Ultrasonic Treatment Induced Fluoride Conversion Coating without Pores for High Corrosion Resistance of Mg Alloy
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
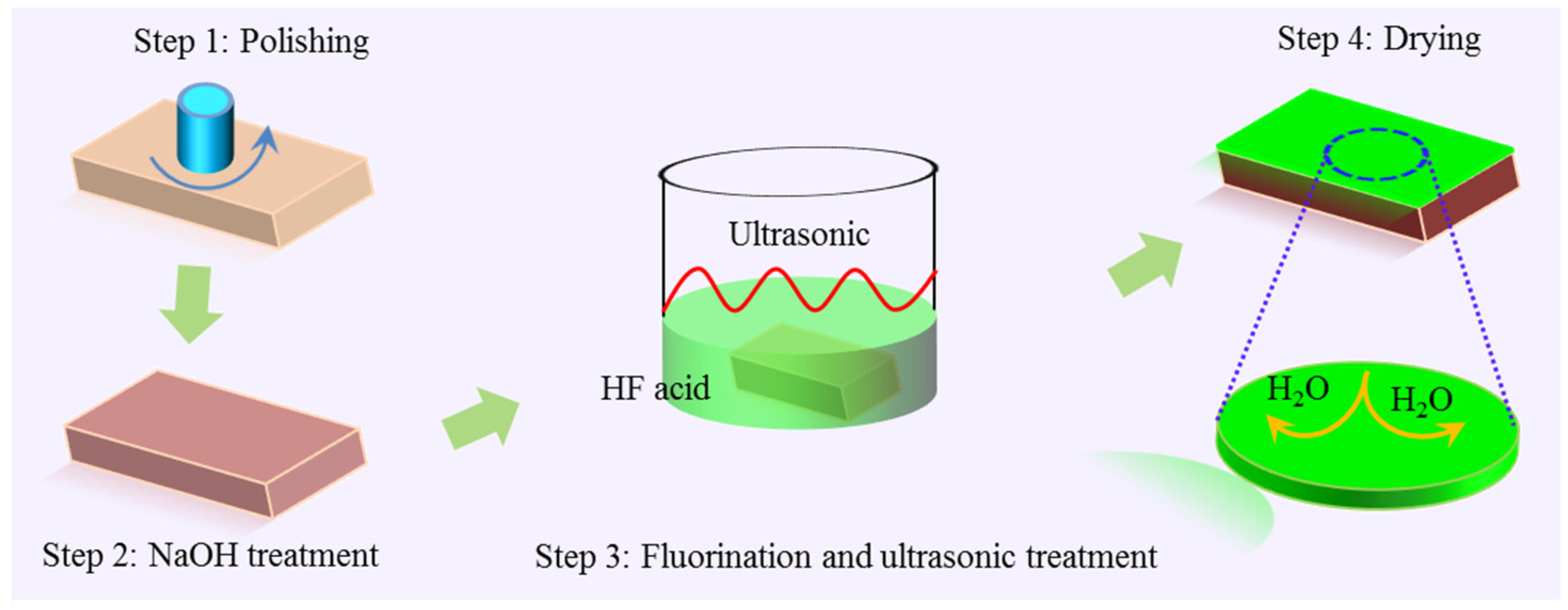
2.2. Fluoride Conversion Coating Preparation
2.3. Characterization
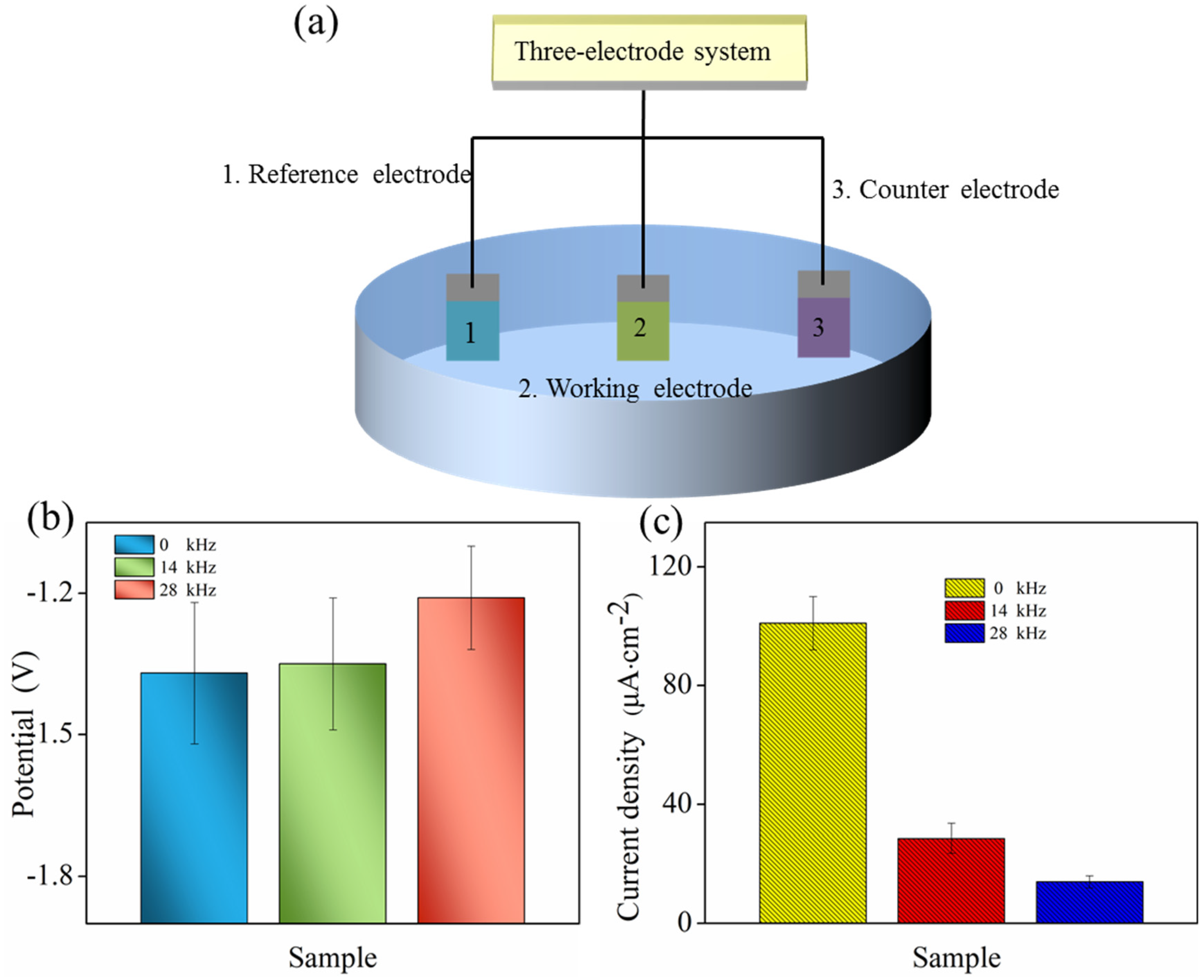
2.4. Electrochemical Properties
2.5. Statistical Analysis
3. Results and Discussion
4. Conclusions
- (1)
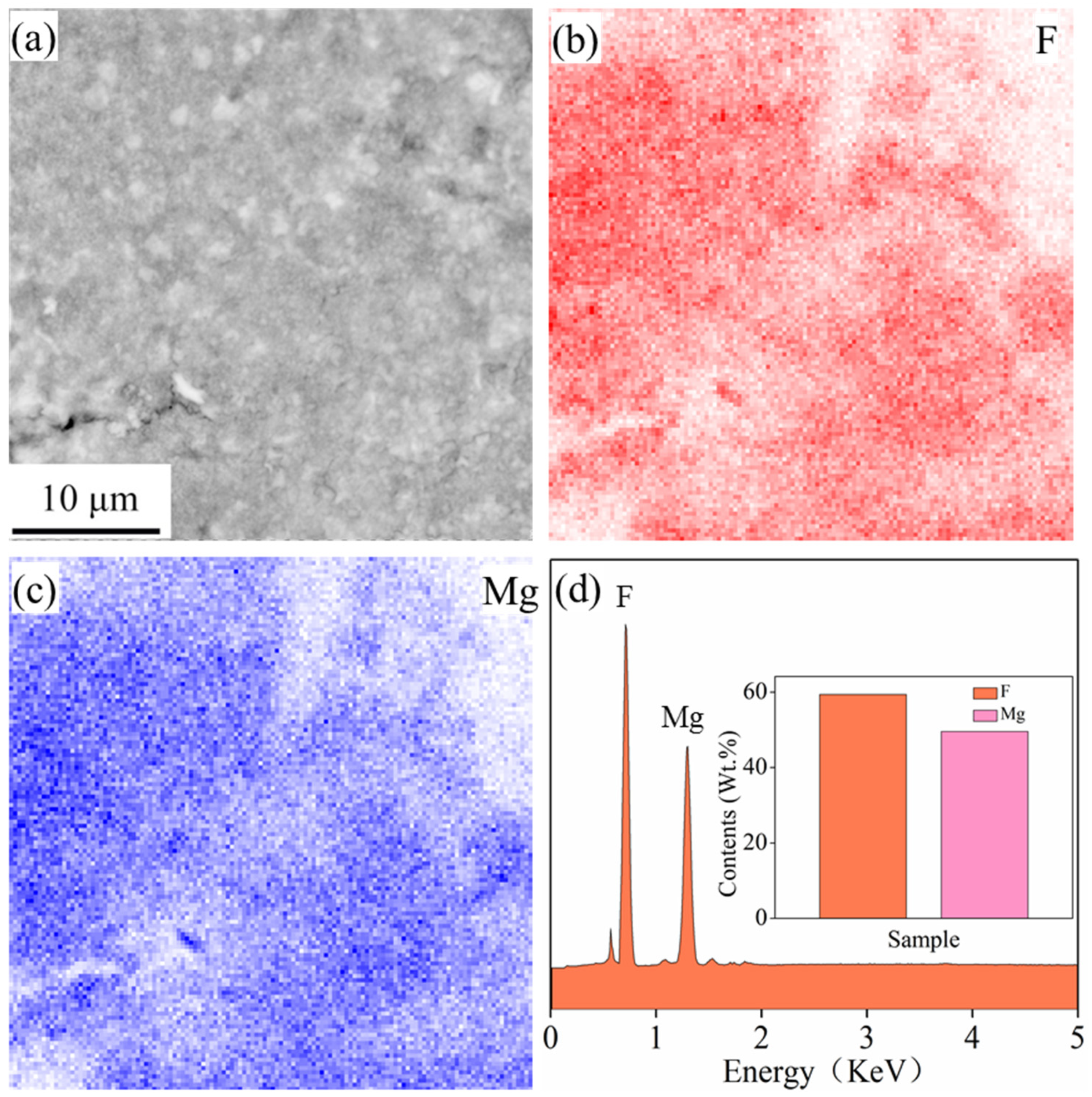
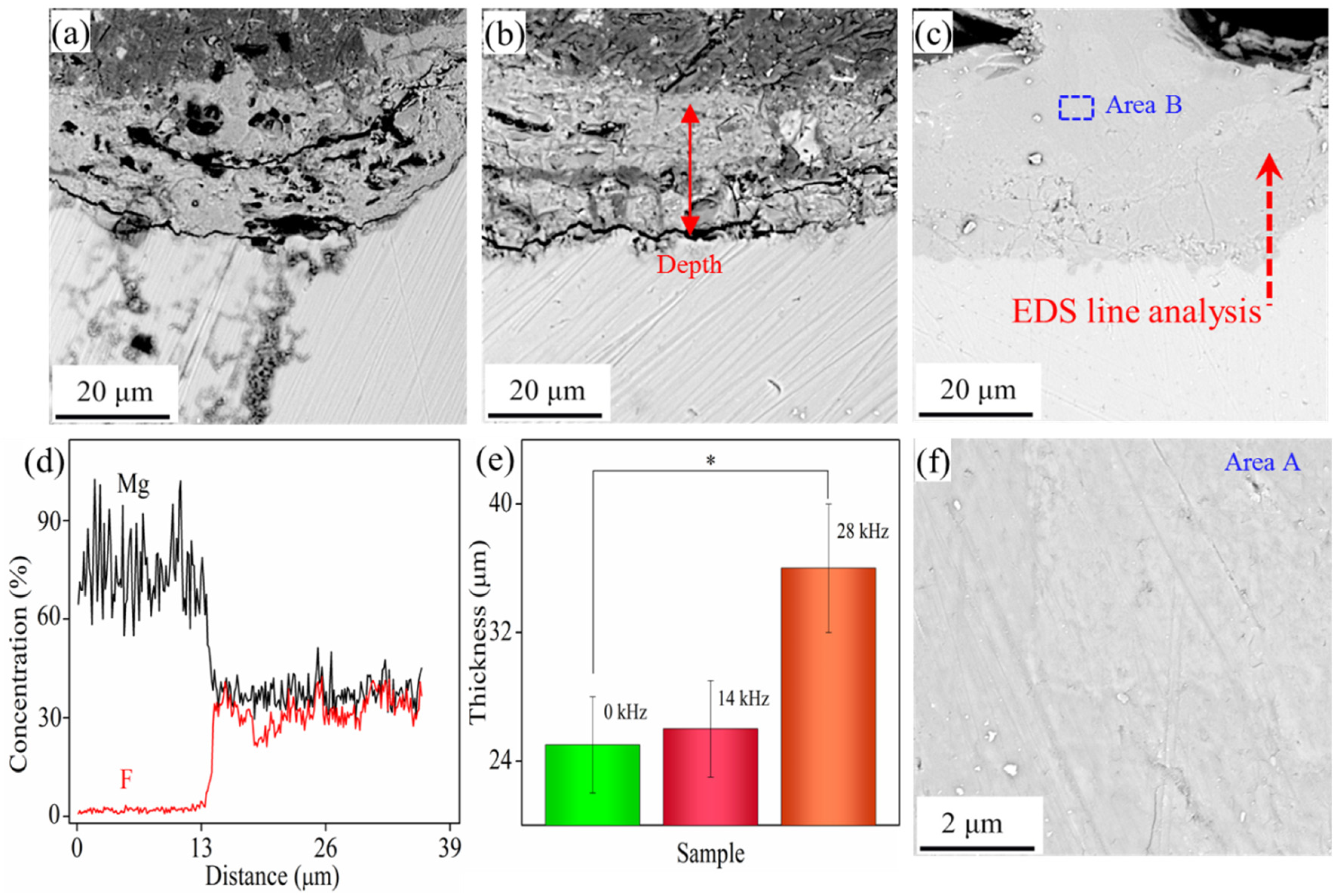
- A dense and compact MgF2 coating was successfully prepared by the combination of fluoride treatment and ultrasonic treatment. When the frequency was 28 kHz during ultrasonic treatment, the surface microstructure was uniform. The chemical compositions were mainly composed of F and Mg elements.
- (2)
- Electrochemical tests in NaCl solution exhibited improved corrosion resistance. The corrosion potential of U-treatment Mg alloy constantly shifted towards the noble direction. The corrosion current density decreased due to the protectiveness of MgF2 coating without pores or cracks.
- (3)
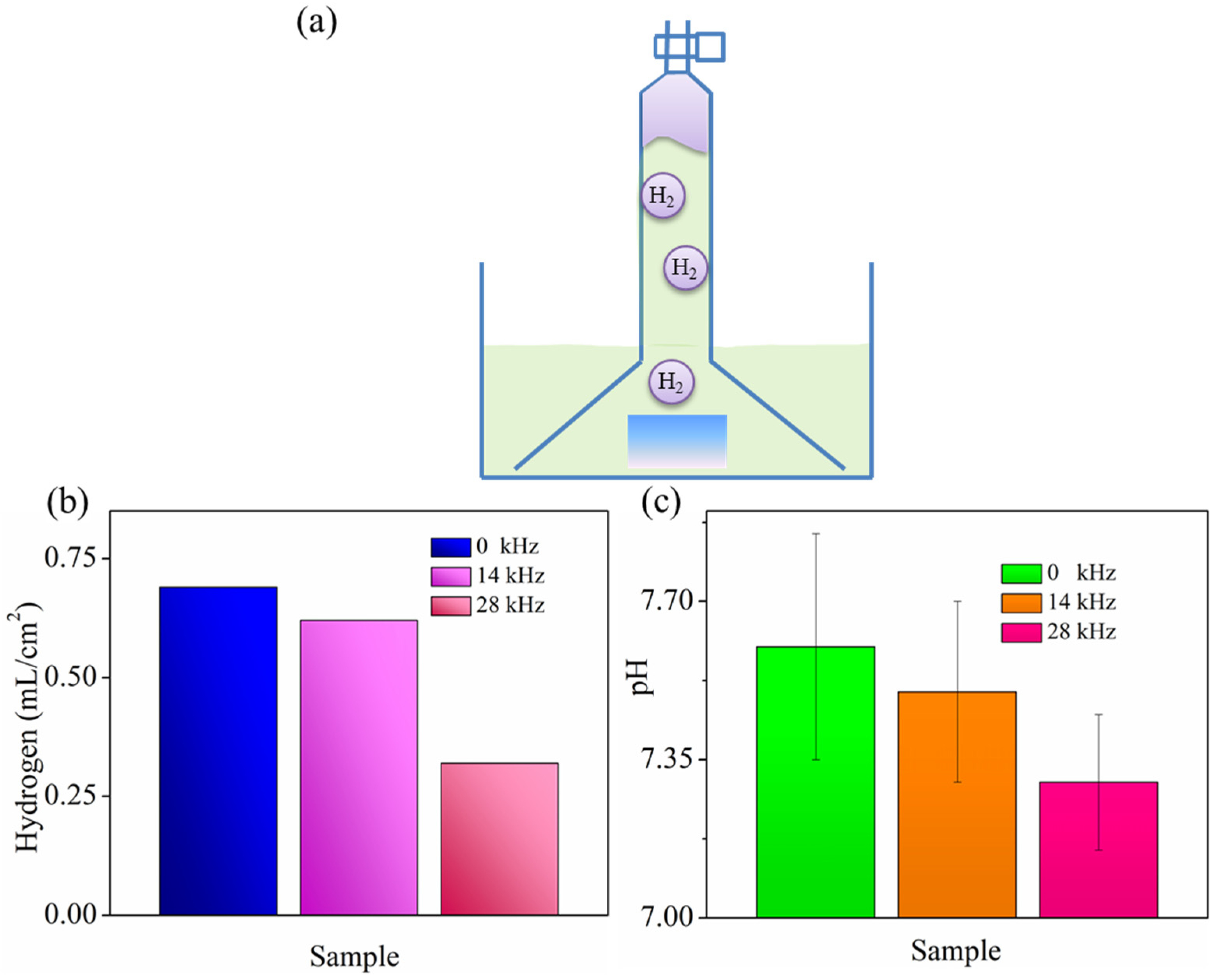
- Immersion tests showed an improvement in the short-term instability of the MgF2 coating after ultrasonic treatment, which provided enough corrosion protection, inhibiting not only pH increase but also gas cavities formation. The improvement in corrosion resistance that MgF2 coating induced on Mg alloy was due to the formation of a compact structure without defects. This reduced the channel of the corrosive medium into the Mg alloy surface and thereby strengthened coating passivation ability. Consequently, it could be concluded that ultrasonically treated fluoride coating had much promise for their use in anti-corrosion applications of Mg alloy, such as automotive, aviation industry and temporary implants.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xiang, D.; Li, S.; Yi, L.-H.; Liu, T.-F.; Liu, J.; Yang, C.-M.; Zhow, L.-H. In Situ Formation of intermetallic compound bonding by laser cladding Al2O3 to enhance the corrosion resistance of AZ31 magnesium alloy. Lasers Eng. 2020, 45, 127. [Google Scholar]
- Li, S.; Yi, L.; Liu, T.; Ji, B.; Yang, C.; Zhou, L. Al2O3 eliminating defects in the Al coating by laser cladding to improve corrosion and wear resistance of Mg alloy. Mater. Corros. 2020, 71, 980. [Google Scholar] [CrossRef]
- Li, S.; Yi, L.; Liu, T.; Deng, H.; Ji, B.; Zhang, K.; Zhou, L. Formation of a protective layer against corrosion on Mg alloy via alkali pretreatment followed by vanillic acid treatment. Mater. Corros. 2020, 21, 991. [Google Scholar] [CrossRef]
- Rahmati, M.; Raeissi, K.; Toroghinejad, M.R.; Hakimizad, A.; Santamaria, M. Effect of pulse current mode on microstructure, composition and corrosion performance of the coatings produced by plasma electrolytic oxidation on AZ31 Mg alloy. Coatings 2019, 9, 688. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, J.; Liu, S.; Li, Z.; Zhang, L.; Wu, R.; Hou, L.; Zhang, M. Hydrothermal synthesis of protective coating on Mg alloy for degradable implant applications. Coatings 2019, 9, 160. [Google Scholar] [CrossRef]
- Mehrjou, B.; Dehghan-Baniani, D.; Shi, M.; Shanaghi, A.; Wang, G.; Liu, L.; Qasim, A.M.; Chu, P.K. Nanopatterned silk-coated AZ31 magnesium alloy with enhanced antibacterial and corrosion properties. Mater. Sci. Eng. C 2020, 18, 111173. [Google Scholar] [CrossRef]
- Vaughan, M.; Karayan, A.; Srivastava, A.; Mansoor, B.; Seitz, J.; Eifler, R.; Karaman, I.; Castaneda, H.; Maier, H. The effects of severe plastic deformation on the mechanical and corrosion characteristics of a bioresorbable Mg-ZKQX6000 alloy. Mater. Sci. Eng. C 2020, 21, 111130. [Google Scholar] [CrossRef]
- Pezzato, L.; Babbolin, R.; Cerchier, P.; Marigo, M.; Dolcet, P.; Dabalà, M.; Brunelli, K. Sealing of PEO coated AZ91 magnesium alloy using solutions containing neodymium. Corros. Sci. 2020, 13, 108741. [Google Scholar] [CrossRef]
- Wei, X.; Liu, P.; Ma, S.; Li, Z.; Peng, X.; Deng, R.; Zhao, Q. Improvement on corrosion resistance and biocompability of ZK60 magnesium alloy by carboxyl ion implantation. Corros. Sci. 2020, 44, 108729. [Google Scholar] [CrossRef]
- Korrapati, V.K.; Scharnagl, N.; Letzig, D.; Zheludkevich, M.L. Self-assembled layers for the temporary corrosion protection of magnesium-AZ31 alloy. Corros. Sci. 2020, 27, 108619. [Google Scholar] [CrossRef]
- Toorani, M.; Aliofkhazraei, M.; Mahdavian, M.; Naderi, R. Effective PEO/Silane pretreatment of epoxy coating applied on AZ31B Mg alloy for corrosion protection. Corros. Sci. 2020, 11, 108608. [Google Scholar] [CrossRef]
- Buzolin, R.H.; Volovitch, P.; Maltseva, A.; Lamaka, S.; Blawert, C.; Mendis, C.L.; Lohmüller, A.; Kainer, K.U.; Hort, N. Thixomolded AZ91D and MRI153M magnesium alloys and their enhanced corrosion resistance. Mater. Corros. 2020, 71, 339–351. [Google Scholar] [CrossRef]
- Xue, Y.; Pang, X.; Jiang, B.; Jahed, H.; Wang, D. Characterization of the corrosion performances of as-cast Mg–Al and Mg–Zn magnesium alloys with microarc oxidation coatings. Mater. Corros. 2020, 71, 992–1006. [Google Scholar] [CrossRef]
- Dvorsky, D.; Kubasek, J.; Vojtech, D. Corrosion protection of WE43 magnesium alloy by fluoride conversion coating. Manuf. Technol. 2017, 17, 440–446. [Google Scholar] [CrossRef]
- Panemangalore, D.B.; Shabadi, R.; Gupta, M.; Ji, G. Effect of fluoride coatings on the corrosion behavior of Mg–Zn–Er alloys. Surf. Interfaces 2019, 14, 72–81. [Google Scholar] [CrossRef]
- Chiu, K.; Wong, M.; Cheng, F.; Man, H. Characterization and corrosion studies of fluoride conversion coating on degradable Mg implants. Surf. Coat. Technol. 2007, 202, 590–598. [Google Scholar] [CrossRef]
- Yan, T.; Tan, L.; Zhang, B.; Yang, K. Fluoride conversion coating on biodegradable AZ31B magnesium alloy. J. Mater. Sci. Technol. 2014, 30, 666–674. [Google Scholar] [CrossRef]
- Saranya, K.; Kalaiyarasan, M.; Chatterjee, S.; Rajendran, N. Dynamic electrochemical impedance study of fluoride conversion coating on AZ31 magnesium alloy to improve bio-adaptability for orthopedic application. Mater. Corros. 2019, 70, 698–710. [Google Scholar] [CrossRef]
- Ma, F.-F.; Zhang, D.; Huang, T.; Zhang, N.; Wang, Y. Ultrasonication-assisted deposition of graphene oxide on electrospun poly (vinylidene fluoride) membrane and the adsorption behavior. Chem. Eng. J. 2019, 358, 1065–1073. [Google Scholar] [CrossRef]
- Zhan, Y.; Wu, J.; Xia, H.; Yan, N.; Fei, G.; Yuan, G. Dispersion and exfoliation of graphene in rubber by an ultrasonically-assisted latex mixing and in situ reduction process. Macromol. Mater. Eng. 2011, 296, 590–602. [Google Scholar] [CrossRef]
- Sun, X.; Liu, M.; Song, J.; Xu, Y. Corrosion behaviors of hybrid ultrasonic phosphating coatings on carbon steel in simulated 150 °C hot-dry-rock fluids. Geothermics 2020, 86, 101807. [Google Scholar] [CrossRef]
- Yang, J.; Kim, J.; Chun, J.S. A study of the effect of ultrasonics on manganese phosphating of steel. Thin Solid Films 1983, 101, 193–200. [Google Scholar] [CrossRef]
- Domnikov, L. The effect of ultrasonics on the phosphating process. Metal Finish. 1967, 65, 55–57. [Google Scholar]
- Qu, L.; Li, M.; Liu, M.; Zhang, E.; Ma, C. Microstructure and corrosion resistance of ultrasonic micro-arc oxidation biocoatings on magnesium alloy. J. Adv. Ceram. 2013, 2, 227–234. [Google Scholar] [CrossRef]
- Saha, A.; Mondal, A.; Maiti, S.; Ghosh, S.C.; Mahanty, S.; Panda, A.B. A facile method for the synthesis of a C@MoO2 hollow yolk–shell structure and its electrochemical properties as a faradaic electrode. Mater. Chem. Front. 2017, 1, 1585–1593. [Google Scholar] [CrossRef]
- Pérez, E.; Carretero, N.; Sandoval, S.; Fuertes, A.; Tobias, G.; Casañ-Pastor, N. Nitro-graphene oxide in iridium oxide hybrids: Electrochemical modulation of N-graphene redox states and charge capacities. Mater. Chem. Front. 2020, 4, 1421–1433. [Google Scholar] [CrossRef]
- Ren, M.; Cai, S.; Liu, T.; Huang, K.; Wang, X.; Zhao, H.; Niu, S.; Zhang, R.; Wu, X. Calcium phosphate glass/MgF2 double layered composite coating for improving the corrosion resistance of magnesium alloy. J. Alloy. Compd. 2014, 591, 34–40. [Google Scholar] [CrossRef]
- Riaz, U.; Rahman, Z.U.; Asgar, H.; Shah, U.; Shabib, I.; Haider, W. An insight into the effect of buffer layer on the electrochemical performance of MgF2 coated magnesium alloy ZK60. Surf. Coat. Technol. 2018, 344, 514–521. [Google Scholar] [CrossRef]
- Zang, D.; Zhu, R.; Zhang, W.; Yu, X.; Lin, L.; Guo, X.; Liu, M.; Jiang, L. Corrosion-resistant superhydrophobic coatings on Mg alloy surfaces inspired by Lotus seedpod. Adv. Funct. Mater. 2017, 27, 1605446. [Google Scholar] [CrossRef]
- Ho, C.-Y.; Huang, S.-M.; Lee, S.-T.; Chang, Y.-J. Evaluation of synthesized graphene oxide as corrosion protection film coating on steel substrate by electrophoretic deposition. Appl. Surf. Sci. 2019, 477, 226–231. [Google Scholar] [CrossRef]
- Da Conceicao, T.F.; Scharnagl, N.; Blawert, C.; Dietzel, W.; Kainer, K. Surface modification of magnesium alloy AZ31 by hydrofluoric acid treatment and its effect on the corrosion behavior. Thin Solid Films 2010, 518, 5209–5218. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, Z.; Liu, S.; Chen, D.; Wang, G.; Wang, Y.; Zhang, M.; Chen, Y. Ultrasonic-assisted electroless Ni-P plating on dual phase Mg-Li alloy. J. Electrochem. Soc. 2014, 162, C64. [Google Scholar] [CrossRef]
- Ismail, N.; Hussain, Z.; Abdalla, K. Effect of strontium pretreatment on magnesium alloy evaluated by immersion test. AIP Conf. Proc. 2020, 2267. [Google Scholar] [CrossRef]
- Toorani, M.; Aliofkhazraei, M. Review of electrochemical properties of hybrid coating systems on Mg with plasma electrolytic oxidation process as pretreatment. Surf. Interfaces 2019, 14, 262–295. [Google Scholar] [CrossRef]
- Kamrani, S.; Fleck, C. Biodegradable magnesium alloys as temporary orthopaedic implants: A review. BioMetals 2019, 32, 185–193. [Google Scholar] [CrossRef]
- Dilshad, T.; Rahim, S.A.; Hanas, T. Polyvinyl alcohol/magnesium phosphate composite coated Mg–Ca Alloy for biodegradable orthopaedic implant applications. Mater. Res. Express 2019, 6, 1165–1167. [Google Scholar]
- Dubey, A.; Jaiswal, S.; Haldar, S.; Roy, P.; Lahiri, D. Functionally gradient magnesium based composite for temporary orthopedic implant with improved corrosion resistance and osteogenic properties. Biomed. Mater. 2020. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Yi, L.; Zhu, X.; Liu, T. Ultrasonic Treatment Induced Fluoride Conversion Coating without Pores for High Corrosion Resistance of Mg Alloy. Coatings 2020, 10, 996. https://doi.org/10.3390/coatings10100996
Li S, Yi L, Zhu X, Liu T. Ultrasonic Treatment Induced Fluoride Conversion Coating without Pores for High Corrosion Resistance of Mg Alloy. Coatings. 2020; 10(10):996. https://doi.org/10.3390/coatings10100996
Chicago/Turabian StyleLi, Sheng, Laihua Yi, Xiongxiang Zhu, and Tongfang Liu. 2020. "Ultrasonic Treatment Induced Fluoride Conversion Coating without Pores for High Corrosion Resistance of Mg Alloy" Coatings 10, no. 10: 996. https://doi.org/10.3390/coatings10100996
APA StyleLi, S., Yi, L., Zhu, X., & Liu, T. (2020). Ultrasonic Treatment Induced Fluoride Conversion Coating without Pores for High Corrosion Resistance of Mg Alloy. Coatings, 10(10), 996. https://doi.org/10.3390/coatings10100996



