Ti–Cu Coatings Deposited by a Combination of HiPIMS and DC Magnetron Sputtering: The Role of Vacuum Annealing on Cu Diffusion, Microstructure, and Corrosion Resistance
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
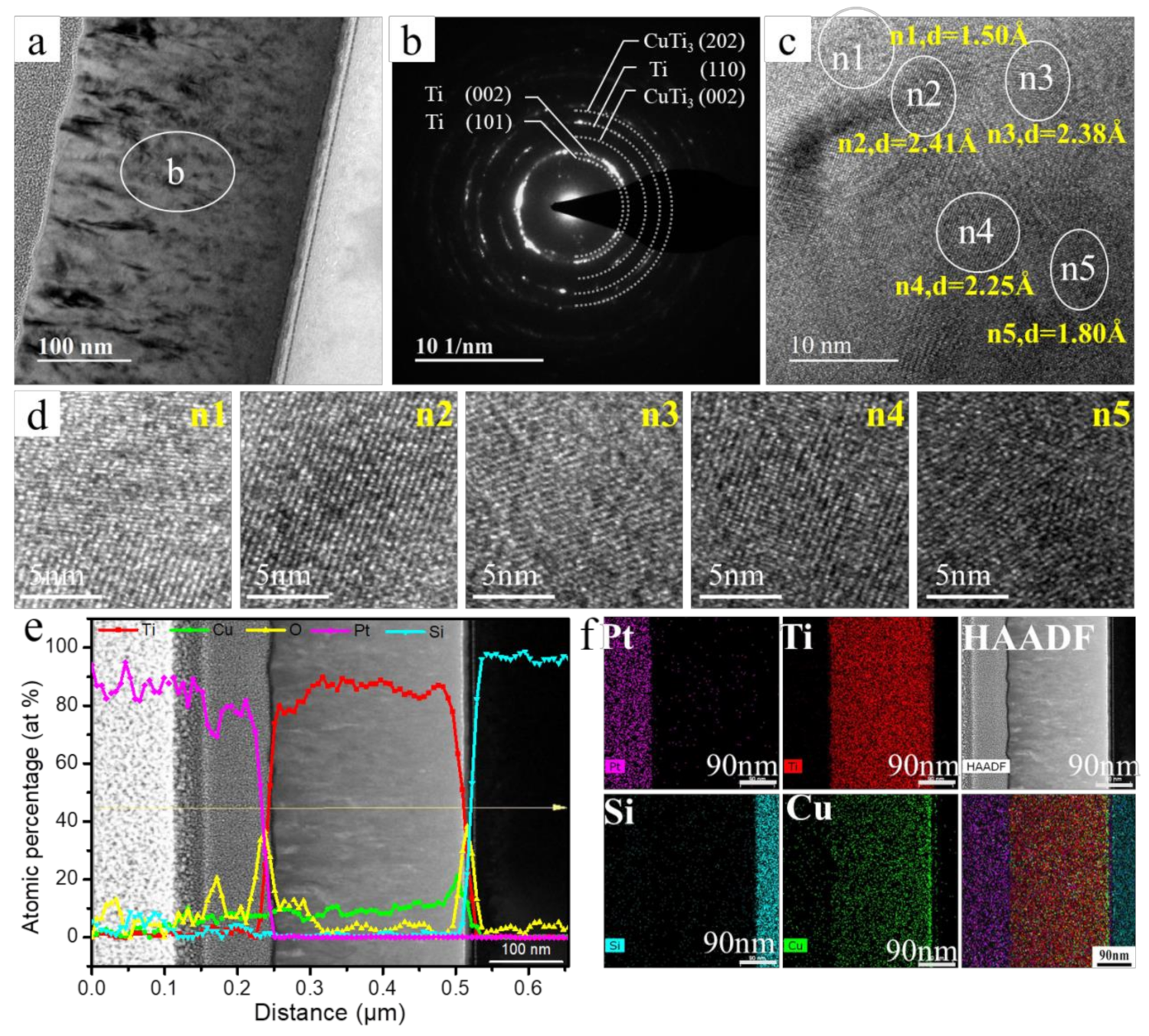
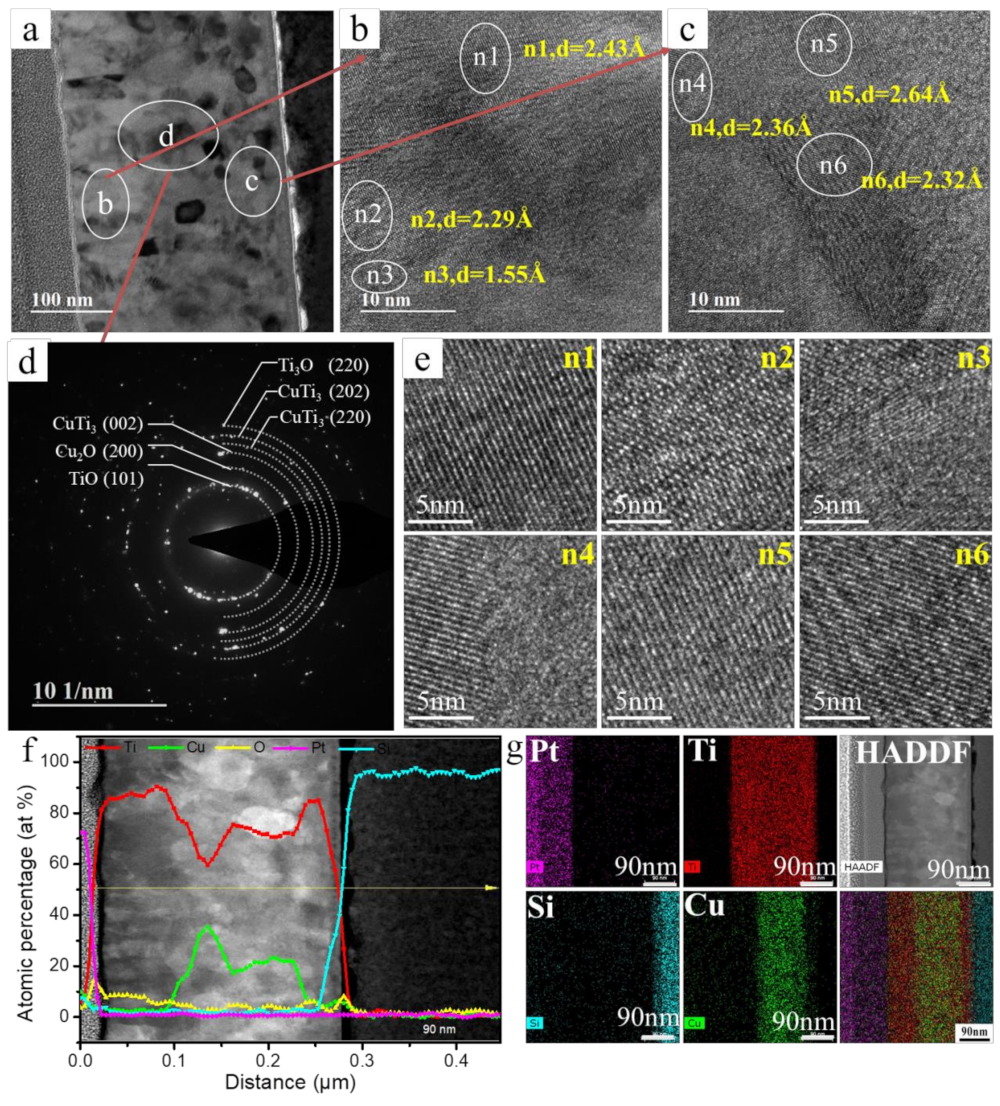
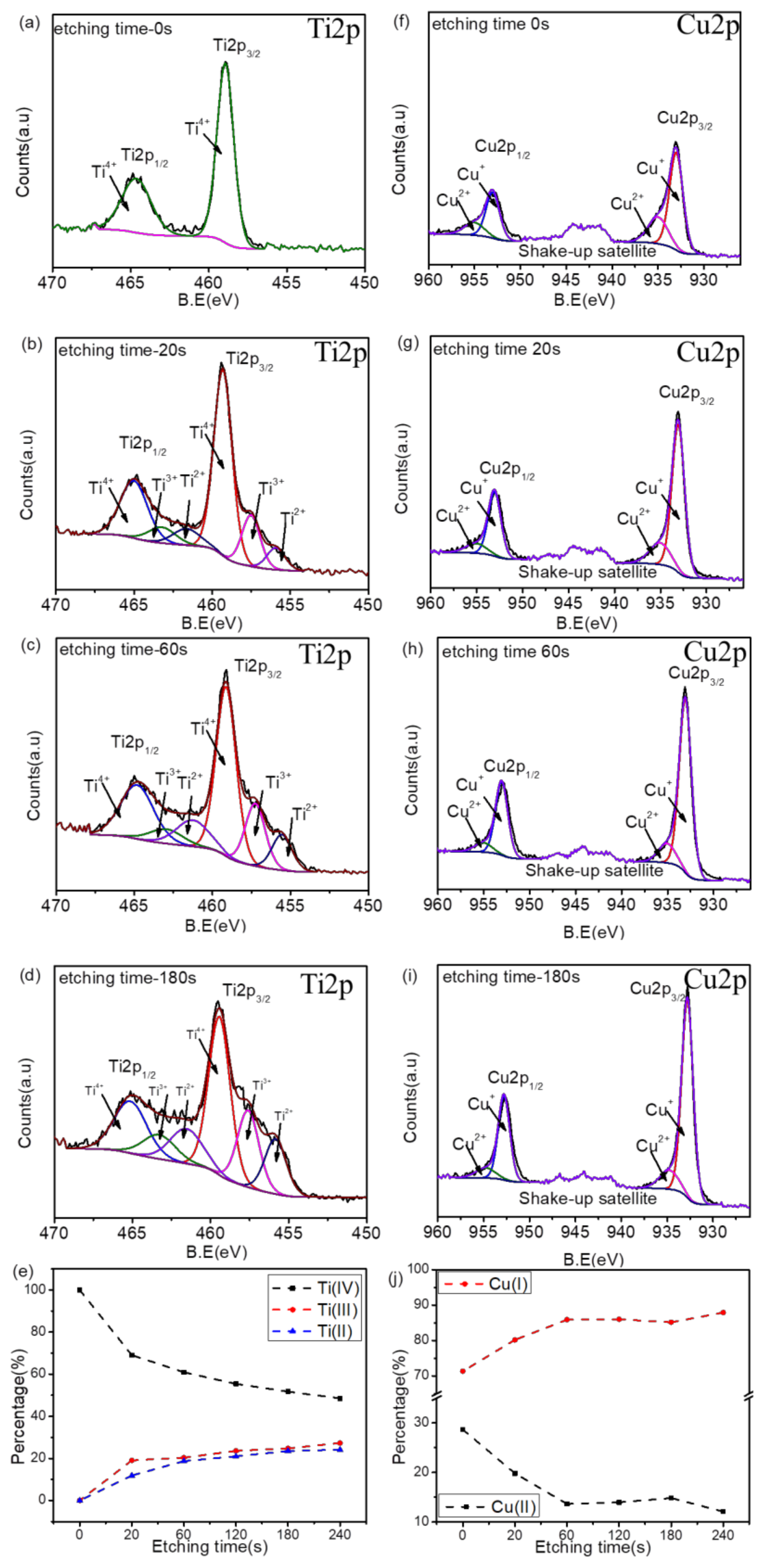
3.1. Surface Chemistry and Structural Analyses of Ti–Cu Coatings
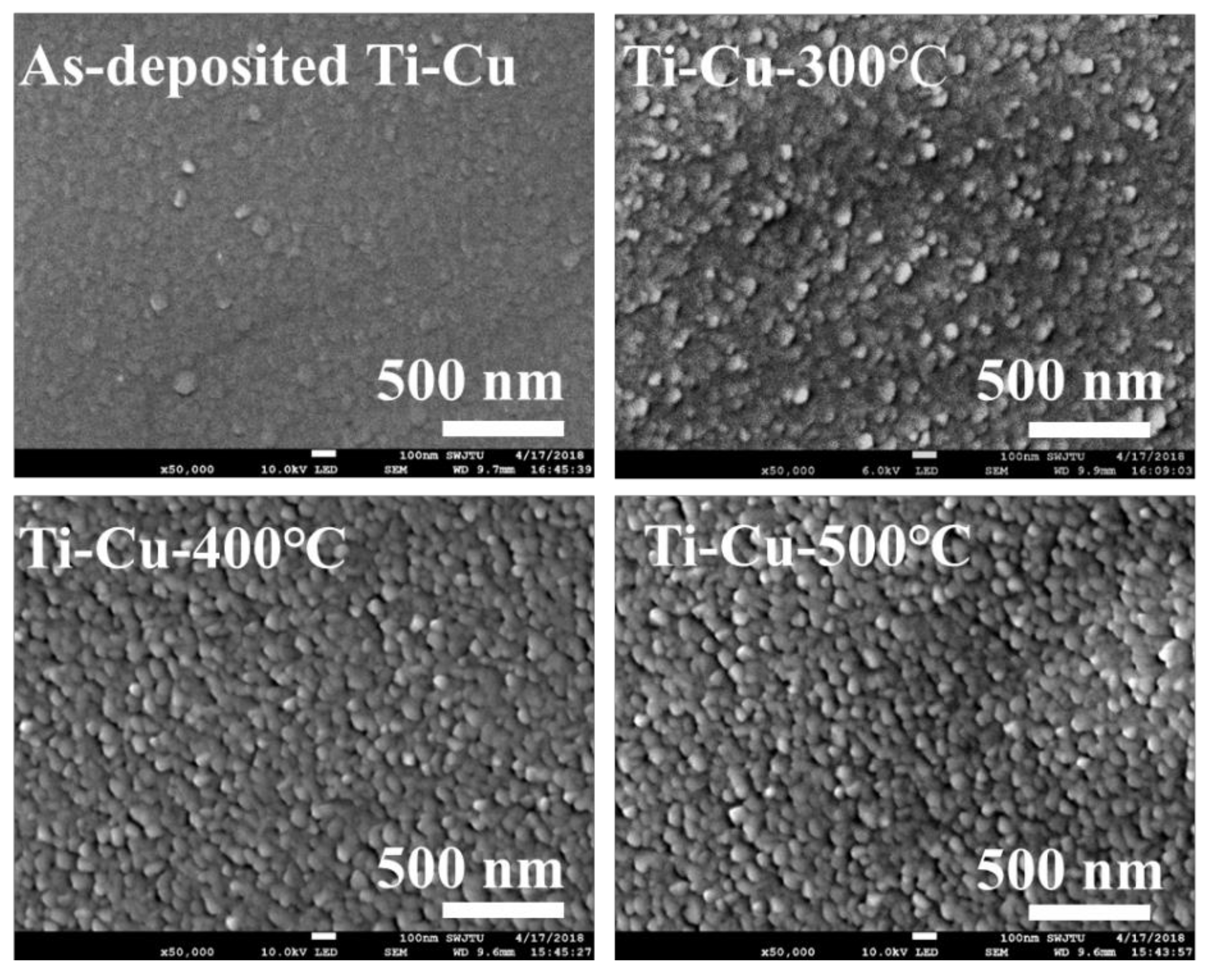
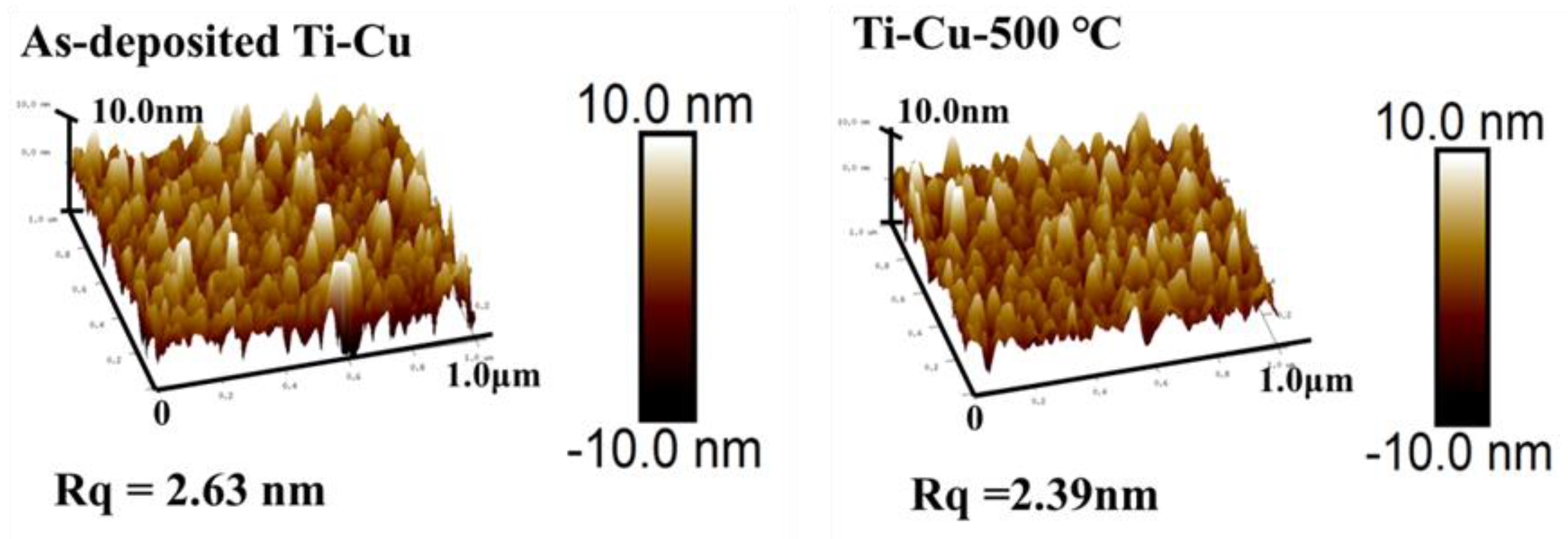
3.2. Surface Morphology
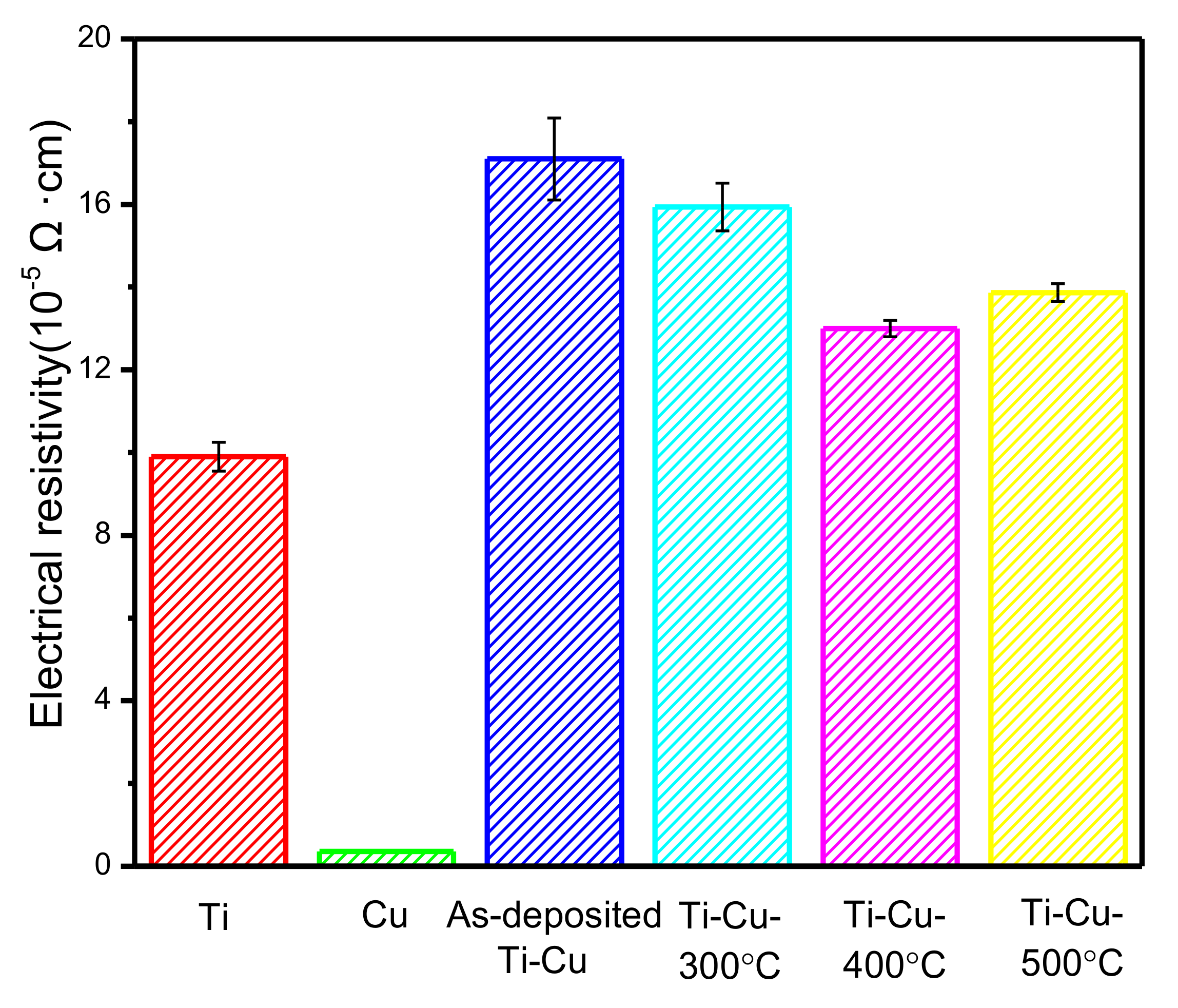
3.3. Electrical Resistivity of Ti–Cu Coatings
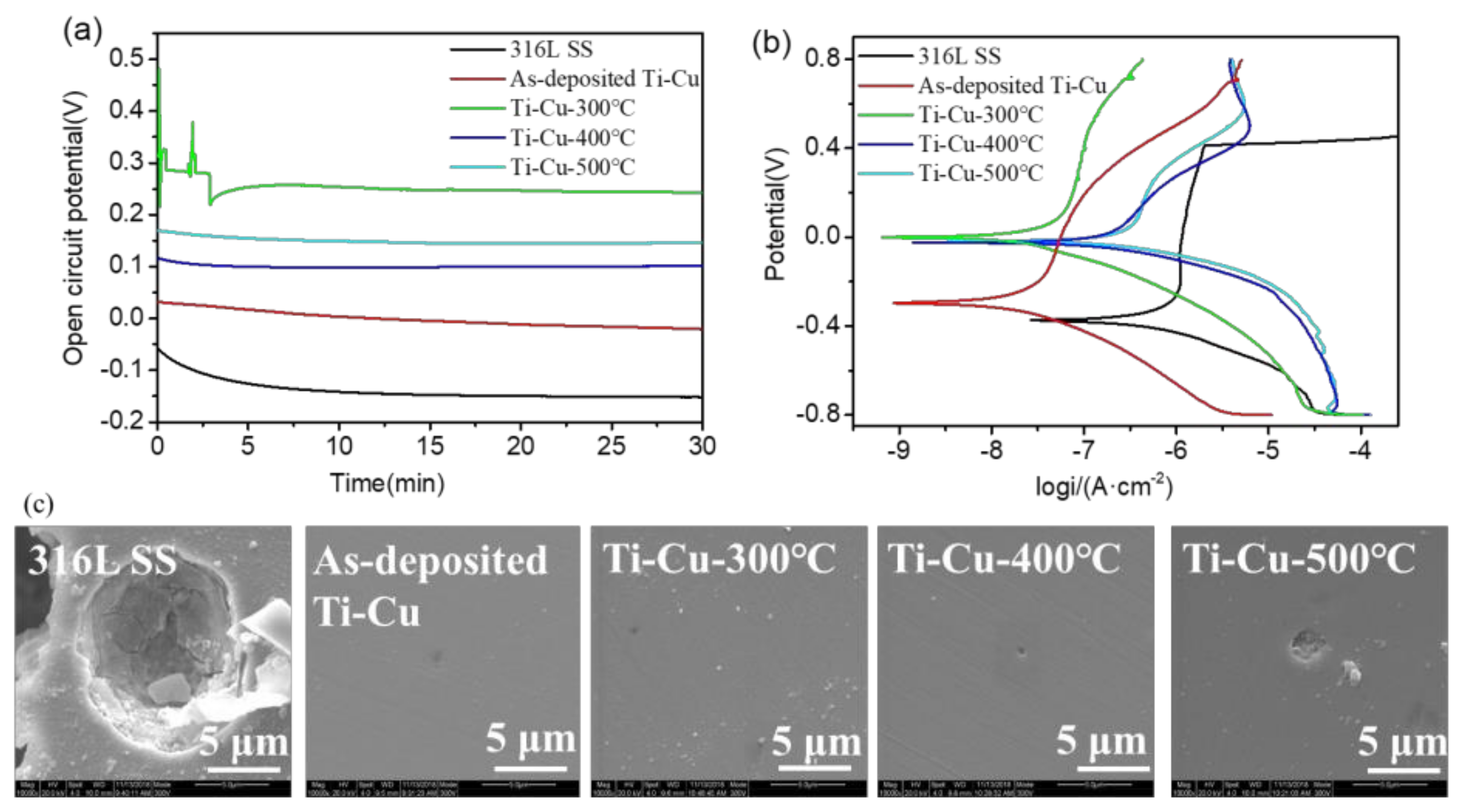
3.4. Electrochemical Corrosion of Ti–Cu Coatings
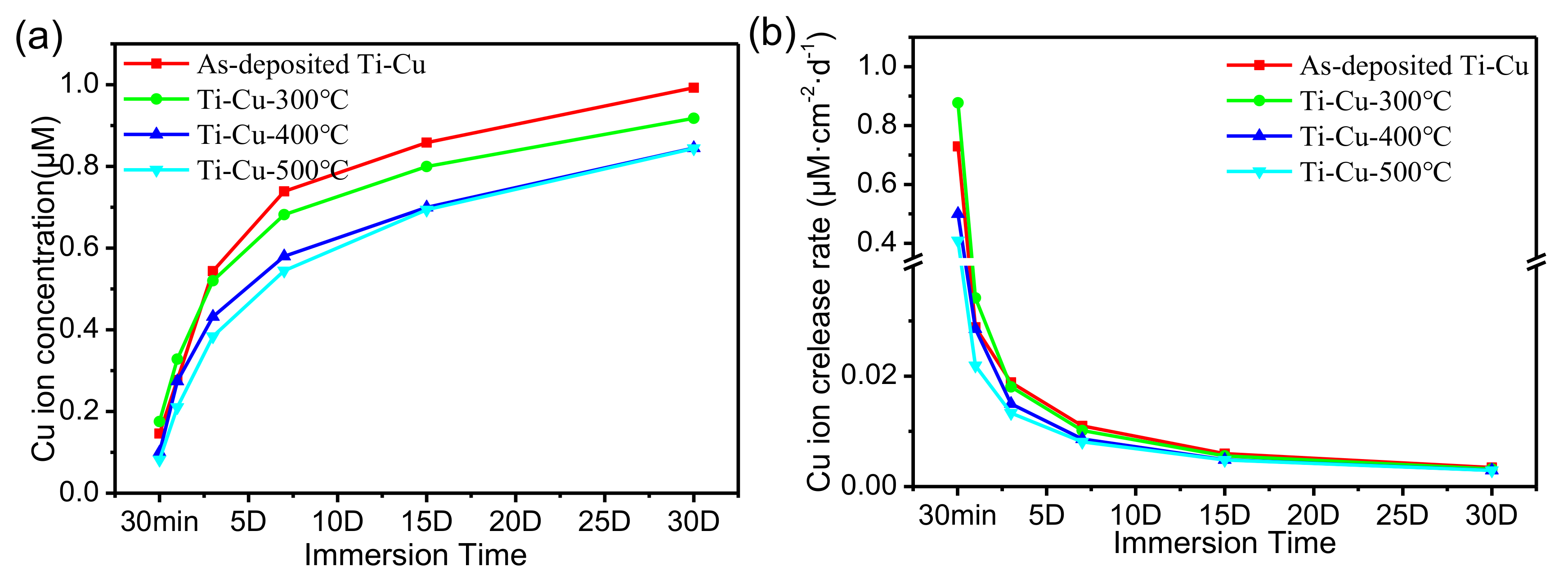
3.5. Copper Ion Release of Ti–Cu Coatings
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kaur, M.; Singh, K. Review on titanium and titanium based alloys as biomaterials for orthopaedic applications. Mater. Sci. Eng. C 2019, 102, 844–862. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.T.; Khan, Z.A.; Siddiquee, A.N. Surface Modifications of Titanium Materials for developing Corrosion Behavior in Human Body Environment: A Review. Procedia Mater. Sci. 2014, 6, 1610–1618. [Google Scholar] [CrossRef]
- Chu, C.; Wang, R.M.; Hu, T.; Yin, L.H.; Pu, Y.P.; Lin, P.H.; Dong, Y.S.; Guo, C.; Chung, C.; Yeung, K.W.K.; et al. XPS and biocompatibility studies of titania film on anodized NiTi shape memory alloy. J. Mater. Sci. Mater. Electron. 2008, 20, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Rezaee, S.; Arman, A.; Jurečka, S.; Korpi, A.G.; Mwema, F.; Luna, C.; Sobola, D.; Kulesza, S.; Shakoury, R.; Bramowicz, M.; et al. Effect of annealing on the micromorphology and corrosion properties of Ti/SS thin films. Superlattices Microstruct. 2020, 146, 106681. [Google Scholar] [CrossRef]
- Williamson, M.A. Geochemistry; Springer: Dordrecht, The Netherlands, 1998; pp. 101–102. [Google Scholar]
- Michels, H.; Noyce, J.; Keevil, C.W. Effects of temperature and humidity on the efficacy of methicillin-resistant Staphylococcus aureus challenged antimicrobial materials containing silver and copper. Lett. Appl. Microbiol. 2009, 49, 191–195. [Google Scholar] [CrossRef]
- Domanskaya, I.K.; Tropnikov, D.L. Prospects of Application of Waste Copper Slags as Fillers for Building Materials. Tsvetnye Met. 2014. Available online: http://www.rudmet.com/journal/1347/article/23061/ (accessed on 1 October 2020).
- Natter, H.; Schmelzer, M.; Hempelmann, R. Nanocrystalline nickel and nickel-copper alloys: Synthesis, characterization, and thermal stability. J. Mater. Res. 1998, 13, 1186–1197. [Google Scholar] [CrossRef]
- Duwez, P.; Willens, R.H.; Klement, W. Continuous Series of Metastable Solid Solutions in Silver-Copper Alloys. J. Appl. Phys. 1960, 31, 1136. [Google Scholar] [CrossRef]
- Bacon, G.E.; Dunmur, I.W.; Smith, J.H.; Street, R. The antiferromagnetism of manganese copper alloys. Proc. R. Soc. London Ser. A Math. Phys. Sci. 1957, 241, 223–238. [Google Scholar] [CrossRef]
- Lopes, C.; Vieira, M.; Borges, J.; Fernandes, J.; Rodrigues, M.; Alves, E.; Barradas, N.; Apreutesei, M.; Steyer, P.; Tavares, C.; et al. Multifunctional Ti–Me (Me=Al, Cu) thin film systems for biomedical sensing devices. Vacuum 2015, 122, 353–359. [Google Scholar] [CrossRef]
- Lin, Q.-J.; Yang, S.; Wang, C.-Y.; Ding, J.-J.; Jiang, Z.-D. Multifractal analysis for Cu/Ti bilayer thin films. Surf. Interface Anal. 2013, 45, 1223–1227. [Google Scholar] [CrossRef]
- Swain, M.; Bhattacharya, D.; Basu, S. Deposition of optical quality Cu/Ti films. In Proceedings of the 58th DAE Solid State Physics Symposium 2013, Punjab, India, 17–21 December 2013. [Google Scholar]
- Jin, X.; Gao, L.; Liu, E.; Yu, F.; Shu, X.; Wang, H. Microstructure, corrosion and tribological and antibacterial properties of Ti–Cu coated stainless steel. J. Mech. Behav. Biomed. Mater. 2015, 50, 23–32. [Google Scholar] [CrossRef]
- Stranak, V.; Wulff, H.; Rebl, H.; Zietz, C.; Arndt, K.; Bogdanowicz, R.; Nebe, B.; Bader, R.; Podbielski, A.; Hubička, Z.; et al. Deposition of thin titanium–copper films with antimicrobial effect by advanced magnetron sputtering methods. Mater. Sci. Eng. C 2011, 31, 1512–1519. [Google Scholar] [CrossRef]
- Huang, B.; Jing, F.; Akhavan, B.; Ji, L.; Leng, Y.; Xie, D.; Bilek, M.; Huang, N. Multifunctional Ti-xCu coatings for cardiovascular interfaces: Control of microstructure and surface chemistry. Mater. Sci. Eng. C 2019, 104, 109969. [Google Scholar] [CrossRef]
- Wang, Q.; Akhavan, B.; Jing, F.; Cheng, D.; Sun, H.; Xie, N.; Leng, Y.; Bilek, M.M.; Huang, N. Catalytic Formation of Nitric Oxide Mediated by Ti–Cu Coatings Provides Multifunctional Interfaces for Cardiovascular Applications. Adv. Mater. Interfaces 2018, 5, 1701487. [Google Scholar] [CrossRef]
- Horton, D.; Ha, H.; Foster, L.L.; Bindig, H.; Scully, J. Tarnishing and Cu Ion release in Selected Copper-Base Alloys: Implications towards Antimicrobial Functionality. Electrochim. Acta 2015, 169, 351–366. [Google Scholar] [CrossRef]
- Qiao, Y.; Ping, Y.; Zhang, H.; Zhou, B.; Liu, F.; Yu, Y. Laser-activatable CuS nanodots to treat multidrug-resistant bacteria and release copper ion to accelerate healing of Iinfected chronic nonhealing wounds. ACS Appl. Mater. Interfaces 2019, 11, 3809–3822. [Google Scholar] [CrossRef]
- Gritsch, L.; Lovell, C.; Goldmann, W.H.; Boccaccini, A.R. Fabrication and characterization of copper(II)-chitosan complexes as antibiotic-free antibacterial biomaterial. Carbohydr. Polym. 2018, 179, 370–378. [Google Scholar] [CrossRef]
- Foley, J.; Blackwell, A. Ion Release from Copper Phosphate Cement and Influence on Streptococcus mutans Growth in vitro: A Comparative Study. Caries Res. 2003, 37, 416–424. [Google Scholar] [CrossRef]
- Christensen, G.D.; Baldassarri, L.; Simpson, W.A. Methods for studying microbial colonization of plastics. Methods Enzymol. 1995, 253, 477–500. [Google Scholar] [CrossRef]
- Chen, W.; Meng, X.; Wu, D.; Yao, D.; Zhang, D. The effect of vacuum annealing on microstructure, adhesion strength and electrochemical behaviors of multilayered AlCrTiSiN coatings. Appl. Surf. Sci. 2019, 468, 391–401. [Google Scholar] [CrossRef]
- Yang, J.; Xiao, S.; Zhang, Q.; Xu, C.; Li, W.; Zheng, B.; Hu, F.; Yin, J.; Song, Z. In-situ synthesis of Ti–Al intermetallic compounds coating on Ti alloy by magnetron sputtering deposition followed by vacuum annealing. Vacuum 2020, 172, 109060. [Google Scholar] [CrossRef]
- Tsukimoto, S.; Morita, T.; Moriyama, M.; Ito, K.; Murakami, M. Formation of Ti diffusion barrier layers in Thin Cu(Ti) alloy films. J. Electron. Mater. 2005, 34, 592–599. [Google Scholar] [CrossRef]
- Sarakinos, K.; Alami, J.; Konstantinidis, S. High power pulsed magnetron sputtering: A review on scientific and engineering state of the art. Surf. Coat. Technol. 2010, 204, 1661–1684. [Google Scholar] [CrossRef]
- Najafi-Ashtiani, H.; Akhavan, B.; Jing, F.; Bilek, M.M.M. Transparent Conductive Dielectric–Metal–Dielectric Structures for Electrochromic Applications Fabricated by High-Power Impulse Magnetron Sputtering. ACS Appl. Mater. Interfaces 2019, 11, 14871–14881. [Google Scholar] [CrossRef]
- Ganesan, R.; Akhavan, B.; Partridge, J.G.; McCulloch, D.G.; McKenzie, D.R.; Bilek, M.M.M. Evolution of target condition in reactive HiPIMS as a function of duty cycle: An opportunity for refractive index grading. J. Appl. Phys. 2017, 121, 171909. [Google Scholar] [CrossRef]
- Akhavan, B.; Ganesan, R.; Stueber, M.; Ulrich, S.; McKenzie, D.R.; Bilek, M.M.M. Carbon films deposited by mixed-mode high power impulse magnetron sputtering for high wear resistance: The role of argon incorporation. Thin Solid Film. 2019, 688, 137353. [Google Scholar] [CrossRef]
- Ganesan, R.; Akhavan, B.; Hiob, M.A.; McKenzie, D.R.; Weiss, A.S.; Bilek, M.M. HiPIMS carbon coatings show covalent protein binding that imparts enhanced hemocompatibility. Carbon 2018, 139, 118–128. [Google Scholar] [CrossRef]
- Gorzalski, A.S.; Donley, C.; Coronell, O. Elemental composition of membrane foulant layers using EDS, XPS, and RBS. J. Membr. Sci. 2017, 522, 31–44. [Google Scholar] [CrossRef]
- Lau, K.; Akhavan, B.; Lord, M.S.; Bilek, M.M.; Rnjak-Kovacina, J. Dry Surface Treatments of Silk Biomaterials and Their Utility in Biomedical Applications. ACS Biomater. Sci. Eng. 2020, 6, 5431–5452. [Google Scholar] [CrossRef]
- Miler, M.; Mirtič, B. Accuracy and precision of EDS analysis for identification of metal-bearing minerals in polished and rough particle samples. Geologija 2013, 56, 5–17. [Google Scholar] [CrossRef]
- Lin, P.C.; Chen, H.; Hsieh, H.-C.; Tseng, T.-H.; Lee, H.Y.; Wu, A.T. Co-sputtered Cu(Ti) thin alloy film for formation of Cu diffusion and chip-level bonding. Mater. Chem. Phys. 2018, 211, 17–22. [Google Scholar] [CrossRef]
- Gouttebaron, R.; Cornelissen, D.; Snyders, R.; Dauchot, J.P.; Wautelet, M.; Hecq, M. XPS study of TiOx thin films prepared by d.c. magnetron sputtering in Ar–O2 gas mixtures. Surf. Interface Anal. 2015, 30, 527–530. [Google Scholar] [CrossRef]
- Hashimoto, S.; Tanaka, A.; Murata, A.; Sakurada, T. Formulation for XPS spectral change of oxides by ion bombardment as a function of sputtering time. Surf. Sci. 2004, 556, 22–32. [Google Scholar] [CrossRef]
- Cano, E.; Tôrres, C.L.; Bastidas, J.M. An XPS study of copper corrosion originated by formic acid vapour at 40% and 80% relative humidity. Mater. Corros. 2001, 52, 667–676. [Google Scholar] [CrossRef]
- Poulston, S.; Parlett, P.M.; Stone, P.; Bowker, M. Surface oxidation and reduction of CuO and Cu2O studied using XPS and XAES. Surf. Interface Anal. 1996, 24, 811–820. [Google Scholar] [CrossRef]
- Luo, B.; Li, K.; Tan, X.; Zhang, J.; Luo, J.; Jiang, X.; Wu, W.; Tang, Y. Influences of in situ annealing on microstructure, residual stress and electrical resistivity for sputter-deposited Be coating. J. Alloys Compd. 2014, 607, 150–156. [Google Scholar] [CrossRef]
- Li, W.Y.; Li, C.-J.; Liao, H. Effect of Annealing Treatment on the Microstructure and Properties of Cold-Sprayed Cu Coating. J. Therm. Spray Technol. 2006, 15, 206–211. [Google Scholar] [CrossRef]
- Turnow, H.; Wendrock, H.; Menzel, S.B.; Gemming, T.; Eckert, J. Structure and properties of sputter deposited crystalline and amorphous Cu–Ti films. Thin Solid Films 2016, 598, 184–188. [Google Scholar] [CrossRef]
- Cacucci, A.; Tsiaoussis, I.; Potin, V.; Imhoff, L.; Martin, N.; Nyberg, T. The interdependence of structural and electrical properties in TiO2/TiO/Ti periodic multilayers. Acta Mater. 2013, 61, 4215–4225. [Google Scholar] [CrossRef][Green Version]
- Ben Saâd, H.; Ajili, M.; Dabbebi, S.; Kamoun, N.T. Investigation on thickness and annealing effects on physical properties and electrical circuit model of CuO sprayed thin films. Superlattices Microstruct. 2020, 142, 106508. [Google Scholar] [CrossRef]
- Wen, Y.; Yunxin, W.; Hai, G. Molecular dynamics simulations of the effects of annealing on the micro residual stress induced by vacancy defects in aluminum crystal. J. Cryst. Growth 2020, 546, 125754. [Google Scholar] [CrossRef]
- Choi, D.; Wang, B.; Chung, S.; Liu, X.; Darbal, A.; Wise, A.; Nuhfer, N.T.; Barmak, K.; Warren, A.P.; Coffey, K.R.; et al. Phase, grain structure, stress, and resistivity of sputter-deposited tungsten films. J. Vac. Sci. Technol. A 2011, 29, 051512. [Google Scholar] [CrossRef]
- Sheu, J.-K.; Shu, K.W.; Lee, M.L.; Tun, C.J.; Chi, G.C. Effect of Thermal Annealing on Ga-Doped ZnO Films Prepared by Magnetron Sputtering. J. Electrochem. Soc. 2007, 154, H521. [Google Scholar] [CrossRef]
- Minhong, J.; Liu, X. Structural, electrical and optical properties of Al–Ti codoped ZnO (ZATO) thin films prepared by RF magnetron sputtering. Appl. Surf. Sci. 2008, 255, 3175–3178. [Google Scholar] [CrossRef]
- Kotyk, J.F.K.; Chen, C.; Sheehan, S.W. Corrosion Potential Modulation on Lead Anodes Using Water Oxidation Catalyst Coatings. Coatings 2018, 8, 246. [Google Scholar] [CrossRef]
- Yang, H.; Guo, X.; Wu, G.; Wang, S.; Ding, W. Continuous intermetallic compounds coatings on AZ91D Mg alloy fabricated by diffusion reaction of Mg–Al couples. Surf. Coat. Technol. 2011, 205, 2907–2913. [Google Scholar] [CrossRef]
- Ren, Q.; Qin, L.; Jing, F.; Cheng, D.; Wang, Y.; Yang, M.; Xie, D.; Leng, Y.; Akhavan, B.; Huang, N. Reactive magnetron co-sputtering of Ti-xCuO coatings: Multifunctional interfaces for blood-contacting devices. Mater. Sci. Eng. C 2020, 116, 111198. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, P.-Z.; Wei, D.; Wang, R.-N.; Chen, X.-H.; Wang, H.-Y. Microstructure and corrosion resistance of pure titanium surface modified by double-glow plasma surface alloying. Mater. Des. 2013, 49, 1042–1047. [Google Scholar] [CrossRef]
- Naka, M.; Hashimoto, K.; Masumoto, T. Corrosion behavior of amorphous and crystalline Cu50Ti50 and Cu50Zr50 alloys. J. Non-Cryst. Solids 1978, 30, 29–36. [Google Scholar] [CrossRef]
- Zietz, C.; Fritsche, A.; Finke, B.; Stranak, V.; Haenle, M.; Hippler, R.; Mittelmeier, W.; Bader, R. Analysis of the Release Characteristics of Cu-Treated Antimicrobial Implant Surfaces Using Atomic Absorption Spectrometry. Bioinorg. Chem. Appl. 2011, 2012, 32. [Google Scholar] [CrossRef]
- Ding, R.; Li, X.; Wang, J.; Li, W.; Wang, X.; Gui, T. Antifouling Properties and Release of Dissolved Copper of Cold Spray Cu/Cu2O Coatings for Ships and Steel Structures in Marine Environment. J. Mater. Eng. Perform. 2018, 27, 5947–5963. [Google Scholar] [CrossRef]
- Finke, B.; Polak, M.; Hempel, F.; Rebl, H.; Zietz, C.; Stranak, V.; Lukowski, G.; Hippler, R.; Bader, R.; Nebe, J.B.; et al. Antimicrobial Potential of Copper-Containing Titanium Surfaces Generated by Ion Implantation and Dual High Power Impulse Magnetron Sputtering. Adv. Eng. Mater. 2011, 14, B224–B230. [Google Scholar] [CrossRef]
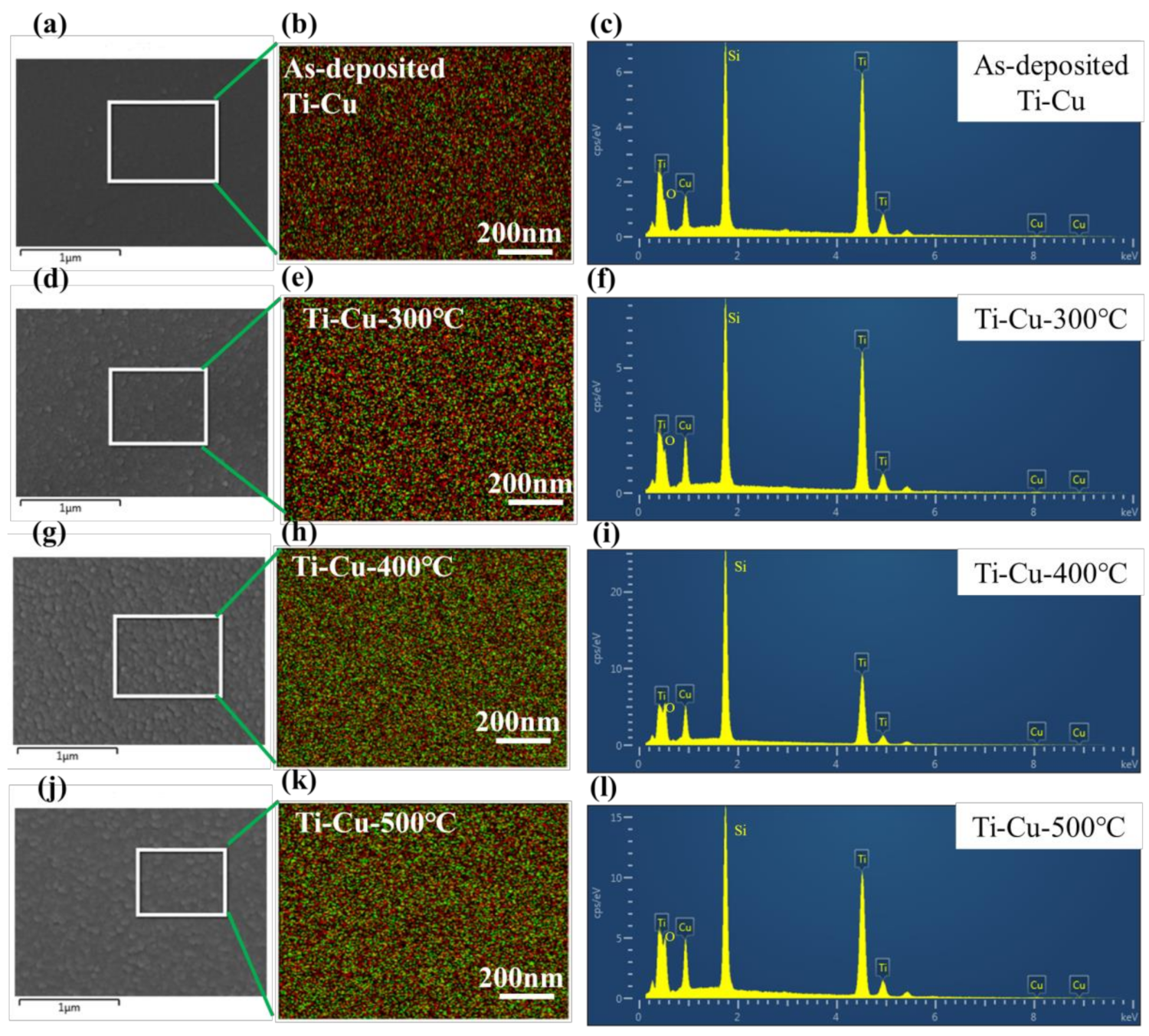
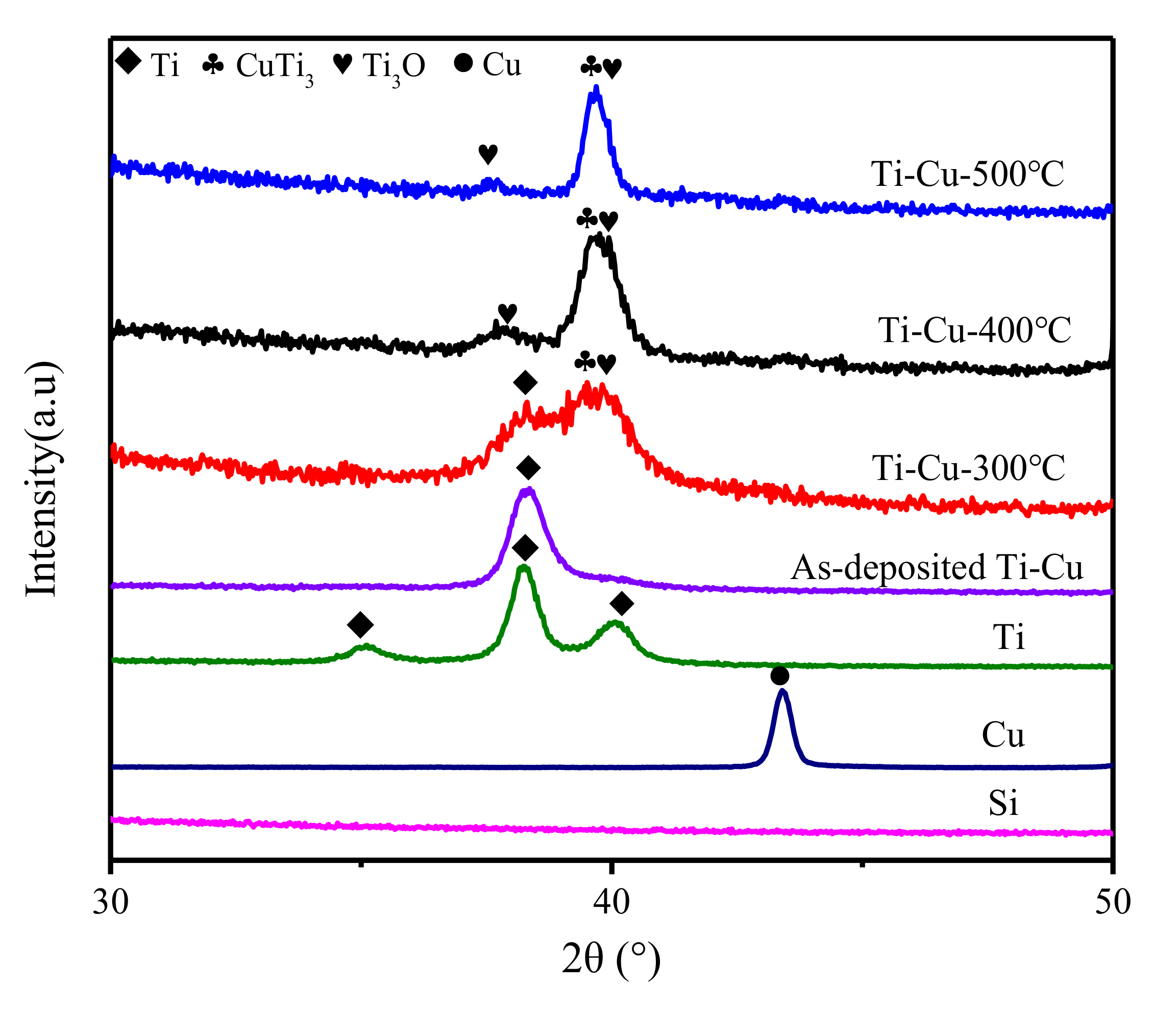
| Samples | As–Deposited Ti-Cu | Ti–Cu-300 °C | Ti–Cu-400 °C | Ti–Cu-500 °C |
|---|---|---|---|---|
| Cu/(Cu + Ti) values (at.%) | 12.1 | 14.9 | 19.5 | 15.4 |
| Sample | Ecorr (V) | Icorr (A·cm−2) |
|---|---|---|
| 316L SS | −0.375 | 3.38 × 10−7 |
| As-deposited Ti-Cu | −0.293 | 1.07 × 10−8 |
| Ti-Cu-300 °C | 0.004 | 1.42 × 10−8 |
| Ti-Cu-400 °C | −0.026 | 1.24 × 10−7 |
| Ti-Cu-500 °C | −0.016 | 1.76 × 10−7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, L.; Ma, D.; Li, Y.; Jing, P.; Huang, B.; Jing, F.; Xie, D.; Leng, Y.; Akhavan, B.; Huang, N. Ti–Cu Coatings Deposited by a Combination of HiPIMS and DC Magnetron Sputtering: The Role of Vacuum Annealing on Cu Diffusion, Microstructure, and Corrosion Resistance. Coatings 2020, 10, 1064. https://doi.org/10.3390/coatings10111064
Qin L, Ma D, Li Y, Jing P, Huang B, Jing F, Xie D, Leng Y, Akhavan B, Huang N. Ti–Cu Coatings Deposited by a Combination of HiPIMS and DC Magnetron Sputtering: The Role of Vacuum Annealing on Cu Diffusion, Microstructure, and Corrosion Resistance. Coatings. 2020; 10(11):1064. https://doi.org/10.3390/coatings10111064
Chicago/Turabian StyleQin, Lina, Donglin Ma, Yantao Li, Peipei Jing, Bin Huang, Fengjuan Jing, Dong Xie, Yongxiang Leng, Behnam Akhavan, and Nan Huang. 2020. "Ti–Cu Coatings Deposited by a Combination of HiPIMS and DC Magnetron Sputtering: The Role of Vacuum Annealing on Cu Diffusion, Microstructure, and Corrosion Resistance" Coatings 10, no. 11: 1064. https://doi.org/10.3390/coatings10111064
APA StyleQin, L., Ma, D., Li, Y., Jing, P., Huang, B., Jing, F., Xie, D., Leng, Y., Akhavan, B., & Huang, N. (2020). Ti–Cu Coatings Deposited by a Combination of HiPIMS and DC Magnetron Sputtering: The Role of Vacuum Annealing on Cu Diffusion, Microstructure, and Corrosion Resistance. Coatings, 10(11), 1064. https://doi.org/10.3390/coatings10111064







