Long-Term Failure Mechanisms of Thermal Barrier Coatings in Heavy-Duty Gas Turbines
Abstract
1. Introduction
2. Long-Term Oxidation of TBCs
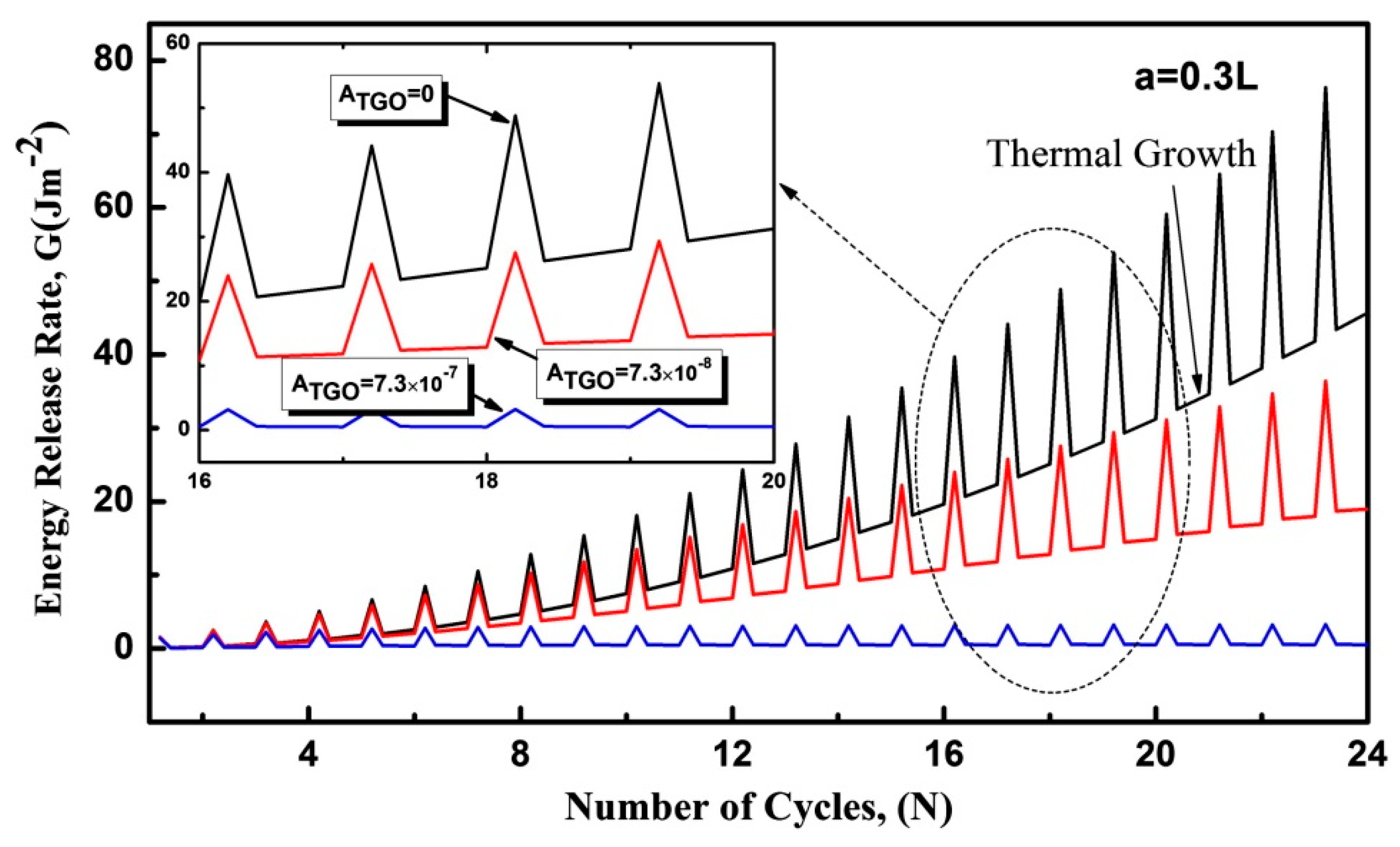
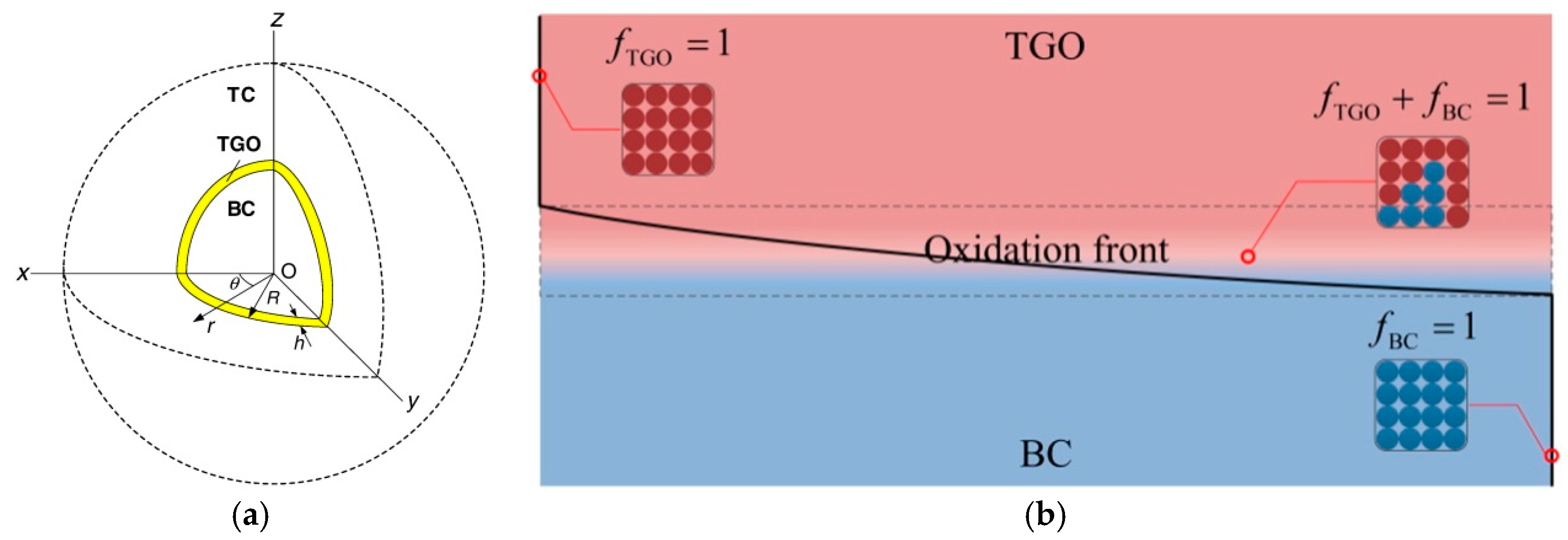
2.1. Research Advance on TGO Growth
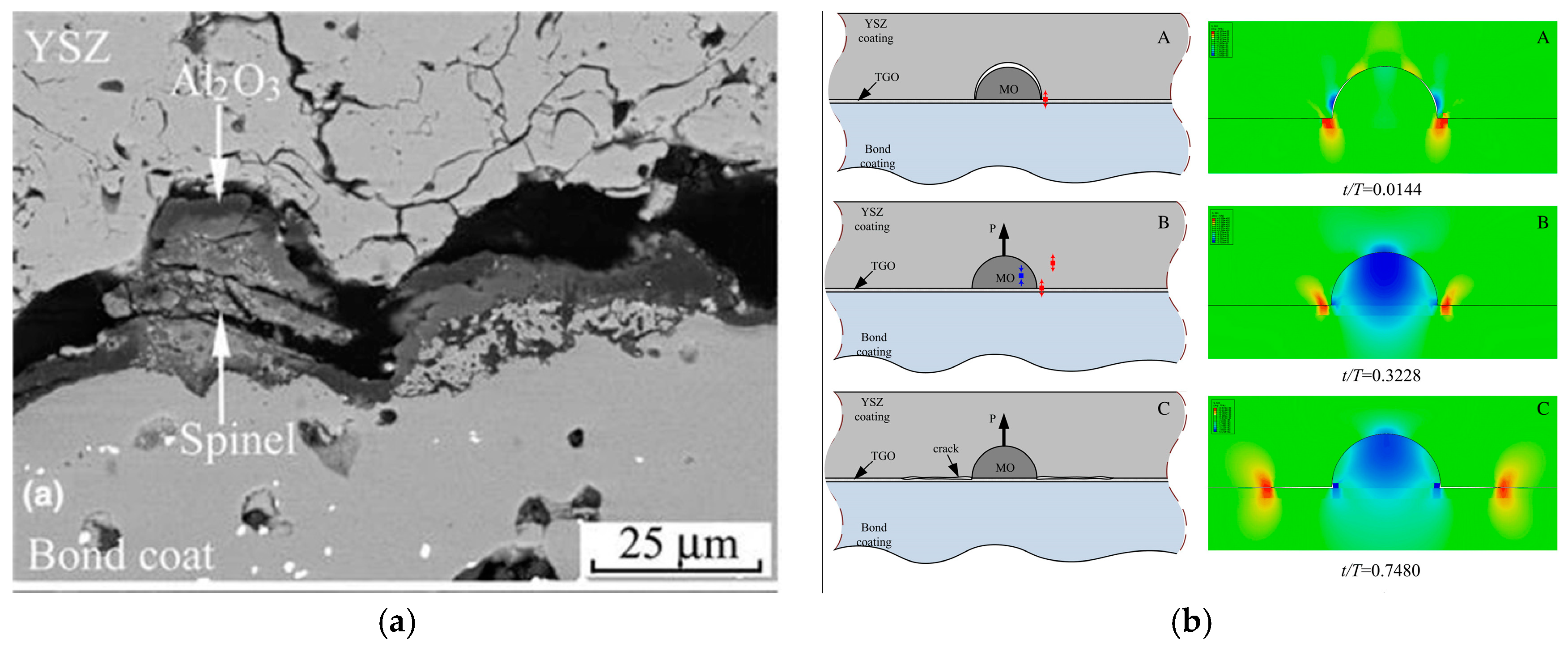
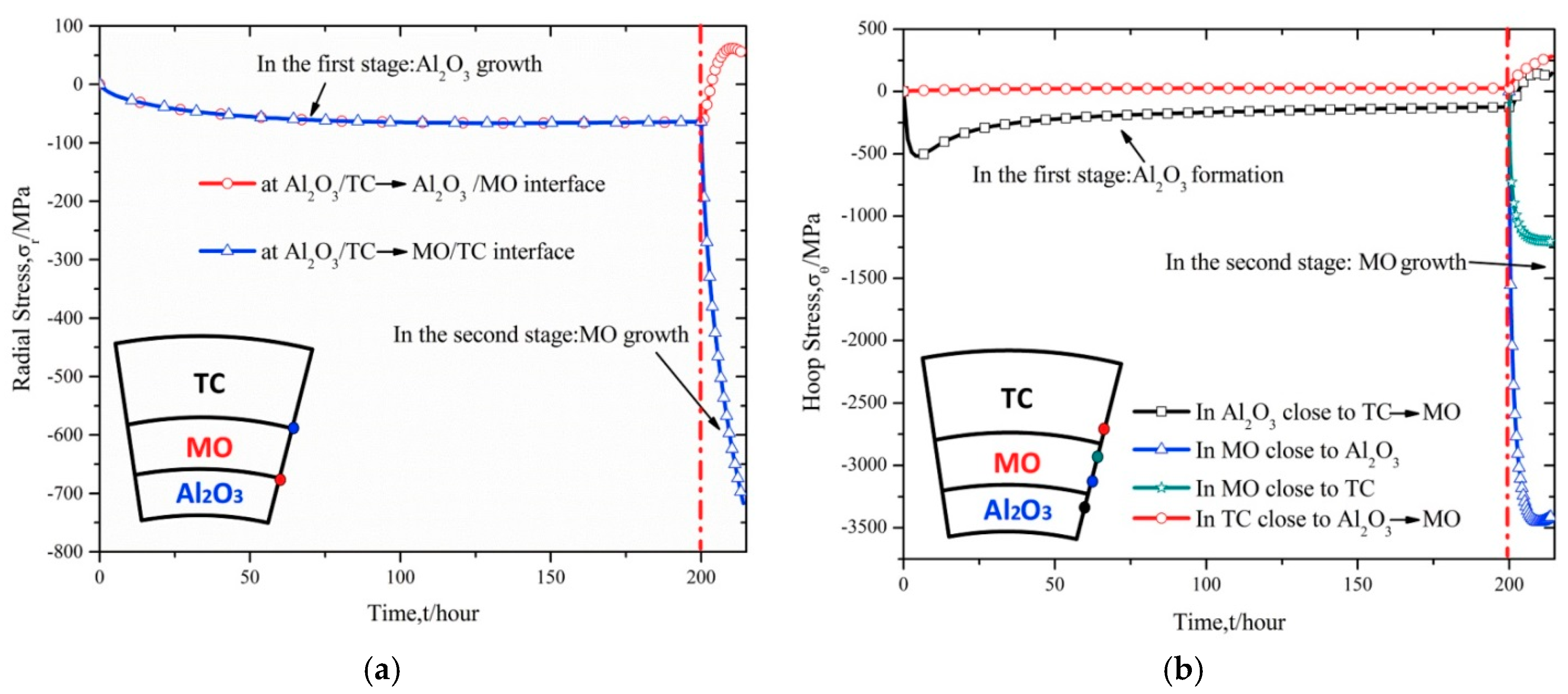
2.2. Failure Mechanism of TBCs Induced by the Growth of Uniform MO
3. Element Diffusion in TBCs
3.1. Research Progress on Diffusion
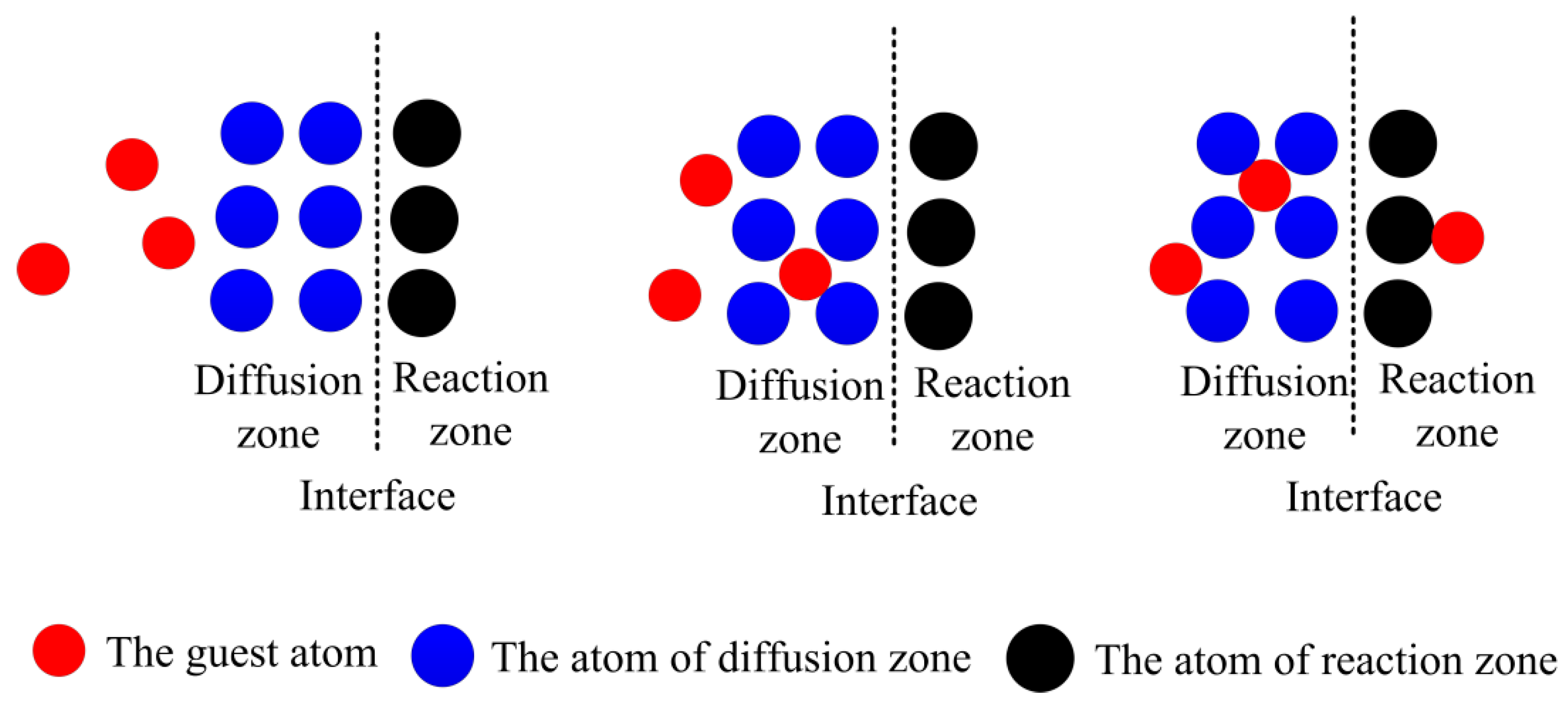
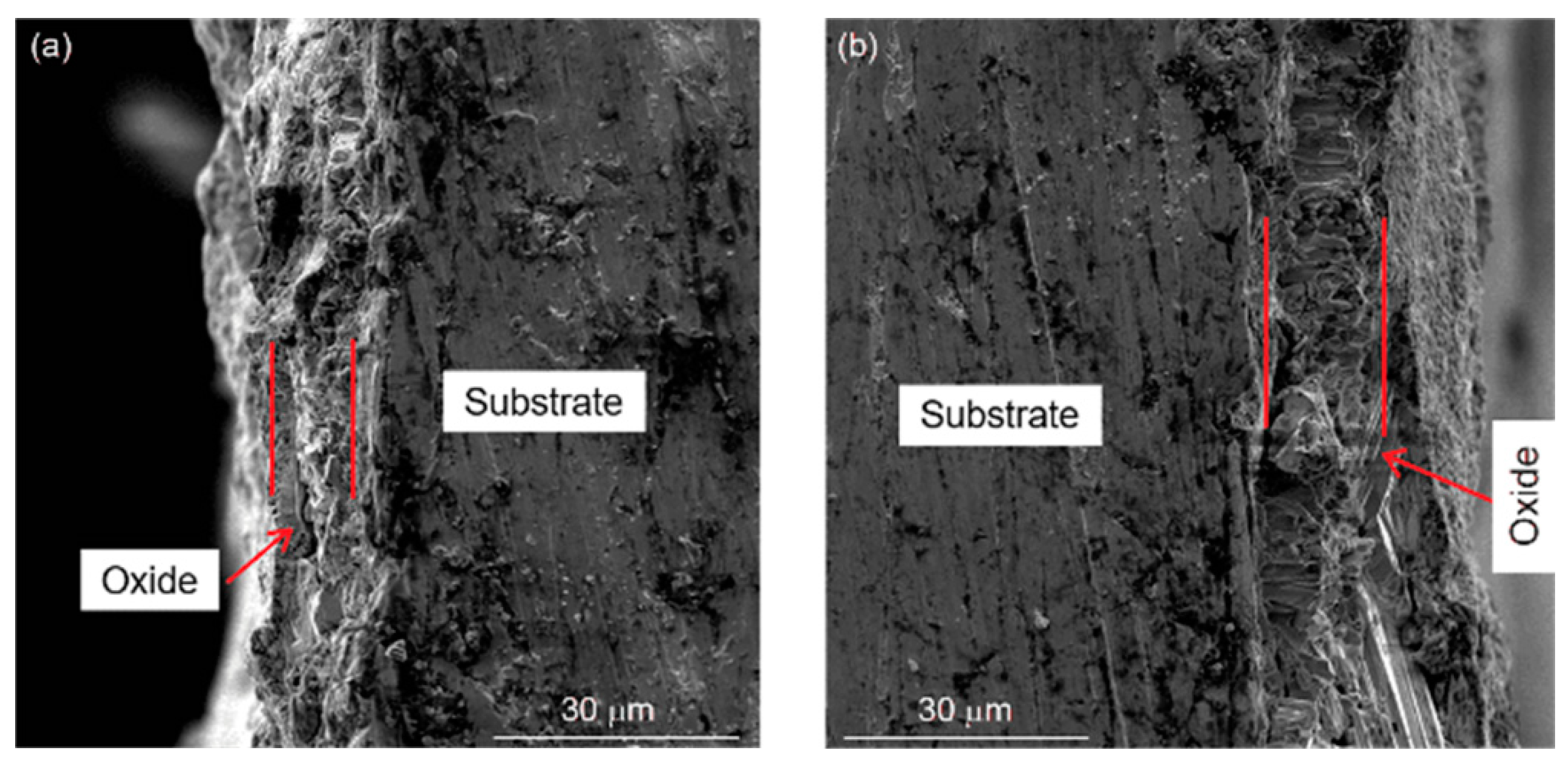
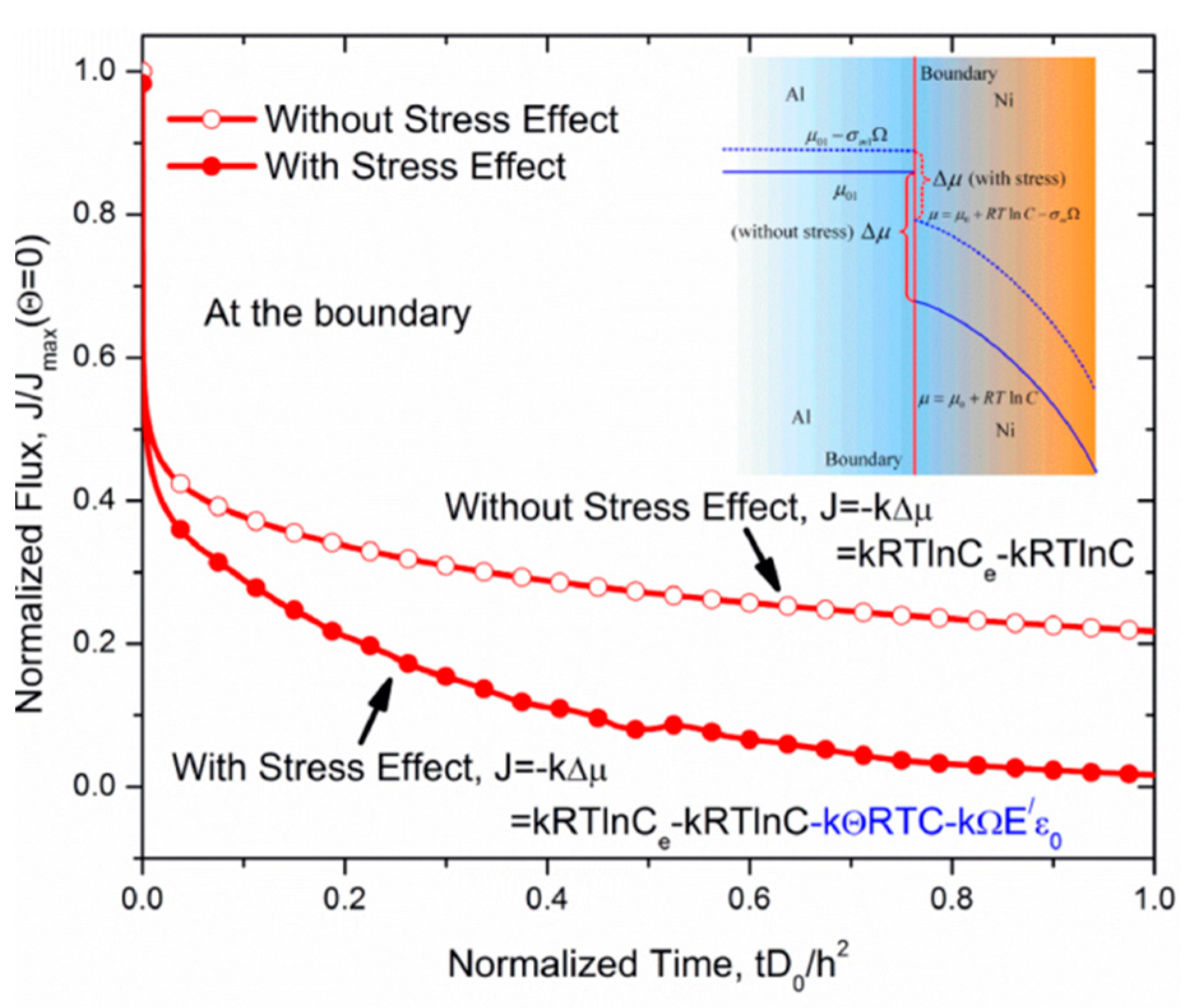
3.2. Failure Mechanism of TBCs Related to Element Diffusion
4. Summary
- a.
- Oxidation and element diffusion are responsible for the long-term failure mechanisms of TBCs. Different from the failures induced by the frequent thermal cycles in aero-engines, for heavy-duty gas turbines, the initial thermal stress can be almost released by material creep, and the long-time oxidation and element diffusion determine the change in performance and service lifetime of TBCs.
- b.
- The catastrophic stress induced by the growth of uniform MO is a key cause for the long-term failure of TBCs. Compared to the slow growth of α-Al2O3, the fast growth and large expansion of MO induce the out-plane tensile stress at the α-Al2O3/MO interface and the in-plane tensile stress in α-Al2O3 and TC layers. Accordingly, interfacial delamination and micro-cracks can appear in TBCs. Especially, once crack occurs in α-Al2O3 layer, its protective function is destroyed, MO growth is further accelerated and then the lifetime of TBCs is reduced significantly.
- c.
- The formations of an interdiffusion region and Kirkendall voids induced by element diffusion also play the key roles in the long-term failures of TBCs, besides controlling TGO growth. The process of element diffusion in TBCs is affected by stress, creep and interface property, etc. Moreover, the interdiffusion of alloy elements, surface oxidation of the BC and residual stress affect the distribution of element in TBCs, and then change the phase component, which leads to change in mechanical performance.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Clarke, D.R.; Oechsner, M.; Padture, N.P. Thermal-barrier coatings for more efficient gas-turbine engines. MRS Bull. 2012, 37, 891–902. [Google Scholar] [CrossRef]
- Padture, N.P.; Gell, M.; Jordan, E.H. Materials science-Thermal barrier coatings for gas-turbine engine applications. Science 2002, 296, 280–284. [Google Scholar] [CrossRef]
- Zhang, W.W.; Li, G.R.; Zhang, Q.; Yang, G.J. Comprehensive damage evaluation of localized spallation of thermal barrier coatings. J. Adv. Ceram. 2017, 6, 230–239. [Google Scholar] [CrossRef]
- Zhou, J.H. The process management and fault treament of 9F gas turbine during A-class maintenance. Intern. Combust. Engine Parts 2017, 60–62. (In Chinese) [Google Scholar]
- Zeng, Z. The experience in formulating the repair cycle of gas turbine. Gas Turbine Technol. 2012, 25, 68–72. (In Chinese) [Google Scholar]
- Rosler, J.; Baker, M.; Aufzug, K. A parametric study of the stress state of thermal barrier coatings-Part I: Creep relaxation. Acta Mater. 2004, 52, 4809–4817. [Google Scholar] [CrossRef]
- Rosler, J.; Baker, M.; Volgmann, M. Stress state and failure mechanisms of thermal barrier coatings: Role of creep in thermally grown oxide. Acta Mater. 2001, 49, 3659–3670. [Google Scholar] [CrossRef]
- Lee, C.H.; Kim, H.K.; Choi, H.S.; Ahn, H.S. Phase transformation and bond coat oxidation behavior of plasma-sprayed zirconia thermal barrier coating. Surf. Coat. Technol. 2000, 124, 1–12. [Google Scholar] [CrossRef]
- Ali, M.S.; Song, S.H.; Xiao, P. Degradation of thermal barrier coatings due to thermal cycling up to 1150 degrees C. J. Mater. Sci. 2002, 37, 2097–2102. [Google Scholar] [CrossRef]
- Ma, K.K.; Schoenung, J.M. Isothermal oxidation behavior of cryomilled NiCrAlY bond coat: Homogeneity and growth rate of TGO. Surf. Coat. Technol. 2011, 205, 5178–5185. [Google Scholar] [CrossRef]
- Xie, F.; Sun, Y.L.; Li, D.J.; Bai, Y.; Zhang, W.X. Modelling of catastrophic stress development due to mixed oxide growth in thermal barrier coatings. Ceram. Int. 2019, 45, 11353–11361. [Google Scholar] [CrossRef]
- Gupta, M.; Eriksson, R.; Sand, U.; Nylen, P. A diffusion-based oxide layer growth model using real interface roughness in thermal barrier coatings for lifetime assessment. Surf. Coat. Technol. 2015, 271, 181–191. [Google Scholar] [CrossRef]
- Soulignac, R.; Maurel, V.; Remy, L.; Koster, A. Cohesive zone modelling of thermal barrier coatings interfacial properties based on three-dimensional observations and mechanical testing. Surf. Coat. Technol. 2013, 237, 95–104. [Google Scholar] [CrossRef]
- Fan, X.L.; Zhang, W.X.; Wang, T.J.; Sun, Q. The effect of thermally grown oxide on multiple surface cracking in air plasma sprayed thermal barrier coating system. Surf. Coat. Technol. 2012, 208, 7–13. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, X.Y.; Jung, Y.G.; Li, L.; Knapp, J. Lanthanum zirconate based thermal barrier coatings: A review. Surf. Coat. Technol. 2017, 323, 18–29. [Google Scholar] [CrossRef]
- Thakare, J.G.; Pandey, C.; Mahapatra, M.M.; Mulik, R.S. Thermal barrier coatings-A state of the art review. Met. Mater. Int. 2020, 1–22. [Google Scholar] [CrossRef]
- Liu, M.J.; Zhang, G.; Lu, Y.H.; Han, J.Q.; Li, G.R.; Li, C.X.; Li, C.J.; Yang, G.J. Plasma spray-physical vapor deposition toward advanced thermal barrier coatings: A review. Rare Met. 2020, 39, 479–497. [Google Scholar] [CrossRef]
- Mehta, A.; Vasudev, H.; Singh, S. Recent developments in the designing of deposition of thermal barrier coatings—A review. Mater. Today Proc. 2020, 26, 1336–1342. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, J.; Lu, Z.; Park, H.Y.; Jung, Y.G.; Park, H.; Koo, D.D.; Sinatra, R.; Zhang, J. Removal and repair techniques for thermal barrier coatings: A review. Trans. IMF 2020, 98, 121–128. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, Q.; Yang, L.; Wu, D.; Mao, W. Failure mechanisms and life prediction of thermal barrier coatings. Acta Mech. Solida Sin. 2010, 31, 504–531. [Google Scholar]
- Miller, R.A. Current status of thermal barrier coatings—An overview. Surf. Coat. Technol. 1987, 30, 1–11. [Google Scholar] [CrossRef]
- Vassen, R.; Jarligo, M.O.; Steinke, T.; Mack, D.E.; Stover, D. Overview on advanced thermal barrier coatings. Surf. Coat. Technol. 2010, 205, 938–942. [Google Scholar] [CrossRef]
- Mehboob, G.; Liu, M.-J.; Xu, T.; Hussain, S.; Mehboob, G.; Tahir, A. A review on failure mechanism of thermal barrier coatings and strategies to extend their lifetime. Ceram. Int. 2020, 46, 8497–8521. [Google Scholar] [CrossRef]
- Hu, Z.-C.; Liu, B.; Wang, L.; Cui, Y.-H.; Wang, Y.-W.; Ma, Y.-D.; Sun, W.-W.; Yang, Y. Research progress of failure mechanism of thermal barrier coatings at high temperature via finite element method. Coatings 2020, 10, 732. [Google Scholar] [CrossRef]
- Wang, L.; Li, D.C.; Yang, J.S.; Shao, F.; Zhong, X.H.; Zhao, H.Y.; Yang, K.; Tao, S.Y.; Wang, Y. Modeling of thermal properties and failure of thermal barrier coatings with the use of finite element methods: A review. J. Eur. Ceram. Soc. 2016, 36, 1313–1331. [Google Scholar] [CrossRef]
- Wang, T.; Fan, X.; Sun, Y.; Su, L.; Song, Y.; Lv, B. The stresses and cracks in thermal barrier coating system: A review. Chin. J. Solid Mech. 2016, 37, 477–517. [Google Scholar]
- Lv, B.W.; Jin, X.C.; Cao, J.; Xu, B.S.; Wang, Y.G.; Fang, D.N. Advances in numerical modeling of environmental barrier coating systems for gas turbines. J. Eur. Ceram. Soc. 2020, 40, 3363–3379. [Google Scholar] [CrossRef]
- Elsass, M.; Frommherz, M.; Oechsner, M. The Influence of the coating deposition process on the interdiffusion behavior between nickel-based superalloys and MCrAlY bond coats. J. Therm. Spray Technol. 2018, 27, 379–390. [Google Scholar] [CrossRef]
- Wei, Z.Y.; Cai, H.N. Comprehensive effects of TGO growth on the stress characteristic and delamination mechanism in lamellar structured thermal barrier coatings. Ceram. Int. 2020, 46, 2220–2237. [Google Scholar] [CrossRef]
- Chai, Y.J.; Lin, C.; Li, Y.M. Effects of creep-plastic behavior on stress development in TBCs during cooling. Ceram. Int. 2017, 43, 11627–11634. [Google Scholar] [CrossRef]
- Yang, S.; Peng, H.; Zhang, T. A damage model to evaluate the mechanical properties in the medium-long life stage for coating system subjected to elevated thermal loads with 3D-DIC technique. J. Alloy. Compd. 2020, 832, 154955. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Hu, X.B. Segregation and microstructural evolution at interfaces of atmospheric plasma sprayed thermal barrier coatings during thermal cycling. J. Alloy. Compd. 2020, 819, 13. [Google Scholar] [CrossRef]
- Yu, Q.M.; Zhou, H.L.; Wang, L.B. Influences of interface morphology and thermally grown oxide thickness on residual stress distribution in thermal barrier coating system. Ceram. Int. 2016, 42, 8338–8350. [Google Scholar] [CrossRef]
- Balint, D.S.; Hutchinson, J.W. An analytical model of rumpling in thermal barrier coatings. J. Mech. Phys. Solids 2005, 53, 949–973. [Google Scholar] [CrossRef]
- Karlsson, A.M.; Hutchinson, J.W.; Evans, A.G. A fundamental model of cyclic instabilities in thermal barrier systems. J. Mech. Phys. Solids 2002, 50, 1565–1589. [Google Scholar] [CrossRef]
- Naumenko, D.; Shemet, V.; Singheiser, L.; Quadakkers, W.J. Failure mechanisms of thermal barrier coatings on MCrAlY-type bondcoats associated with the formation of the thermally grown oxide. J. Mater. Sci. 2009, 44, 1687–1703. [Google Scholar] [CrossRef]
- Rabiei, A.; Evans, A.G. Failure mechanisms associated with the thermally grown oxide in plasma-sprayed thermal barrier coatings. Acta Mater. 2000, 48, 3963–3976. [Google Scholar] [CrossRef]
- Chen, W.R.; Wu, X.; Marple, B.R.; Nagy, D.R.; Patnaik, P.C. TGO growth behaviour in TBCs with APS and HVOF bond coats. Surf. Coat. Technol. 2008, 202, 2677–2683. [Google Scholar] [CrossRef]
- Tang, J.J.; Bai, Y.; Liu, K.; Zhang, P.; Li, B.Q.; Yang, J.F. Microstructural evolution of SAPS/HVOF CoNiCrAlY alloy coating during thermal cycling test. Oxid. Met. 2016, 86, 75–87. [Google Scholar] [CrossRef]
- Hutchinson, J.W.; He, M.Y.; Evans, A.G. The influence of imperfections on the nucleation and propagation of buckling driven delaminations. J. Mech. Phys. Solids 2000, 48, 709–734. [Google Scholar] [CrossRef]
- Evans, A.G.; Mumm, D.R.; Hutchinson, J.W.; Meier, G.H.; Pettit, F.S. Mechanisms controlling the durability of thermal barrier coatings. Prog. Mater. Sci. 2001, 46, 505–553. [Google Scholar] [CrossRef]
- Mumm, D.R.; Evans, A.G.; Spitsberg, I.T. Characterization of a cyclic displacement instability for a thermally grown oxide in a thermal barrier system. Acta Mater. 2001, 49, 2329–2340. [Google Scholar] [CrossRef]
- He, M.Y.; Hutchinson, J.W.; Evans, A.G. Simulation of stresses and delamination in a plasma-sprayed thermal barrier system upon thermal cycling. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2003, 345, 172–178. [Google Scholar] [CrossRef]
- Tolpygo, V.K.; Clarke, D.R. Surface rumpling of a (Ni, Pt)Al bond coat induced by cyclic oxidation. Acta Mater. 2000, 48, 3283–3293. [Google Scholar] [CrossRef]
- Tolpygo, V.K.; Clarke, D.R. Wrinkling of alpha-alumina films grown by thermal oxidation-I. Quantitative studies on single crystals of Fe-Cr-Al alloy. Acta Mater. 1998, 46, 5153–5166. [Google Scholar] [CrossRef]
- Su, L.; Zhang, W.; Sun, Y.; Wang, T.J. Effect of TGO creep on top-coat cracking induced by cyclic displacement instability in a thermal barrier coating system. Surf. Coat. Technol. 2014, 254, 410–417. [Google Scholar] [CrossRef]
- Song, J.N.; Li, S.L.; Yang, X.G.; Qi, H.Y.; Shi, D.Q. Numerical investigation on the cracking behaviors of thermal barrier coating system under different thermal cycle loading waveforms. Surf. Coat. Technol. 2018, 349, 166–176. [Google Scholar] [CrossRef]
- Jiang, J.S.; Zou, Z.H.; Wang, W.Z.; Zhao, X.F.; Liu, Y.Z.; Cao, Z.M. Effect of internal oxidation on the interfacial morphology and residual stress in air plasma sprayed thermal barrier coatings. Surf. Coat. Technol. 2018, 334, 215–226. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, Z.B.; Yang, L.; Zhou, Y.C.; Wei, Y.G. Spallation of thermal barrier coatings with real thermally grown oxide morphology under thermal stress. Mater. Des. 2018, 146, 180–193. [Google Scholar] [CrossRef]
- Wei, Z.Y.; Cai, H.N.; Li, C.J. Comprehensive dynamic failure mechanism of thermal barrier coatings based on a novel crack propagation and TGO growth coupling model. Ceram. Int. 2018, 44, 22556–22566. [Google Scholar] [CrossRef]
- Han, Y.J.; Ye, F.X.; Lu, G.X.; Liu, C.; Hao, L.J. Residual stress evolution of thermally grown oxide in thermal barrier coatings deposited onto nickel-base superalloy and iron-base alloy with thermal exposure ageing. J. Alloy. Compd. 2014, 584, 19–27. [Google Scholar] [CrossRef]
- Cen, L.; Qin, W.Y.; Yu, Q.M. Finite element analysis of interface undulation and interface delamination in the MCrAlY coating system under thermal cycling: Considering oxide thickness and top-coat effects. J. Therm. Spray Technol. 2020, 29, 597–610. [Google Scholar] [CrossRef]
- Chen, Z.; Huang, H.M.; Zhao, K.; Jia, W.B.; Fang, L. Influence of inhomogeneous thermally grown oxide thickness on residual stress distribution in thermal barrier coating system. Ceram. Int. 2018, 44, 16937–16946. [Google Scholar] [CrossRef]
- Chai, Y.J.; Lin, C.; Wang, X.; Li, Y.M. Study on Stress Development in the phase transition layer of thermal barrier coatings. Materials 2016, 9, 14. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, C.; Xuan, J.; Liu, B.; Yang, G.; Gao, Y.; Luo, H. Effect of TGO evolution and element diffusion on the life span of YSZ/Pt–Al and YSZ/NiCrAlY coatings at high temperature. Ceram. Int. 2020, 46, 813–823. [Google Scholar] [CrossRef]
- Lin, C.; Li, Y.M. A coupled mechanical-chemical model for reflecting the influence of stress on oxidation reactions in thermal barrier coating. J. Appl. Phys. 2018, 123, 10. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Zheng, S.J.; Zhu, Y.L.; Wei, H.; Ma, X.L. Microstructural evolution at interfaces of thermal barrier coatings during isothermal oxidation. J. Eur. Ceram. Soc. 2016, 36, 1765–1774. [Google Scholar] [CrossRef]
- Shen, Q.; Yang, L.; Zhou, Y.C.; Wei, Y.G.; Wang, N.G. Models for predicting TGO growth to rough interface in TBCs. Surf. Coat. Technol. 2017, 325, 219–228. [Google Scholar] [CrossRef]
- Sun, Y.L.; Zhang, W.X.; Li, J.G.; Wang, T.J. Local stress around cap-like portions of anisotropically and nonuniformly grown oxide layer in thermal barrier coating system. J. Mater. Sci. 2013, 48, 5962–5982. [Google Scholar] [CrossRef]
- Zhao, X.; Hashimoto, T.; Xiao, P. Effect of the top coat on the phase transformation of thermally grown oxide in thermal barrier coatings. Scr. Mater. 2006, 55, 1051–1054. [Google Scholar] [CrossRef]
- Evans, A.G.; He, M.Y.; Hutchinson, J.W. Mechanics-based scaling laws for the durability of thermal barrier coatings. Prog. Mater. Sci. 2001, 46, 249–271. [Google Scholar] [CrossRef]
- Clarke, D.R. The lateral growth strain accompanying the formation of a thermally grown oxide. Acta Mater. 2003, 51, 1393–1407. [Google Scholar] [CrossRef]
- Sun, Y.; Li, J.; Zhang, W.; Wang, T.J. Local stress evolution in thermal barrier coating system during isothermal growth of irregular oxide layer. Surf. Coat. Technol. 2013, 216, 237–250. [Google Scholar] [CrossRef]
- Lin, C.; Li, Y.M. Interface stress evolution considering the combined creep–plastic behavior in thermal barrier coatings. Mater. Des. 2016, 89, 245–254. [Google Scholar] [CrossRef]
- Shen, Q.; Yang, L.; Zhou, Y.C.; Wei, Y.G.; Zhu, W. Effects of growth stress in finite-deformation thermally grown oxide on failure mechanism of thermal barrier coatings. Mech. Mater. 2017, 114, 228–242. [Google Scholar] [CrossRef]
- Chai, Y.; Yang, X.; Li, Y.; Ogawa, K. Stress development in thermal barrier coatings with morphology-controlled thermally grown oxide. Ceram. Int. 2019, 45, 20435–20445. [Google Scholar] [CrossRef]
- Loeffel, K.; Anand, L. A chemo-thermo-mechanically coupled theory for elastic-viscoplastic deformation, diffusion, and volumetric swelling due to a chemical reaction. Int. J. Plast. 2011, 27, 1409–1431. [Google Scholar] [CrossRef]
- Loeffel, K.; Anand, L.; Gasem, Z.M. On modeling the oxidation of high-temperature alloys. Acta Mater. 2013, 61, 399–424. [Google Scholar] [CrossRef]
- Lv, B.W.; Xie, H.; Xu, R.; Fan, X.L.; Zhang, W.X.; Wang, T.J. Effects of sintering and mixed oxide growth on the interface cracking of air-plasma-sprayed thermal barrier coating system at high temperature. Appl. Surf. Sci. 2016, 360, 461–469. [Google Scholar] [CrossRef]
- Dong, H.; Yang, G.; Luo, X.; Li, C. Effects of mixed oxides on thermal cyclic lifetime of plasma-sprayed thermal barrier coatings. China Surf. Eng. 2015, 28, 21–28. [Google Scholar]
- Yang, G.J.; Xiang, X.D.; Xing, L.K.; Li, D.J.; Li, C.J.; Li, C.X. Isothermal oxidation behavior of NiCoCrAlTaY coating deposited by high velocity air-fuel spraying. J. Therm. Spray Technol. 2012, 21, 391–399. [Google Scholar] [CrossRef]
- Busso, E.P.; Evans, H.E.; Qian, Z.Q.; Taylor, M.P. Effects of breakaway oxidation on local stresses in thermal barrier coatings. Acta Mater. 2010, 58, 1242–1251. [Google Scholar] [CrossRef]
- Li, Y.; Li, C.J.; Zhang, Q.; Yang, G.J.; Li, C.X. Influence of TGO Composition on the thermal shock lifetime of thermal barrier coatings with cold-sprayed MCrAlY bond coat. J. Therm. Spray Technol. 2010, 19, 168–177. [Google Scholar] [CrossRef]
- Zhang, W.X.; Sun, Y.L.; Wang, T.J. Effect of spinel growth on the delamination of thermal barrier coatings. Key Eng. Mater. 2011, 462–463, 389–394. [Google Scholar] [CrossRef]
- Xu, R.; Fan, X.L.; Zhang, W.X.; Wang, T.J. Interfacial fracture mechanism associated with mixed oxides growth in thermal barrier coating system. Surf. Coat. Technol. 2014, 253, 139–147. [Google Scholar] [CrossRef]
- Zhang, B.Y.; Shi, J.; Yang, G.J.; Li, C.X.; Li, C.J. Healing of the interface between splashed particles and underlying bulk coating and its influence on isothermal oxidation behavior of LPPS MCrAlY bond coat. J. Therm. Spray Technol. 2015, 24, 611–621. [Google Scholar] [CrossRef]
- Tang, J.J.; Bai, Y.; Zhang, J.C.; Liu, K.; Liu, X.Y.; Zhang, P.; Wang, Y.; Zhang, L.; Liang, G.Y.; Gao, Y.; et al. Microstructural design and oxidation resistance of CoNiCrAlY alloy coatings in thermal barrier coating system. J. Alloy. Compd. 2016, 688, 729–741. [Google Scholar] [CrossRef]
- Bai, Y.; Ding, C.H.; Li, H.Q.; Han, Z.H.; Ding, B.J.; Wang, T.J.; Yu, L. Isothermal oxidation behavior of supersonic atmospheric plasma-sprayed thermal barrier coating system. J. Therm. Spray Technol. 2013, 22, 1201–1209. [Google Scholar] [CrossRef]
- Lim, L.Y.; Meguid, S.A. Modeling and characterisation of depletion of aluminium in bond coat and growth of mixed oxides in thermal barrier coatings. Int. J. Mech. Mater. Des. 2019, 1–17. [Google Scholar] [CrossRef]
- Mahalingam, S.; Yunus, S.M.; Manap, A.; Afandi, N.M.; Zainuddin, R.A.; Kadir, N.F. Crack propagation and effect of mixed oxides on TGO growth in thick La-Gd-YSZ thermal barrier coating. Coatings 2019, 9, 719. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, W.X.; Li, H.M.; Fan, X.L. Modelling of alloying process of cold sprayed Ni/Al coating. Mech. Mater. 2019, 135, 129–143. [Google Scholar] [CrossRef]
- Evans, A.G.; Clarke, D.R.; Levi, C.G. The influence of oxides on the performance of advanced gas turbines. J. Eur. Ceram. Soc. 2008, 28, 1405–1419. [Google Scholar] [CrossRef]
- Elsass, M.; Frommherz, M.; Scholz, A.; Oechsner, M. Interdiffusion in MCrAlY coated nickel-base superalloys. Surf. Coat. Technol. 2016, 307, 565–573. [Google Scholar] [CrossRef]
- Zhao, G.; Xu, B.; Ren, K.; Shao, G.; Wang, Y. Oxygen diffusion through environmental barrier coating materials. Ceram. Int. 2020, 46, 19545–19549. [Google Scholar] [CrossRef]
- Fick, A.V. On liquid diffusion. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1855, 10, 30–39. [Google Scholar] [CrossRef]
- Darken, L.S. Diffusion of carbon in austenite with a discontinuity in composition. Trans. Am. Inst. Min. Metall. Eng. 1949, 180, 430–438. [Google Scholar]
- Darken, L.S. Diffusion, mobility and their interrelation through free energy in binary metallic systems. Trans. Am. Inst. Min. Metall. Eng. 1948, 175, 184–201. [Google Scholar]
- Yang, F.Q. Interaction between diffusion and chemical stresses. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2005, 409, 153–159. [Google Scholar] [CrossRef]
- Wang, W.L.; Lee, S.; Chen, J.R. Effect of chemical stress on diffusion in a hollow cylinder. J. Appl. Phys. 2002, 91, 9584–9590. [Google Scholar] [CrossRef]
- Zhang, X.C.; Shyy, W.; Sastry, A.M. Numerical simulation of intercalation-induced stress in Li-ion battery electrode particles. J. Electrochem. Soc. 2007, 154, A910–A916. [Google Scholar] [CrossRef]
- Suo, Y.H.; Shen, S.P. Coupling diffusion-reaction-mechanics model for oxidation. Acta Mech. 2015, 226, 3375–3386. [Google Scholar] [CrossRef]
- Wang, X.K.; Fan, X.L.; Sun, Y.L.; Xu, R.; Jiang, P. Modelling and analysis of the oxide growth coupling behaviour of thermal barrier coatings. J. Mater. Sci. 2019, 54, 10270–10283. [Google Scholar] [CrossRef]
- Haftbaradaran, H.; Song, J.; Curtin, W.A.; Gao, H.J. Continuum and atomistic models of strongly coupled diffusion, stress, and solute concentration. J. Power Sources 2011, 196, 361–370. [Google Scholar] [CrossRef]
- Dong, X.L.; Fang, X.F.; Feng, X.; Hwang, K.C. Diffusion and stress coupling effect during oxidation at high temperature. J. Am. Ceram. Soc. 2013, 96, 44–46. [Google Scholar] [CrossRef]
- Fang, X.F.; Li, Y.; Yue, M.K.; Feng, X. Chemo-mechanical coupling effect on high temperature oxidation: A review. Sci. China-Technol. Sci. 2019, 62, 1297–1321. [Google Scholar] [CrossRef]
- Suo, Z. A continuum theory that couples creep and self-diffusion. J. Appl. Mech.-Trans. ASME 2004, 71, 646–651. [Google Scholar] [CrossRef]
- Gao, Y.F.; Cho, M.; Zhou, M. Stress relaxation through interdiffusion in amorphous lithium alloy electrodes. J. Mech. Phys. Solids 2013, 61, 579–596. [Google Scholar] [CrossRef]
- Sethuraman, V.A.; Chon, M.J.; Shimshak, M.; Van Winkle, N.; Guduru, P.R. In situ measurement of biaxial modulus of Si anode for Li-ion batteries. Electrochem. Commun. 2010, 12, 1614–1617. [Google Scholar] [CrossRef]
- Sethuraman, V.A.; Chon, M.J.; Shimshak, M.; Srinivasan, V.; Guduru, P.R. In situ measurements of stress evolution in silicon thin films during electrochemical lithiation and delithiation. J. Power Sources 2010, 195, 5062–5066. [Google Scholar] [CrossRef]
- Brassart, L.; Suo, Z.G. Reactive flow in solids. J. Mech. Phys. Solids 2013, 61, 61–77. [Google Scholar] [CrossRef]
- Huang, S.; Fan, F.; Li, J.; Zhang, S.; Zhu, T. Stress generation during lithiation of high-capacity electrode particles in lithium ion batteries. Acta Mater. 2013, 61, 4354–4364. [Google Scholar] [CrossRef]
- Bower, A.F.; Guduru, P.R.; Sethuraman, V.A. A finite strain model of stress, diffusion, plastic flow, and electrochemical reactions in a lithium-ion half-cell. J. Mech. Phys. Solids 2011, 59, 804–828. [Google Scholar] [CrossRef]
- Zhao, K.J.; Pharr, M.; Cai, S.Q.; Vlassak, J.J.; Suo, Z.G. Large Plastic Deformation in high-capacity lithium-ion batteries caused by charge and discharge. J. Am. Ceram. Soc. 2011, 94, S226–S235. [Google Scholar] [CrossRef]
- Zhao, K.J.; Pharr, M.; Wan, Q.; Wang, W.L.; Kaxiras, E.; Vlassak, J.J.; Suo, Z.G. Concurrent reaction and plasticity during initial lithiation of crystalline silicon in lithium-ion batteries. J. Electrochem. Soc. 2012, 159, A238–A243. [Google Scholar] [CrossRef]
- Zhao, K.J.; Tritsaris, G.A.; Pharr, M.; Wang, W.L.; Okeke, O.; Suo, Z.G.; Vlassak, J.J.; Kaxiras, E. Reactive flow in silicon electrodes assisted by the insertion of lithium. Nano Lett. 2012, 12, 4397–4403. [Google Scholar] [CrossRef] [PubMed]
- Di Leo, C.V.; Rejovitzky, E.; Anand, L. Diffusion-deformation theory for amorphous silicon anodes: The role of plastic deformation on electrochemical performance. Int. J. Solids Struct. 2015, 67–68, 283–296. [Google Scholar] [CrossRef]
- Moon, J.; Cho, K.; Cho, M. Ab-initio study of silicon and tin as a negative electrode materials for lithium-ion batteries. Int. J. Precis. Eng. Manuf. 2012, 13, 1191–1197. [Google Scholar] [CrossRef]
- Yang, F.Q. Diffusion-induced stress in inhomogeneous materials: Concentration-dependent elastic modulus. Sci. China-Phys. Mech. Astron. 2012, 55, 955–962. [Google Scholar] [CrossRef]
- Yang, F.Q. Entropy change-induced elastic softening of lithiated materials. Theor. Appl. Mech. Lett. 2015, 5, 255–257. [Google Scholar] [CrossRef]
- Hong, C.S.; Qaiser, N.; Nam, H.G.; Han, S.M. Effect of Li concentration-dependent material properties on diffusion induced stresses of a Sn anode. Phys. Chem. Chem. Phys. 2019, 21, 9581–9589. [Google Scholar] [CrossRef]
- Chang, S.; Moon, J.; Cho, M. Stress-diffusion coupled multiscale analysis of Si anode for Li-ion battery. J. Mech. Sci. Technol. 2015, 29, 4807–4816. [Google Scholar] [CrossRef]
- Lu, Y.J.; Che, Q.; Song, X.; Wang, F.H.; Zhao, X. Stress self-relaxation arising from diffusion-induced creep in bilayer lithium-ion battery electrode. Scr. Mater. 2018, 150, 164–167. [Google Scholar] [CrossRef]
- Xu, C.Q.; Deng, Q.F.; Weng, Z.D.; Chen, B.B.; Zhou, J.Q. Lithium-assisted creep deformation behavior of Sn nanoparticle electrode with fracture-resistant ability. J. Mater. Res. 2019, 34, 3887–3898. [Google Scholar] [CrossRef]
- Pinson, M.B.; Bazant, M.Z. Theory of SEI formation in rechargeable batteries: Capacity fade, accelerated aging and lifetime prediction. J. Electrochem. Soc. 2013, 160, A243–A250. [Google Scholar] [CrossRef]
- Zhang, B.Y.; Meng, G.H.; Yang, G.J.; Li, C.X.; Li, C.J. Dependence of scale thickness on the breaking behavior of the initial oxide on plasma spray bond coat surface during vacuum pre-treatment. Appl. Surf. Sci. 2017, 397, 125–132. [Google Scholar] [CrossRef]
- Li, N.W.; Yin, Y.X.; Yang, C.P.; Guo, Y.G. An artificial solid electrolyte interphase layer for stable lithium metal anodes. Adv. Mater. 2016, 28, 1853–1858. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Lin, D.C.; Yuen, P.Y.; Liu, K.; Xie, J.; Dauskardt, R.H.; Cui, Y. An artificial solid electrolyte interphase with high Li-ion conductivity, mechanical strength, and flexibility for stable lithium metal anodes. Adv. Mater. 2017, 29, 8. [Google Scholar] [CrossRef]
- De la Hoz, J.M.M.; Soto, F.A.; Balbuena, P.B. Effect of the electrolyte composition on SEI reactions at Si anodes of Li-ion batteries. J. Phys. Chem. C 2015, 119, 7060–7068. [Google Scholar] [CrossRef]
- Hu, Z.L.; Zhang, S.; Dong, S.M.; Li, W.J.; Li, H.; Cui, G.L.; Chen, L.Q. Poly(ethyl alpha-cyanoacrylate)-based artificial solid electrolyte interphase layer for enhanced interface stability of Li metal anodes. Chem. Mat. 2017, 29, 4682–4689. [Google Scholar] [CrossRef]
- Khorramirad, M.M.; Rahimipour, M.R.; Hadavi, S.M.M.; Shirvani, K. Preoxidation of bond coat in IN-738LC/NiCrAlY/LaMgAl11O19 thermal barrier coating system. Ceram. Int. 2018, 44, 22080–22091. [Google Scholar] [CrossRef]
- Song, P.; Yu, X.; Huang, T.H.; He, X.; Ji, Q.; Zang, J.J.; Chen, R.; Lu, J.G.; Lu, J.S. Evolution of in-situ pores and high-temperature thermal-barrier performance of Al-Si coating on NiCoCrAl alloy. Surf. Coat. Technol. 2018, 344, 489–498. [Google Scholar] [CrossRef]
- Wang, J.L.; Chen, M.H.; Yang, L.L.; Zhu, S.L.; Wang, F.H. Comparative study of oxidation and interdiffusion behavior of AIP NiCrAlY and sputtered nanocrystalline coatings on a nickel-based single-crystal superalloy. Corros. Sci. 2015, 98, 530–540. [Google Scholar] [CrossRef]
- Wang, R.L.; Gong, X.Y.; Peng, H.; Ma, Y.; Guo, H.B. Interdiffusion behavior between NiAlHf coating and Ni-based single crystal superalloy with different crystal orientations. Appl. Surf. Sci. 2015, 326, 124–130. [Google Scholar] [CrossRef]
- Wen, J.X.; Cao, R.; Gao, Y.F. Mysterious failure in load-free superalloys under repeated thermal shocks. Acta Mater. 2020, 194, 276–282. [Google Scholar] [CrossRef]
- Yang, L.L.; Chen, M.H.; Wang, J.L.; Qiao, Y.X.; Guo, P.Y.; Zhu, S.L.; Wang, F.H. Microstructure and composition evolution of a single-crystal superalloy caused by elements interdiffusion with an overlay NiCrAlY coating on oxidation. J. Mater. Sci. Technol. 2020, 45, 49–58. [Google Scholar] [CrossRef]
- Smigelskas, A.D.; Kirkendall, E.O. Zinc diffusion in alpha-brass. Trans. Am. Inst. Min. Metall. Eng. 1947, 171, 130–142. [Google Scholar]
- Texier, D.; Monceau, D.; Hervier, Z.; Andrieu, E. Effect of interdiffusion on mechanical and thermal expansion properties at high temperature of a MCrAlY coated Ni-based superalloy. Surf. Coat. Technol. 2016, 307, 81–90. [Google Scholar] [CrossRef]
- Qi, W.Y.; Peng, H.; He, J.; Li, S.S.; Gong, S.K.; Guo, H.B. Cyclic oxidation and interdiffusion behavior of Pt modified NiAlHfCrSi coatings on single crystal superalloy containing Mo. Surf. Coat. Technol. 2014, 259, 426–433. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, F.; Li, D.; Zhang, W. Long-Term Failure Mechanisms of Thermal Barrier Coatings in Heavy-Duty Gas Turbines. Coatings 2020, 10, 1022. https://doi.org/10.3390/coatings10111022
Xie F, Li D, Zhang W. Long-Term Failure Mechanisms of Thermal Barrier Coatings in Heavy-Duty Gas Turbines. Coatings. 2020; 10(11):1022. https://doi.org/10.3390/coatings10111022
Chicago/Turabian StyleXie, Feng, Dingjun Li, and Weixu Zhang. 2020. "Long-Term Failure Mechanisms of Thermal Barrier Coatings in Heavy-Duty Gas Turbines" Coatings 10, no. 11: 1022. https://doi.org/10.3390/coatings10111022
APA StyleXie, F., Li, D., & Zhang, W. (2020). Long-Term Failure Mechanisms of Thermal Barrier Coatings in Heavy-Duty Gas Turbines. Coatings, 10(11), 1022. https://doi.org/10.3390/coatings10111022




