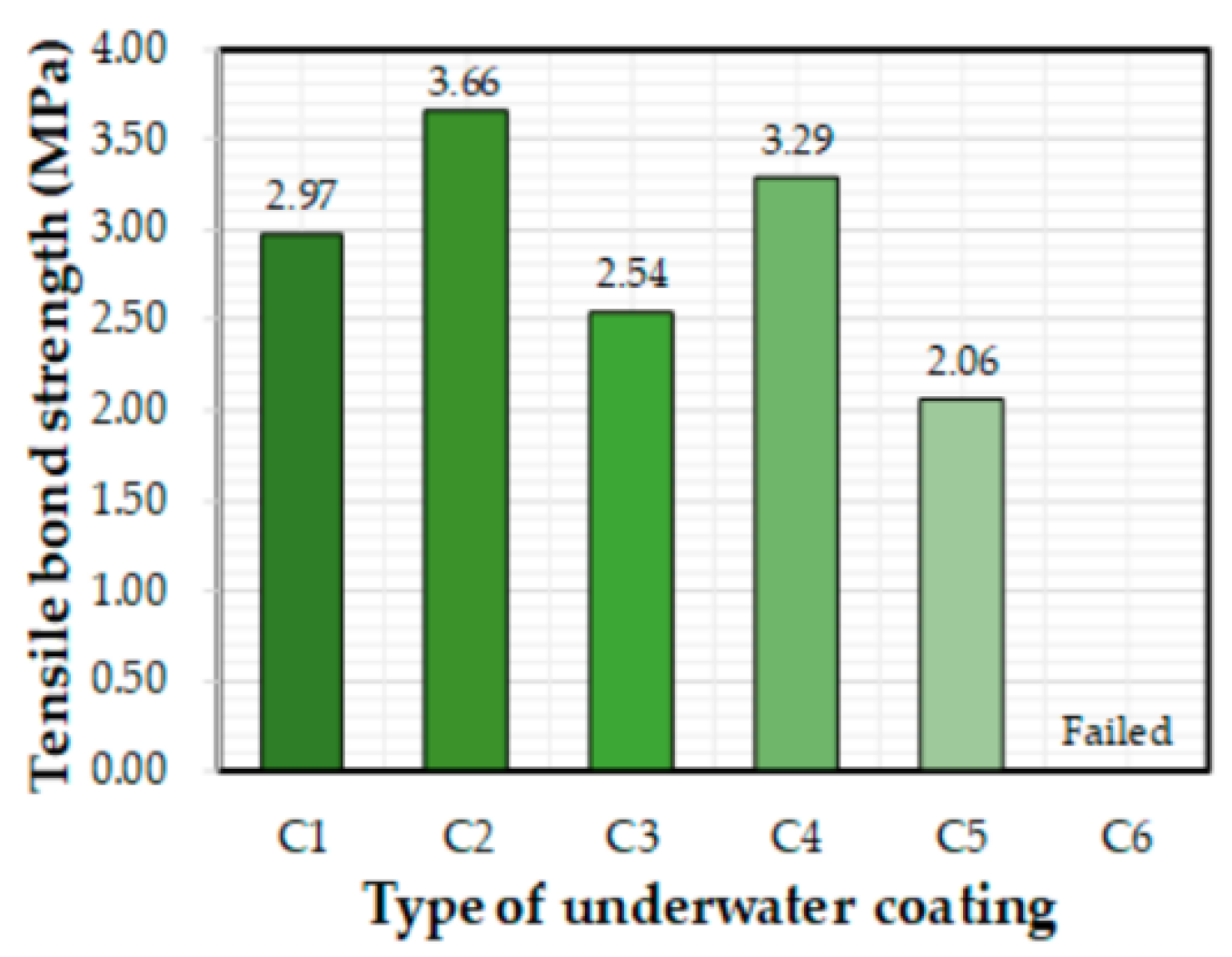
- Kim, M.O.; Jeong, Y.; Kang, S.H.; Moon, J.; Yi, J.H. Tensile bond characteristics between underwater coating materials and concrete substrate. J. Korean Soc. Coast. Ocean Eng. 2018, 30, 298–305. [Google Scholar] [CrossRef]
- Hammer, P.; dos Santos, F.C.; Cerrutti, B.M.; Pulcinelli, S.H.; Santilli, C.V. Carbon nanotube-reinforced siloxane-PMMA hybrid coatings with high corrosion resistance. Prog. Org. Coat. 2013, 76, 601–608. [Google Scholar] [CrossRef]
- Won, B.; Kim, M.O.; Park, S.; Yi, J. Effects of water exposure on the interfacial bond between an epoxy resin coating and a concrete substrate. Materials 2019, 12, 3715. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Hong, H.; Han, T.H.; Kim, M.O. Early-age tensile bond characteristics of epoxy coatings for underwater applications. Coatings 2019, 9, 757. [Google Scholar] [CrossRef]
- Global Wind Energy Council, Global offshore Wind Report 2020. Available online: https://www.rivieramm.com/news-content-hub/news-content-hub/offshore-wind-to-surge-to-more-than-234-gw-by-2030-60496 (accessed on 2 October 2020).
- Stehly, T.; Beiter, P. 2018 Cost of Wind Energy Review; Technical Report; NREL: Golden, CO, USA, 2019.
- Bugnot, A.B.; Mayer-Pinto, M.; Airoldi, L.; Heery, E.C.; Johnston, E.L.; Critchley, L.P.; Strain, E.M.A.; Morris, R.L.; Loke, L.H.L.; Bishop, M.J.; et al. Current and projected global extent of marine built structures. Nat. Sustain. 2020. [Google Scholar] [CrossRef]
- Kanwal, S.; Ali, N.Z.; Hussain, R.; Shah, F.U.; Akhter, Z. Poly-thiourea formaldehyde based anticorrosion marine coatings on type 304 stainless steel. J. Mater. Res. Technol. 2020, 9, 2146–2153. [Google Scholar] [CrossRef]
- Ates, M. A review on conducting polymer coatings for corrosion protection. J. Adhes. Sci. Technol. 2016, 30, 1510–1536. [Google Scholar] [CrossRef]
- Abbas, M.; Shafiee, M. An overview of maintenance management strategies for corroded steel structures in extreme marine environments. Mar. Struct. 2020, 71, 102718. [Google Scholar] [CrossRef]
- Nazarov, A.; Bozec, N.L.; Thierry, D. Scanning Kelvin probe assessment of steel corrosion protection by marine paints containing Zn-rich primer. Prog. Org. Coat. 2018, 125, 61–72. [Google Scholar] [CrossRef]
- Zhao, Z.; Tang, J.; Tariq, N.H.; Wang, J.; Cui, X.; Xiong, T. Microstructure and corrosion behavior of cold-sprayed Zn-Al composite coating. Coatings 2020, 10, 931. [Google Scholar] [CrossRef]
- Aggoun, K.; Chaal, L.; Creus, J.; Sabot, R.; Saidani, B.; Jeannin, M. Marine corrosion resistance of CeO2/Mg(OH)2 mixed coating on a low alloyed steel. Surf. Coat. Technol. 2019, 372, 410–421. [Google Scholar] [CrossRef]
- Ibrahim, M.; Kannan, K.; Parangusan, H.; Eldeib, S.; Shehata, O.; Ismail, M.; Zarandah, R.; Sadasivuni, K.K. Enhanced corrosion protection of epoxy/ZnO-NiO nanocomposite coatings on steel. Coatings 2020, 10, 783. [Google Scholar] [CrossRef]
- Singh, A.A.M.M.; Franco, P.A.; Binoj, J.S. Enhancement of corrosion resistance on plasma spray coated mild steel substrate exposed to marine environment. Mater. Today Proc. 2019, 15, 84–89. [Google Scholar] [CrossRef]
- Sambyal, P.; Ruhi, G.; Dhawan, S.K.; Bisht, B.M.S.; Gairola, S.P. Enhanced anticorrosive properties of tailored poly(aniline-anisidine)/chitosan/SiO2 composite for protection of mild steel in aggressive marine conditions. Prog. Org. Coat. 2018, 119, 203–213. [Google Scholar] [CrossRef]
- Kamburova, K.; Boshkova, N.; Tabakova, N.; Boshkov, N.; Radeva, T.S. Application of polymeric modified polyaniline-silica particles for improved corrosion resistance of hybrid zinc coatings. Colloids Surf. A 2020, 592, 124546. [Google Scholar] [CrossRef]
- Mohan, S.; Nair, S.S.; Ajay, A.V.; Saravanan, M.S.S.; Vishnu, B.R.; Sivapirakasam, S.P.; Surianarayann, M. Corrosion behaviour of ZrO2–TiO2 nano composite coating on stainless steel under simulated marine environment. Mater. Today Proc. 2020, 27, 2492–2497. [Google Scholar] [CrossRef]
- Zhai, Y.; Pan, K.; Zhang, E. Anti-corrosive coating of carbon-steel assisted by polymer-camphorsulfonic acid embedded within graphene. Coatings 2020, 10, 879. [Google Scholar] [CrossRef]
- Acero-Gutierrez, A.K.; Perez-Rlores, A.L.; Gorinez-Salcedo, J.G.; Moreno-Palmerin, J.; Morales-Ramirez, A.J. Corrosion protection of A36 Steel with SnO2 nanoparticles integrated into SiO2 coatings. Coatings 2020, 10, 385. [Google Scholar] [CrossRef]
- Verma, C.; Olasunkanmi, L.O.; Akpan, E.D.; Quraishi, M.A.; Dagdag, O.; El Gouri, M.; Sherif, E.M.; Ebenso, E.E. Epoxy resins as anticorrosive polymeric materials: A review. React. Funct. Polym. 2020, 156, 104741. [Google Scholar] [CrossRef]
- Atta, A.M.; Al-Hodan, H.A.; Hameed, R.S.A.; Ezzat, A.O. Preparation of green cardanol-based epoxy and hardener as primer coatings for petroleum and gas steel in marine environment. Prog. Org. Coat. 2017, 111, 283–293. [Google Scholar] [CrossRef]

| Epoxy Resins (Nomenclature) | Bond Strength (MPa) | Density (g/cm3) | Working Time (min) | Won et al. [11] | Kim et al. [12] |
|---|---|---|---|---|---|
| M1 | 16.6 | 1.60 ± 0.1 | 30 at 30 °C | C4 | S2 |
| M2 | 6.9 | 1.55 | 45–60 at 20 °C | C2 | S4 |
| M3 | 17.0 | 1.82 | 45–60 at 20 °C | C1 | S5 |
| Previous EQ_G | Problem | Improvement | EQ_G |
|---|---|---|---|
 | 1. Pulled out wool brush → hard to treat a surface | 1. Apply a new PTFE brush |  |
| 2. Adhesion of coating material between piston and equipment body → hard to wash | 2. Use PTFE coating material | ||
| 3. Only one angled grip → hard to handle | 3. Attach dual grips and a strap | ||
| 4. Frequently malfunctioning spring → hard to jet | 4. Replace the spring type with a screw type and apply a one-way pulley |
| Previous EQ_H | Problem | Improvement | EQ_H |
|---|---|---|---|
 | 1. Adhesion of coating materials and small inlet diameter → hard to wash inside | 1. Use polytetrafluoroethylene (PTFE) coating material and bigger inlet diameter |  |
| 2. Stiff material brush → hard to treat surface | 2. Apply a new PTFE brush | ||
| 3. Unfavorable gauge position → low visibility | 3. Change positions of the gauges | ||
| 4. Heavy weight → hard to handle | 4. Apply a new PTFE brush | ||
| 5. Adhesion of coating material at U-shaped tube → hard to wash and replace | 5. Apply new PTFE tubes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Hong, H.; Park, J.K.; Park, S.; Choi, S.I.; Kim, M.O. Effect of Exposure Conditions on the Interfacial Bond Properties of SS400 Plate Coated with Various Epoxy Resins. Coatings 2020, 10, 1159. https://doi.org/10.3390/coatings10121159
Kim S, Hong H, Park JK, Park S, Choi SI, Kim MO. Effect of Exposure Conditions on the Interfacial Bond Properties of SS400 Plate Coated with Various Epoxy Resins. Coatings. 2020; 10(12):1159. https://doi.org/10.3390/coatings10121159
Chicago/Turabian StyleKim, Sungwon, Hyemin Hong, Jun Kil Park, Sangmin Park, Seoung Ik Choi, and Min Ook Kim. 2020. "Effect of Exposure Conditions on the Interfacial Bond Properties of SS400 Plate Coated with Various Epoxy Resins" Coatings 10, no. 12: 1159. https://doi.org/10.3390/coatings10121159
APA StyleKim, S., Hong, H., Park, J. K., Park, S., Choi, S. I., & Kim, M. O. (2020). Effect of Exposure Conditions on the Interfacial Bond Properties of SS400 Plate Coated with Various Epoxy Resins. Coatings, 10(12), 1159. https://doi.org/10.3390/coatings10121159






