Deposition of Titanium Dioxide Coating by the Cold-Spray Process on Annealed Stainless Steel Substrate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Process
2.2. Materials
2.3. Characterization
2.3.1. Tensile-Strength Testing
2.3.2. Coatings Evaluation
2.3.3. Micro-Vickers Hardness
2.3.4. Substrate Oxide Evaluations
2.3.5. Wipe Test
2.3.6. TEM Testing
3. Results
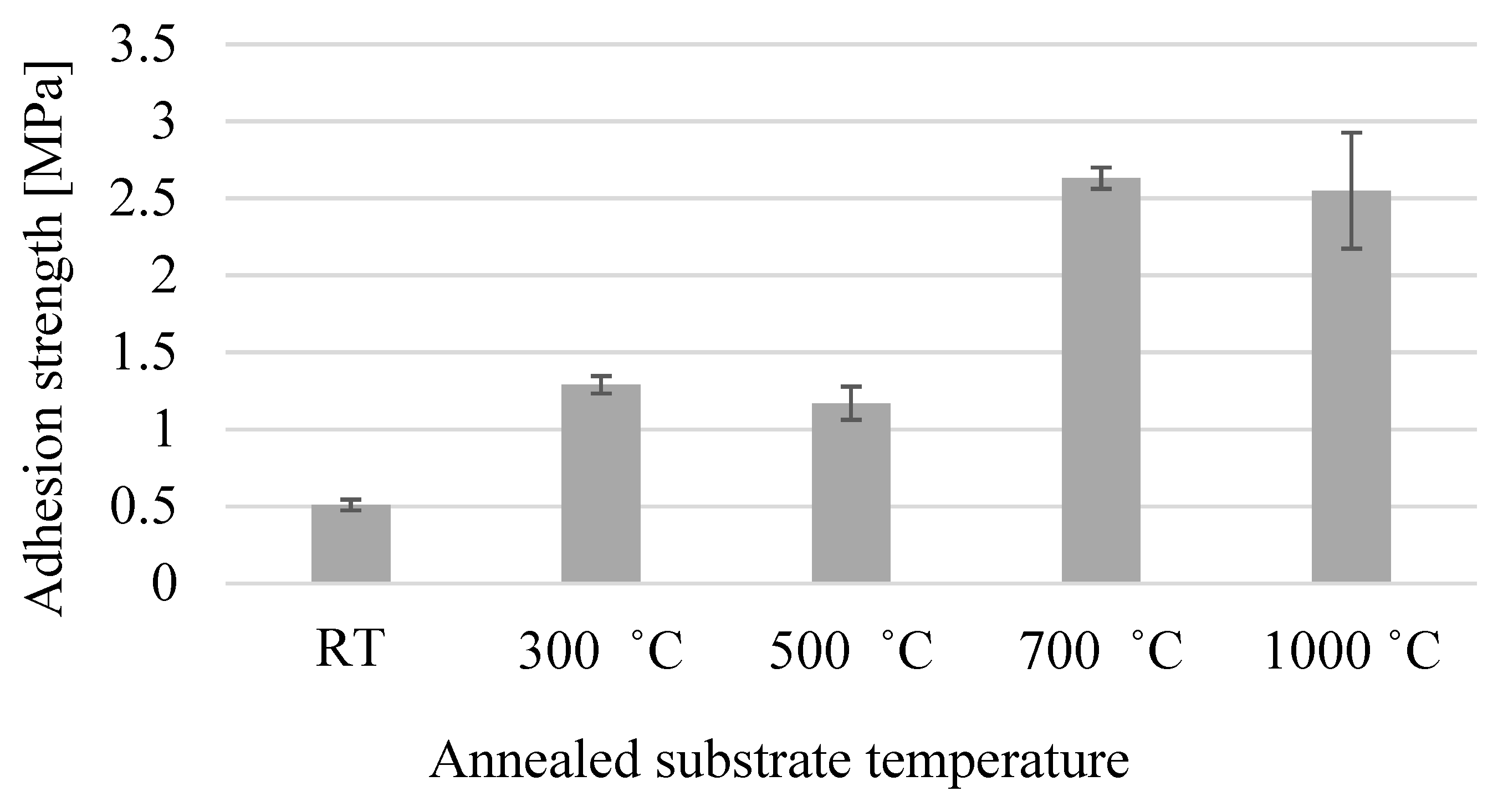
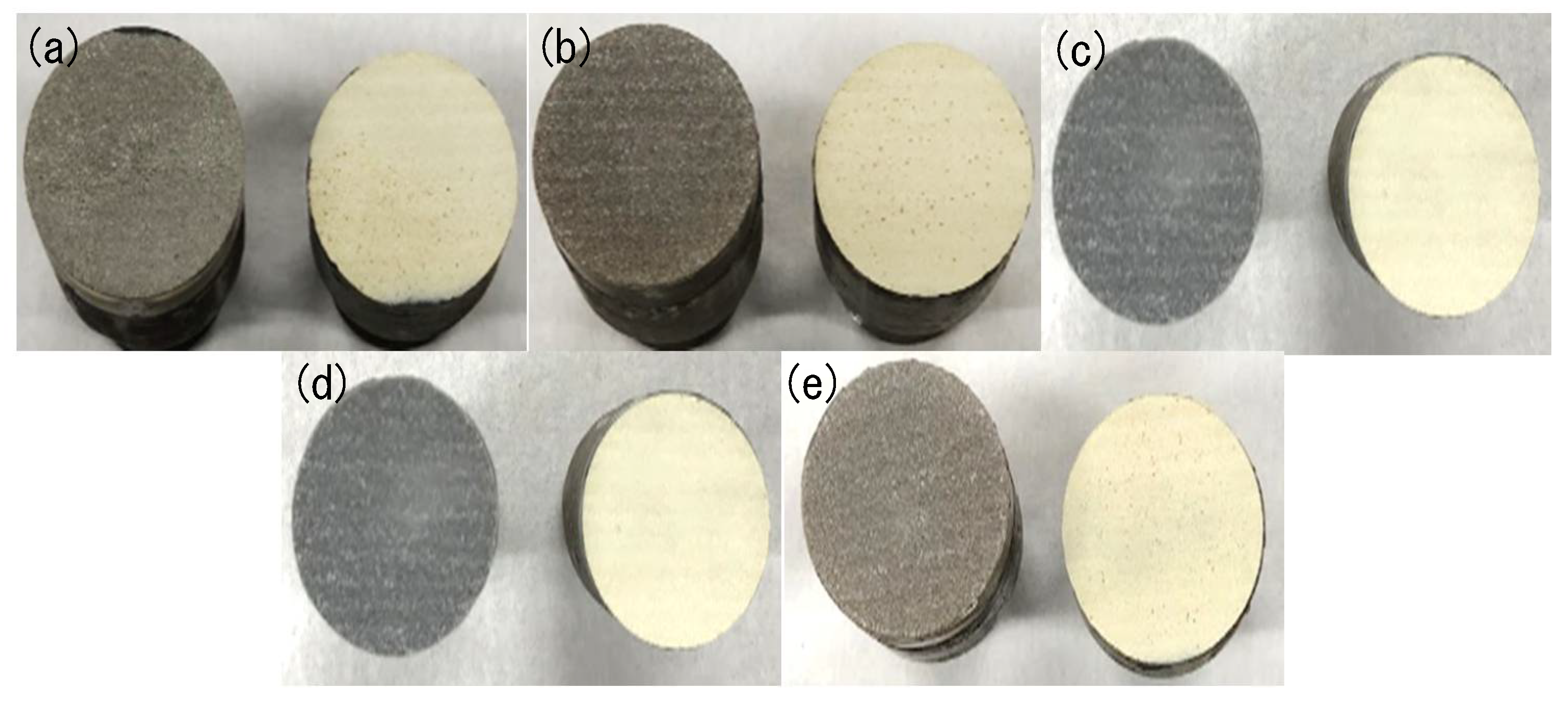
3.1. Strength of Adhesion
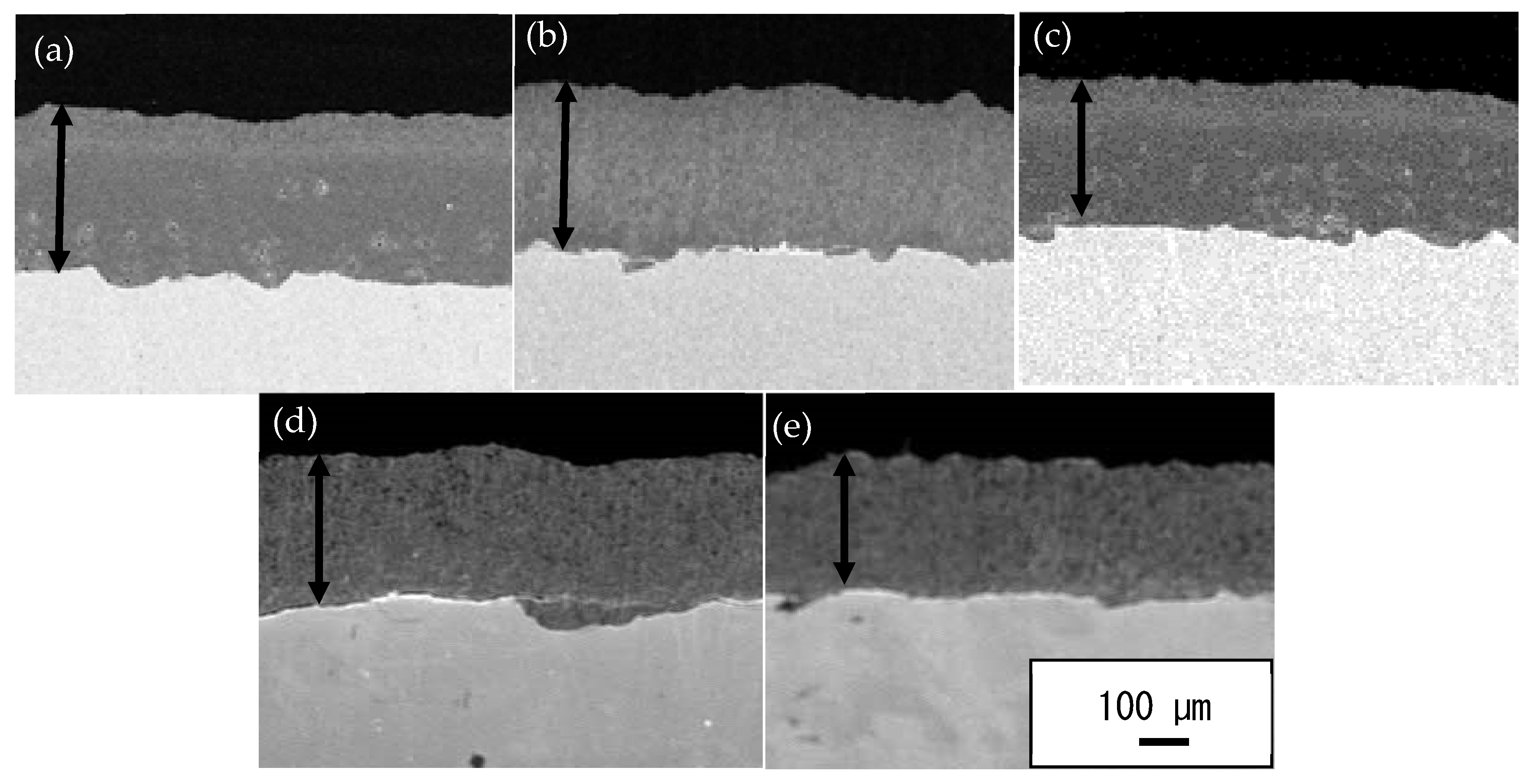
3.2. SEM Cross-Section Microstructure of TiO2 Coatings on Annealed SUS 304 Substrates
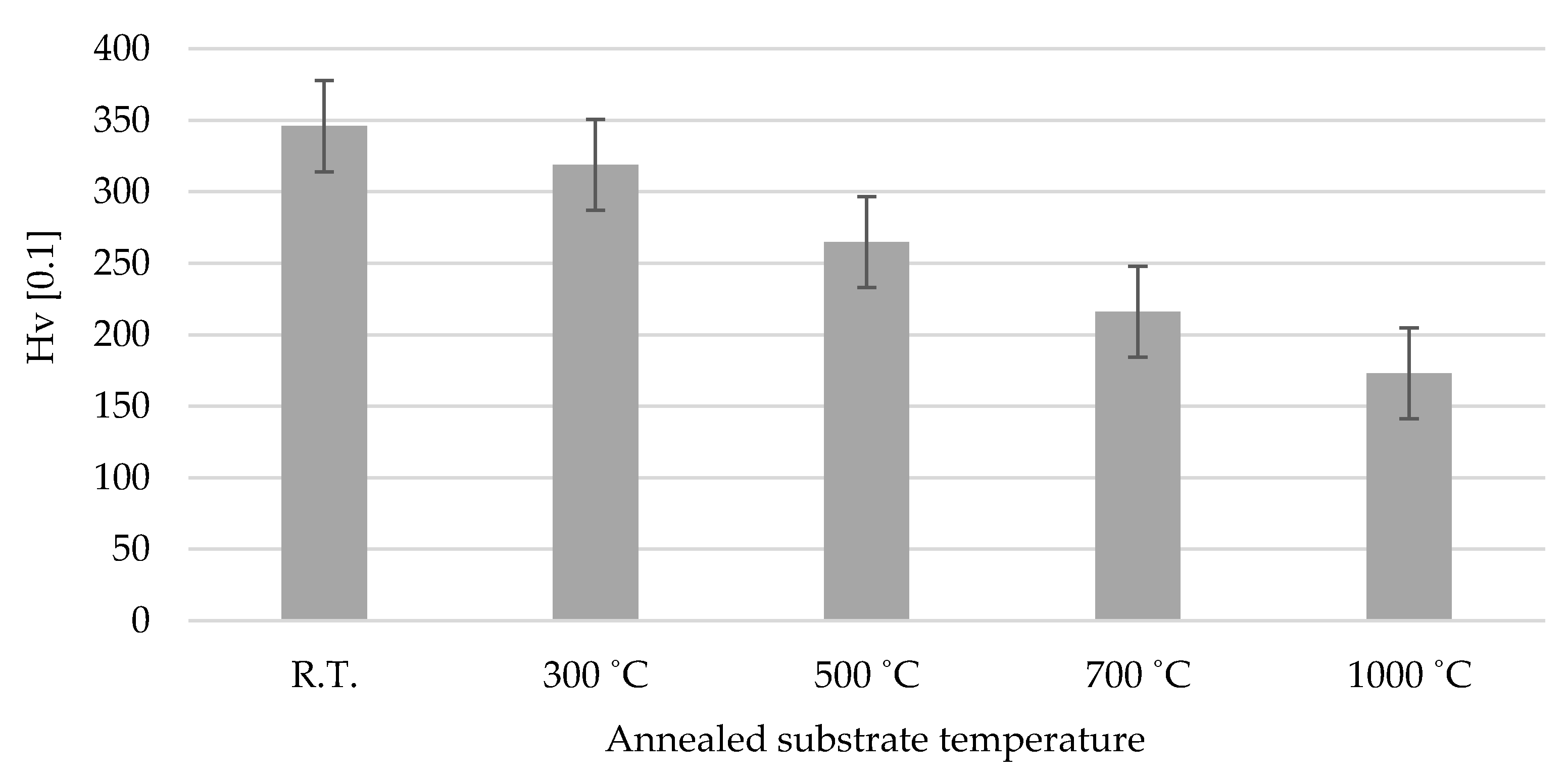
3.3. Substrate Vickers Microhardness
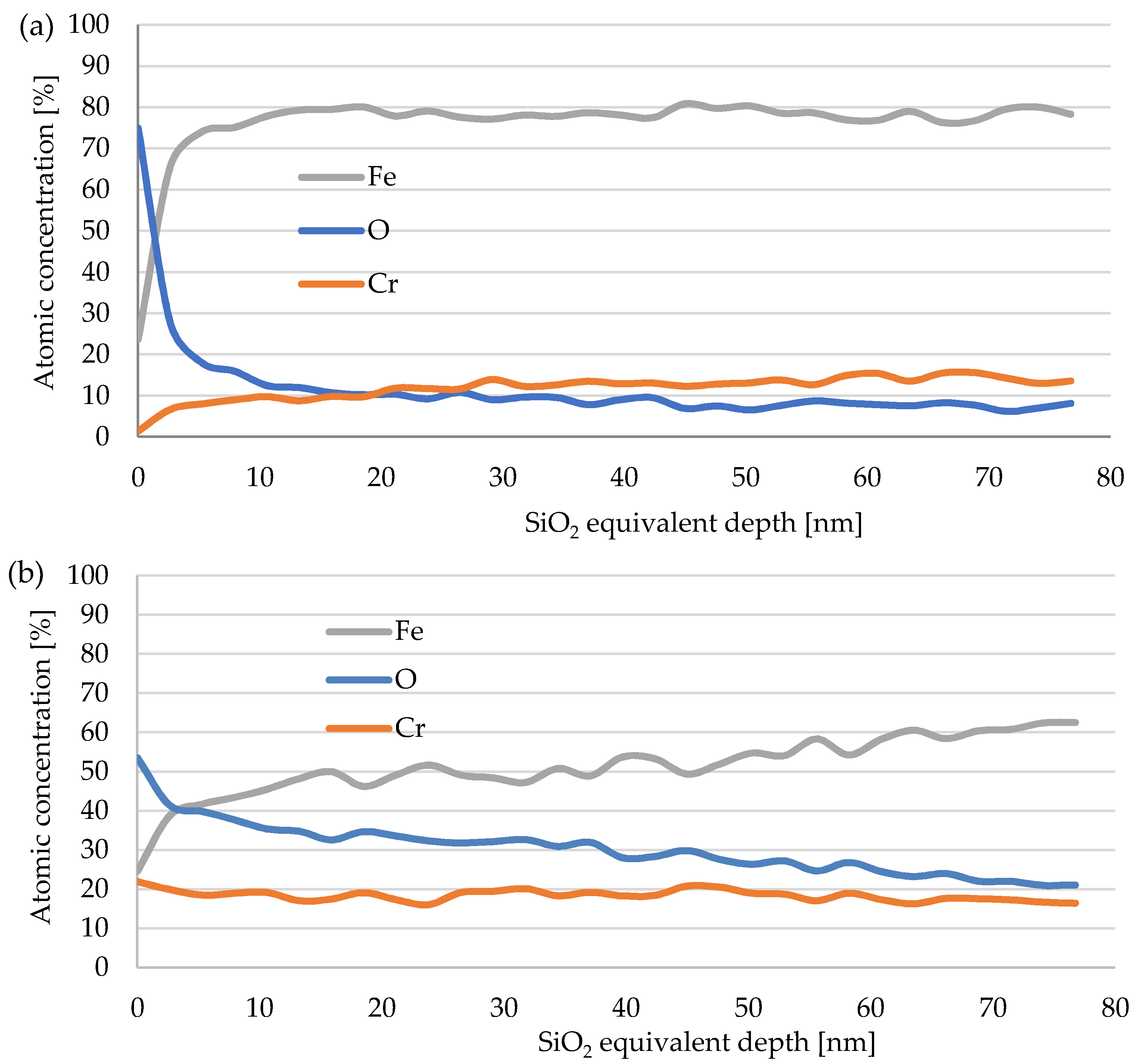
3.4. Depth Profile of the Oxide Layer
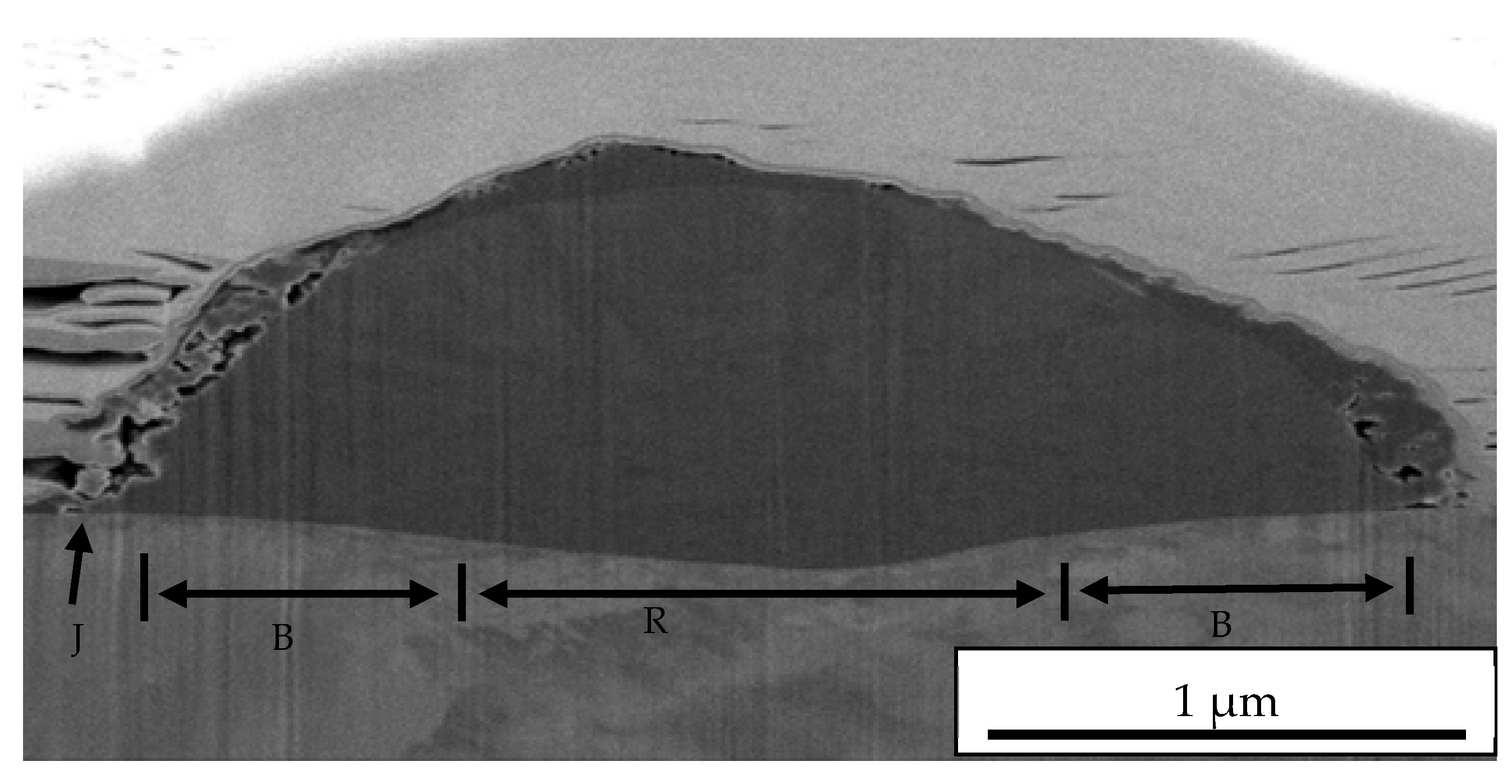
3.5. FIB Splat TiO2 Particle on 1000 °C Annealed Substrates
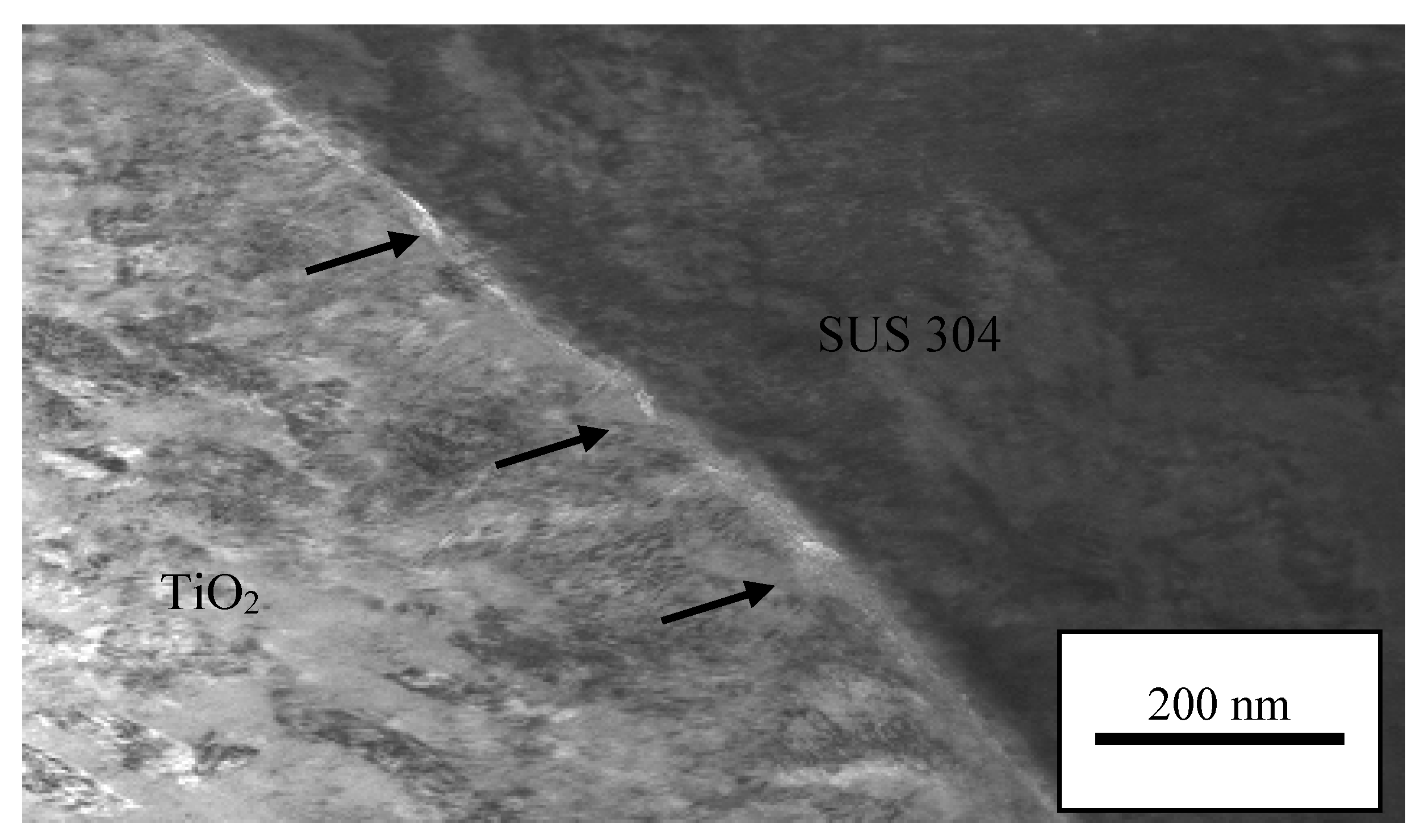
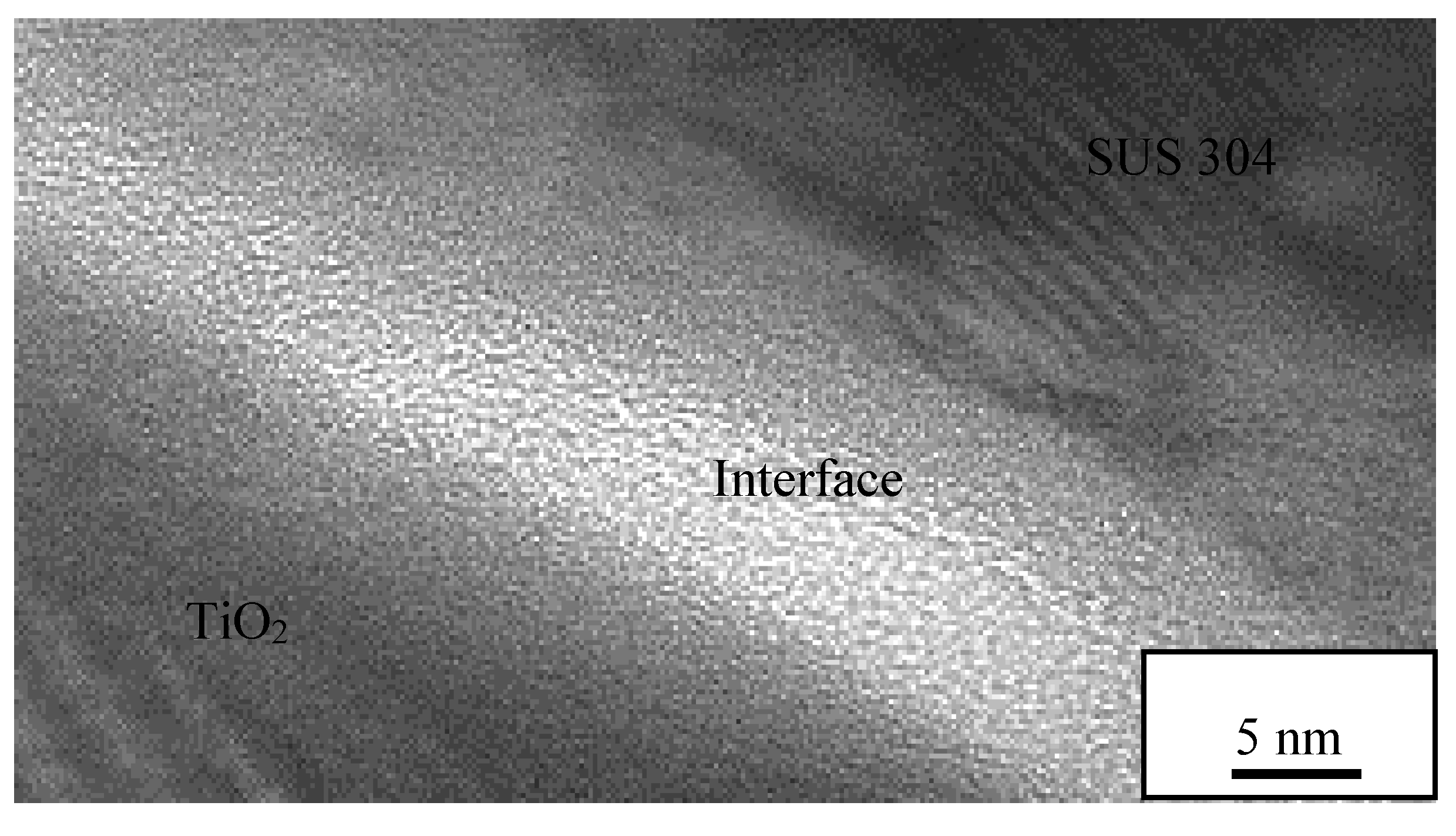
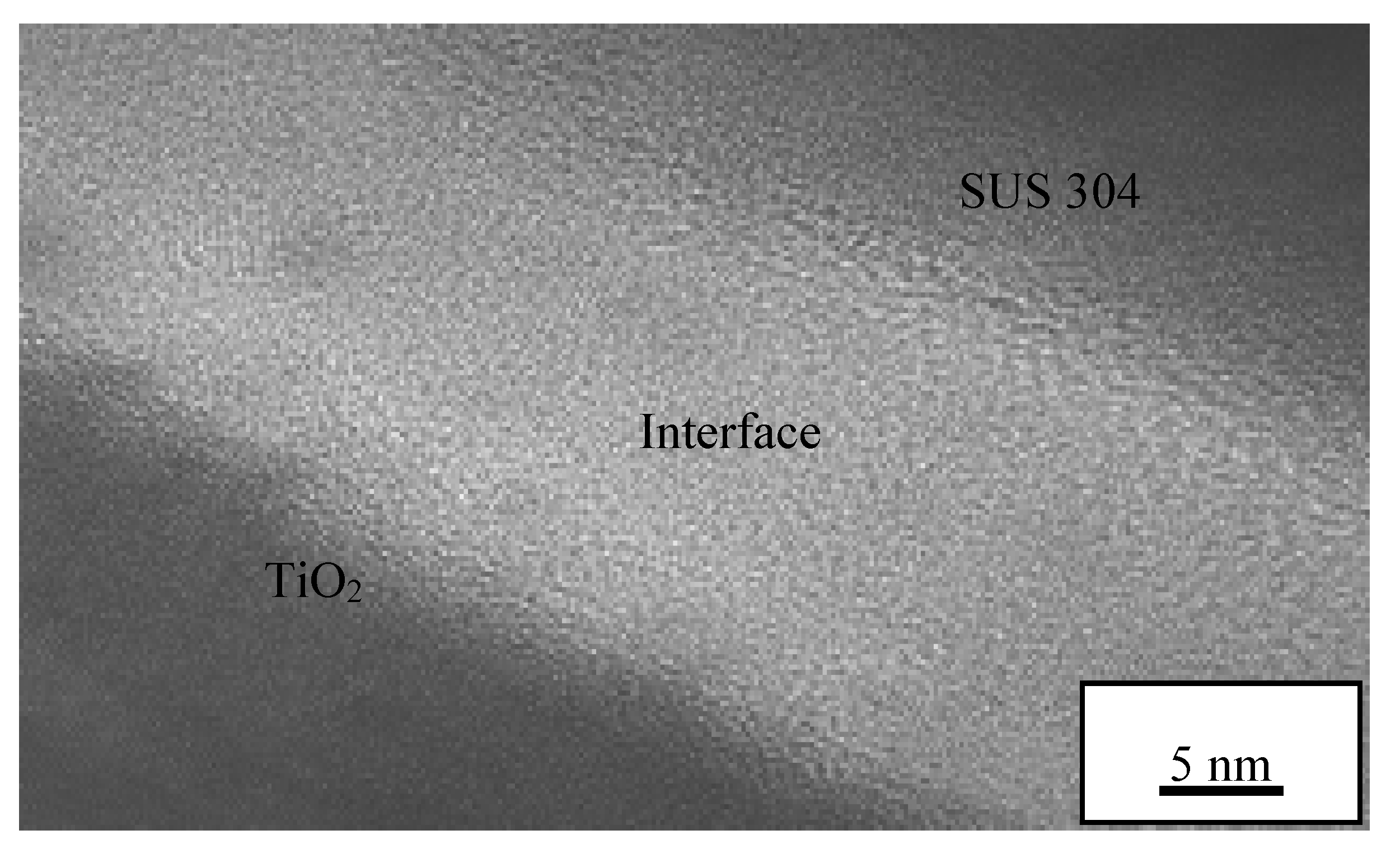
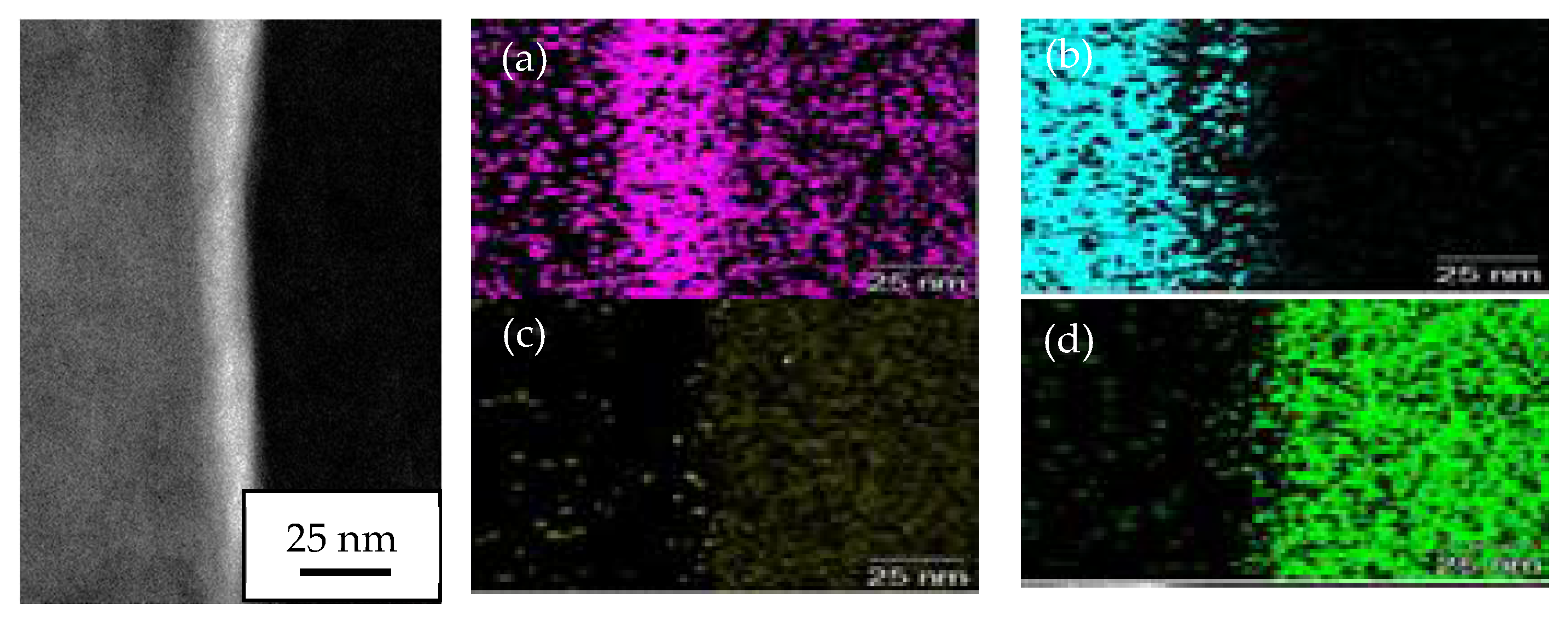
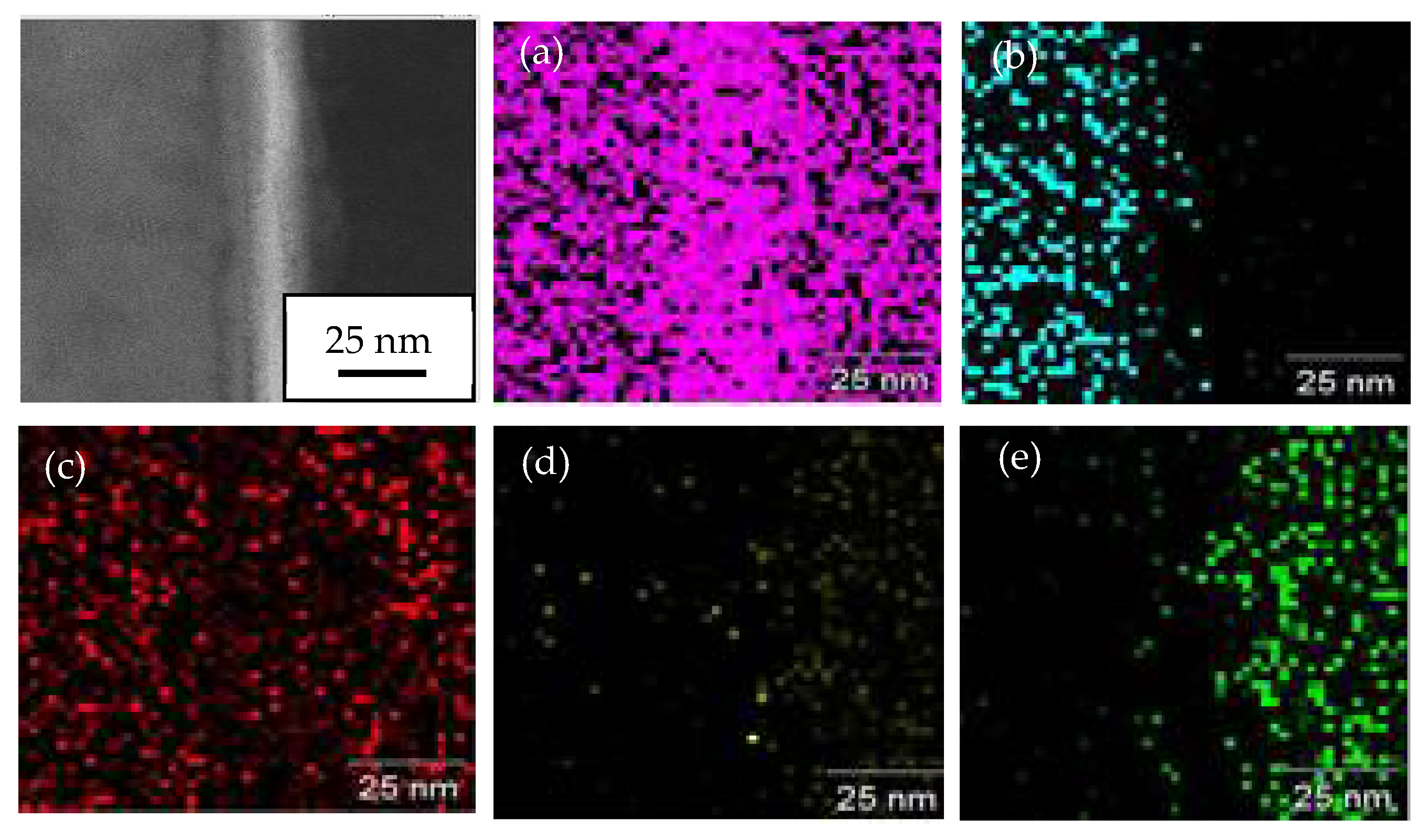
3.6. TEM Analysis on Interface Oxide Layer between TiO2 Particle on 1000 °C Annealed Substrates
4. Conclusions
- The annealing process plays an important role in the induced ductility of the austenitic stainless steel, SUS 304 especially when annealed at a high temperature such as 1000 °C. This will lead to a decrease in the hardness of the substrate and will make it softer. When the cold-sprayed TiO2 particle is impacted with a high velocity on the annealed 1000 °C SUS 304 surface, the plastic deformation of the substrate occurs and provides a large continuous contact zone between the particles and the substrate, resulting in bonding. Therefore, the adhesion strength of the TiO2 coating is high on the annealed 1000 °C SUS 304 substrate.
- The oxide layer of austenitic stainless steel, SUS 304 grows thicker as the annealed temperature of the substrate increases. The TEM/EDX result shows that the existence of the remaining interface of the amorphous layer is approximately 10 nm for the rebound region, R and 15 nm for the bonded region, B between the TiO2 particles and 1000 °C annealed SUS 304. Due to the restructuring of the interfacial layer upon a high-velocity particle impact, the adhesion between the brittle TiO2 particle and the stainless steel SUS 304 metal was attributed to a high-velocity collision and resulted in limited amorphization and atomic intermixing. Atomic intermixing of Ti/Fe/Cr at the interface may produce a strong adhesive bond between them due to chemical adhesion forces.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Champagne, V.K. The Cold Spray Materials Deposition Process Fundamentals and Applications, 1st ed.; Victor, K.C., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2007; Introduction; pp. 1–2. [Google Scholar]
- Ko, K.; Choi, J.; Lee, H. Intermixing and interfacial morphology of cold-sprayed Al coatings on steel. Mater. Lett. 2014, 136, 45–47. [Google Scholar] [CrossRef]
- Xie, Y.; Planche, M.-P.; Raoelison, R.; Liao, H.; Suo, X.; Hervé, P. Effect of Substrate preheating on adhesive strength of SS 316L cold spray coatings. J. Therm. Spray Technol. 2015, 25, 123–130. [Google Scholar] [CrossRef]
- Yamada, M.; Isago, H.; Nakano, H.; Fukumoto, M. Cold spraying of TiO2 photocatalyst coating with nitrogen process gas. J. Therm. Spray Technol. 2010, 19, 1218–1223. [Google Scholar] [CrossRef]
- Winnicki, M.; Baszczuk, A.; Jasiorski, M.; Borak, B.; Małachowska, A. Preliminary studies of TiO2 nanopowder deposition onto metallic substrate by low pressure cold spraying. Surf. Coatings Technol. 2019, 371, 194–202. [Google Scholar] [CrossRef]
- Winnicki, M.; Jasiorski, M.; Baszczuk, A.; Borak, B. Preliminary studies of TiO2 coatings by deposition onto ABS polymer substrates by low pressure cold spraying. In Proceedings of the Les Rencontres Internationales sur la Projection Thermique—RIPT 9, Jülich, Germany, 11–13 December 2019. [Google Scholar]
- Hajipour, H.; Abdollah-Zadeh, A.; Assadi, H.; Taheri-Nassaj, E.; Jahed, H. Effect of feedstock powder morphology on cold-sprayed titanium dioxide coatings. J. Therm. Spray Technol. 2018, 27, 1542–1550. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, K.; Buhl, S.; Davoudi, N.; Godard, C.; Merz, R.; Raid, I.; Kerscher, E.; Kopnarski, M.; Renno, C.M.; Ripperger, S.; et al. Ti surface modification by cold spraying with TiO2 mircoparticles. Surf. Coat. Technol. 2017, 309, 749–758. [Google Scholar] [CrossRef]
- Kliemann, J.-O.; Gutzmann, H.; Gärtner, F.; Hübner, H.; Borchers, C.; Klassen, T. Formation of cold-sprayed ceramic titanium dioxide layers on metal surfaces. J. Therm. Spray Technol. 2010, 20, 292–298. [Google Scholar] [CrossRef] [Green Version]
- Gutzmann, H.; Freese, S.; Gartner, F.; Klassen, T. Cold gas spraying of ceramics using the example of titanium dioxide. In Proceedings of the nternational Thermal Spray Conference, ITSC, Hamburg, Germany, 27–29 September 2011; pp. 391–396. [Google Scholar]
- Gardon, M.; Fernández-Rodíguez, C.; Garzón Sousa, D.; Doña-RodRíguez, J.M.; Dosta, S.; Cano, I.G.; Guilemany, J.M. Photocatalytic activity of nanostructured anatase coatings obtained by cold gas spray. J. Therm. Spray Technol. 2014, 23, 1135–1140. [Google Scholar] [CrossRef] [Green Version]
- Salim, N.T.; Yamada, M.; Nakano, H.; Shima, K.; Isago, H.; Fukumoto, M. The effect of post-treatments on the powder morphology of titanium dioxide (TiO2) powders synthesized for cold spray. Surf. Coatings Technol. 2011, 206, 366–371. [Google Scholar] [CrossRef]
- Clayton, C.R. Materials science and engineering: An introduction. Mater. Sci. Eng. 1987, 94, 266–267. [Google Scholar] [CrossRef]
- Trompetter, B.; Hyland, M.; McGrouther, D.; Munroe, P.; Markwitz, A. Effect of substrate hardness on splat morphology in high-velocity thermal spray coatings. J. Therm. Spray Technol. 2006, 15, 663–669. [Google Scholar] [CrossRef]
- Yin, S.; Wang, X.; Suo, X.; Liao, H.; Guo, Z.; Li, W.; Coddet, C. Deposition behavior of thermally softened copper particles in cold spraying. Acta Mater. 2013, 61, 5105–5118. [Google Scholar] [CrossRef]
- Assadi, H.; Gärtner, F.; Stoltenhoff, T.; Kreye, H. Bonding mechanism in cold gas spraying. Acta Mater. 2003, 51, 4379–4394. [Google Scholar] [CrossRef]
- Grujicic, M.; Zhao, C.I.; De Rosset, W.S.; Helfritch, D. Adabatic shear instability based mechanism for particle/substrate bonding in the cold-gas dynamic-spray process. Mater. Des. 2004, 25, 681–688. [Google Scholar] [CrossRef]
- Bae, G.; Xiong, Y.; Kumar, S.; Kang, K.; Lee, C. General aspects of interface bonding in kinetic sprayed coatings. Acta Mater. 2008, 56, 4858–4868. [Google Scholar] [CrossRef]
- Yin, S.; Wang, X.-F.; Li, W.Y.; Jie, H.-E. Effect of substrate hardness on the deformation behavior of subsequently incident particles in cold spraying. Appl. Surf. Sci. 2011, 257, 7560–7565. [Google Scholar] [CrossRef]
- Kim, K.; Li, W.; Guo, X. Detection of oxygen at the interface and its effect on strain, stress, and temperature at the interface between cold sprayed aluminum and steel substrate. Appl. Surf. Sci. 2015, 357, 1720–1726. [Google Scholar] [CrossRef]
- Kim, K.; Kuroda, S. Amorphous oxide film formed by dynamic oxidation during kinetic spraying of titanium at high temperature and its role in subsequent coating formation. Scr. Mater. 2010, 63, 215–218. [Google Scholar] [CrossRef]
- Ko, K.; Choi, J.; Lee, H. The interfacial restructuring to amorphous: A new adhesion mechanism of cold-sprayed coatings. Mater. Lett. 2016, 175, 13–15. [Google Scholar] [CrossRef]

| Element | Fe | Cr | Ni | Mo | S | P | Mn | Si | C |
|---|---|---|---|---|---|---|---|---|---|
| SUS 304 | Bal | 18 | 11 | – | 0.030 | 0.045 | 2.00 | 1.00 | 0.08 |
| Measured Regions | Fe 2p, O 1s, Cr 2p |
|---|---|
| Measured X-Ray output [W] | 10 |
| Probe diameter[µm] | 50 |
| Time per step [ms] | 30 |
| Pass energy | 140 |
| Cycle | 30 |
| Element | Atomic% |
|---|---|
| O | 96.33 |
| Ti | 2.35 |
| Fe | 0.95 |
| Cr | 0.38 |
| Total | 100 |
| Element | Atomic% |
|---|---|
| O | 91.88 |
| Ti | 4.22 |
| Fe | 2.77 |
| Cr | 0.83 |
| N | 0.30 |
| Total | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omar, N.i.; Selvami, S.; Kaisho, M.; Yamada, M.; Yasui, T.; Fukumoto, M. Deposition of Titanium Dioxide Coating by the Cold-Spray Process on Annealed Stainless Steel Substrate. Coatings 2020, 10, 991. https://doi.org/10.3390/coatings10100991
Omar Ni, Selvami S, Kaisho M, Yamada M, Yasui T, Fukumoto M. Deposition of Titanium Dioxide Coating by the Cold-Spray Process on Annealed Stainless Steel Substrate. Coatings. 2020; 10(10):991. https://doi.org/10.3390/coatings10100991
Chicago/Turabian StyleOmar, Noor irinah, Santirraprahkash Selvami, Makoto Kaisho, Motohiro Yamada, Toshiaki Yasui, and Masahiro Fukumoto. 2020. "Deposition of Titanium Dioxide Coating by the Cold-Spray Process on Annealed Stainless Steel Substrate" Coatings 10, no. 10: 991. https://doi.org/10.3390/coatings10100991
APA StyleOmar, N. i., Selvami, S., Kaisho, M., Yamada, M., Yasui, T., & Fukumoto, M. (2020). Deposition of Titanium Dioxide Coating by the Cold-Spray Process on Annealed Stainless Steel Substrate. Coatings, 10(10), 991. https://doi.org/10.3390/coatings10100991






