Influence of Element Penetration Region on Adhesion and Corrosion Performance of Ni-Base Coatings
Abstract
1. Introduction
2. Experimental
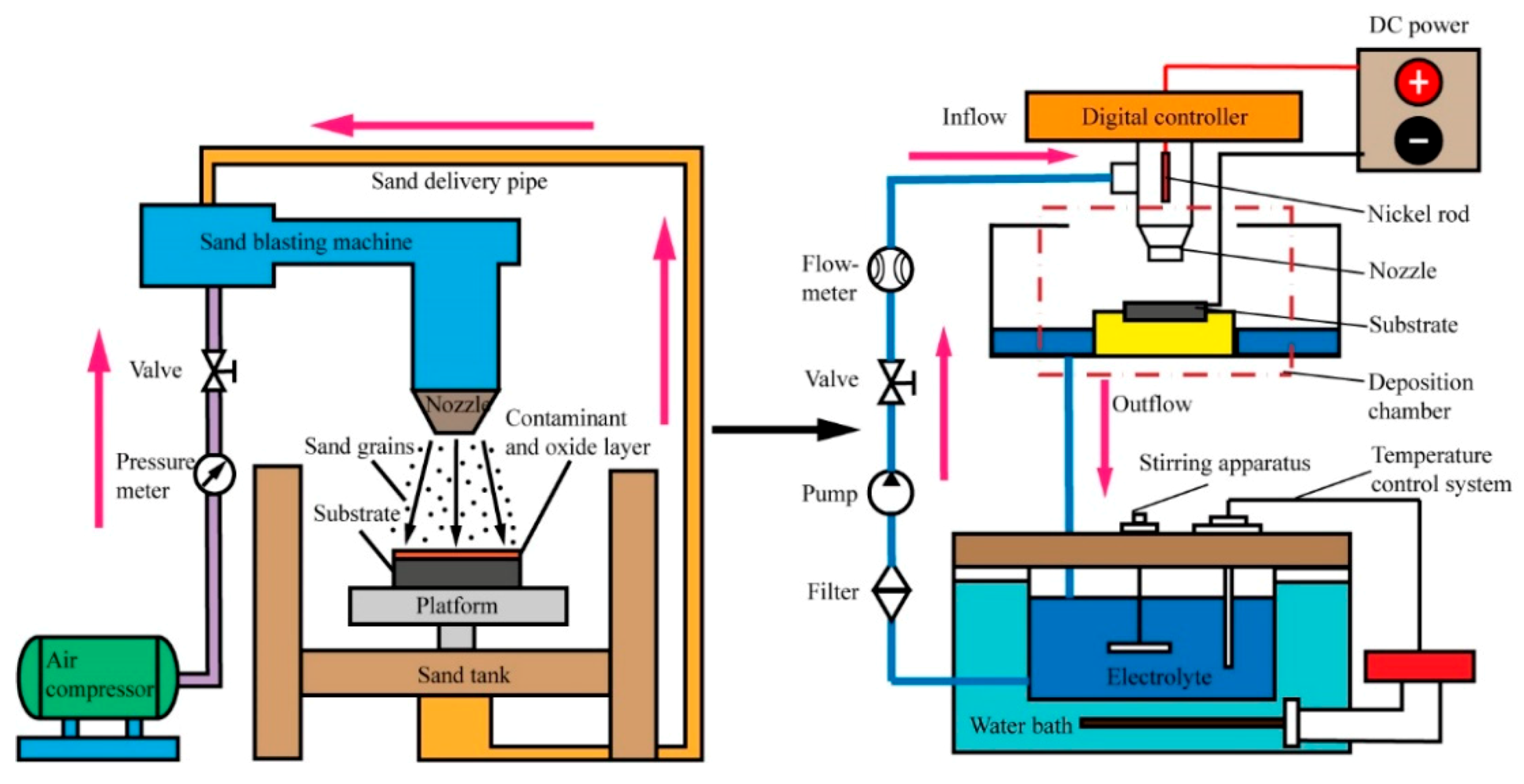
2.1. Experimental Procedures
2.2. Experimental Details
2.3. Experimental Principle
2.4. Instruments and Characterization
3. Results and Discussion
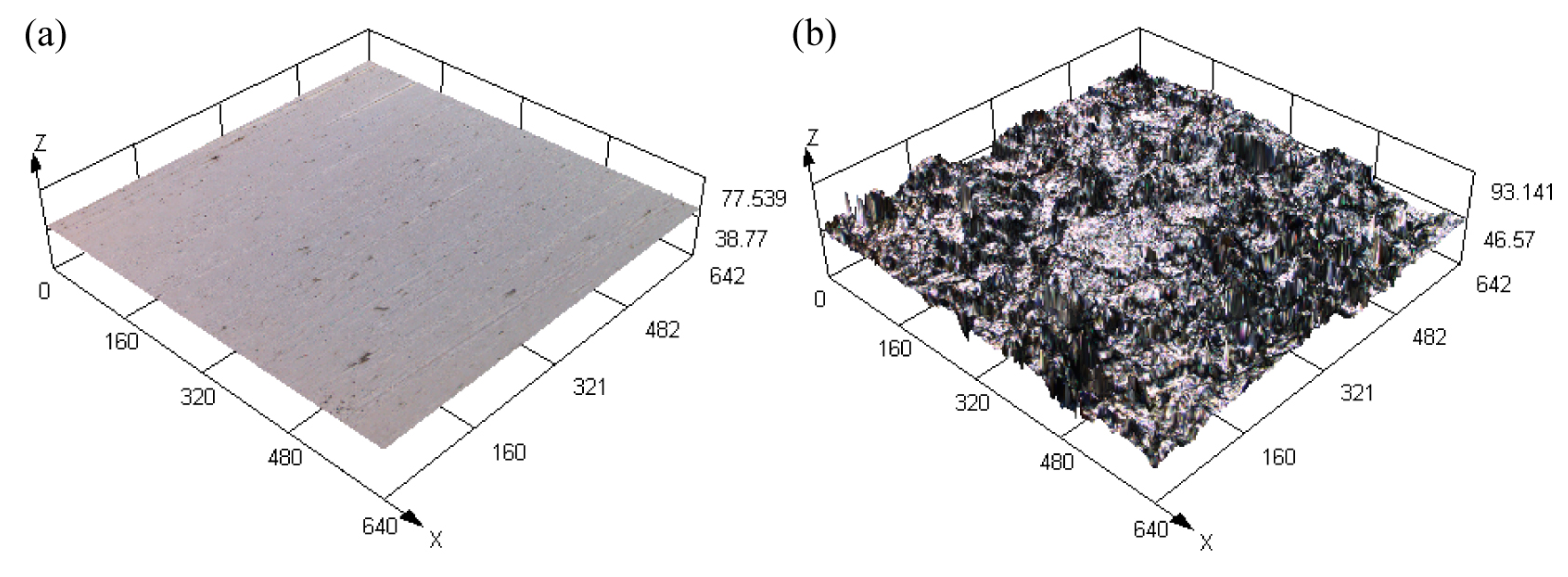
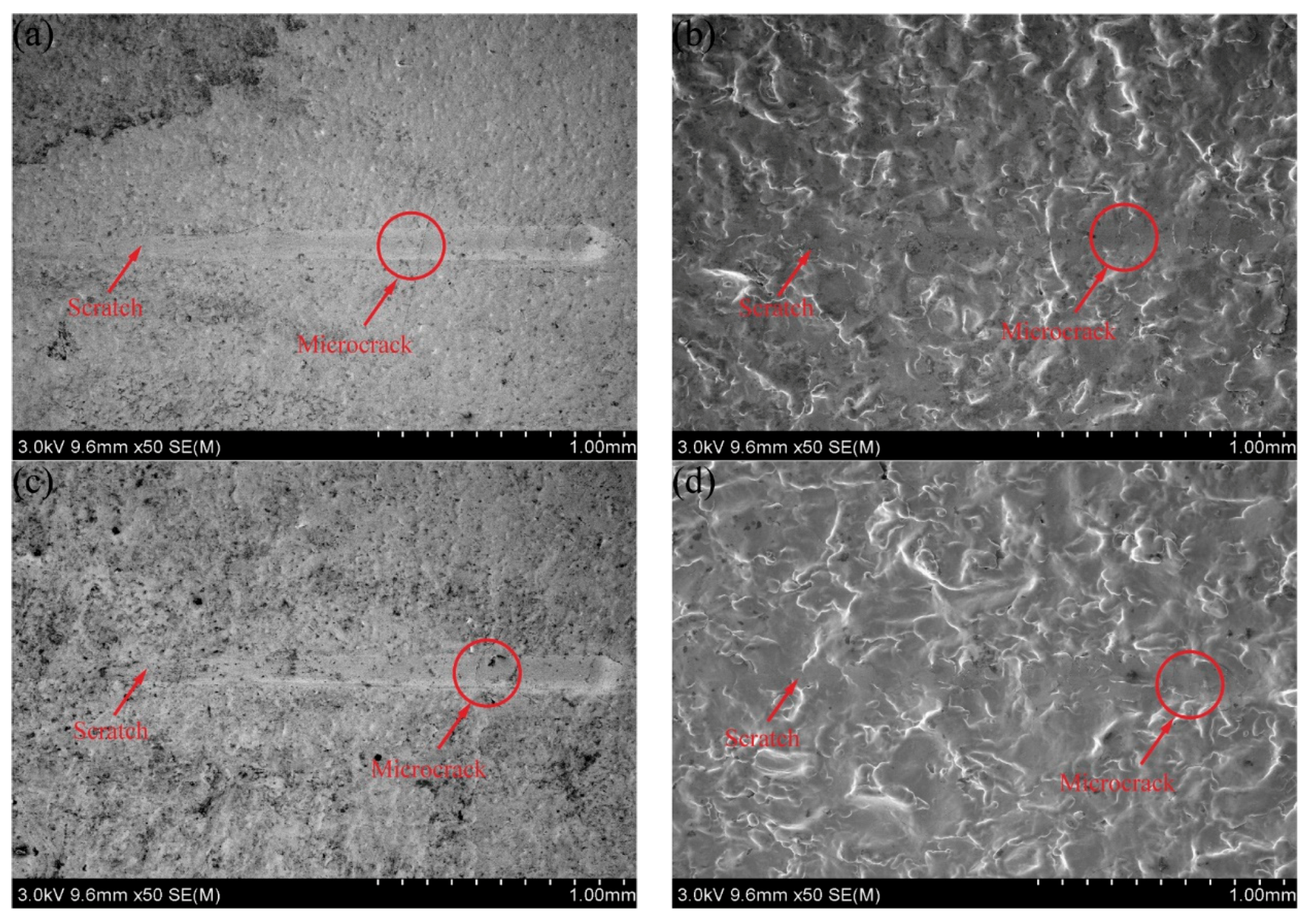
3.1. Substrate Surface Morphology
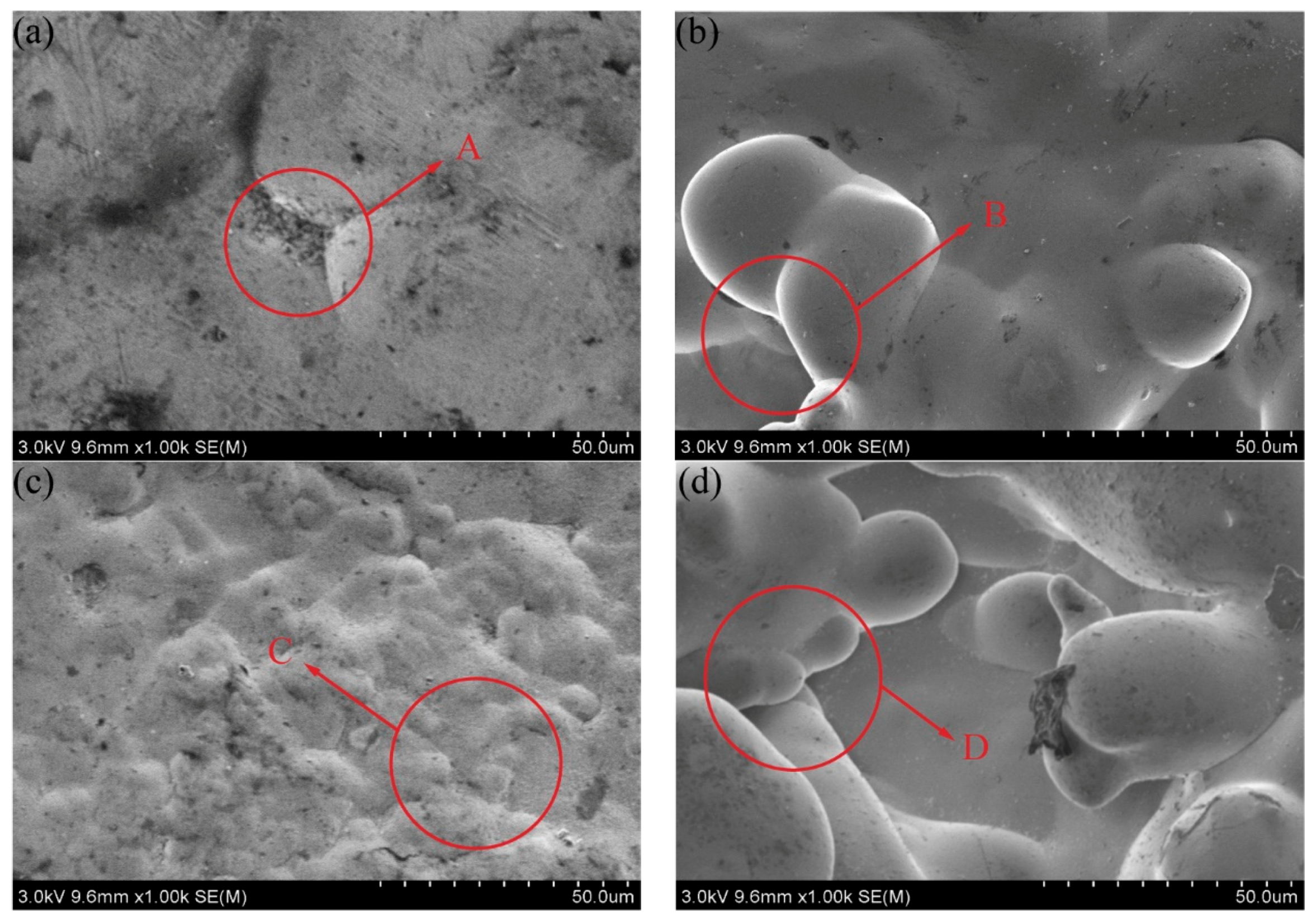
3.2. Coating Morphology
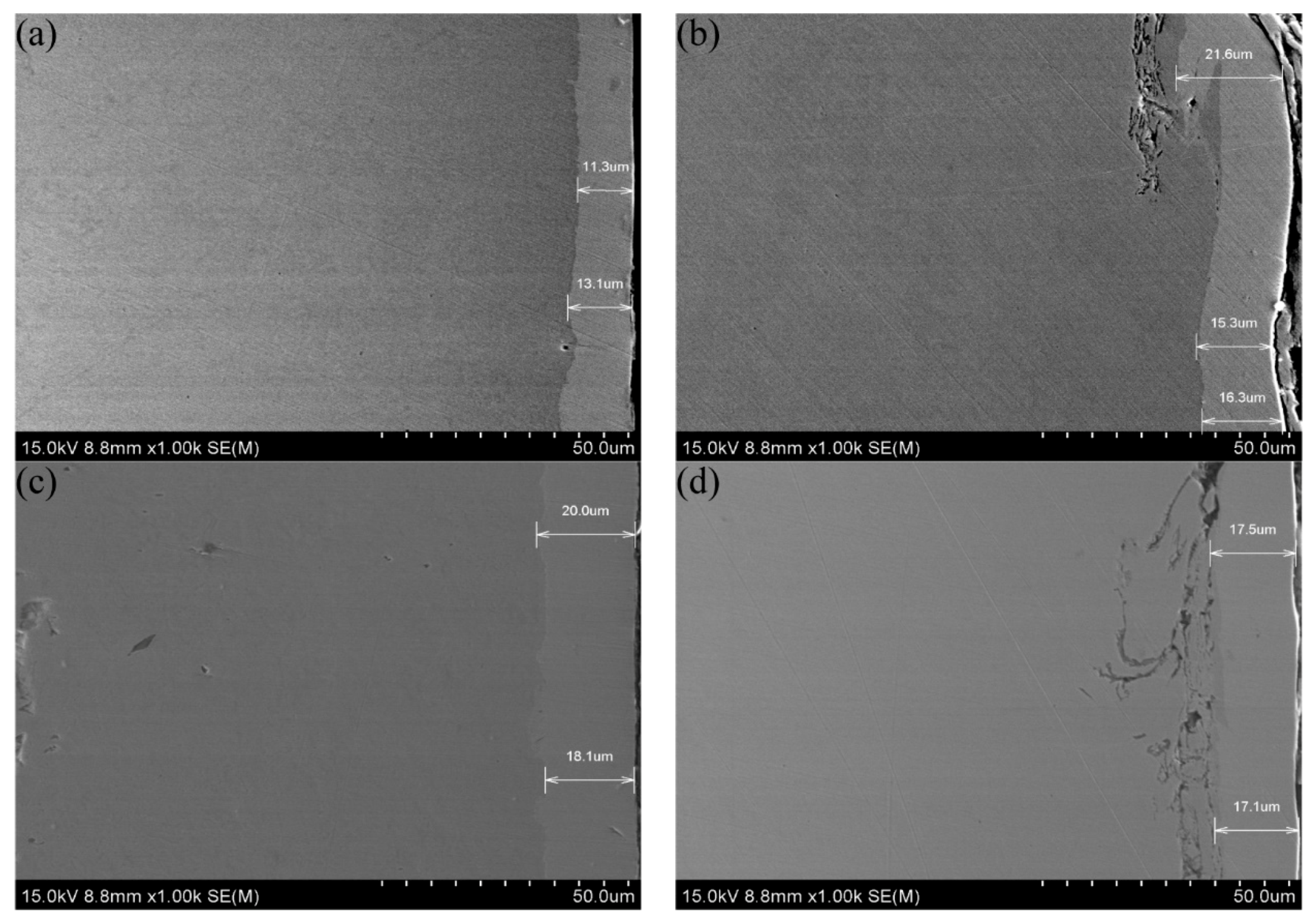
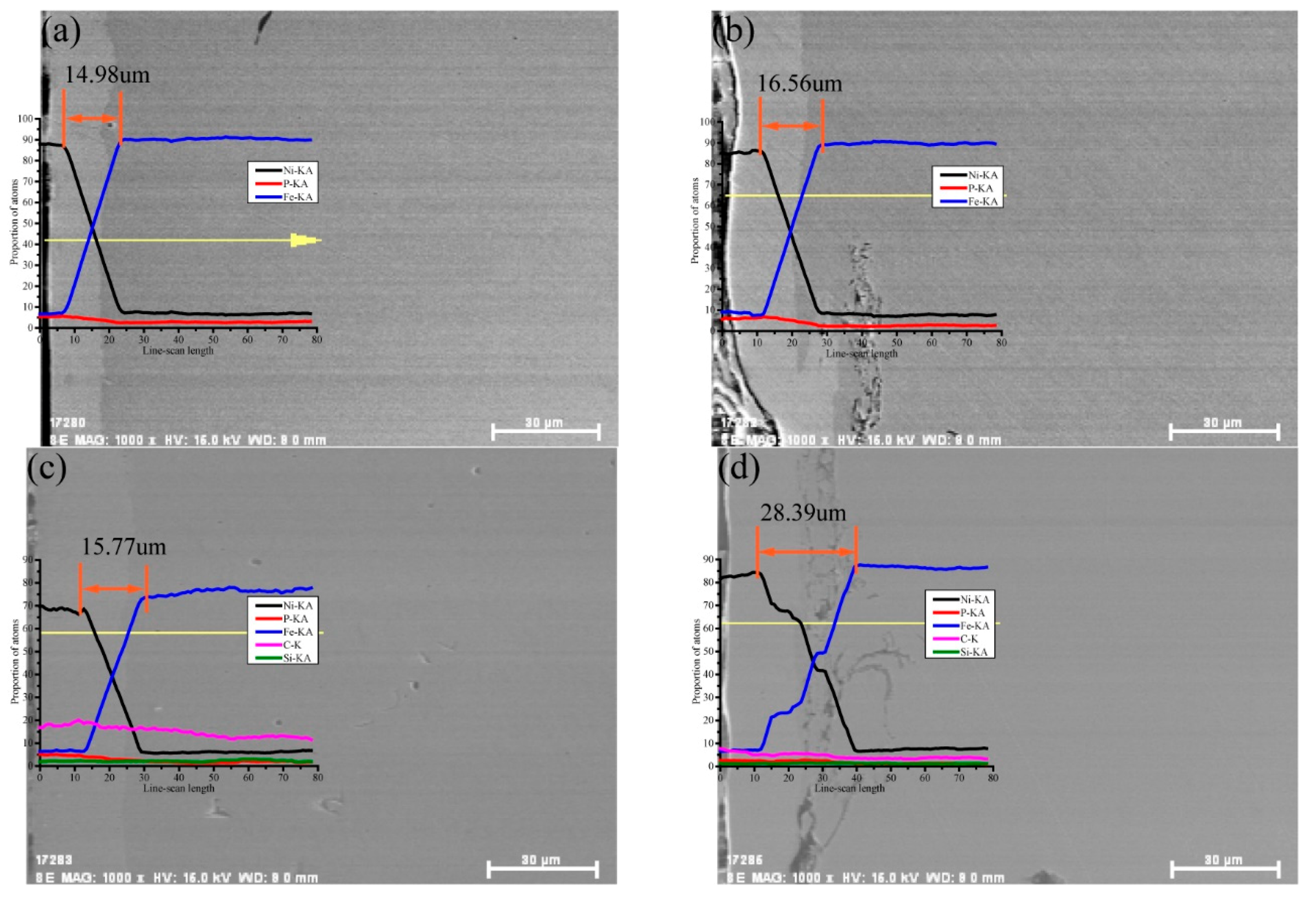
3.3. Section Morphology and Element Analysis
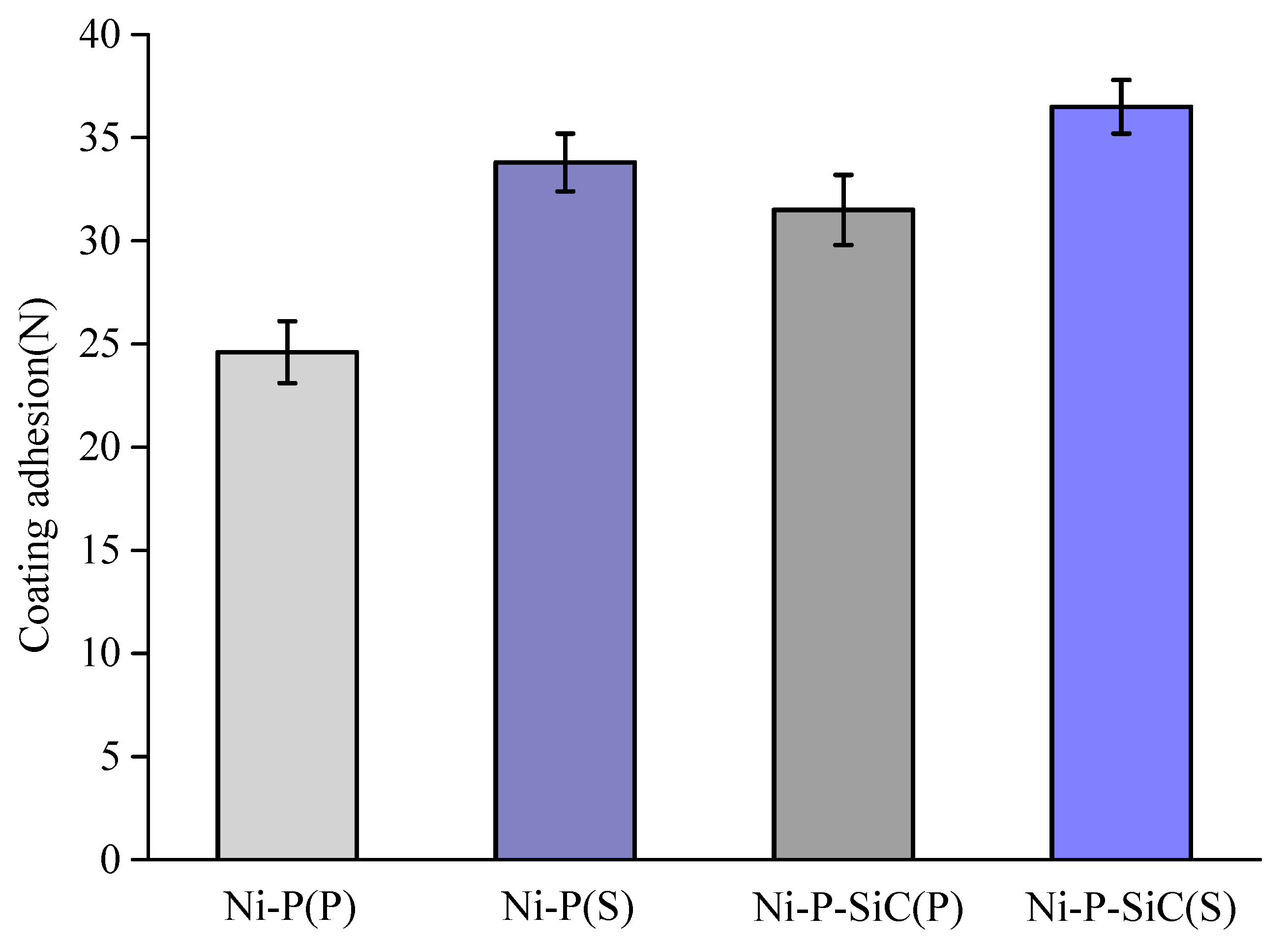
3.4. Coating Adhesion
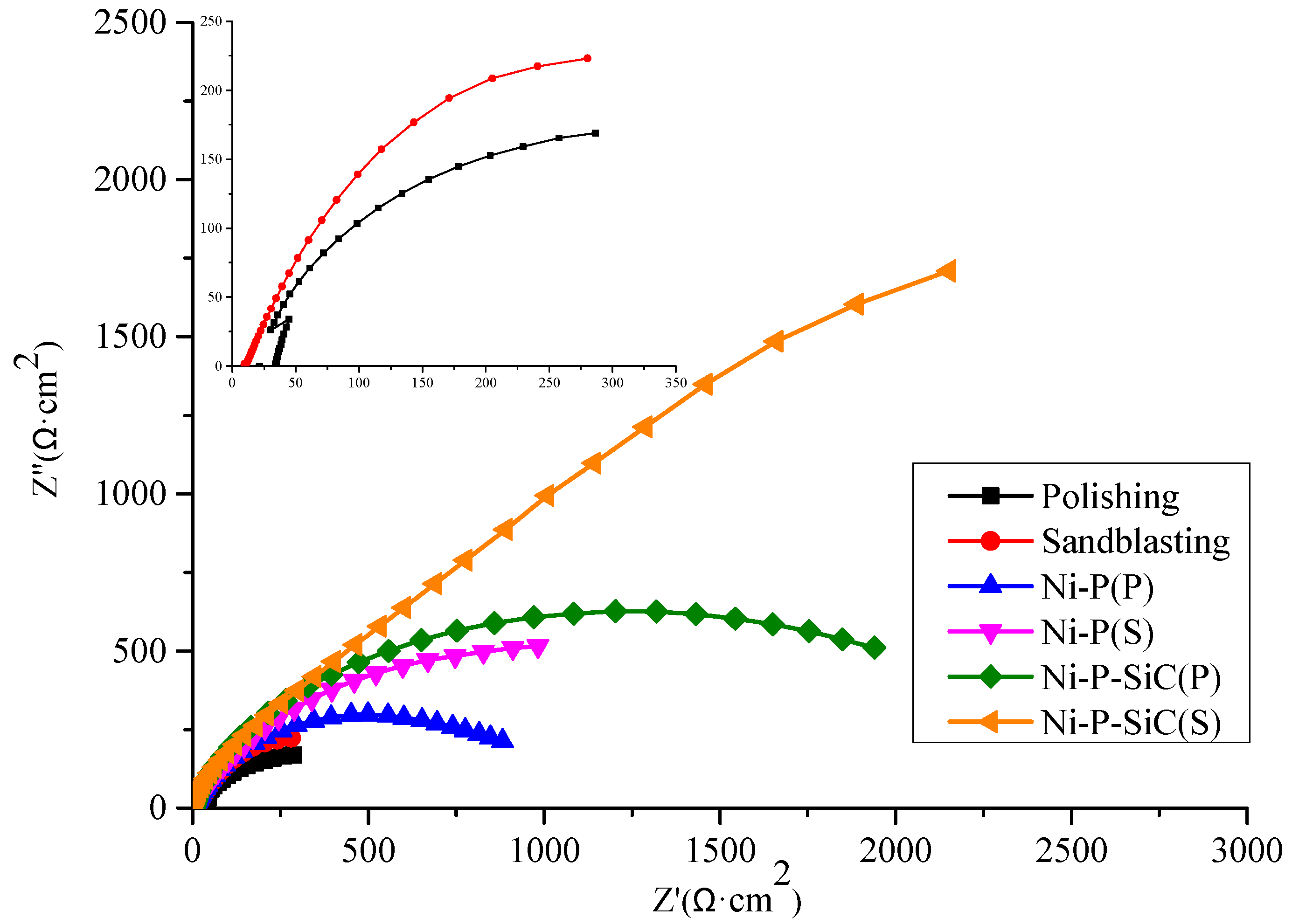
3.5. Corrosion Resistance
4. Conclusions
- The surface square-root roughness values of the 45 steel substrates subjected to polishing and sandblasting pretreatments were 0.006 ± 0.0011 μm and 0.804 ± 0.027 μm, respectively, and the root-mean-square roughness values were 0.008 ± 0.0007 μm and 1.055 ± 0.066 μm, respectively. Compared with the polishing pretreatment, the sandblasting pretreatment could quickly impart a rough appearance on the substrate surface.
- The sandblasting pretreatment helped increase the number of nucleation points of the cellular structure on the coating surface and impart a dense cellular structure distribution. The addition of the SiC nanoparticles could refine the Ni–P coating, improve the cathode polarization, and promote the nucleation of Ni2+. Defects on the coating surfaced were clearly improved.
- An analysis of the section morphology after the sandblasting pretreatment showed that the coating and the rough substrate surface adhered tightly. The high roughness of the surface led to an increase in the number of nucleation points in the coating, thus increasing the coating thickness. After adding the SiC nanoparticles, the coating section was more compact, the adhesion between the substrate was higher, and the coating thickness increased. An element penetration region was formed between the coating and the substrate, as observed by scanning the section elements. The sandblasting pretreatment and SiC nanoparticle addition could promote the interpenetration of the elements and increase the range of the element penetration region. The Ni–P–SiC coating prepared by scanning electrodeposition with the sandblasting pretreatment exhibited the largest element permeating region, up to 28.39 ± 0.07 µm.
- The sandblasting pretreatment increased the real surface area of contact between the substrate and the coating, so that the adhesion between the coating and the substrate formed a mechanical interlock. The addition of SiC nanoparticles refined the Ni–P coating structure. The expansion of the element penetration region could improve the coating adhesion. After the sandblasting pretreatment, the Ni–P–SiC coating adhesion was the highest, reaching 36.5 N.
- Compared with the polished samples, the corrosion potential of the sandblasted substrate increased from −1.13 to −0.91 V, and the current density decreased from 4.67 × 10−4 to 2.15 × 10−5 A·cm−2. The element penetration region between the coating and the substrate could alleviate the impact of corrosion on the substrate. The Ni–P–SiC coating prepared on the sandblasted substrate exhibited the highest corrosion potential, reaching −0.30 V, and the lowest corrosion current density, reaching 8.45 × 10−7 A·cm−2.
Author Contributions
Funding
Conflicts of Interest
References
- Miao, B.; Zhao, X.B.; Song, L.; Wei, K.X.; Hu, J. Raising the Corrosion Resistance of Plasma-Nitrided Steel 45 (AISI 1045) by Preliminary Sandblasting. Met. Sci. Heat Treat. 2019, 60, 808–813. [Google Scholar] [CrossRef]
- Lu, Y.Z.; Li, H.G.; Zhang, H.; Huang, G.H.; Xu, H.D.; Qin, Z.X.; Lu, X. Zr-Based Metallic Glass Coating for Corrosion Resistance Improvement of 45 Steel. Mater. Trans. 2019, 58, 1319–1321. [Google Scholar] [CrossRef]
- Luo, X.X.; Yao, Z.J.; Zhang, P.Z.; Zhou, K.Y. Corrosion Resistance of Al-Cr Composite Strengthening Layer on the Surface of 45(#) steel. Rare Met. Mater. Eng. 2018, 47, 3127–3133. [Google Scholar]
- Huang, G.K.; Qu, L.D.; Lu, Y.Z.; Wang, Y.Z.; Li, H.G.; Qin, Z.X.; Lu, X. Corrosion resistance improvement of 45 steel by Fe-based amorphous coating. Vacuum 2018, 153, 39–42. [Google Scholar] [CrossRef]
- Li, G.; Gan, Y.Y.; Liu, C.H.; Shi, Y.; Zhao, Y.C.; Kou, S.Z. Corrosion and Wear Resistance of Fe-Based Amorphous Coatings. Coatings 2020, 10, 73. [Google Scholar] [CrossRef]
- Dudkina, N.G. Corrosion Resistance of Steel 45 Substrate to Electromechanical Treatment and Surface Plastic Deformation. Met. Sci. Heat Treat. 2018, 59, 584–587. [Google Scholar] [CrossRef]
- Ding, H.Q.; Jiang, S.Y.; Xu, J. Effect of chemical heat treatment on cavitation erosion resistance of stainless steel. Proc. Inst. Mech. Eng. Part J 2019, 233, 1753–1762. [Google Scholar] [CrossRef]
- Li, Y.L.; Wu, Q.; Liu, C.S. Effects of chemical composition and heat treatment on wear properties of backup rolls steel. Mater. Express. 2019, 9, 764–772. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, N.N.; Zhang, B.C.; Sun, F.; Bolot, R.; Planche, M.P.; Liao, H.L.; Coddet, C. Very low pressure plasma sprayed alumina and yttria-stabilized zirconia thin dense coatings using a modified transferred arc plasma torch. Appl. Surf. Sci. 2011, 258, 1422–1428. [Google Scholar] [CrossRef]
- Yu, Z.Y.; Zheng, Y.; Chen, J.M.; Wu, C.F.; Xu, J.J.; Lu, H.; Yu, C. Effect of laser remelting processing on microstructure and mechanical properties of 17-4 PH stainless steel during laser direct metal deposition. J. Mater. Process. Technol. 2020, 284, 116738. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, D.H.; Wu, G.L.; Chen, K.Y.; Wu, H.; Zhang, Q.L.; Yao, J.H. Effect of laser surface remelting pretreatment with different energy density on MAO bioceramic coating. Surf. Coat. Technol. 2020, 393, 125815. [Google Scholar] [CrossRef]
- Bello, M.; Shanmugan, S. Achievements in mid and high -temperature selective absorber coatings by physical vapor deposition (PVD) for solar thermal Application -A review. J. Alloys Compd. 2020, 839, 15510. [Google Scholar] [CrossRef]
- Bobzin, K.; Brogelmann, T.; Kalscheuer, C.; Liang, T. Post-annealing of (Ti,Al,Si)N coatings deposited by high speed physical vapor deposition (HS-PVD). Surf. Coat. Technol. 2019, 375, 752–762. [Google Scholar] [CrossRef]
- Wei, X.D.; Chen, W.F.; Liu, N.; Fan, H.R. Dendritic FeNi3 phase grown on copper foil by electrodeposition as electrocatalyst for efficient oxygen evolution reaction. J. Alloys Compd. 2020, 830, 154708. [Google Scholar] [CrossRef]
- Arai, S.; Murakami, I.; Shimizu, M.; Oshigane, A. Fabrication of CNT/Cu Composite Yarn via Single-Step Electrodeposition. J. Electrochem. Soc. 2020, 167, 102509. [Google Scholar] [CrossRef]
- Malyshev, V.N.; Volkhin, A.M. Antifriction properties increasing of ceramic MAO-coatings. Proc. Inst. Mech. Eng. Part J 2014, 228, 435–444. [Google Scholar] [CrossRef]
- Panchuk, D.A.; Sadakbaeva, Z.K.; Bagrov, D.V.; Bol’shakova, A.V.; Yarysheva, L.M.; Meshkov, I.B.; Muzafarov, A.M.; Volynskii, A.L.; Bakeev, N.F. Estimating the Strain-Strength Characteristics of Nanothick Nonmetallic Coatings Deposited onto Poly (ethylene terephthalate) Films. Polym. Sci. Ser. A 2011, 53, 303–310. [Google Scholar] [CrossRef]
- Boda, M.A.; Cirak, B.B.; Demir, Z.; Cirak, C. Facile synthesis of hybrid ZnO nanostructures by combined electrodeposition and chemical bath deposition for improved performance of dye-sensitized solar cell. Mater. Lett. 2019, 248, 143–145. [Google Scholar] [CrossRef]
- Zhang, F.; Yao, Z.J.; Moliar, O.; Tao, X.W.; Yang, C. Nanocrystalline Ni coating prepared by a novel electrodeposition. J. Alloys Compd. 2020, 830, 153785. [Google Scholar] [CrossRef]
- Xu, Y.K.; Fan, M.Y.; Luo, Y.Q.; Chen, Y.N.; Hao, J.M.; Hou, X.H. Tribology and corrosion properties investigation of a pulse electrodeposition duplex hard-particle-reinforced Ni-Mo nanocomposite coating. Surf. Coat. Technol. 2020, 393, 125797. [Google Scholar] [CrossRef]
- Wang, G.F.; Shen, L.D.; Dou, L.M.; Tian, Z.J.; Liu, Z.D. Porous Metal and Method for Preparation by the Scanning and Swinging Jet Electrodeposition. Int. J. Electrochem. Sci. 2014, 9, 3319–3326. [Google Scholar]
- Wang, Z.W.; Shen, L.D.; Jiang, W.; Fan, M.Z.; Liu, D.C.; Zhao, J.F. Superhydrophobic nickel coatings fabricated by scanning electrodeposition on stainless steel formed by selective laser melting. Surf. Coat. Technol. 2019, 377, 124886. [Google Scholar] [CrossRef]
- Wang, Z.W.; Shen, L.D.; Qiu, M.B.; Jiang, W.; Chen, Y.; Zhao, J.F. Study on the properties of superhydrophobic coating prepared by scanning electrodeposition on SLM substrate. Mater. Res. Express. 2019, 6, 106409. [Google Scholar] [CrossRef]
- Shen, L.D.; Xu, M.Y.; Jiang, W.; Qiu, M.B.; Fan, M.Z.; Ji, G.B.; Tian, Z.J. A novel superhydrophobic Ni/Nip coating fabricated by magnetic field induced selective scanning electrodeposition. Appl. Surf. Sci. 2019, 489, 25–33. [Google Scholar] [CrossRef]
- Li, Y.F.; Zhang, K.; Zhang, M.M.; Zhang, Y.Q.; Wu, T.T.; Zhao, H.Y.; Su, J.X.; Wang, Z.K.; Wang, J.F. Preparation of Sol-Enhanced Ni-P-Al2O3 Nanocomposite Coating by Electrodeposition. J. Nanomater. 2020, 2020, 5239474. [Google Scholar] [CrossRef]
- Safavi, M.S.; Rasooli, A. Ni-P-TiO2 nanocomposite coatings with uniformly dispersed Ni3Ti intermetallics: Effects of TiO2 nanoparticles concentration. Surf. Eng. 2019, 35, 1070–1080. [Google Scholar] [CrossRef]
- Du, Y.C.; Zhang, X.M.; Wei, L.Q.; Yu, B.; Ma, D.Q.; Ye, S.F. Electrodeposition of a Ni-P-TiO2/Ti3C2Tx Coating with In Situ Grown Nanoparticles TiO2 on Ti3C2Tx Sheets. Coatings 2019, 9, 750. [Google Scholar] [CrossRef]
- Cheon, H.U.; Lee, J.H. Effects of Bath Composition and P Contents on the Mechanical Properties of Ni-P Electroplating. Sci. Adv. Mater. 2020, 12, 605–608. [Google Scholar] [CrossRef]
- Jiang, W.; Shen, L.D.; Xu, M.Y.; Wang, Z.W.; Tian, Z.J. Mechanical properties and corrosion resistance of Ni-Co-SiC composite coatings by magnetic field-induced jet electrodeposition. J. Alloys Compd. 2019, 791, 847–855. [Google Scholar] [CrossRef]
- Ahmadkhaniha, D.; Zanella, C. The Effects of Additives, Particles Load and Current Density on Codeposition of SiC Particles in NiP Nanocomposite Coatings. Coatings 2019, 9, 554. [Google Scholar] [CrossRef]
- Jiang, W.; Shen, L.D.; Wang, K.; Wang, Z.W.; Tian, Z.J. Wear resistance of Ni-Co/SiC composite coating by jet electrodeposition in the presence of magnetic field. Proc. Inst. Mech. Eng. Part B 2019, 243, 431–438. [Google Scholar] [CrossRef]
- Kazimierczak, H.; Szymkiewicz, K.; Bobrowski, P.; Swiatek, Z.; Rogal, L.; Gileadi, E.; Eliaz, N. The Effect of SiC Nanoparticle Size on the Electrodeposition of Zn-SiC Nanocomposite Coatings from Citrate Bath. J. Electrochem. Soc. 2018, 165, D774–D782. [Google Scholar] [CrossRef]
- Cheng, A.Y.; Sheu, H.H.; Liu, Y.M.; Hou, K.H.; Hsieh, P.Y.; Ger, M.D. Effect of Pretreatment Process on the Adhesion and Corrosion Resistance of Nickel-Boron Coatings Deposited on 8620H Alloy Steel. Int. J. Electrochem. Sci. 2020, 15, 61–89. [Google Scholar] [CrossRef]
- Zhang, P.J.; Xu, G.Q.; Liu, J.Q.; Yi, X.F.; Wu, Y.C.; Chen, J.W. Effect of pretreating technologies on the adhesive strength and anticorrosion property of Zn coated NdFeB specimens. Appl. Surf. Sci. 2016, 363, 499–506. [Google Scholar] [CrossRef]
- Sheu, H.H.; Chen, Y.R.; Lin, T.T.; Ger, M.D. Effect of Pretreatment Process on the Adhesion and Corrosion Resistance of Chromium-Carbon Coatings Deposited on Copper Substrates. Int. J. Electrochem. Sci. 2019, 14, 3281–3290. [Google Scholar] [CrossRef]
- Shin, J.; Kim, T.; Lim, K. Effects of steel type and sandblasting pretreatment on the solid-liquid compound casting characteristics of zinc-coated steel/aluminum bimetals. J. Alloys Compd. 2019, 778, 170–185. [Google Scholar] [CrossRef]



| Composition | C | Si | Mn | Cr | Ni | Cu |
|---|---|---|---|---|---|---|
| Content (%) | 0.42–0.50 | 0.17–0.37 | 0.50–0.80 | ≤0.25 | ≤0.30 | ≤0.25 |
| Composition | Content/(g·L−1) |
|---|---|
| NiSO4·6H2O | 200 |
| NiCl2·6H2O | 30 |
| H3PO3 | 20 |
| H3BO3 | 30 |
| C6H8O7 | 60 |
| C12H25SO4Na | 0.08 |
| CH4N2S | 1 |
| SiC (silicon carbide) | 3 |
| Specimens | Polishing | Sandblasting |
|---|---|---|
| Sa (μm) | 0.006 ± 0.0011 | 0.804 ± 0.028 |
| Sq (μm) | 0.008 ± 0.0007 | 1.055 ± 0.066 |
| Samples | Ecorr (V) (CV) | icorr (A·cm−2) (CV) | Corrosion Rate (mm·a−1) (CV) |
|---|---|---|---|
| P | −1.1365 (0.20%) | 4.67 × 10−4 (4.13%) | 5.89154 (2.12%) |
| S | −0.9134 (0.31%) | 2.15 × 10−5 (4.98%) | 0.18865 (4.19%) |
| Ni–P(P) | −0.5420 (0.22%) | 1.67 × 10−5 (3.21%) | 0.20156 (5.73%) |
| Ni–P(S) | −0.4093 (0.60%) | 6.06 × 10−6 (5.47%) | 0.073395 (2.21%) |
| Ni–P–SiC(P) | −0.3718 (0.55%) | 5.88 × 10−6 (2.38%) | 0.071158 (3.80%) |
| Ni–P–SiC(S) | −0.3000 (0.45%) | 8.45 × 10−7 (2.59%) | 0.041128 (2.91%) |
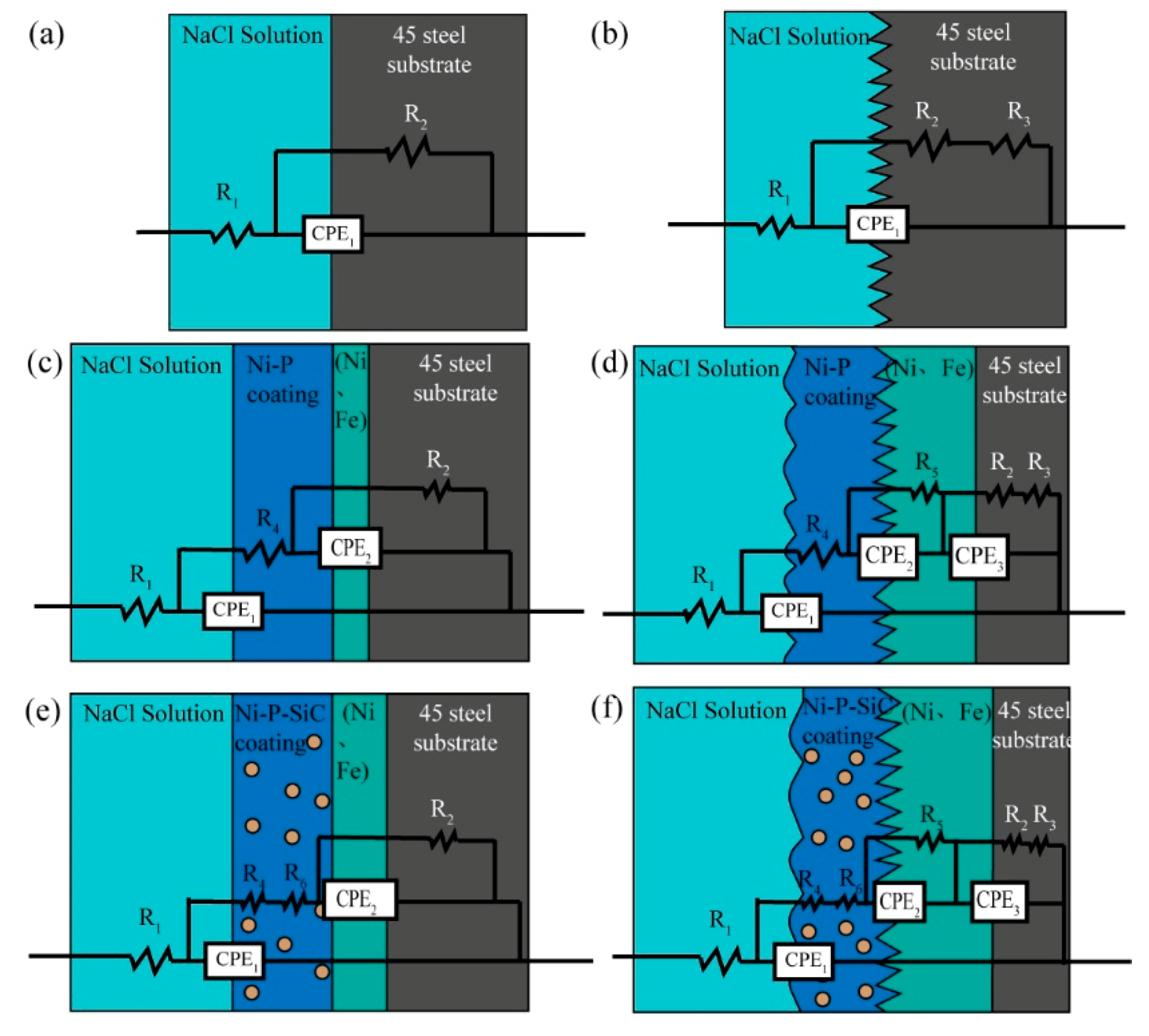
| R1/Ω | R2/Ω | R3/Ω | R4/Ω | R5/Ω | R6/Ω | CPE1/F | CPE2/F | CPE3/F | |
|---|---|---|---|---|---|---|---|---|---|
| P | 12.85 | 331.6 | 3.60 × 10−4 | ||||||
| S | 35.13 | 518 | 1.31 × 104 | 2.85 × 10−4 | |||||
| Ni–P(P) | 3.18 | 708.7 | 46.57 | 1.62 × 10−5 | 3.89 × 10−5 | ||||
| Ni–P(S) | 5.29 | 963.4 | 1.86 × 106 | 32.41 | 221.9 | 1.19 × 10−6 | 1.31 × 10−5 | 2.89 × 10−6 | |
| Ni–P–SiC(P) | 3.82 | 1501 | 93.24 | 0.01097 | 7.20 × 10−6 | 2.09 × 10−5 | |||
| Ni–P–SiC(S) | 1.52 | 3108 | 4.73 × 108 | 56.1 | 516.4 | 0.4105 | 1.00 × 10−6 | 2.35 × 10−6 | 1.84 × 10−5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, X.; Shen, Z.; Chen, X.; Lin, J.; Cao, H. Influence of Element Penetration Region on Adhesion and Corrosion Performance of Ni-Base Coatings. Coatings 2020, 10, 895. https://doi.org/10.3390/coatings10090895
Fu X, Shen Z, Chen X, Lin J, Cao H. Influence of Element Penetration Region on Adhesion and Corrosion Performance of Ni-Base Coatings. Coatings. 2020; 10(9):895. https://doi.org/10.3390/coatings10090895
Chicago/Turabian StyleFu, Xiuqing, Zhenyu Shen, Xinxin Chen, Jinran Lin, and Hongbing Cao. 2020. "Influence of Element Penetration Region on Adhesion and Corrosion Performance of Ni-Base Coatings" Coatings 10, no. 9: 895. https://doi.org/10.3390/coatings10090895
APA StyleFu, X., Shen, Z., Chen, X., Lin, J., & Cao, H. (2020). Influence of Element Penetration Region on Adhesion and Corrosion Performance of Ni-Base Coatings. Coatings, 10(9), 895. https://doi.org/10.3390/coatings10090895





