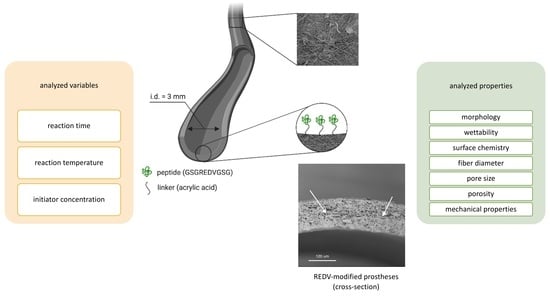
Physicochemical and Mechanical Properties of Blow Spun Nanofibrous Prostheses Modified with Acrylic Acid and REDV Peptide
Abstract
1. Introduction
2. Materials and Methods
2.1. Fibrous Material Fabrication
2.2. Surface Modification of Flat Nanofibrous Mats
2.3. Characterization of Modified Nanofibrous Mats
2.3.1. Fiber Morphology, Fiber Diameter and Pore Size
2.3.2. Surface Wettability
2.4. Surface Modification of Cylindrical Nanofibrous Prostheses in a Flow System
Modification Process
2.5. Characterization of Cylindrical Prostheses Modified in a Flow System
2.5.1. Fiber Morphology, Fiber Diameter and Pore Size
2.5.2. Surface Wettability
2.5.3. Porosity Measurements
2.5.4. Mechanical Testing
2.5.5. Spectroscopic Analysis
3. Results
3.1. Mechanism of the Modification Process
3.2. Modification of Nanofibrous Mats—The Influence of Process Parameters on the Fiber Morphology and Wettability
3.3. Modification of Cylindrical Nanofibrous Prostheses in a Flow System
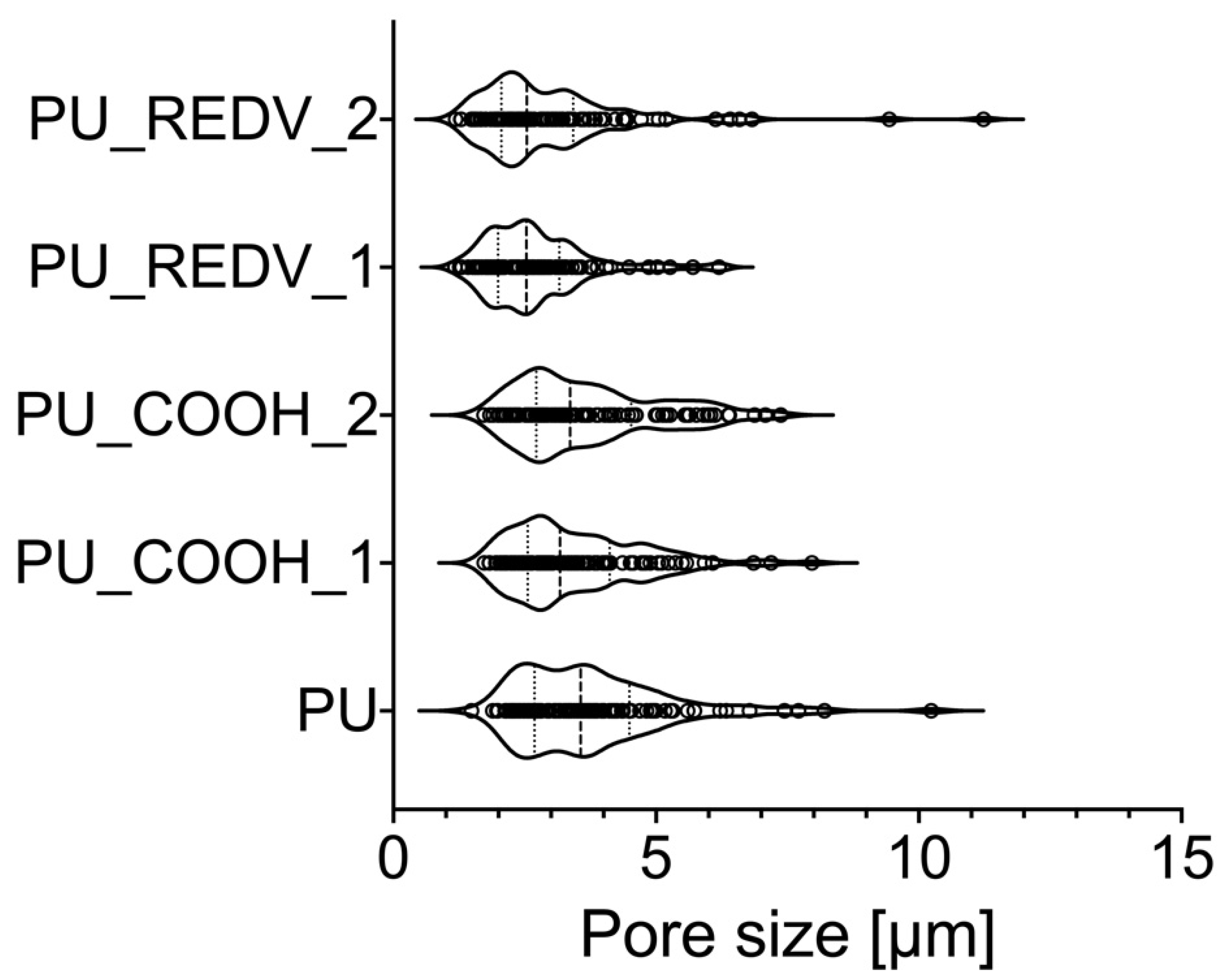
3.3.1. Morphology, Wettability, Pore Size and Porosity
3.3.2. Mechanical Properties
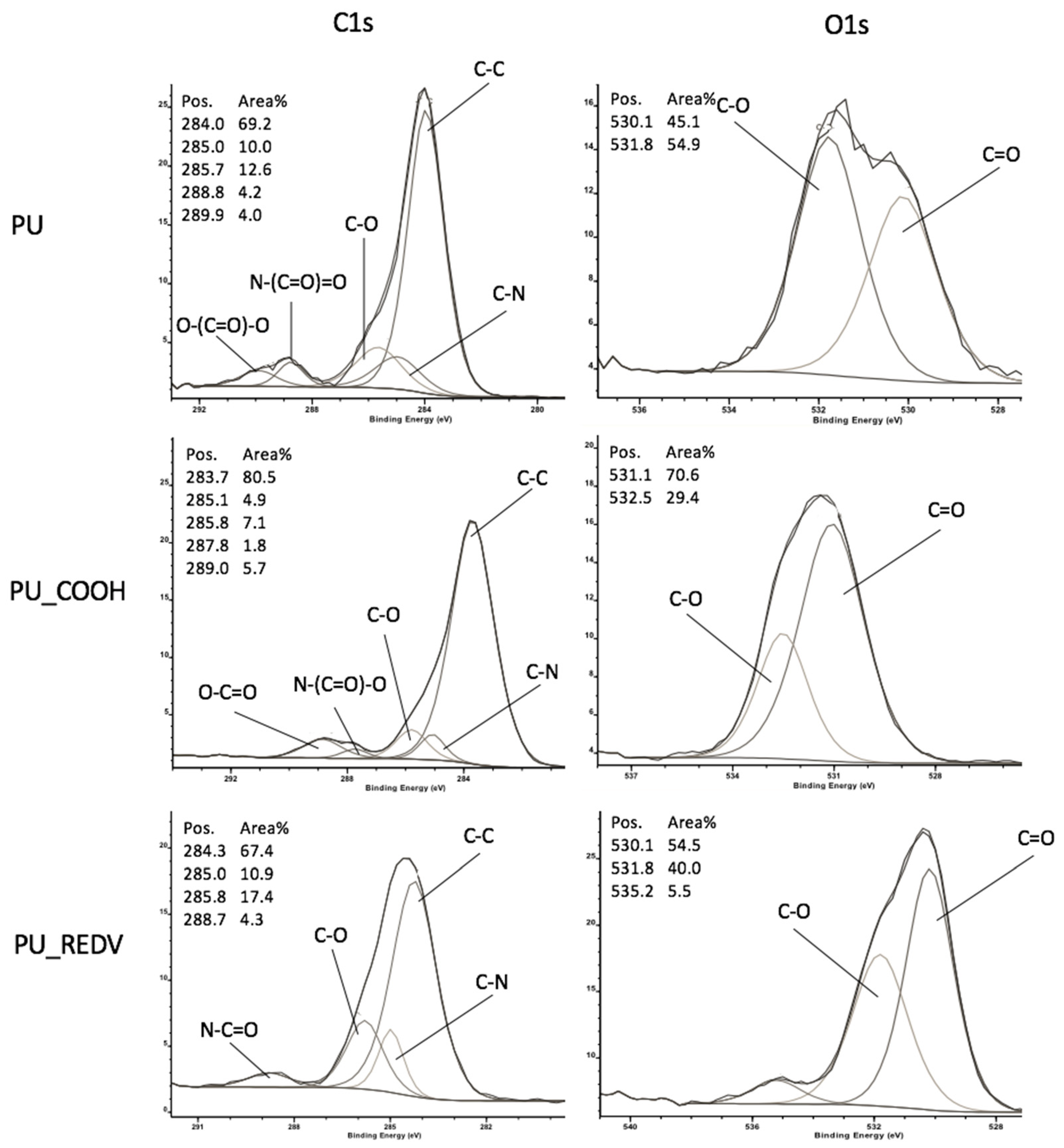
3.3.3. Spectroscopic Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xue, L.; Greisler, H.P. Biomaterials in the development and future of vascular grafts. J. Vasc. Surg. 2003, 37, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Taggart, D.P. Current status of arterial grafts for coronary artery bypass grafting. Ann. Cardiothorac. Surg. 2013, 2, 427–430. [Google Scholar] [PubMed]
- Ju, Y.M.; Choi, J.S.; Atala, A.; Yoo, J.J.; Lee, S.J. Bilayered scaffold for engineering cellularized blood vessels. Biomaterials 2010, 31, 4313–4321. [Google Scholar] [CrossRef] [PubMed]
- Milleret, V.; Hefti, T.; Hall, H.; Vogel, V.; Eberli, D. Influence of the fiber diameter and surface roughness of electrospun vascular grafts on blood activation. Acta Biomater. 2012, 8, 4349–4356. [Google Scholar] [CrossRef]
- Goins, A.; Webb, A.R.; Allen, J.B. Multi-layer approaches to scaffold-based small diameter vessel engineering: A review. Mater. Sci. Eng. C 2019, 97, 896–912. [Google Scholar] [CrossRef]
- Nakielski, P.; Pierini, F. Blood interactions with nano-and microfibers: Recent advances, challenges and applications in nano- and microfibrous hemostatic agents. Acta Biomater. 2019, 84, 63–76. [Google Scholar] [CrossRef]
- Hu, J.J.; Chao, W.C.; Lee, P.Y.; Huang, C.H. Construction and characterization of an electrospun tubular scaffold for small-diameter tissue-engineered vascular grafts: A scaffold membrane approach. J. Mech. Behav. Biomed. Mater. 2012, 13, 140–155. [Google Scholar] [CrossRef]
- Van Wachem, P.B.; Schakenraad, J.M.; Feijen, J.; Beugeling, T.; van Aken, W.G.; Blaauw, E.H.; Nieuwenhuis, P.; Molenaar, I. Adhesion and spreading of cultured endothelial cells on modified and unmodified poly (ethylene terephthalate): A morphological study. Biomaterials 1989, 10, 532–539. [Google Scholar] [CrossRef]
- Dubey, G.; Mequanint, K. Conjugation of fibronectin onto three-dimensional porous scaffolds for vascular tissue engineering applications. Acta Biomater. 2011, 7, 1114–1125. [Google Scholar] [CrossRef]
- Zhang, Y.; Chai, C.; Jiang, X.S.; Teoh, S.H.; Leong, K.W. Fibronectin immobilized by covalent conjugation or physical adsorption shows different bioactivity on aminated-PET. Mater. Sci. Eng. C 2007, 27, 213–219. [Google Scholar] [CrossRef]
- Dejana, E.; Lampugnani, M.G.; Giorgi, M.; Gaboli, M.; Marchisio, P.C. Fibrinogen induces endothelial cell adhesion and spreading via the release of endogenous matrix proteins and the recruitment of more than one integrin receptor. Blood 1990, 75, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Browning, M.B.; Dempsey, D.; Guiza, V.; Becerra, S.; Rivera, J.; Russell, B.; Höök, M.; Clubb, F.; Miller, M.; Fossum, T.; et al. Multilayer vascular grafts based on collagen-mimetic proteins. Acta Biomater. 2012, 8, 1010–1021. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.S.; Wang, S.S.; Chung, T.W.; Wang, Y.H.; Chiou, S.H.; Hsu, J.J.; Chou, N.-K.; Hsieh, K.-H.; Chu, S.-H. Growth of endothelial cells on different concentrations of Gly-Arg-Gly-Asp photochemically grafted in polyethylene glycol modified polyurethane. Artif. Organs 2001, 25, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.S.; Bae, J.W.; Lim, H.R.; Joung, Y.K.; Park, J.-C.; Kwon, I.K.; Dong, P.K. RGD peptide-immobilized electrospun matrix of polyurethane for enhanced endothelial cell affinity. Biomed. Mater. 2008, 30, 44104. [Google Scholar] [CrossRef]
- Walluscheck, K.P.; Steinhoff, G.; Kelm, S.; Haverich, A. Improved endothelial cell attachment on ePTFE vascular grafts pretreated with synthetic RGD-containing peptides. Eur. J. Vasc. Endovasc. Surg. 1996, 12, 321–330. [Google Scholar] [CrossRef]
- Liu, J.C.; Heilshorn, S.C.; Tirrell, D.A. Comparative cell response to artificial extracellular matrix proteins containing the RGD and CS5 cell-binding domains. Biomacromolecules 2004, 5, 497–504. [Google Scholar] [CrossRef]
- Shin, H.; Jo, S.; Mikos, A.G. Biomimetic materials for tissue engineering. Biomaterials 2003, 24, 4353–4364. [Google Scholar] [CrossRef]
- Ji, Y.; Wei, Y.; Liu, X.; Wang, J.; Ren, K.; Ji, J. Zwitterionic polycarboxybetaine coating functionalized with REDV peptide to improve selectivity for endothelial cells. J. Biomed. Mater. Res. A 2012, 100, 1387–1397. [Google Scholar] [CrossRef]
- Castellanos, M.I.; Zenses, A.S.; Grau, A.; Rodríguez-Cabello, J.C.; Gil, F.J.; Manero, J.M.; Pegueroles, M. Biofunctionalization of REDV elastin-like recombinamers improves endothelialization on CoCr alloy surfaces for cardiovascular applications. Colloids Surf. B Biointerfaces 2015, 127, 22–32. [Google Scholar] [CrossRef]
- Wei, Y.; Ji, Y.; Xiao, L.; Lin, Q.; Ji, J. Different complex surfaces of polyethyleneglycol (PEG) and REDV ligand to enhance the endothelial cells selectivity over smooth muscle cells. Colloids Surf B Biointerfaces 2011, 84, 369–378. [Google Scholar] [CrossRef]
- Wang, W.; Guo, L.; Yu, Y.; Chen, Z.; Zhou, R.; Yuan, Z. Peptide REDV-modified polysaccharide hydrogel with endothelial cell selectivity for the promotion of angiogenesis. J. Biomed. Mater. Res. Part A 2015, 103, 1703–1712. [Google Scholar] [CrossRef] [PubMed]
- Massia, S.P.; Hubbell, J.A. Vascular endothelial cell adhesion and spreading promoted by the peptide REDV of the IIICS region of plasma fibronectin is mediated by integrin alpha 4 beta 1. J. Biol. Chem. 1992, 267, 14019–14026. [Google Scholar] [PubMed]
- Zhou, F.; Wen, M.; Zhou, P.; Zhao, Y.; Jia, X.; Fan, Y. Materials Science & Engineering C Electrospun membranes of PELCL/PCL-REDV loading with miRNA-126 for enhancement of vascular endothelial cell adhesion and proliferation. Mater. Sci. Eng. C 2018, 85, 37–46. [Google Scholar] [CrossRef]
- Butruk-Raszeja, B.A.; Kuźminska, A.; Ciach, T.; Adipurnama, I.; Yang, M.-C. Endothelial cell growth on polyurethane modified with acrylic acid and REDV peptide. Surf. Innov. 2019, 8, 1–17. [Google Scholar] [CrossRef]
- Wojasiński, M.; Pilarek, M.; Ciach, T. Comparative studies of electrospinning and solution blow spinning processes for the production of nanofibrous poly(L-lactic acid) materials for biomedical engineering. Pol. J. Chem. Technol. 2014, 16, 43–50. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Butruk-Raszeja, B.A.; Trzaskowska, P.A.; Kuźminska, A.; Ciach, T. Polyurethane modification with acrylic acid by Ce(IV)-initiated graft polymerization. Open Chem. 2016, 14, 206–214. [Google Scholar] [CrossRef]
- Pranantyo, D.; Xu, L.Q.; Neoh, K.G.; Kang, E.T.; Yang, W.; Teo, S.L.M. Photoinduced anchoring and micropatterning of macroinitiators on polyurethane surfaces for graft polymerization of antifouling brush coatings. J. Mater. Chem. B 2014, 2, 398–408. [Google Scholar] [CrossRef]
- Qu, X.H.; Wu, Q.; Liang, J.; Qu, X.; Wang, S.G.; Chen, G.Q. Enhanced vascular-related cellular affinity on surface modified copolyesters of 3-hydroxybutyrate and 3-hydroxyhexanoate (PHBHHx). Biomaterials 2005, 26, 6991–7001. [Google Scholar] [CrossRef]
- Moulder, J.; Stickle, W.; Sobol, P.; Bomben, K. Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer Corporation: Waltham, MA, USA, 1992. [Google Scholar]
- Gardner, S.D.; Singamsetty, C.S.K.; Booth, G.L.; He, G.R.; Pittman, C.U. Surface characterization of carbon fibers using angle-resolved XPS and ISS. Carbon 1995, 33, 587–595. [Google Scholar] [CrossRef]
- Davoudi, P.; Assadpour, S.; Derakhshan, M.A.; Ai, J.; Solouk, A.; Ghanbari, H. Biomimetic modification of polyurethane-based nanofibrous vascular grafts: A promising approach towards stable endothelial lining. Mater. Sci. Eng. C 2017, 80, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Antonova, L.V.; Silnikov, V.N.; Sevostyanova, V.V.; Yuzhalin, A.E.; Koroleva, L.S.; Velikanova, E.A.; Mironov, A.V.; Godovikova, T.S.; Kutikhin, A.G.; Glushkova, T.V.; et al. Biocompatibility of small-diameter vascular grafts in different modes of RGD modification. Polymers 2019, 11, 174. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.S.; Liu, X.; Xu, Z.; Lin, T.; Wang, X. Elastin and collagen enhances electrospun aligned polyurethane as scaffolds for vascular graft. J. Mater. Sci. Mater. Med. 2013, 24, 1865–1874. [Google Scholar] [CrossRef] [PubMed]
- Karimi, A.; Rahmati, S.M.; Sera, T.; Kudo, S.; Navidbakhsh, M. A combination of experimental and numerical methods to investigate the role of strain rate on the mechanical properties and collagen fiber orientations of the healthy and atherosclerotic human coronary arteries. Bioengineered 2017, 8, 154–170. [Google Scholar] [CrossRef]



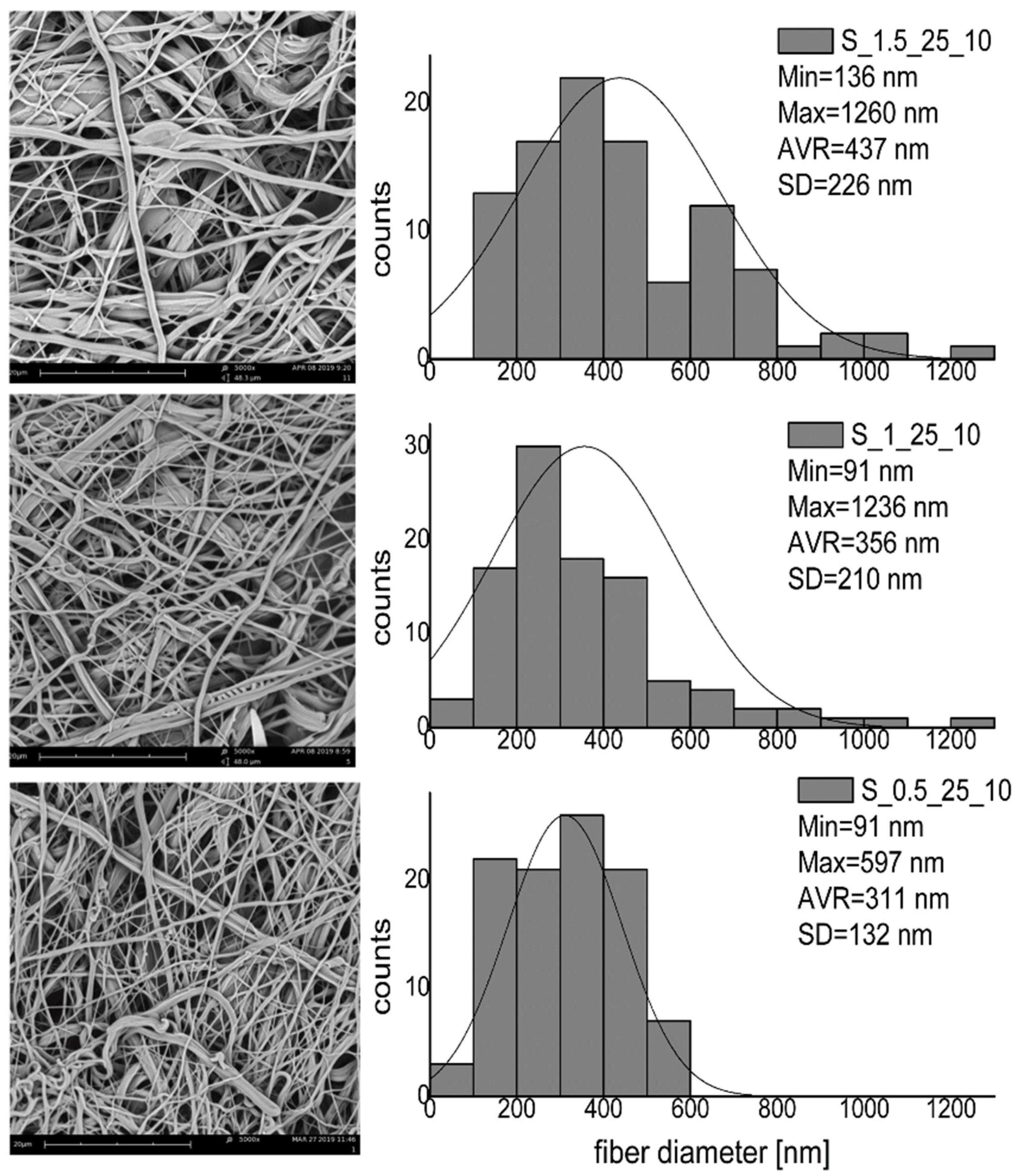
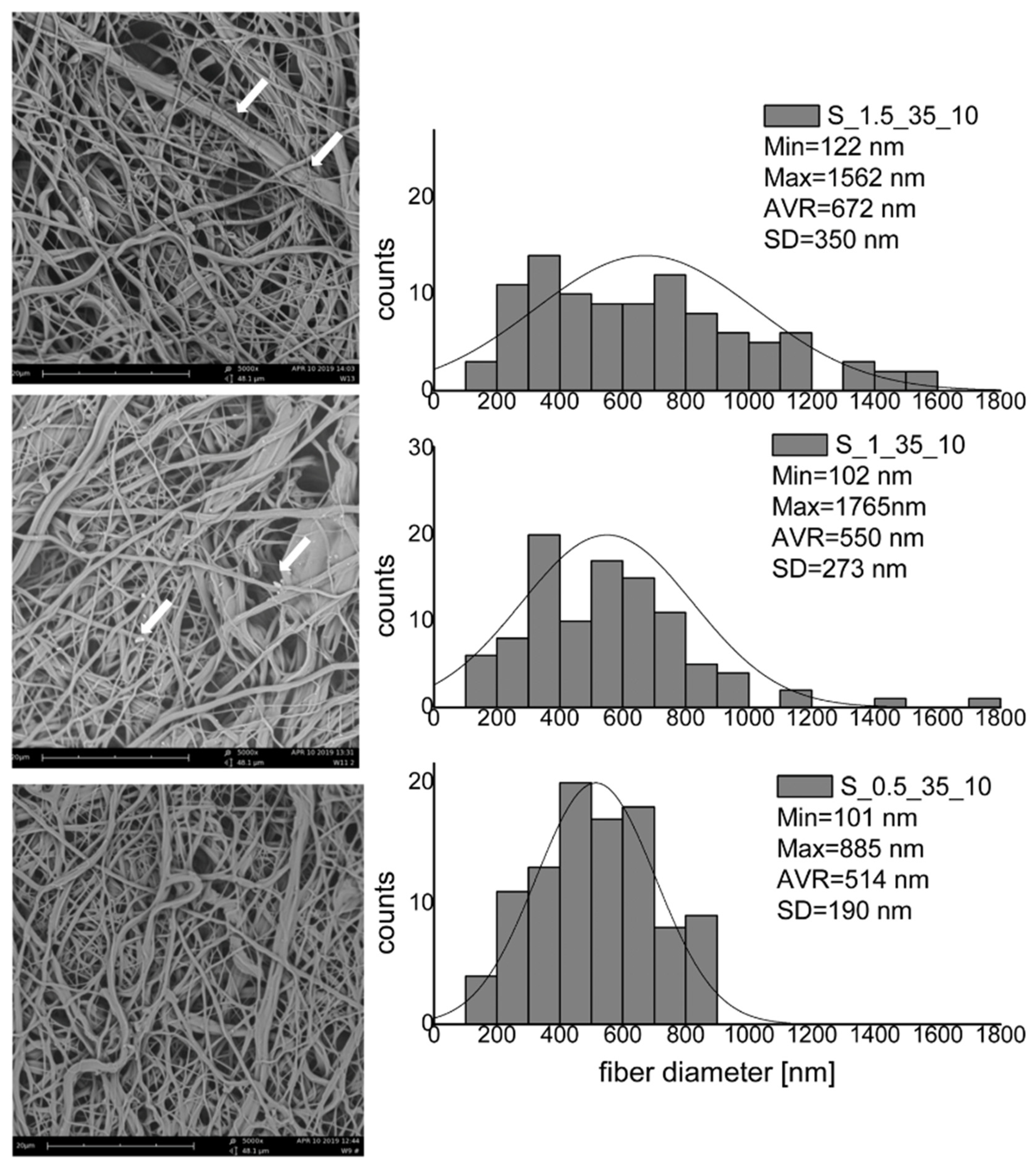



| Material | Propan-2-ol [%] | Reaction Time [h] | Temperature [°C] | (NH4)4Ce(SO3)4 [%] | Acrylic Acid [%] |
|---|---|---|---|---|---|
| S_0 | - | 0 | - | - | - |
| S_0.5_25_10 | 100 | 0.5 | 25 | 0.01 | 1 |
| S_1_25_10 | 100 | 1 | 25 | 0.01 | 1 |
| S_1.5_25_10 | 100 | 1.5 | 25 | 0.01 | 1 |
| S_0.5_35_10 | 100 | 0.5 | 35 | 0.01 | 1 |
| S_1_35_10 | 100 | 1 | 35 | 0.01 | 1 |
| S_1.5_35_10 | 100 | 1.5 | 35 | 0.01 | 1 |
| S_0.5_25_100 | 100 | 0.5 | 25 | 0.1 | 1 |
| S_0.5_25_50 | 100 | 0.5 | 25 | 0.05 | 1 |
| Contact Angle [°] | |
|---|---|
| S_0 | 134 ± 1 |
| S_0.5_25_10 | 104 ± 15 * |
| S_1_25_10 | 97 ± 9 * |
| S_1.5_25_10 | 94 ± 9 * |
| S_0.5_35_10 | 76 ± 5 * |
| S_1_35_10 | 106 ± 12 * |
| S_1.5_35_10 | 95 ± 11 * |
| S_0.5_25_100 | 111 ± 26 |
| S_0.5_25_50 | 82 ± 11 * |
| Before Modification (PU) | After Modification (PU_REDV) | |
|---|---|---|
| wall thickness [μm] | 271 ± 27 | 174 ± 34 |
| CA [°] | 134 ± 1 | 52 ± 3 (1) * 54 ± 1(2) * |
| average fiber diameter [nm] | 231 ± 89 | 318 ± 99 (1) * 311 ± 110 (2) * |
| max fiber diameter [nm] | 493 | 640 (1) 840 (2) |
| min fiber diameter [nm] | 97 | 121 (1) 164 (2) |
| average pore size [μm] | 3.8 ± 1.5 | 2.7 ± 1.0 (1) * 3.0 ± 1.6 (2) * |
| max pore size [μm] | 10.2 | 6.2 (1) 11.2 (2) |
| min pore size [μm] | 1.5 | 1.2 (1) 1.2 (2) |
| porosity [%] | 53.5 ± 2.9 | 48.4 ± 8.6 |
| Young’s modulus [MPa] | 1.8 ± 0.3 | 4.0 ± 0.4 |
| tensile strength [MPa] | 2.1 ± 0.3 | 12.7 ± 1.7 |
| elongation at break [mm/mm] | 1.5 ± 0.1 | 2.5 ± 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butruk-Raszeja, B.A.; Kuźmińska, A.; Wojasiński, M.; Piotrowska, Z. Physicochemical and Mechanical Properties of Blow Spun Nanofibrous Prostheses Modified with Acrylic Acid and REDV Peptide. Coatings 2020, 10, 1110. https://doi.org/10.3390/coatings10111110
Butruk-Raszeja BA, Kuźmińska A, Wojasiński M, Piotrowska Z. Physicochemical and Mechanical Properties of Blow Spun Nanofibrous Prostheses Modified with Acrylic Acid and REDV Peptide. Coatings. 2020; 10(11):1110. https://doi.org/10.3390/coatings10111110
Chicago/Turabian StyleButruk-Raszeja, Beata A., Aleksandra Kuźmińska, Michał Wojasiński, and Zuzanna Piotrowska. 2020. "Physicochemical and Mechanical Properties of Blow Spun Nanofibrous Prostheses Modified with Acrylic Acid and REDV Peptide" Coatings 10, no. 11: 1110. https://doi.org/10.3390/coatings10111110
APA StyleButruk-Raszeja, B. A., Kuźmińska, A., Wojasiński, M., & Piotrowska, Z. (2020). Physicochemical and Mechanical Properties of Blow Spun Nanofibrous Prostheses Modified with Acrylic Acid and REDV Peptide. Coatings, 10(11), 1110. https://doi.org/10.3390/coatings10111110