Direct and Indirect Evidence of the Microbially Induced Pitting Corrosion of Steel Structures in Humid Environments
Abstract
:1. Introduction
2. Materials and Methods
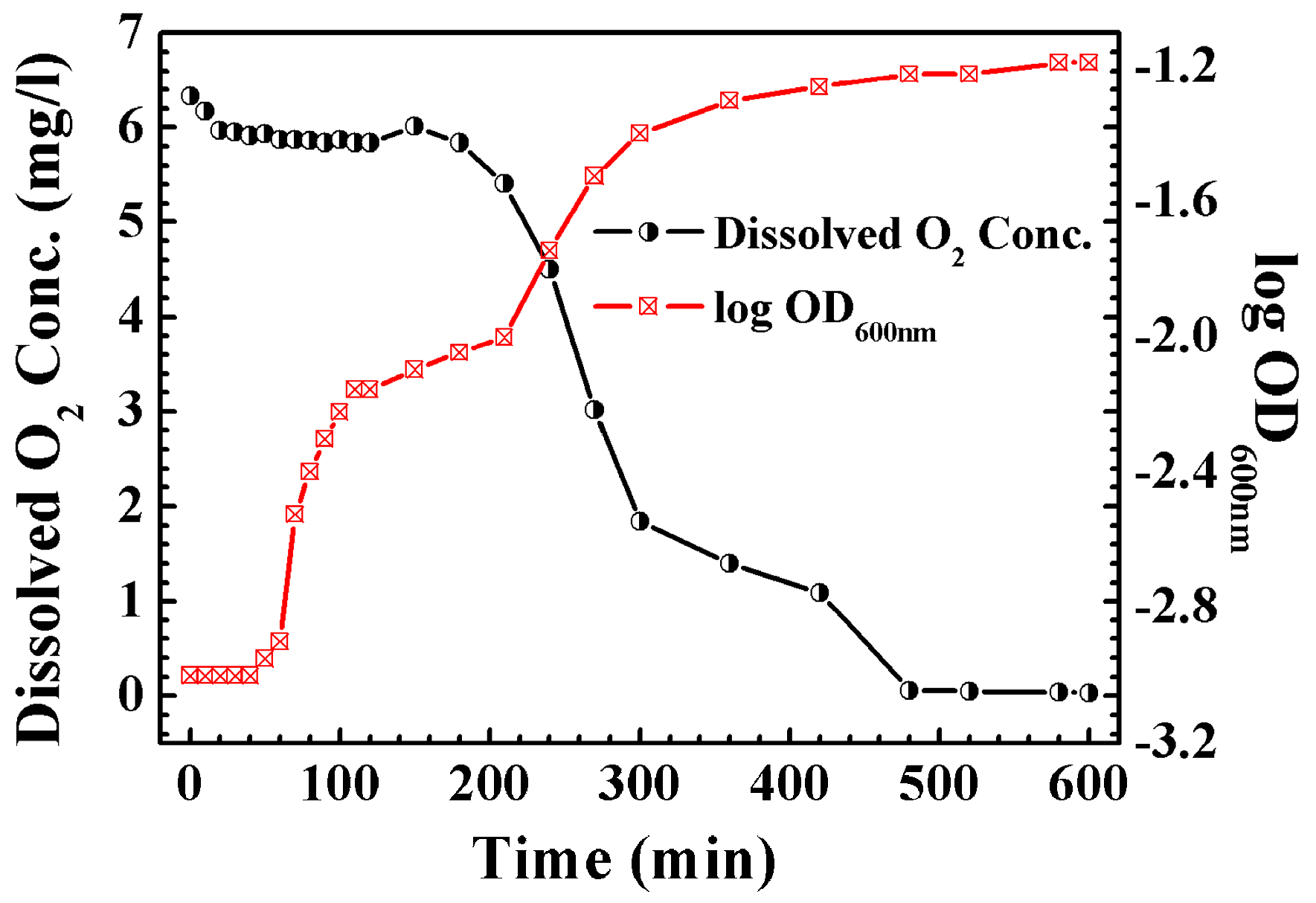
3. Results and Discussion
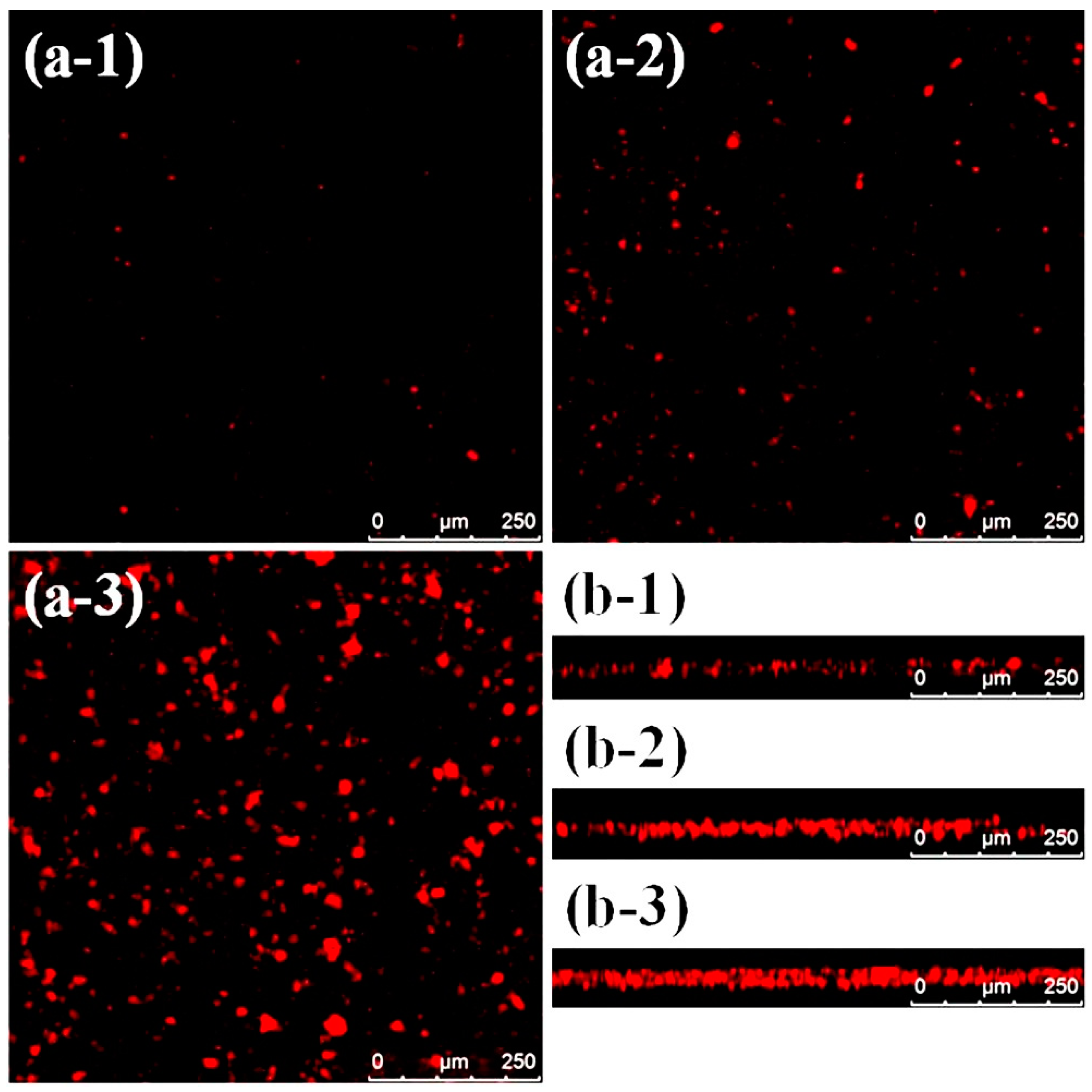
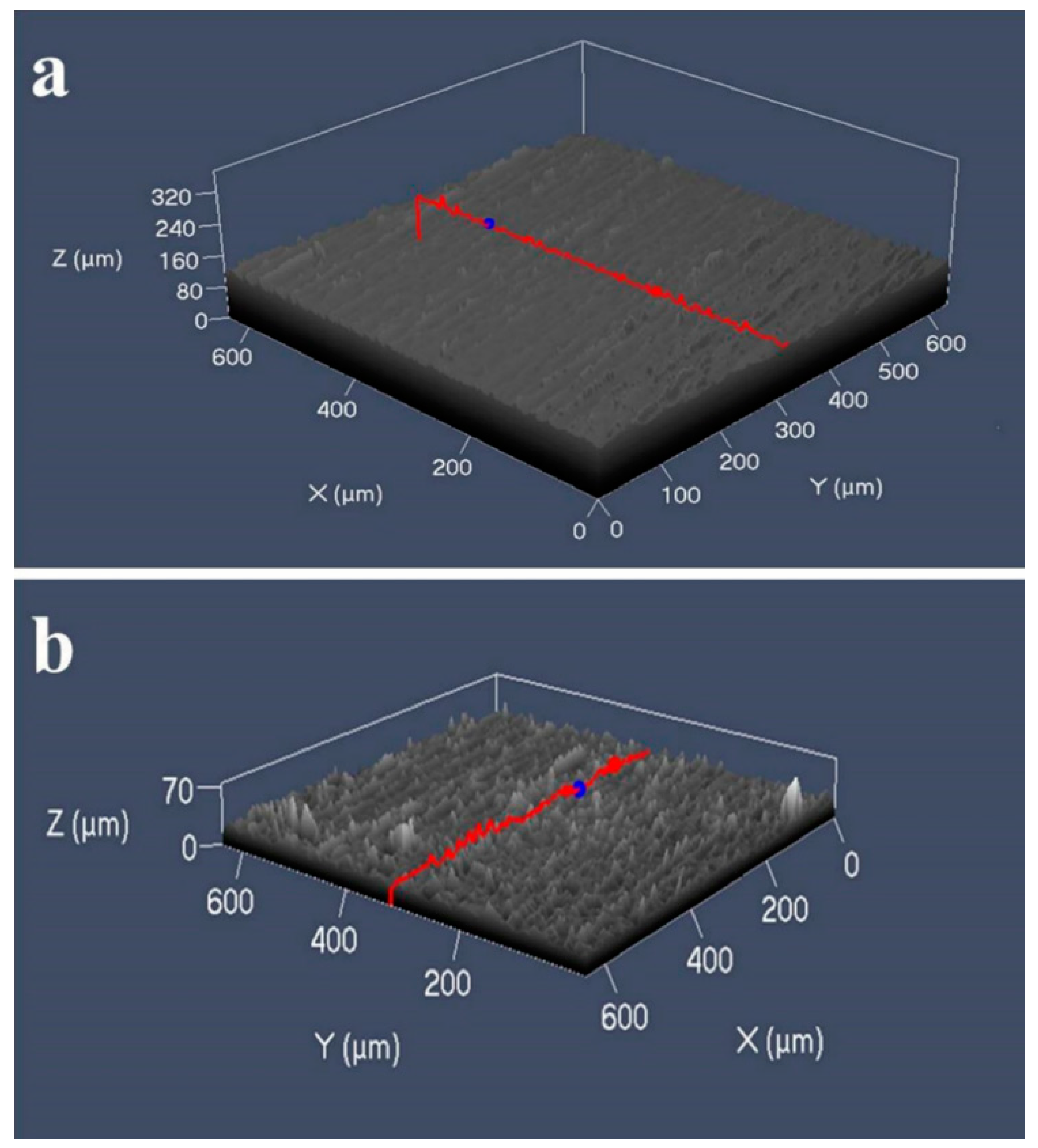
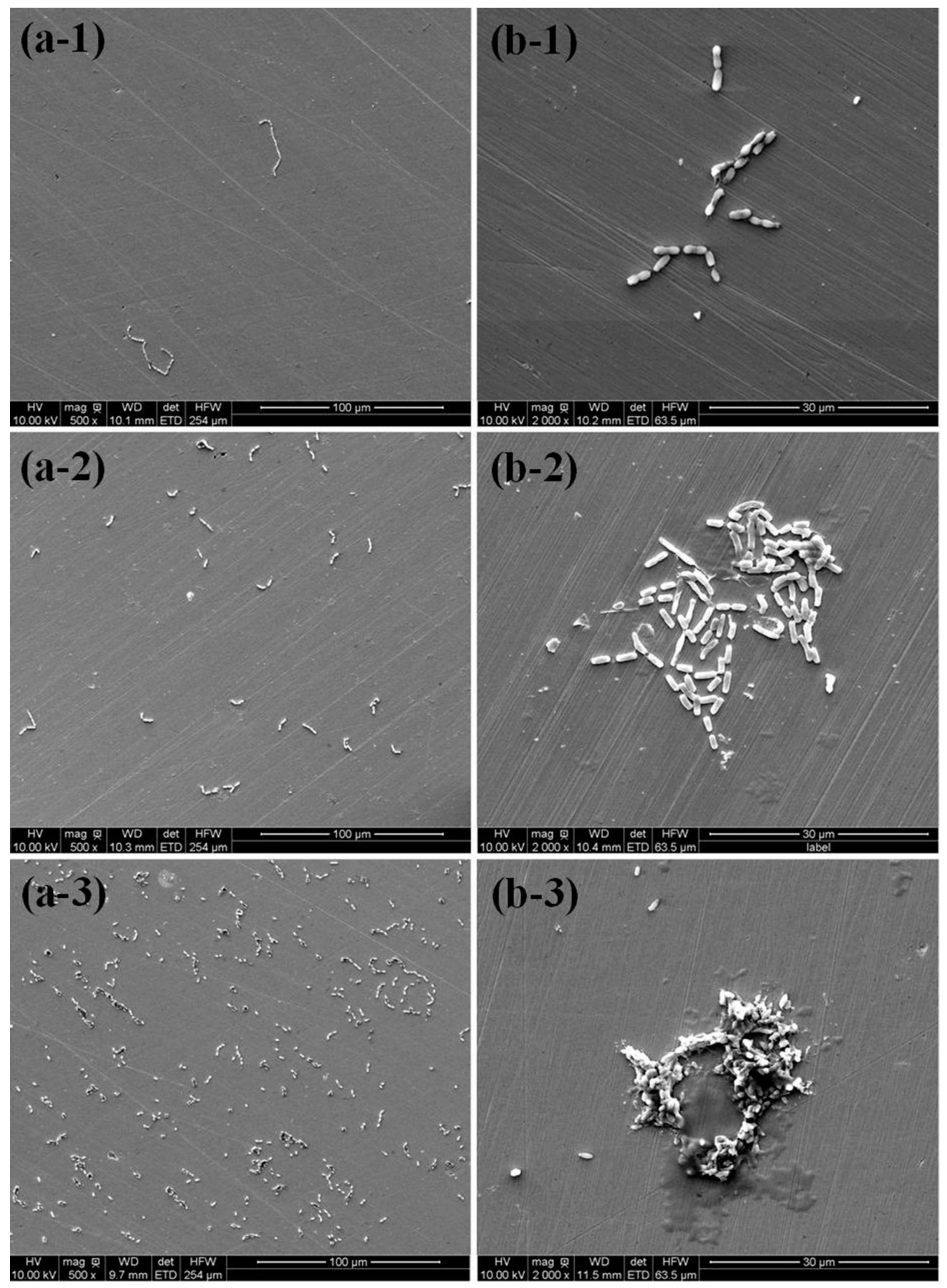
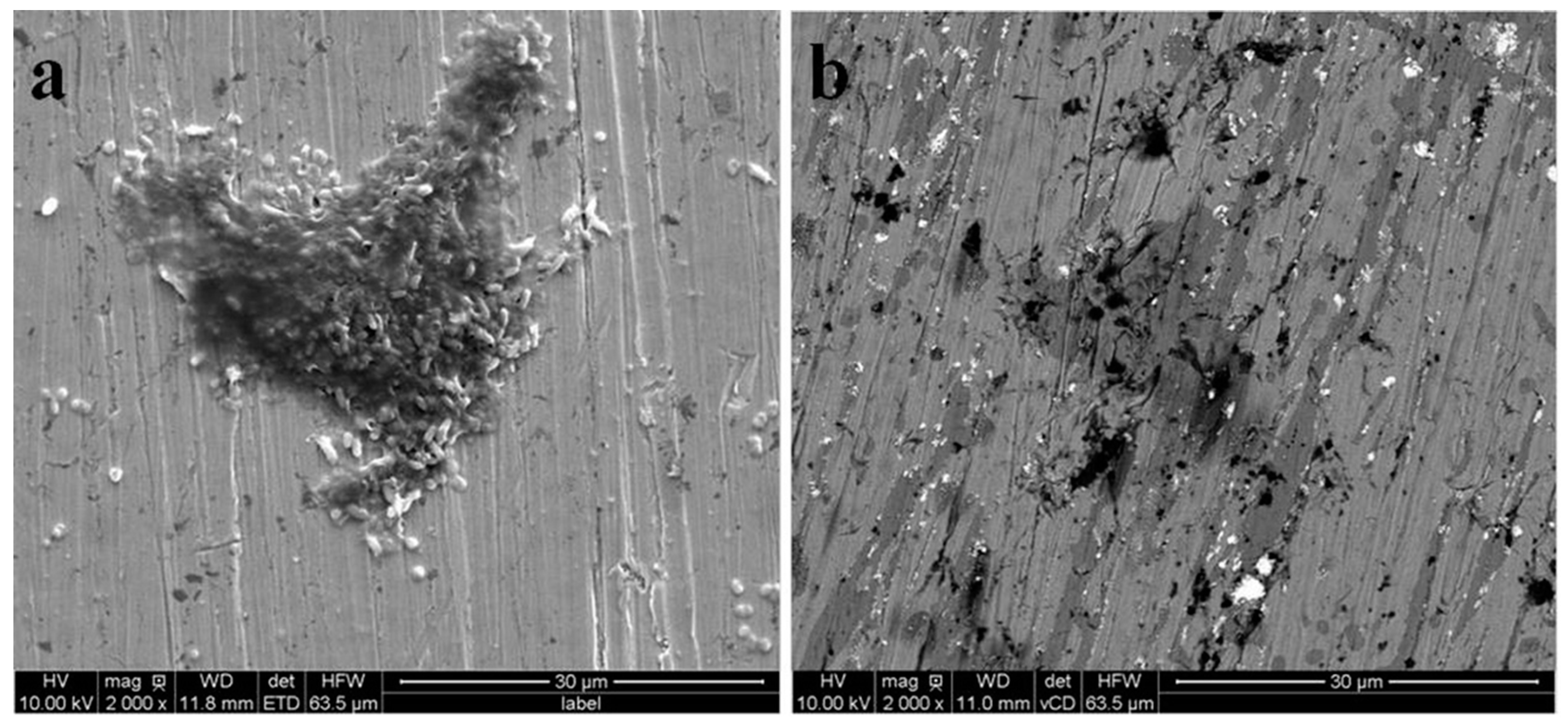
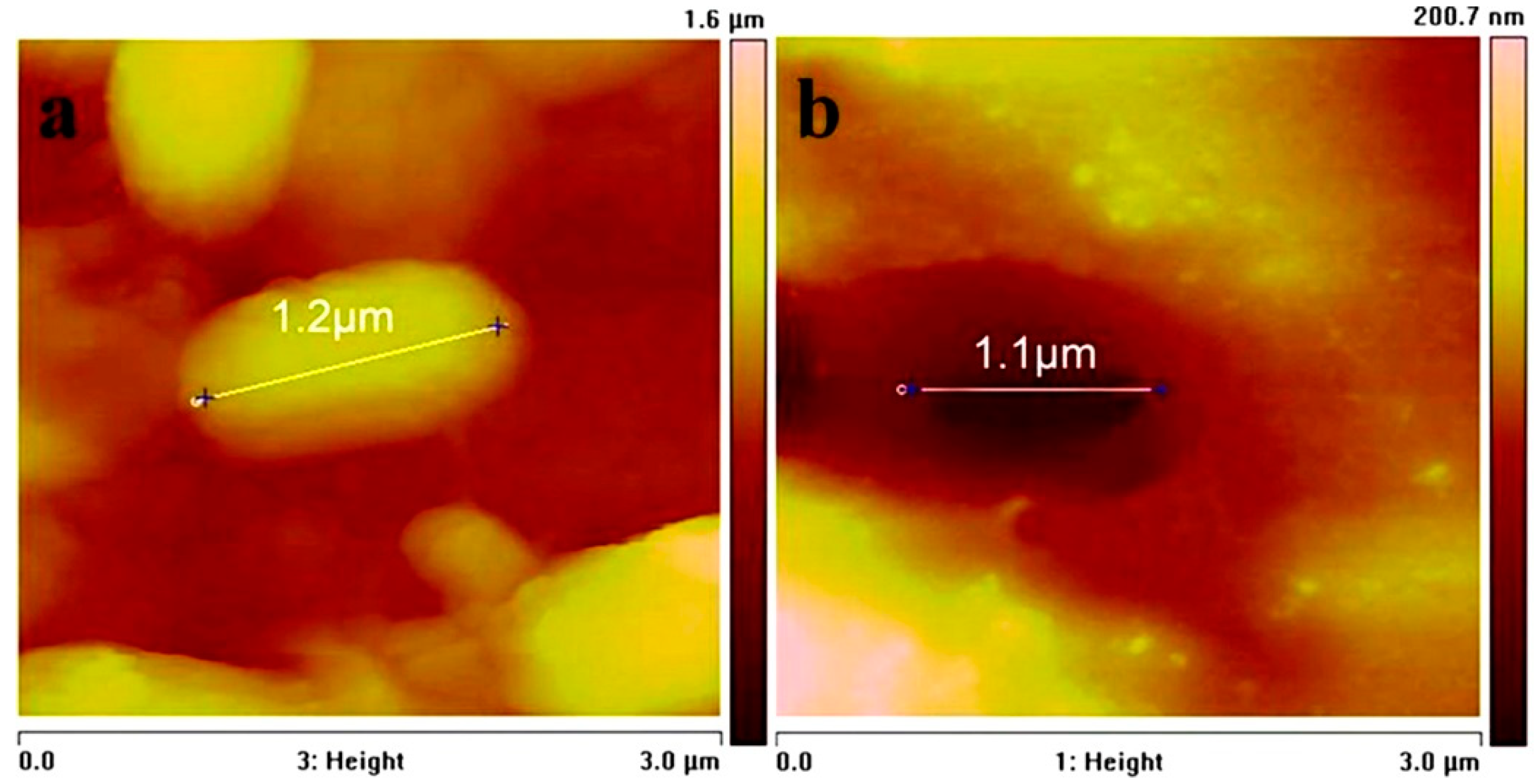
3.1. Surface Morphology and Bacterial Adhesion
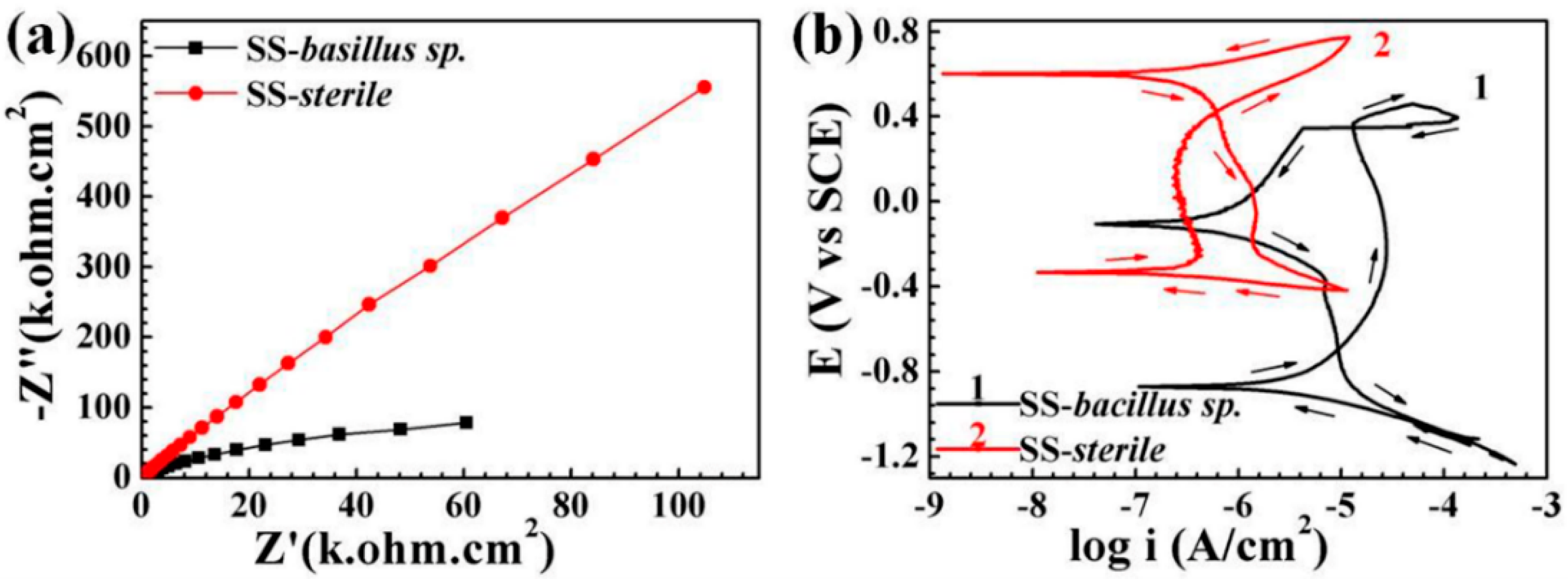
3.2. Electrochemical Analysis
3.3. Direct Observation of Bacteria-Induced Pitting Corrosion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dang, H.; Lovell, C.R. Microbial surface colonization and biofilm development in marine environments. Microbiol. Mol. Biol. Rev. 2015, 80, 91–138. [Google Scholar] [CrossRef] [Green Version]
- Jeffery, B.; Peppler, M.; Lima, R.S.; Mcdonald, A. Bactericidal effects of HOVF-sprayed nanostructured TiO2 on pseudomonas aeruginosa. J. Spray Technol. 2009, 19, 344–349. [Google Scholar] [CrossRef]
- George, N.; Mahon, M.; Mcdonald, A. Bactericidal performance of flame-sprayed nanostructured titania-copper composite coatings. J. Spray Technol. 2010, 19, 1042–1053. [Google Scholar] [CrossRef]
- Callow, J.A.; Callow, M.E. Trends in the development of environmentally friendly fouling-resistant marine coatings. Nat. Commun. 2011, 2, 244. [Google Scholar] [CrossRef] [PubMed]
- Posadas, E.; Garcia-Encina, P.; Soltau, A.; Dominguez, A.; Diaz, I.; Munoz, R. Carbon and nutrient removal from centrates and domestic wastewater using algal-bacterial biofilm bioreactors. Bioresour. Technol. 2013, 139, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Chambers, L.D.; Stokes, K.R.; Walsh, F.C.; Wood, R.J.K. Modern approaches to marine antifouling coatings. Surf. Coat. Tech. 2006, 201, 3642–3652. [Google Scholar] [CrossRef] [Green Version]
- Gottenbos, B.; Mei, H.C.; Busscher, H.J. Initial adhesion and surface growth of staphylococcus epidermidis and pseudomonas aeruginosa on biomedical polymers. J. Biomed. Mater. Res. 2000, 50, 208–214. [Google Scholar] [CrossRef]
- Chatterjee, S.; Biswas, N.; Datta, A.; Dey, R.; Maiti, P. Atomic force microscopy in biofilm study. Microscopy 2014, 63, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Hirai, N.; Mun, M.K.; Masuda, T.; Itoh, H.; Kanematsu, H. Atomic force MICROSCOPY-JPN analysis of biofilms formed on different plastics. Mater. Technol. 2014, 30, B57–B60. [Google Scholar] [CrossRef]
- Cheng, C.; Zhang, Z.; Li, R.; Zhang, L.; Zhang, D.J.; Cao, T.; Min, X.; Zhao, J. Effect of temperature on pitting corrosion of 430 stainless steel under dry and wet cycle of droplet. Surf. Technol. 2019, 48, 245–251. [Google Scholar]
- Huang, K.; McLandsborough, L.A.; Goddard, J.M. Adhesion and removal kinetics of bacillus cereus biofilms on Ni-PTFE modified stainless steel. Biofouling 2016, 32, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.J.; Pehkonen, S.O. Microbiologically influenced corrosion of 304 stainless steel by aerobic pseudomonas NCIMB 2021 bacteria: AFM and XPS study. Colloids Surf. B 2007, 59, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, J.; Khan, F.; Abbassi, R.; Garaniya, V.; Ojeda, R. Modelling of pitting corrosion in marine and offshore steel structure—A technical review. J. Loss Prev. Proc. 2015, 37, 39–62. [Google Scholar] [CrossRef]
- Krishna, N.G.; Thinaharan, C.; George, R.P.; Parvathavarthini, N.; Mudali, U.K. Surface modification of type 304 stainless steel with duplex coatings for corrosion resistance in sea water environments. Surf. Eng. 2014, 31, 39–47. [Google Scholar] [CrossRef]
- Antony, P.J.; Chongdar, S.; Kumar, P.; Raman, R. Corrosion of 2205 duplex stainless steel in chloride medium containing sulfate-reducing bacteria. Electrochim. Acta 2007, 52, 3985–3994. [Google Scholar] [CrossRef]
- Cheng, S.; Tian, J.; Chen, S.; Lei, Y.; Chang, X.; Liu, T.; Yin, Y. Microbially influenced corrosion of stainless steel by marine bacterium vibrionatriegens: (I) Corrosion behavior. Mat. Sci. Eng. C 2009, 29, 751–755. [Google Scholar] [CrossRef]
- Yin, Y.; Cheng, S.; Chen, S.; Tian, J.; Liu, T.; Chang, X. Microbially influenced corrosion of 303 stainless steel by marine bacterium vibrionatriegens: (II) Corrosion mechanism. Mat. Sci. Eng. C 2009, 29, 756–760. [Google Scholar] [CrossRef]
- Xu, D.; Xia, J.; Zhou, E.; Zhang, D.; Li, H.; Yang, C.; Li, Q.; Lin, H.; Li, X.; Yang, K. Accelerated corrosion of 2205 duplex stainless steel caused by marine aerobic pseudomonas aeruginosa biofilm. Bioelectrochemistry 2017, 113, 1–8. [Google Scholar] [CrossRef]
- Rizzo, F.; Di, L.G.; Formisano, A.; Landolfo, R.A. time-dependent corrosion wastage model for wrought iron structures. J. Mater. Civ. Eng. 2019, 31, 04019165. [Google Scholar] [CrossRef]
- Munoz, A.I.; Anton, J.G.; Guinon, J.L.; Herranz, P. Inhibition effect of chromate on the passivation and pitting corrosion of a duplex stainless steel in LiBr solutions using electrochemical techniques. Corros. Sci. 2007, 49, 3200–3225. [Google Scholar] [CrossRef]
- Guo, P.; Plante, E.C.; Wang, B.; Chen, X.; Balonis, M.; Bauchy, M.; Sant, G. Direct observation of pitting corrosion evolutions on carbon steel surfaces at the nano-to-micro-scales. Sci. Rep. UK 2018, 8, 7990. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Ooij, W.J. Corrosion protection of AA 2024-T3 by bis-[3-(triethoxysilyl)propyl]tetrasulfide in sodium chloride solution: Part 2: Mechanism for corrosion protection. Corros. Sci. 2003, 45, 2177–2197. [Google Scholar] [CrossRef]
- Pidaparti, R.M.; Aghazadeh, B.S.; Whitfield, A.; Rao, A.S.; Mercier, G.P. Classification of corrosion defects in NiAl bronze through image analysis. Corros. Sci. 2010, 52, 3661–3666. [Google Scholar] [CrossRef]
- Pan, C.; Liu, L.; Li, Y.; Wang, F. Pitting corrosion of 304ss nanocrystalline thin film. Corros. Sci. 2013, 73, 32–43. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, R.; Kato, M.; Nakasa, K. Observation by atomic force microscope of corrosion product during pitting corrosion on SUS304 stainless steel. Scr. Mater. 2005, 52, 227–230. [Google Scholar] [CrossRef]
- Abdolahi, A.; Hamzah, E.; Ibrahim, Z.; Hashim, S. Microbially influenced corrosion of steels by pseudomonas aeruginosa. Corros. Rev. 2014, 32, 129–141. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Liu, J.; Yu, M. Influence of thiobacillus ferroxidans biofilm on the corrosion behavior of steel A3. Int. J. Mod. Phys. B 2010, 24, 3083–3088. [Google Scholar] [CrossRef]
- Abdoli, L.; Suo, X.; Li, H. Distinctive colonization of Bacillus sp. bacteria and the influence of the bacterial biofilm on electrochemical behaviors of aluminum coatings. Colloids Surf. B 2010, 145, 688–694. [Google Scholar] [CrossRef]
- Hasan, J.; Jain, S.; Radmarajan, R.; Purighalla, S.; Sambandamurthy, V.K.; Chatterjee, K. Multi-scale surface topography to minimize adherence and viability of nosocomial drug-resistant bacteria. Mater. Des. 2018, 140, 332–344. [Google Scholar] [CrossRef]
- Liu, L.; Ercan, B.; Sun, L.; Ziemer, K.S.; Webster, T.J. Understanding the role of polymer surface nanoscale topography on inhibiting bacteria adhesion and growth. Acs Biomater. Sci. Eng. 2016, 2, 122–130. [Google Scholar] [CrossRef]
- Pereira, M.A.; Alves, M.M.; Azeredo, J.; Mota, M.; Oliveira, R. Influence of physico-chemical properties of porous microcarriers on the adhesion of an anaerobic consortium. J. Ind. Microbiol. Biotechnol. 2000, 23, 181–186. [Google Scholar] [CrossRef] [Green Version]
- Wassmann, T.; Kreis, S.; Behr, M.; Buergers, R. The influence of surface texture and wettability on initial bacterial adhesion on titanium and zirconium oxide dental implants. Int. J. Implant. Dent. 2017, 3, 3642–3652. [Google Scholar] [CrossRef] [PubMed]
- Paramonova, E.; Jong, E.D.; Krom, B.P.; Mei, H.C.; Busscher, H.J.; Sharma, P.K. Low-load compression testing: A novel way of measuring biofilm thickness. Appl. Environ. Microb. 2007, 73, 7023–7028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Ding, C.; Liao, J.; Lan, T.; Li, F.; Zhang, D.; Yang, J.; Yang, Y.; Luo, S.; Tang, J.; et al. Biosorption of uranium on Bacillus sp. dwc-2: Preliminary investigation on mechanism. J. Environ. Radioact. 2014, 135, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.; Sato, M.; Ruedy, R.; Lo, K.; Lea, D.W.; Elizade, M. Global temperature change. Proc. Natl. Acad. Sci. USA 2006, 103, 14288–14293. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.; Avci, R.; Geiser, M.; Lewandowski, Z. Comparative study in chemistry of microbially and electrochemically induced pitting of 316L stainless steel. Corros. Sci. 2003, 45, 2577–2595. [Google Scholar] [CrossRef]
- Han, D.; Jiang, Y.; Shi, C.; Deng, B.; Li, J. Effect of temperature, chloride ion and pH on the crevice corrosion behavior of SAF 2205 duplex stainless steel in chloride solutions. J. Mater. Sci. 2012, 47, 1018–1025. [Google Scholar] [CrossRef]
- Deberry, D.W.; Peyton, G.R.; Clark, W.S. Evaluation of corrosion inhibitors in SO2 scrubber solutions. Corrosion 1984, 40, 250–256. [Google Scholar] [CrossRef]
- Lin, J.; Ballim, R. Biocorrosion control: Current strategies and promising alternatives. Afr. J. Biotechnol. 2012, 11, 15736–15747. [Google Scholar] [CrossRef] [Green Version]
- Sheng, X.; Ting, Y.; Pehkonen, S.O. The influence of sulphate-reducing bacteria biofilm on the corrosion of stainless steel AISI 316. Corros. Sci. 2007, 49, 2159–2176. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Sun, K.; Huang, S.; He, X.; Hu, Z.; Li, W. Direct and Indirect Evidence of the Microbially Induced Pitting Corrosion of Steel Structures in Humid Environments. Coatings 2020, 10, 983. https://doi.org/10.3390/coatings10100983
Zhou J, Sun K, Huang S, He X, Hu Z, Li W. Direct and Indirect Evidence of the Microbially Induced Pitting Corrosion of Steel Structures in Humid Environments. Coatings. 2020; 10(10):983. https://doi.org/10.3390/coatings10100983
Chicago/Turabian StyleZhou, Jingzhong, Kuoteng Sun, Songqiang Huang, Xuemin He, Zhaowei Hu, and Wenge Li. 2020. "Direct and Indirect Evidence of the Microbially Induced Pitting Corrosion of Steel Structures in Humid Environments" Coatings 10, no. 10: 983. https://doi.org/10.3390/coatings10100983



