Two-Dimensional As/BlueP van der Waals Hetero-Structure as a Promising Photocatalyst for Water Splitting: A DFT Study
Abstract
1. Introduction
2. Methods
3. Results and Discussion
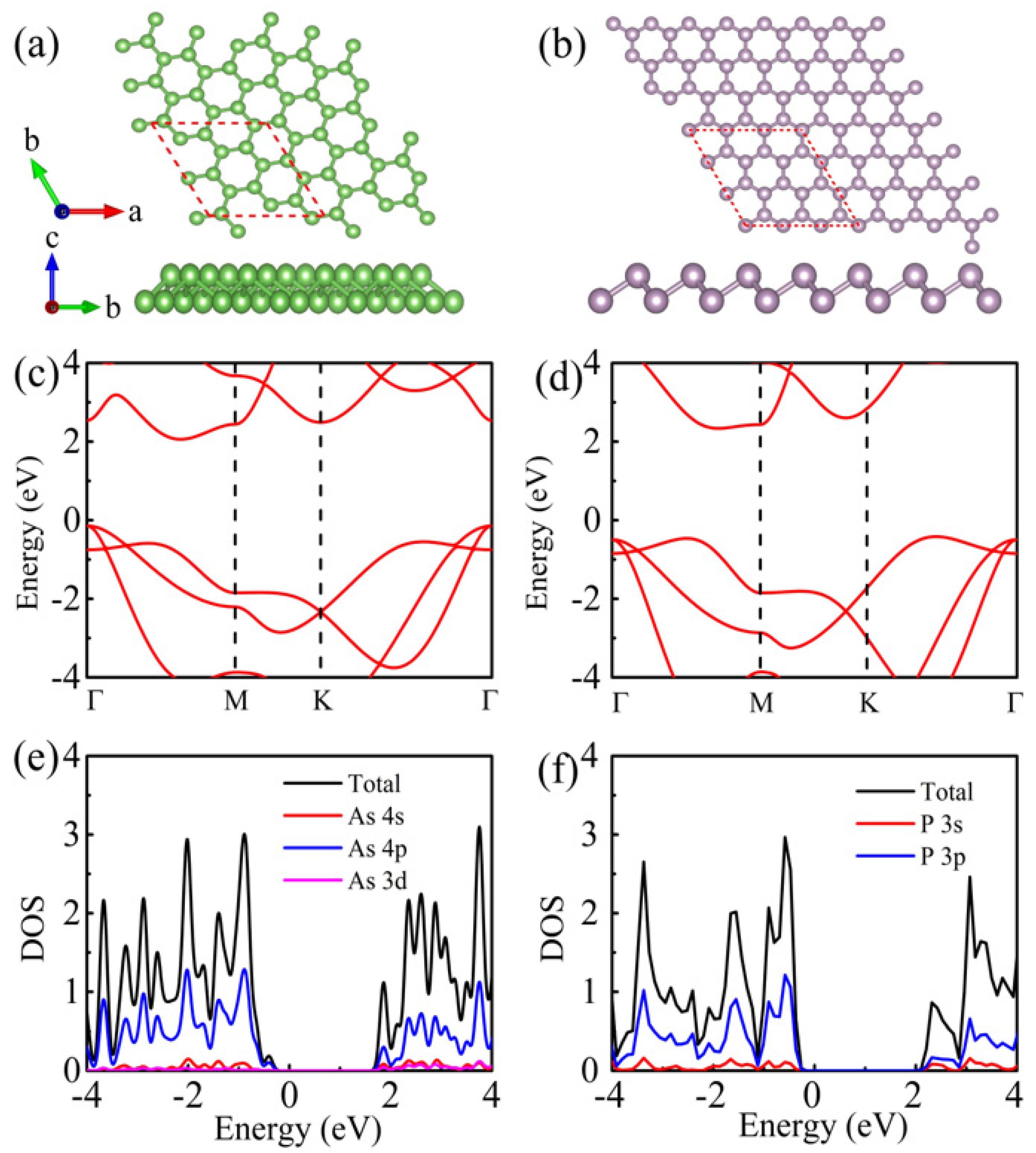
3.1. Structural and Electronic Properties of Monolayer As and BlueP
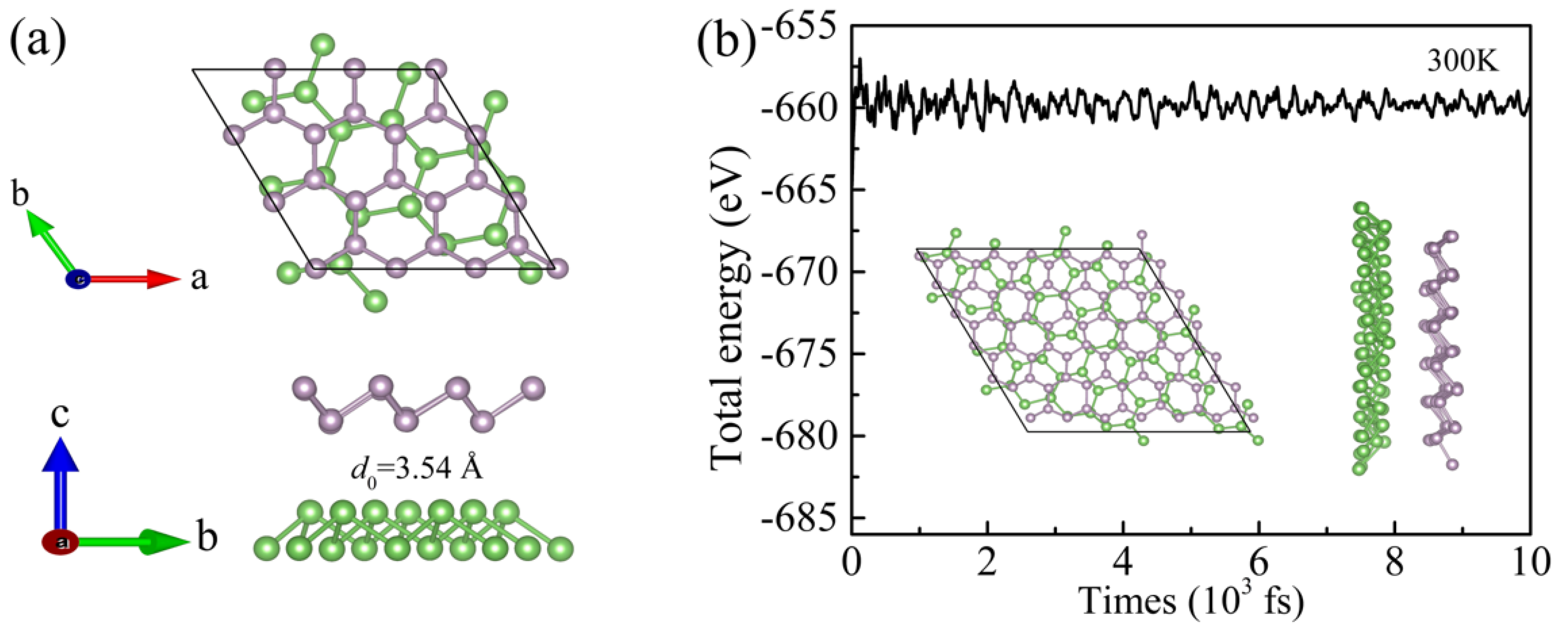
3.2. Structural Stability of the As/BlueP Hetero-Structure
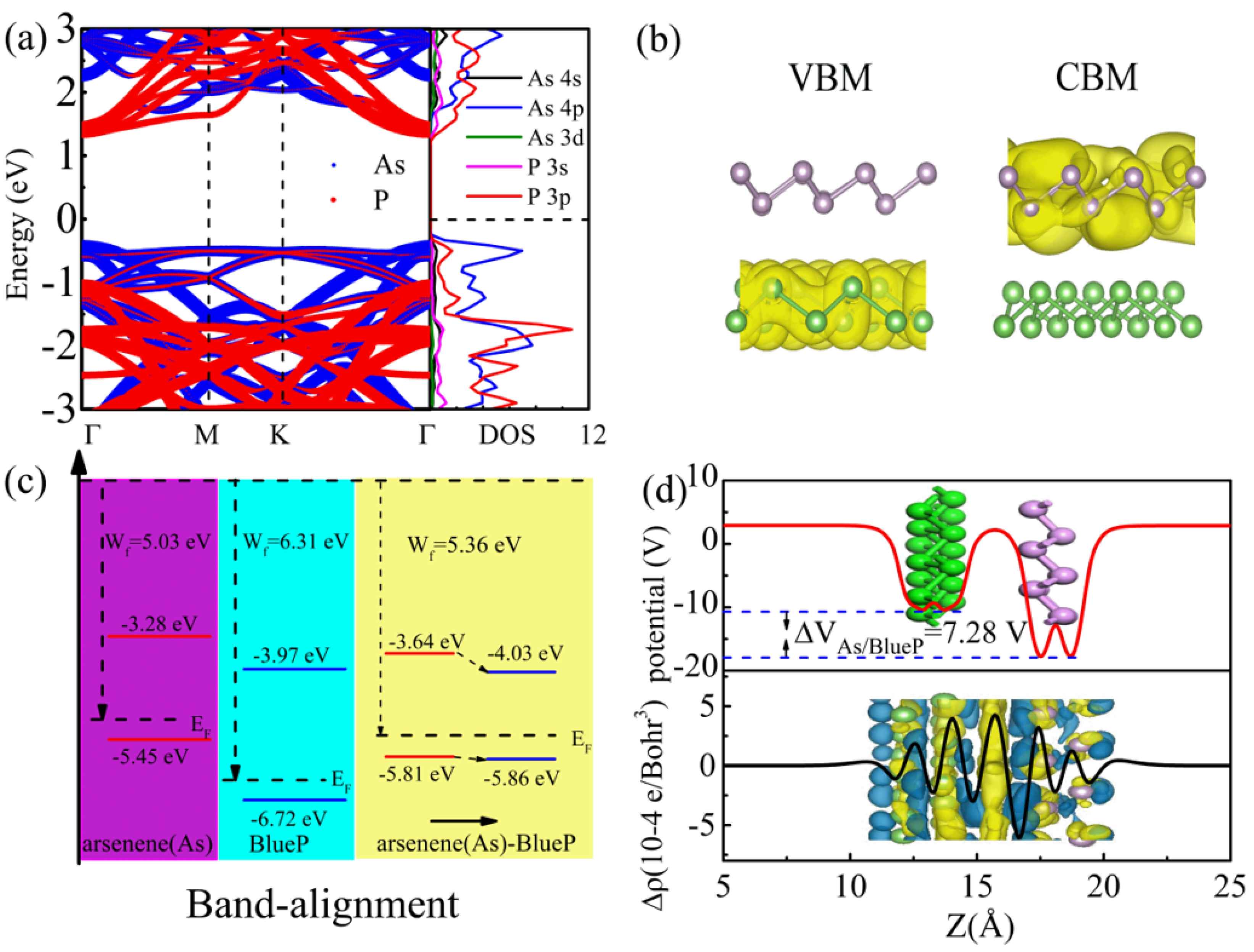
3.3. Electronic Properties of the As/BlueP Hetero-Structure
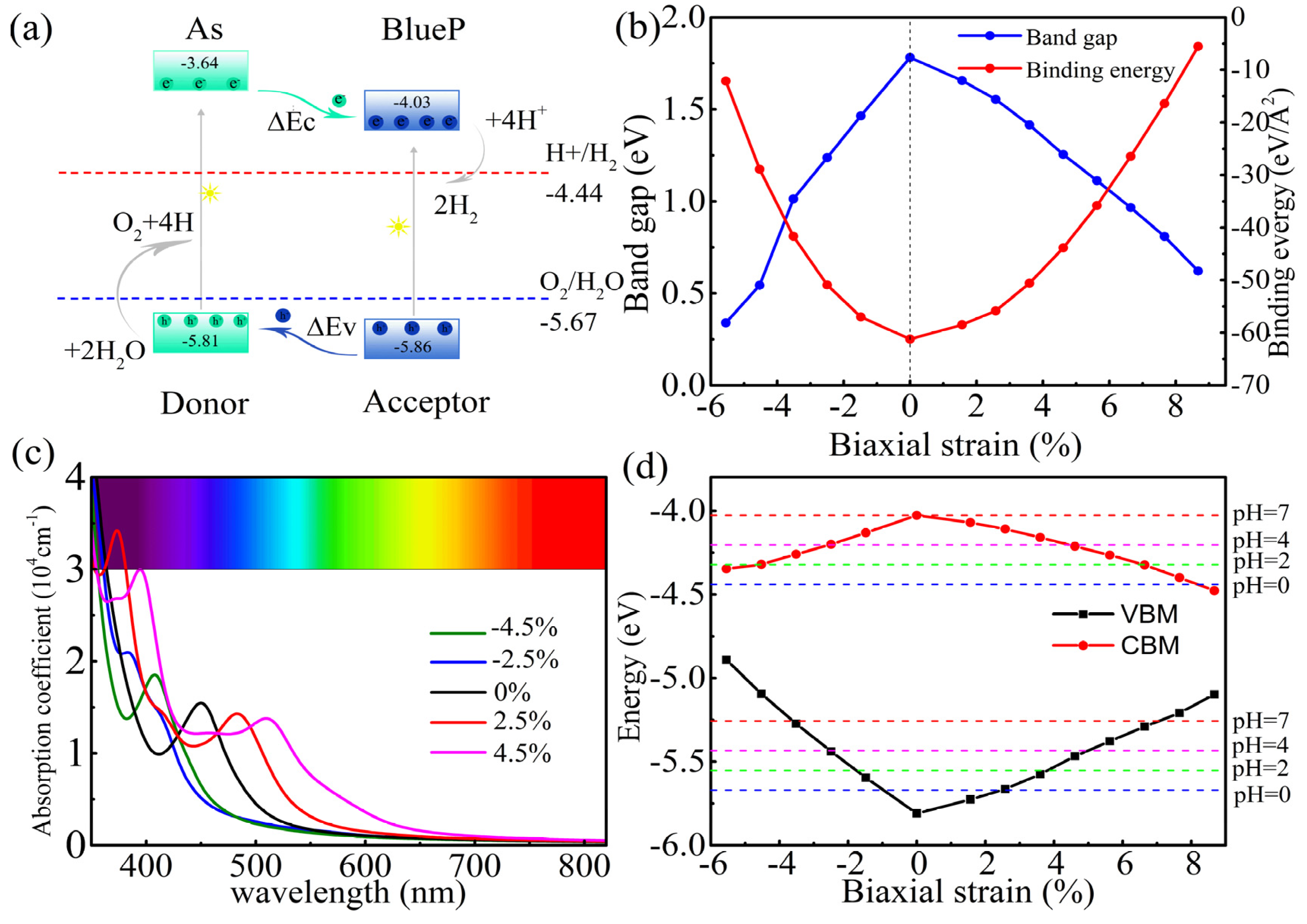
3.4. Optical Absorption Spectrum
3.5. Photocatalytic Properties of the As/BlueP Hetero-Structure
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Steven, C.; Arun, M. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar]
- Seyed, E.H.; Mazlan, A.W. Hydrogen production from renewable and sustainable energy resources: Promising green energy carrier for clean development. Renew. Sustain. Energy Rev. 2016, 57, 850–866. [Google Scholar]
- Zhou, Y.N.; Zhu, Y.R.; Chen, X.Y.; Dong, B.; Li, Q.Z.; Chai, Y.M. Carbonebased transition metal sulfides/selenides nanostructures for electrocatalytic water splitting. J. Alloy. Compd. 2021, 852, 156810. [Google Scholar] [CrossRef]
- Philipp, A.; Hummel, P.; Schilling, T.; Feicht, P.; Rosenfeldt, S.; Ertl, M.; Schottle, M.; Lechner, A.M.; Xu, Z.; Gao, C.; et al. Anisotropic thermal transport in spray-coated single-phase two-dimensional materials: Synthetic clay versus graphene oxide. ACS Appl. Mater. Interfaces 2020, 12, 18785–18791. [Google Scholar] [CrossRef] [PubMed]
- Vovusha, H.; Amorim, R.G.; Scheicher, R.H.; Sanyal, B. Controlling the orientation of nucleobases by dipole moment interaction with graphene/h-BN interfaces. RSC Adv. 2018, 8, 6527. [Google Scholar] [CrossRef]
- Da, H.X.; Song, Q.; Dai, H.W.; Dong, P.; Bao, Q.L.; Ye, H.P.; An, Y.P.; Chen, J. Electrically controllable magneto-optic effects in a two-dimensional hexagonal organometallic lattice. Phys. Rev. B 2020, 101, 035423. [Google Scholar] [CrossRef]
- Wang, Q.; Li, J.W.; Yan, L.; Wang, B. KAgSe: A new two-dimensional efficient photovoltaic material with layer-independent behaviors. ACS Appl. Mater. Interfaces 2018, 10, 41670–41677. [Google Scholar] [CrossRef]
- Liu, Y.P.; Zeng, C.; Zhong, J.H.; Ding, J.N.; Wang, Z.M.M.; Liu, Z.W. Spintronics in two-dimensional materials. Nano-Micro Lett. 2020, 12, 1–26. [Google Scholar] [CrossRef]
- Choudhuri, I.; Bhauriyal, P.; Pathak, B. Recent advances in graphene-like 2D materials for spintronics applications. Chem. Mater. 2019, 31, 8260–8285. [Google Scholar] [CrossRef]
- Li, X.Y.; Lin, B.N.; Li, H.B.; Yu, Q.; Ge, Y.; Jin, X.; Liu, X.H. Carbon doped hexagonal BN as a highly efficient metal-free base catalyst for Knoevenagel condensation reaction. Appl. Catal. B 2018, 239, 254–259. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, L.; Sheng, J.P.; Tang, F.Y.; Zeng, K.; Wang, L.Q.; Liang, K.X. Photostable core-shell CdS/ZIF-8 composite for enhanced photocatalytic reduction of CO2. Appl. Surf. Sci. 2019, 498, 143899. [Google Scholar] [CrossRef]
- Mohanty, B.; Chattopadhyay, A.; Nayak, J. Band gap engineering and enhancement of electrical conductivity in hydrothermally synthesized CeO2–PbS nanocomposites for solar cell applications. J. Alloy. Compd. 2021, 850, 156735. [Google Scholar] [CrossRef]
- Sainbileg, B.; Lai, Y.R.; Chen, L.C.; Hayashi, M. The dual-defective SnS2 monolayers: Promising 2D photocatalysts for overall water splitting. Phys. Chem. Chem. Phys. 2019, 21, 26292. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, J.W.; Liang, Y.; Wang, B.; Ning, Y.H. BX1–BX2 (X1, X2 = P, As, Sb) lateral heterostructure: Novel and efficient two-dimensional photovoltaic materials with ultra-high carrier mobilities. J. Mater. Chem. A 2019, 7, 10684. [Google Scholar] [CrossRef]
- Fang, Q.L.; Huang, Y.H.; Miao, Y.P.; Xu, K.W.; Li, Y.; Ma, F. Interfacial defect engineering on electronic states of two-dimensional AlN/MoS2 heterostructure. J. Phys. Chem. C 2017, 121, 6605–6613. [Google Scholar] [CrossRef]
- Yao, H.; Wang, Q.; Li, J.W.; Cai, W.S.; Wei, Y.D.; Wang, B.; Wang, J. Two-dimensional few-layered PC 3 as a promising photocatalyst for overall water splitting. Phys. Chem. Chem. Phys. 2020, 22, 9477–9486. [Google Scholar] [CrossRef]
- Obeid, M.M.; Bafekry, A.; Rehman, S.U.; Nguyen, C.V. A type-II GaSe/HfS2 van der Waals heterostructure as promising photocatalyst with high carrier mobility. Appl. Surf. Sci. 2020, 534, 147607. [Google Scholar] [CrossRef]
- Wang, G.Z.; Li, Z.F.; Wu, W.K.; Guo, H.; Chen, C.; Yuan, H.K.; Yang, S.Y.A. Two-dimensional h-BN/C2N heterostructure as a promising metal-free photocatalyst for overall water-splitting. Phys. Chem. Chem. Phys. 2020, 22, 24446–24454. [Google Scholar] [CrossRef]
- Azusa, M.; Mikiya, F.; Kenji, M.; Hiroki, M.; Hiroaki, B.; Hideo, O.; Shinzaburo, I.; Koichi, Y. Investigations on the charge transfer mechanism at donor/acceptor interfaces in the quest for descriptors of organic solar cell performance. Phys. Chem. Chem. Phys. 2018, 20, 12193. [Google Scholar]
- Zhang, J.L.; Zhao, S.T.; Han, C.; Wang, Z.Z.; Zhong, S.; Sun, S.; Guo, R.; Zhou, X.; Cheng, D.G.; Yuan, K.D.; et al. Epitaxial growth of single layer blue phosphorus: A new phase of two-dimensional phosphorus. Nano Lett. 2016, 16, 4903–4908. [Google Scholar] [CrossRef]
- Kaewmaraya, T.; Srepusharawoot, P.; Hussian, T.; Amornkitbamrung, V. Electronic Properties of h-BCN-Blue Phosphorene van der Waals Heterostructures. Chem. Phys. Chem. 2018, 19, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Radisavljevic, B.; Radenovic, A.; Brivio, J.; Glacometti, V.; Kis, A. Single-layer MoS2 transistors. Nat. Nanotechnol. 2011, 6, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.L.; Tang, W.C.; Ren, Q.Q.; Wang, S.K.; Yu, J.; Du, Y.H. A first-principles study of light non-metallic atom substituted blue phosphorene. Appl. Surf. Sci. 2015, 356, 110–114. [Google Scholar] [CrossRef]
- Kamal, C.; Ezawa, M. Arsenene: Two-dimensional buckled and puckered honeycomb arsenic systems. Phys. Rev. B 2015, 91, 085423. [Google Scholar] [CrossRef]
- Fang, L.Z.; Li, X.P.; Geng, Z.D.; Wang, T.X.; Xia, C.X. Band alignment tuning in GeS/arsenene staggered hetero-structures. J. Alloy. Compd. 2019, 793, 283–288. [Google Scholar] [CrossRef]
- Jamdagni, P.; Thakur, A.; Kumar, A.; Ahluwalia, P.K.; Pandey, R. Two dimensional allotropes of arsenene with a wide range of high and anisotropic carrier mobility. Phys. Chem. Chem. Phys. 2018, 20, 29939. [Google Scholar] [CrossRef]
- Wang, C.; Xia, Q.L.; Nie, Y.Z.; Rahman, M. Strain engineering band gap, effective mass and anisotropic Dirac-like cone in monolayer arsenene. AIP Adv. 2016, 6, 035204. [Google Scholar] [CrossRef]
- Wang, Y.L.; Ding, Y. Electronic structure and carrier mobilities of arsenene and antimonene nanoribbons: A first-principle study. Nanoscale Res. Lett. 2015, 10, 254. [Google Scholar] [CrossRef]
- Zhang, S.L.; Yan, Z.; Li, Y.F.; Chen, Z.F.; Zeng, H.B. Atomically thin Arsenene and antimonene: Semimetal–semiconductor and indirect–direct band-gap transitions. Angew. Chem. Int. Ed. 2015, 127, 1–5. [Google Scholar]
- Huang, L.; Li, J.B. Tunable electronic structure of black phosphorus/blue phosphorus van der Waals p-n hetero-structure. Appl. Phys. Lett. 2016, 108, 083101. [Google Scholar] [CrossRef]
- Wang, B.J.; Li, X.H.; Cai, X.L.; Yu, W.Y.; Zhang, L.W.; Zhao, R.Q.; Ke, S.H. Blue Phosphorus/Mg(OH)2 van der Waals hetero-structures as Promising Visible-Light Photocatalysts for Water Splitting. J. Phys. Chem. C 2018, 122, 7075–7080. [Google Scholar] [CrossRef]
- Guo, J.J.; Zhou, G.P.; Li, H.H.; Wang, H.Y.; Liu, C. Tuning electronic properties of blue phosphorene/graphene-like GaN van der Waals hetero-structures by vertical external electric field. Nanoscale Res. Lett. 2019, 14, 174. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Wang, S.; Luo, Y.; Xu, Y.J.; Sun, M.L.; Yu, J.; Tang, W.C. Strain-enhanced properties of van der Waals hetero-structure based on blue phosphorus and g-GaN as a visible-light-driven photocatalyst for water splitting. RSC Adv. 2019, 9, 4816. [Google Scholar] [CrossRef]
- Li, W.; Wang, T.X.; Dai, X.Q.; Ma, Y.Q.; Tang, Y.N. Effects of electric field on the electronic structures of MoS2/arsenene van der Waals heterostructure. J. Alloy. Compd. 2017, 705, 486–491. [Google Scholar] [CrossRef]
- Zeng, H.; Zhao, J.; Cheng, A.Q.; Zhang, L.; He, Z.; Chen, R.S. Tuning electronic and optical properties of arsenene/C3N van der Waals heterostructure by vertical strain and external electric field. Nanotechnology 2018, 29, 075201. [Google Scholar] [CrossRef]
- Li, Q.F.; Ma, X.F.; Lei, Z.; Wan, X.G.; Rao, W.F. Theoretical design of blue phosphorene/arsenene lateral heterostructures with superior electronic properties. J. Phys. D Appl. Phys. 2018, 51, 255304. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J.; Hafner, J. Theory of the crystal structures of selenium and tellurium: The effect of generalized-gradient corrections to the local-density approximation. Phys. Rev. B 1994, 50, 17953. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficient iterative schemes for Ab initio total-energy calculations using a plane-Wave Basis Set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Heyd, J.; Scuseria, G.E. Hybrid functional based on a screened coulomb potential. J. Chem. Phys. 2003, 118, 8207. [Google Scholar] [CrossRef]
- Paier, J.; Marsman, M.; Hummer, K.; Kresse, G. Screened hybrid density functionals applied to solids. J. Chem. Phys. 2006, 124, 154709. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 15. [Google Scholar] [CrossRef]
- Alfonso, D.R.; Ulloa, S.E. Molecular-dynamics simulations of methyl-radical deposition on diamond (100) surfaces. Phys. Rev. B 1993, 48, 16. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.J.; Li, X.H.; Zhao, R.Q.; Cai, X.L.; Yu, W.Y.; Li, W.B.; Liu, Z.S.; Zhang, L.W.; Ke, S.H. Electronic structures and enhanced photocatalytic properties of blue phosphorene/BSe van der Waals heterostructures. J. Mater. Chem. A 2018, 6, 8923. [Google Scholar] [CrossRef]
- Xie, Z.F.; Sun, F.W.; Yao, R.; Zhang, Y.; Zhang, Y.H.; Zhang, Z.H.; Fan, J.B.; Ni, L.; Duan, L. Tuning electronic properties of InSe/arsenene heterostructure by external electric field and uniaxial strain. Appl. Surf. Sci. 2019, 475, 839–846. [Google Scholar] [CrossRef]
- Zhou, H.; Cai, W.S.; Li, J.W.; Liu, X.Y.; Xiong, W.; Zhou, Y.; Xu, Z.; Wang, B.; Ye, C. C2N/BlueP van der Waals hetero-structure: An efficient photocatalytic water splitting 2D material. Phys. Chem. Chem. Phys. 2020, 22, 1485–1492. [Google Scholar] [CrossRef]
- Li, X.H.; Wang, B.J.; Cai, X.L.; Zhang, L.W.; Wang, G.D.; Ke, S.H. Tunable electronic properties of arsenene/GaS van der Waals heterostructures. RSC Adv. 2017, 7, 28393. [Google Scholar] [CrossRef]
- Sun, M.L.; Chou, J.P.; Gao, J.F.; Cheng, Y.; Hu, A.; Tang, W.C.; Zhang, G. Exceptional optical Absorption of buckled arsenene covering a broad spectral range by molecular doping. ACS Omega 2018, 3, 8514–8520. [Google Scholar] [CrossRef]
- Liu, N.S.; Zhou, S. Gas adsorption on monolayer blue phosphorus: Implications for environmental stability and gas sensors. Nanotechnology 2017, 28, 175708. [Google Scholar] [CrossRef]
- Li, G.; Zhao, Y.C.; Zeng, S.M.; Ni, J. The realization of half-metal and spin-semiconductor for metal adatoms on arsenene. Appl. Surf. Sci. 2016, 390, 60–67. [Google Scholar] [CrossRef]
- Kuzmenko, A.B. Kramers–Kronig constrained variational analysis of optical spectra. Rev. Sci. Instrum. 2005, 76, 083108. [Google Scholar] [CrossRef]
- Ma, X.G.; Lv, Y.H.; Xu, J.; Liu, Y.F.; Zhang, R.Q.; Zhu, Y.F. A strategy of enhancing the photoactivity of g-C3N4 via doping of nonmetal elements: A first-principles study. J. Phys. Chem. C 2012, 116, 23485–23493. [Google Scholar] [CrossRef]
- Singh, A.K.; Mathew, K.; Zhuang, H.L.; Henning, R.G. Computational screening of 2D materials for photocatalysis. J. Phys. Chem. Lett. 2015, 6, 1087–1098. [Google Scholar] [CrossRef] [PubMed]
- Farkous, M.; Bikerouin, M.; Thuan, D.V.; Benhouria, Y.; El-Yadri, M.; Feddi, E.; Erguig, H.; Dujardin, F.; Nguyen, C.V.; Hieu, N.V.; et al. Strain effects on the electronic and optical properties of Van der Waals hetero-structure MoS2/WS2: A first-principles study. Phys. E 2020, 116, 113799. [Google Scholar] [CrossRef]
- Bai, Y.J.; Zhang, Q.F.; Xu, N.; Deng, K.M.; Kan, E. Efficient carrier separation and band structure tuning of two-dimensional C2N/GaTe van der Waals hetero-structure. J. Phys. Chem. C 2018, 122, 15892–15902. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Jiang, B.; Liu, Y.; Liu, L.; Xia, T.; Zhang, X.; Ye, C.; Yu, Y.; Wang, B. Two-Dimensional As/BlueP van der Waals Hetero-Structure as a Promising Photocatalyst for Water Splitting: A DFT Study. Coatings 2020, 10, 1160. https://doi.org/10.3390/coatings10121160
Liu X, Jiang B, Liu Y, Liu L, Xia T, Zhang X, Ye C, Yu Y, Wang B. Two-Dimensional As/BlueP van der Waals Hetero-Structure as a Promising Photocatalyst for Water Splitting: A DFT Study. Coatings. 2020; 10(12):1160. https://doi.org/10.3390/coatings10121160
Chicago/Turabian StyleLiu, Xinyi, Bei Jiang, Yanxin Liu, Lei Liu, Tian Xia, Xin Zhang, Cong Ye, Yunjin Yu, and Bin Wang. 2020. "Two-Dimensional As/BlueP van der Waals Hetero-Structure as a Promising Photocatalyst for Water Splitting: A DFT Study" Coatings 10, no. 12: 1160. https://doi.org/10.3390/coatings10121160
APA StyleLiu, X., Jiang, B., Liu, Y., Liu, L., Xia, T., Zhang, X., Ye, C., Yu, Y., & Wang, B. (2020). Two-Dimensional As/BlueP van der Waals Hetero-Structure as a Promising Photocatalyst for Water Splitting: A DFT Study. Coatings, 10(12), 1160. https://doi.org/10.3390/coatings10121160



