Osteoconductive and Osteoinductive Surface Modifications of Biomaterials for Bone Regeneration: A Concise Review
Abstract
1. Bone Regenerative Medicine
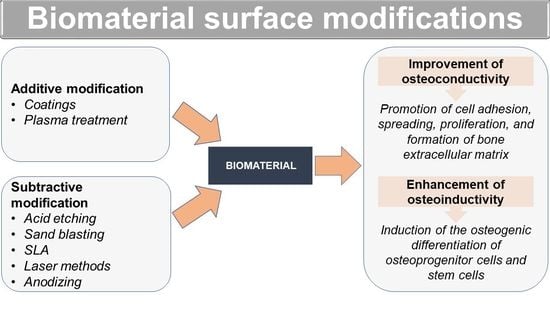
2. Surface Modifications of Biomaterials to Improve Their Osteoconductivity and Osteoinductivity
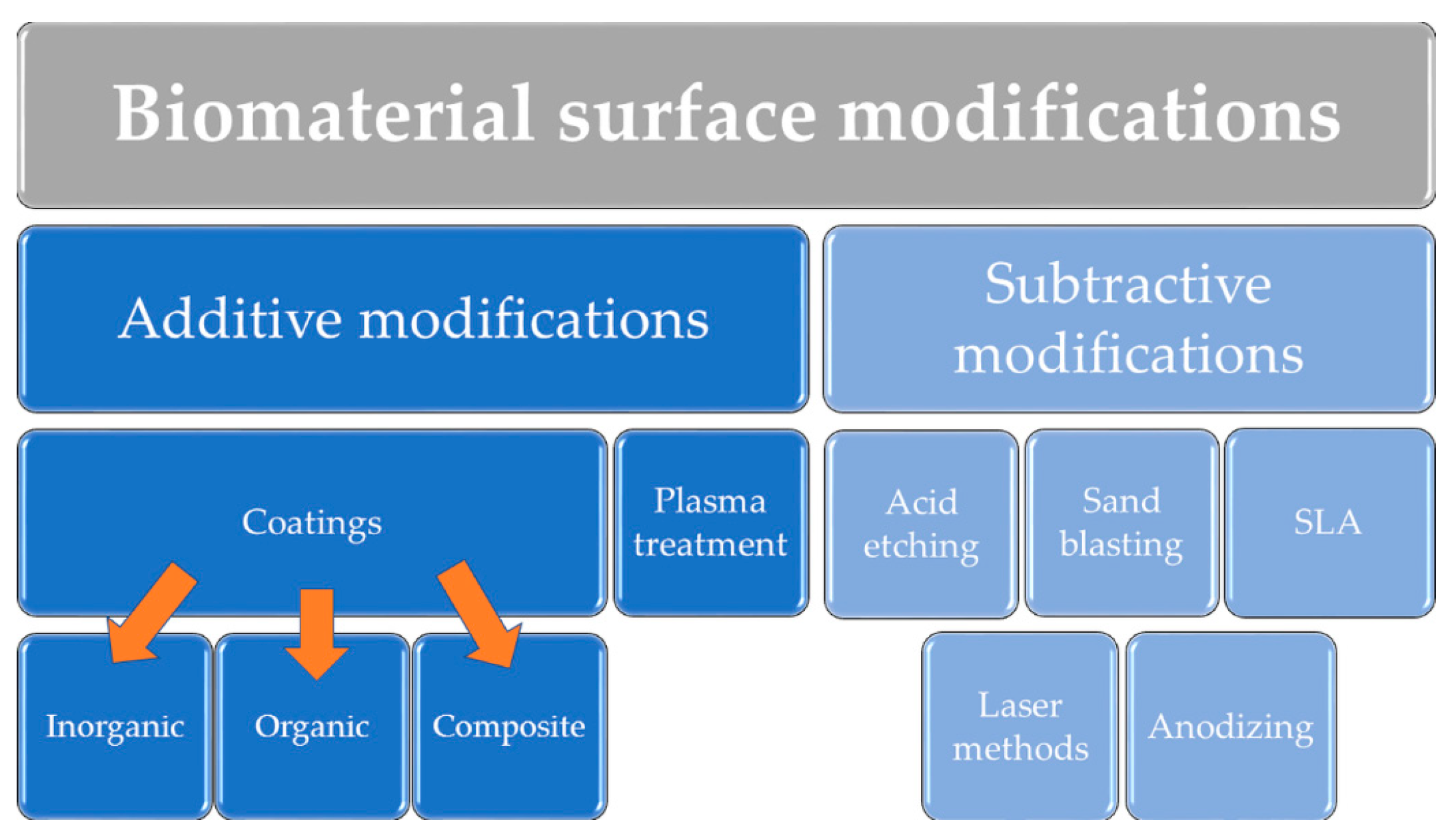
2.1. Additive Modifications of Biomaterial Surface
2.1.1. Inorganic and Composite Coatings
2.1.2. Organic Coatings
2.1.3. Plasma Modifications
2.1.4. Magnetron Sputtering Modifications
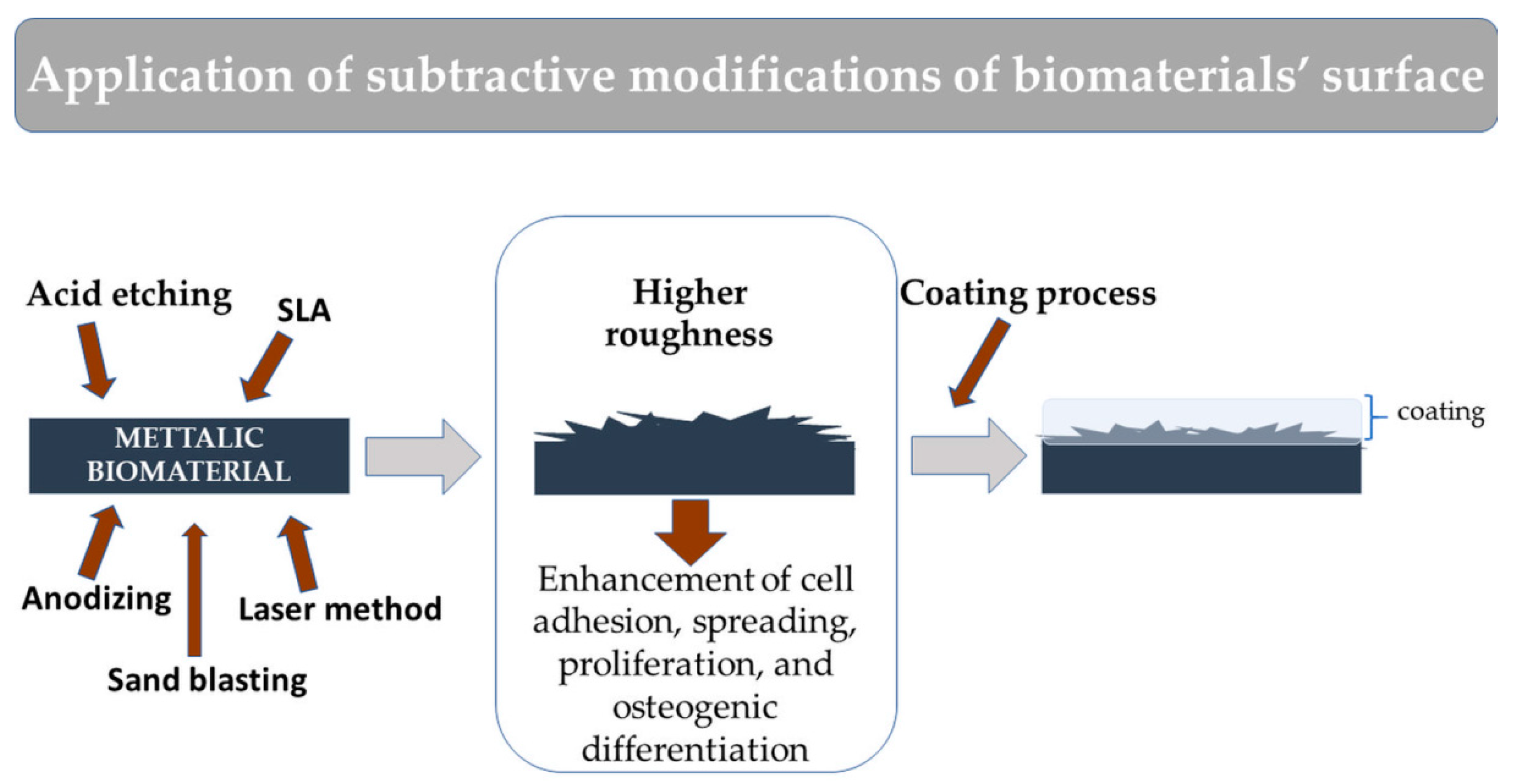
2.2. Subtractive Modifications of Biomaterial Surface
3. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Arvidson, K.; Abdallah, B.M.; Applegate, L.A.; Baldini, N.; Cenni, E.; Gomez-Barrena, E.; Granchi, D.; Kassem, M.; Konttinen, Y.T.; Mustafa, K.; et al. Bone regeneration and stem cells. J. Cell. Mol. Med. 2011, 15, 718–746. [Google Scholar] [CrossRef]
- Zivic, F.; Affatato, S.; Trajanovic, M.; Schnabelrauch, M.; Grujovic, N.; Choy, K.L. Biomaterials in Clinical Practice. Advances in Clinical Research and Medical Devices; Springer International Publishing: Cham, Switzerland, 2018; ISBN 978-3-319-68025-5. [Google Scholar]
- Oryan, A.; Alidadi, S.; Moshiri, A.; Maffulli, N. Bone regenerative medicine: Classic options, novel strategies and future directions. J. Orthop. Surg. Res. 2014, 9, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Rose, F.R.A.J.; Oreffo, R.O.C. Bone tissue engineering: Hope vs hype. Biochem. Biophys. Res. Commun. 2002, 292, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Przekora, A. The summary of the most important cell-biomaterial interactions that need to be considered during in vitro biocompatibility testing of bone scaffolds for tissue engineering applications. Mater. Sci. Eng. C 2019, 97, 1036–1051. [Google Scholar] [CrossRef] [PubMed]
- Dolcimascolo, A.; Calabrese, G.; Conoci, S.; Parenti, R. Innovative biomaterials for tissue engineering. In Biomaterial-Supported Tissue Reconstruction or Regeneration; Barbeck, M., Jung, O., Smeets, R., Koržinskas, T., Eds.; IntechOpen: London, UK, 2019; pp. 1–18. [Google Scholar]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Mour, M.; Das, D.; Winkler, T.; Hoenig, E.; Mielke, G.; Morlock, M.M.; Schilling, A.F. Advances in porous biomaterials for dental and orthopaedic applications. Materials 2010, 3, 2947–2974. [Google Scholar] [CrossRef]
- Prasadh, S.; Wong, R.C.W. Unraveling the mechanical strength of biomaterials used as a bone scaffold in oral and maxillofacial defects. Oral Sci. Int. 2018, 15, 48–55. [Google Scholar] [CrossRef]
- Tang, Z.; Li, X.; Tan, Y.; Fan, H.; Zhang, X. The material and biological characteristics of osteoinductive calcium phosphate ceramics. Regen. Biomater. 2018, 5, 43–59. [Google Scholar] [CrossRef]
- Fillingham, Y.; Jacobs, J. Bone grafts and their substitues. Bone Jt. J. 2016, 98, 6–9. [Google Scholar] [CrossRef]
- Morais, J.M.; Papadimitrakopoulos, F.; Burgess, D.J. Biomaterials/tissue interactions: Possible solutions to overcome foreign body response. Am. Assoc. Pharm. Sci. 2010, 12, 188–196. [Google Scholar] [CrossRef]
- Stevens, M.M. Biomaterials for bone tissue engineering. Mater. Today 2008, 11, 18–25. [Google Scholar] [CrossRef]
- Kazimierczak, P.; Benko, A.; Palka, K.; Canal, C.; Kolodynska, D.; Przekora, A. Novel synthesis method combining a foaming agent with freeze-drying to obtain hybrid highly macroporous bone scaffolds. J. Mater. Sci. Technol. 2020, 43, 52–63. [Google Scholar] [CrossRef]
- Xie, Y.; Hu, C.; Feng, Y.; Li, D.; Ai, T.; Huang, Y.; Chen, X.; Huang, L.; Tan, J. Osteoimmunomodulatory effects of biomaterial modification strategies on macrophage polarization and bone regeneration. Regen. Biomater. 2020, 7, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, K.; Nakajima, H. Metallic scaffolds for bone regeneration. Materials 2009, 2, 790–832. [Google Scholar] [CrossRef]
- Hanawa, T. Titanium-tissue interface reaction and its control with surface treatment. Front. Bioeng. Biotechnol. 2019, 7, 170. [Google Scholar] [CrossRef]
- Qiu, Z.Y.; Chen, C.; Wang, X.M.; Lee, I.S. Advances in the surface modification techniques of bone-related implants for last 10 years. Regen. Biomater. 2014, 1, 67–79. [Google Scholar] [CrossRef]
- Dehghanghadikolaei, A.; Fotovvati, B. Coating techniques for functional enhancement of metal implants for bone replacement: A review. Materials 2019, 12, 1795. [Google Scholar] [CrossRef]
- Yadav, D.; Garg, R.K.; Ahlawat, A.; Chhabra, D. 3D printable biomaterials for orthopedic implants: Solution for sustainable and circular economy. Resour. Policy 2020, 68, 101767. [Google Scholar] [CrossRef]
- Diekjürgen, D.; Grainger, D.W. Polysaccharide matrices used in 3D in vitro cell culture systems. Biomaterials 2017, 141, 96–115. [Google Scholar] [CrossRef]
- Sachot, N.; Engel, E.; Castano, O. Hybrid organic-inorganic scaffolding biomaterials for regenerative therapies. Curr. Org. Chem. 2014, 18, 2299–2314. [Google Scholar] [CrossRef]
- Ullah, S.; Chen, X. Fabrication, applications and challenges of natural biomaterials in tissue engineering. Appl. Mater. Today 2020, 20, 100656. [Google Scholar] [CrossRef]
- Przekora, A.; Palka, K.; Ginalska, G. Chitosan/β-1,3-glucan/calcium phosphate ceramics composites—Novel cell scaffolds for bone tissue engineering application. J. Biotechnol. 2014, 182, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Przekora, A. Current trends in fabrication of biomaterials for bone and cartilage regeneration: Materials modifications and biophysical stimulations. Int. J. Mol. Sci. 2019, 20, 435. [Google Scholar] [CrossRef] [PubMed]
- Mano, J.F.; Silva, G.A.; Azevedo, H.S.; Malafaya, P.B.; Sousa, R.A.; Silva, S.S.; Boesel, L.F.; Oliveira, J.M.; Santos, T.C.; Marques, A.P.; et al. Natural origin biodegradable systems in tissue engineering and regenerative medicine: Present status and some moving trends. J. R. Soc. Interface 2007, 4, 999–1030. [Google Scholar] [CrossRef]
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for bone tissue engineering: State of the art and new perspectives. Mater. Sci. Eng. C 2017, 78, 1246–1262. [Google Scholar] [CrossRef]
- Nouri, A.; Wen, C. Introduction to surface coating and midification for metallic biomaterials. In Surface Coating and Modification of Metallic Biomaterials; Wen, C., Ed.; Woodhead Publishing: Cambridge, UK, 2015; pp. 3–60. ISBN 9781782423164. [Google Scholar]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 2018, 3, 278–314. [Google Scholar] [CrossRef] [PubMed]
- Pagel, M.; Beck-Sickinger, A.G. Multifunctional biomaterial coatings: Synthetic challenges and biological activity. Biol. Chem. 2017, 398, 3–22. [Google Scholar] [CrossRef]
- Bose, S.; Robertson, S.F.; Bandyopadhyay, A. Surface modification of biomaterials and biomedical devices using additive manufacturing. Acta Biomater. 2018, 66, 143–169. [Google Scholar] [CrossRef]
- Arjunan, A.; Robinson, J.; Al Ani, E.; Heaselgrave, W.; Baroutaji, A.; Wang, C. Mechanical performance of additively manufactured pure silver antibacterial bone scaffolds. J. Mech. Behav. Biomed. Mater. 2020, 112, 104090. [Google Scholar] [CrossRef]
- Oshida, Y.; Guven, Y. Biocompatible coatings for metallic biomaterials. In Surface Coating and Modification of Metallic Biomaterials; Wen, C., Ed.; Woodhead Publishing: Cambridge, UK, 2015; pp. 287–343. ISBN 978-1-78242-303-4. [Google Scholar]
- Sergi, R.; Bellucci, D.; Cannillo, V. A comprehensive review of bioactive glass coatings: State of the art, challenges and future perspectives. Coatings 2020, 10, 757. [Google Scholar] [CrossRef]
- Ahn, T.-K.; Lee, D.H.; Kim, T.; chol Jang, G.; Choi, S.; Oh, J.B.; Ye, G.; Lee, S. Modification of titanium implant and titanium dioxide for bone tissue engineering. In Novel Biomaterials for Regenerative Medicine; Chun, H., Park, K., Kim, C., Khang, G., Eds.; Springer: Singapore, 2018; pp. 355–368. ISBN 9789811309465. [Google Scholar]
- Surmenev, R.A.; Surmeneva, M.A.; Ivanova, A.A. Significance of calcium phosphate coatings for the enhancement of new bone osteogenesis—A review. Acta Biomater. 2014, 10, 557–579. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, P.G.F.P.; de Melo Soares, M.S.; Silveira e Souza, A.M.M.; Taba, M.; Palioto, D.B.; Messora, M.R.; Ghiraldini, B.; Nunes, F.A.D.S.; de Souza, S.L.S. Influence of nano-hydroxyapatite coating implants on gene expression of osteogenic markers and micro-CT parameters. An in vivo study in diabetic rats. J. Biomed. Mater. Res. Part A 2020. [Google Scholar] [CrossRef] [PubMed]
- Popkov, A.V.; Gorbach, E.N.; Kononovich, N.A.; Popkov, D.A.; Tverdokhlebov, S.I.; Shesterikov, E.V. Bioactivity and osteointegration of hydroxyapatite-coated stainless steel and titanium wires used for intramedullary osteosynthesis. Strat. Trauma Limb Reconstr. 2017, 12, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Liu, P.; Zhao, D.; Yuan, B.; Xiao, Z.; Zhou, Y.; Yang, X.; Zhu, X.; Tu, C.; Zhang, X. Effects of nanotopography regulation and silicon doping on angiogenic and osteogenic activities of hydroxyapatite coating on titanium implant. Int. J. Nanomed. 2020, 15, 4171–4189. [Google Scholar] [CrossRef] [PubMed]
- Mumith, A.; Cheong, V.S.; Fromme, P.; Coathup, M.J.; Blunn, G.W. The effect of strontium and silicon substituted hydroxyapatite electrochemical coatings on bone ingrowth and osseointegration of selective laser sintered porous metal implants. PLoS ONE 2020, 15, e0227232. [Google Scholar] [CrossRef] [PubMed]
- Mokabber, T.; Cao, H.T.; Norouzi, N.; Van Rijn, P.; Pei, Y.T. Antimicrobial electrodeposited silver-containing calcium phosphate coatings. ACS Appl. Mater. Interfaces 2020, 12, 5531–5541. [Google Scholar] [CrossRef]
- Marzban, K.; Rabiee, S.M.; Zabihi, E.; Bagherifard, S. Nanostructured akermanite glass-ceramic coating on Ti6Al4V for orthopedic applications. J. Appl. Biomater. Funct. Mater. 2019, 17, 1–8. [Google Scholar] [CrossRef]
- Zhang, R.; Xu, N.; Liu, X.; Yang, X.; Yan, H.; Ma, J.; Feng, Q.; Shen, Z. Micro/nanostructured TiO2/ZnO coating enhances osteogenic activity of SaOS-2 cells. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2838–2845. [Google Scholar] [CrossRef]
- Schitea, R.I.; Nitu, A.; Ciobota, A.A.; Munteanu, A.L.; David, I.M.; Miu, D.; Raileanu, M.; Bacalum, M.; Busuioc, C. Pulsed laser deposition derived bioactive glass-ceramic coatings for enhancing the biocompatibility of scaffolding materials. Materials 2020, 13, 2615. [Google Scholar] [CrossRef]
- Huang, Z.; He, Y.; Chang, X.; Liu, J.; Yu, L.; Wu, Y.; Li, Y.; Tian, J.; Kang, L.; Wu, D.; et al. A magnetic iron oxide/polydopamine coating can improve osteogenesis of 3D-printed porous titanium scaffolds with a static magnetic field by upregulating the TGF β -Smads pathway. Adv. Healthc. Mater. 2020, 9, 1–13. [Google Scholar] [CrossRef]
- Fan, B.; Guo, Z.; Li, X.; Li, S.; Gao, P.; Xiao, X.; Wu, J.; Shen, C.; Jiao, Y.; Hou, W. Electroactive barium titanate coated titanium scaffold improves osteogenesis and osseointegration with low-intensity pulsed ultrasound for large segmental bone defects. Bioact. Mater. 2020, 5, 1087–1101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, L.; Zeng, J.; Zhou, K.; Zhang, D. Aligned porous barium titanate/hydroxyapatite composites with high piezoelectric coefficients for bone tissue engineering. Mater. Sci. Eng. C 2014, 39, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Ehterami, A.; Kazemi, M.; Nazari, B.; Saraeian, P.; Azami, M. Fabrication and characterization of highly porous barium titanate based scaffold coated by Gel/HA nanocomposite with high piezoelectric coefficient for bone tissue engineering applications. J. Mech. Behav. Biomed. Mater. 2018, 79, 195–202. [Google Scholar] [CrossRef]
- Scarisoreanu, N.D.; Craciun, F.; Ion, V.; Birjega, R.; Bercea, A.; Dinca, V.; Dinescu, M.; Sima, L.E.; Icriverzi, M.; Roseanu, A.; et al. Lead-free piezoelectric (Ba,Ca)(Zr,Ti)O3 thin films for biocompatible and flexible devices. ACS Appl. Mater. Interfaces 2017, 9, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Zlotnik, S.; Maltez-Da Costa, M.; Barroca, N.; Hortigüela, M.J.; Singh, M.K.; Fernandes, M.H.V.; Vilarinho, P.M. Functionalized-ferroelectric-coating-driven enhanced biomineralization and protein-conformation on metallic implants. J. Mater. Chem. B 2019, 7, 2177–2189. [Google Scholar] [CrossRef]
- Bacakova, L.; Vandrovcova, M.; Kopova, I.; Jirka, I. Applications of zeolites in biotechnology and medicine-a review. Biomater. Sci. 2018, 6, 974–989. [Google Scholar] [CrossRef]
- Qing, Y.; Li, K.; Li, D.; Qin, Y. Antibacterial effects of silver incorporated zeolite coatings on 3D printed porous stainless steels. Mater. Sci. Eng. C 2020, 108, 110430. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, X.; Huang, C.; Cai, H.; Hu, S.; Wan, Q.; Pei, X.; Wang, J. Osteogenic activity and antibacterial effect of porous titanium modified with metal-organic framework films. J. Biomed. Mater. Res. Part A 2017, 105, 834–846. [Google Scholar] [CrossRef]
- Zhang, X.; Li, H.; Liu, J.; Wang, H.; Sun, W.; Lin, K.; Wang, X.; Shen, S.G. Amorphous carbon modification on implant surface: A general strategy to enhance osteogenic differentiation for diverse biomaterials via FAK/ERK1/2 signaling pathways. J. Mater. Chem. B 2019, 7, 2518–2533. [Google Scholar] [CrossRef]
- Rifai, A.; Tran, N.; Lau, D.W.; Elbourne, A.; Zhan, H.; Stacey, A.D.; Mayes, E.L.H.; Sarker, A.; Ivanova, E.P.; Crawford, R.J.; et al. Polycrystalline diamond coating of additively manufactured titanium for biomedical applications. ACS Appl. Mater. Interfaces 2018, 10, 8474–8484. [Google Scholar] [CrossRef]
- Tien, H.W.; Lee, C.Y.; Lin, I.N.; Chen, Y.C. Long term: In vivo functional stability and encapsulation reliability of using ultra-nanocrystalline diamond as an insulating coating layer for implantable microchips. J. Mater. Chem. B 2017, 5, 3706–3717. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.D.; Kim, T.H.; Mandakhbayar, N.; Singh, R.K.; Jang, J.H.; Lee, J.H.; Kim, H.W. Coating biopolymer nanofibers with carbon nanotubes accelerates tissue healing and bone regeneration through orchestrated cell- and tissue-regulatory responses. Acta Biomater. 2020, 108, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Ogura, Y.; Enomoto, K.; Hara, M.; Maurstad, G.; Stokke, B.T.; Kitamura, S. Dense carbon-nanotube coating scaffolds stimulate osteogenic differentiation of mesenchymal stem cells. PLoS ONE 2020, 15, e0225589. [Google Scholar] [CrossRef] [PubMed]
- Przekora, A.; Benko, A.; Nocun, M.; Wyrwa, J.; Blazewicz, M.; Ginalska, G. Titanium coated with functionalized carbon nanotubes—A promising novel material for biomedical application as an implantable orthopaedic electronic device. Mater. Sci. Eng. C 2014, 45, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Benko, A.; Przekora, A.; Wesełucha-Birczyńska, A.; Nocuń, M.; Ginalska, G.; Błażewicz, M. Fabrication of multi-walled carbon nanotube layers with selected properties via electrophoretic deposition: Physicochemical and biological characterization. Appl. Phys. A Mater. Sci. Process. 2016, 122, 447. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Crook, J.M.; Wallace, G.G. 3D printing of cytocompatible graphene/alginate scaffolds for mimetic tissue constructs. Front. Bioeng. Biotechnol. 2020, 8, 1–11. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Jiang, X.; Yamaguchi, M.; Ito, A.; Bando, Y.; Golberg, D. Boron nitride nanotube-enhanced osteogenic differentiation of mesenchymal stem cells. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 323–329. [Google Scholar] [CrossRef]
- Özmeriç, A.; Tanoğlu, O.; Ocak, M.; Çelik, H.H.; Fırat, A.; Kaymaz, F.F.; Koca, G.; Şenes, M.; Alemdaroğlu, K.B.; İltar, S.; et al. Intramedullary implants coated with cubic boron nitride enhance bone fracture healing in a rat model. J. Trace Elem. Med. Biol. 2020, 62, 126599. [Google Scholar] [CrossRef]
- Yu, L.; Silva Santisteban, T.; Liu, Q.; Hu, C.; Bi, J.; Wei, M. Effect of three-dimensional porosity gradients of biomimetic coatings on their bonding strength and cell behavior. J. Biomed. Mater. Res. Part A 2020. [Google Scholar] [CrossRef]
- Stevanović, M.; Djošić, M.; Janković, A.; Nešović, K.; Kojić, V.; Stojanović, J.; Grujić, S.; Matić Bujagić, I.; Rhee, K.Y.; Mišković-Stanković, V. Assessing the bioactivity of gentamicin-preloaded hydroxyapatite/chitosan composite coating on titanium substrate. ACS Omega 2020, 5, 15433–15445. [Google Scholar] [CrossRef]
- Lin, Q.; Zhou, Y.; Yin, M.; Cheng, Y.; Wei, Y.; Hu, Y.; Lian, X.; Chen, W.; Huang, D. Hydroxyapatite/tannic acid composite coating formation based on Ti modified by TiO2 nanotubes. Colloids Surf. B Biointerfaces 2020, 196, 111304. [Google Scholar] [CrossRef]
- Gorgin Karaji, Z.; Jahanmard, F.; Mirzaei, A.; van der Wal, B.; Amin Yavari, S. Multifunctional silk coating on additively manufactured porous titanium to prevent implant-associated infection and stimulate bone regeneration. Biomed. Mater. 2020. [Google Scholar] [CrossRef] [PubMed]
- Harb, S.V.; Uvida, M.C.; Trentin, A.; Oliveira Lobo, A.; Webster, T.J.; Pulcinelli, S.H.; Santilli, C.V.; Hammer, P. PMMA-silica nanocomposite coating: Effective corrosion protection and biocompatibility for a Ti6Al4V alloy. Mater. Sci. Eng. C 2020, 110, 110713. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Xu, W.; Chen, F.; Qi, C.; Lu, B.Q.; Zhang, H.; Wu, J.; Qian, Q.R.; Zhu, Y.J. Amorphous calcium phosphate nanospheres/polylactide composite coated tantalum scaffold: Facile preparation, fast biomineralization and subchondral bone defect repair application. Colloids Surf. B Biointerfaces 2014, 123, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Bougas, K.; Stenport, V.F.; Tengvall, P.; Currie, F. Laminin coating promotes calcium phosphate precipitation on titanium discs in vitro. J. Oral Maxillofac. Res. 2011, 2, 1–10. [Google Scholar] [CrossRef]
- Bougas, K.; Jimbo, R.; Xue, Y.; Mustafa, K.; Wennerberg, A. Novel implant coating agent promotes gene expression of osteogenic markers in rats during early osseointegration. Int. J. Biomater. 2012, 2012, 579274. [Google Scholar] [CrossRef]
- Rabe, R.; Hempel, U.; Martocq, L.; Keppler, J.K.; Aveyard, J.; Douglas, T.E.L. Dairy-inspired coatings for bone implants from whey protein isolate-derived self-assembled fibrils. Int. J. Mol. Sci. 2020, 21, 5544. [Google Scholar] [CrossRef]
- Zhao, M.; Anouz, R.; Groth, T. Effect of microenvironment on adhesion and differentiation of murine C3H10T1/2 cells cultured on multilayers containing collagen I and glycosaminoglycans. J. Tissue Eng. 2020, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, J.; Gao, C.; Wang, Z.; Zhou, X.; Tang, M.; Yu, K.; Deng, Y. Enhanced osteoinductivity and corrosion resistance of dopamine/gelatin/rhBMP-2–coated β-TCP/Mg-Zn orthopedic implants: An in vitro and in vivo study. PLoS ONE 2020, 15, e0228247. [Google Scholar] [CrossRef]
- Abdal-Hay, A.; Dewidar, M.; Lim, J.K. Biocorrosion behavior and cell viability of adhesive polymer coated magnesium based alloys for medical implants. Appl. Surf. Sci. 2012, 261, 536–546. [Google Scholar] [CrossRef]
- Albano, C.S.; Moreira Gomes, A.; da Silva Feltran, G.; da Costa Fernandes, C.J.; Trino, L.D.; Zambuzzi, W.F.; Lisboa-Filho, P.N. Biofunctionalization of titanium surfaces with alendronate and albumin modulates osteoblast performance. Heliyon 2020, 6, e04455. [Google Scholar] [CrossRef] [PubMed]
- Ashwin, B.; Abinaya, B.; Prasith, T.P.; Chandran, S.V.; Yadav, L.R.; Vairamani, M.; Patil, S.; Selvamurugan, N. 3D-poly (lactic acid) scaffolds coated with gelatin and mucic acid for bone tissue engineering. Int. J. Biol. Macromol. 2020, 162, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Khojasteh, A.; Fahimipour, F.; Eslaminejad, M.B.; Jafarian, M.; Jahangir, S.; Bastami, F.; Tahriri, M.; Karkhaneh, A.; Tayebi, L. Development of PLGA-coated β-TCP scaffolds containing VEGF for bone tissue engineering. Mater. Sci. Eng. C 2016, 69, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Liu, Z.; Zhu, X.; Jiang, L.; Shi, W.; Wang, Y.; Xu, N.; Gang, F.; Wang, X.; Zhao, L.; et al. Dual directions to address the problem of aseptic loosening via electrospun PLGA @ aspirin nanofiber coatings on titanium. Biomaterials 2020, 257, 120237. [Google Scholar] [CrossRef]
- Ding, X.; Wang, S.; Jin, W.; Liu, X.; Chen, J.; Chen, S. Encapsulation of a nanoporous simvastatin-chitosan composite to enhance osteointegration of hydroxyapatite-coated polyethylene terephthalate ligaments. Int. J. Nanomed. 2019, 14, 4881–4893. [Google Scholar] [CrossRef]
- Coelho, P.G.; Giro, G.; Teixeira, H.S.; Marin, C.; Witek, L.; Thompson, V.P.; Tovar, N.; Silva, N.R.F.A. Argon-based atmospheric pressure plasma enhances early bone response to rough titanium surfaces. J. Biomed. Mater. Res. Part A 2012, 100, 1901–1906. [Google Scholar] [CrossRef]
- Jacobs, T.; Morent, R.; De Geyter, N.; Dubruel, P.; Leys, C. Plasma surface modification of biomedical polymers: Influence on cell-material interaction. Plasma Chem. Plasma Process. 2012, 32, 1039–1073. [Google Scholar] [CrossRef]
- Gadow, R.; Killinger, A.; Rauch, J. Introduction to high-velocity suspension flame spraying (HVSFS). J. Therm. Spray Technol. 2008, 17, 655–661. [Google Scholar] [CrossRef]
- Sobolev, A.; Valkov, A.; Kossenko, A.; Wolicki, I.; Zinigrad, M.; Borodianskiy, K. Bioactive coating on Ti alloy with high osseointegration and antibacterial Ag nanoparticles. ACS Appl. Mater. Interfaces 2019, 11, 39534–39544. [Google Scholar] [CrossRef]
- Santos-Coquillat, A.; Martínez-Campos, E.; Vargas-Alfredo, N.; Arrabal, R.; Rodríguez-Hernández, J.; Matykina, E. Hierarchical functionalized polymeric-ceramic coatings on Mg–Ca alloys for biodegradable implant applications. Macromol. Biosci. 2019, 19, 1–9. [Google Scholar] [CrossRef]
- Ong, J.; Zhao, J.; Levy, G.K.; Macdonald, J.; Justin, A.W.; Markaki, A.E. Functionalisation of a heat-derived and bio-inert albumin hydrogel with extracellular matrix by air plasma treatment. Sci. Rep. 2020, 10, 12429. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Visalakshan, R.M.; Guo, J.; Wei, F.; Zhang, L.; Chen, L.; Lin, Z.; Vasilev, K.; Xiao, Y. Plasma deposited poly-oxazoline nanotextured surfaces dictate osteoimmunomodulation towards ameliorative osteogenesis. Acta Biomater. 2019, 96, 568–581. [Google Scholar] [CrossRef] [PubMed]
- Mas-Vinyals, A.; Gilabert-Porres, J.; Figueras-Esteve, L.; Borrós, S. Improving linking interface between collagen-based hydrogels and bone-like substrates. Colloids Surf. B Biointerfaces 2019, 181, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Hagiwara, K.; Hasebe, T.; Hotta, A. Surface modification of polymers by plasma treatments for the enhancement of biocompatibility and controlled drug release. Surf. Coat. Technol. 2013, 233, 99–107. [Google Scholar] [CrossRef]
- Xing, H.; Li, R.; Wei, Y.; Ying, B.; Li, D.; Qin, Y. Improved osteogenesis of selective-laser-melted titanium alloy by coating strontium-doped phosphate with high-efficiency air-plasma treatment. Front. Bioeng. Biotechnol. 2020, 8, 367. [Google Scholar] [CrossRef]
- Liu, L.; Wang, X.; Zhou, Y.; Cai, M.; Lin, K.; Fang, B.; Xia, L. The synergistic promotion of osseointegration by nanostructure design and silicon substitution of hydroxyapatite coatings in a diabetic model. J. Mater. Chem. B 2020, 8, 2754–2767. [Google Scholar] [CrossRef]
- Lu, R.J.; Wang, X.; He, H.X.; Ling-Ling, E.; Li, Y.; Zhang, G.L.; Li, C.J.; Ning, C.Y.; Liu, H.C. Tantalum-incorporated hydroxyapatite coating on titanium implants: Its mechanical and in vitro osteogenic properties. J. Mater. Sci. Mater. Med. 2019, 30, 111. [Google Scholar] [CrossRef]
- Longo, G.; Ioannidu, C.A.; D’Abusco, A.S.; Superti, F.; Misiano, C.; Zanoni, R.; Politi, L.; Mazzola, L.; Iosi, F.; Mura, F.; et al. Improving osteoblast response in vitro by a nanostructured thin film with titanium carbide and titanium oxides clustered around graphitic carbon. PLoS ONE 2016, 11, e0152566. [Google Scholar] [CrossRef]
- Veronesi, F.; Giavaresi, G.; Fini, M.; Longo, G.; Ioannidu, C.A.; Scotto d’Abusco, A.; Superti, F.; Panzini, G.; Misiano, C.; Palattella, A.; et al. Osseointegration is improved by coating titanium implants with a nanostructured thin film with titanium carbide and titanium oxides clustered around graphitic carbon. Mater. Sci. Eng. C 2017, 70, 264–271. [Google Scholar] [CrossRef]
- Polo, T.O.B.; da Silva, W.P.; Momesso, G.A.C.; Lima-Neto, T.J.; Barbosa, S.; Cordeiro, J.M.; Hassumi, J.S.; da Cruz, N.C.; Okamoto, R.; Barão, V.A.R.; et al. Plasma electrolytic oxidation as a feasible surface treatment for biomedical applications: An in vivo study. Sci. Rep. 2020, 10, 10000. [Google Scholar] [CrossRef]
- He, J.; Feng, W.; Zhao, B.-H.; Zhang, W.; Lin, Z. In vivo effect of titanium implants with porous zinc-containing coatings prepared by plasma electrolytic oxidation method on osseointegration in rabbits. Int. J. Oral Maxillofac. Implant. 2018, 33, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Yi, L.; Jiang, L.; Ma, Y.; Lin, H.; Dong, J. Osteogenic activity and antibacterial ability on titanium surfaces modified with magnesium-doped titanium dioxide coating. Nanomedicine 2019, 14, 1109–1133. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Gao, P.; Zhang, H.; Guo, Z.; Zheng, Y.; Han, Y. Osteoimmunomodulation, osseointegration, and in vivo mechanical integrity of pure Mg coated with HA nanorod/pore-sealed MgO bilayer. Biomater. Sci. 2018, 6, 3202–3218. [Google Scholar] [CrossRef] [PubMed]
- Santos-Coquillat, A.; Esteban-Lucia, M.; Martinez-Campos, E.; Mohedano, M.; Arrabal, R.; Blawert, C.; Zheludkevich, M.L.; Matykina, E. PEO coatings design for Mg–Ca alloy for cardiovascular stent and bone regeneration applications. Mater. Sci. Eng. C 2019, 105, 110026. [Google Scholar] [CrossRef]
- Michalska, J.; Sowa, M.; Piotrowska, M.; Widziołek, M.; Tylko, G.; Dercz, G.; Socha, R.P.; Osyczka, A.M.; Simka, W. Incorporation of Ca ions into anodic oxide coatings on the Ti–13Nb–13Zr alloy by plasma electrolytic oxidation. Mater. Sci. Eng. C 2019, 104, 109957. [Google Scholar] [CrossRef]
- Meng, G.; Wu, X.; Yao, R.; He, J.; Yao, W.; Wu, F. Effect of zinc substitution in hydroxyapatite coating on osteoblast and osteoclast differentiation under osteoblast/osteoclast co-culture. Regen. Biomater. 2019, 6, 349–359. [Google Scholar] [CrossRef]
- Zhang, W.; Gu, J.; Li, K.; Zhao, J.; Ma, H.; Wu, C.; Zhang, C.; Xie, Y.; Yang, F.; Zheng, X. A hydrogenated black TiO2 coating with excellent effects for photothermal therapy of bone tumor and bone regeneration. Mater. Sci. Eng. C 2019, 102, 458–470. [Google Scholar] [CrossRef]
- Vu, A.A.; Robertson, S.F.; Ke, D.; Bandyopadhyay, A.; Bose, S. Mechanical and biological properties of ZnO, SiO2, and Ag2O doped plasma sprayed hydroxyapatite coating for orthopaedic and dental applications. Acta Biomater. 2019, 92, 325–335. [Google Scholar] [CrossRef]
- Ke, D.; Vu, A.A.; Bandyopadhyay, A.; Bose, S. Compositionally graded doped hydroxyapatite coating on titanium using laser and plasma spray deposition for bone implants. Acta Biomater. 2019, 84, 414–423. [Google Scholar] [CrossRef]
- Croes, M.; Akhavan, B.; Sharifahmadian, O.; Fan, H.; Mertens, R.; Tan, R.P.; Chunara, A.; Fadzil, A.A.; Wise, S.G.; Kruyt, M.C.; et al. A multifaceted biomimetic interface to improve the longevity of orthopedic implants. Acta Biomater. 2020, 110, 266–279. [Google Scholar] [CrossRef]
- Ankha, M.d.V.E.A.; Silva, A.d.M.; Do Prado, R.F.; Camalionte, M.P.; De Vasconcellos, L.M.R.; Radi, P.A.; Sobrinho, A.S.D.S.; Vieira, L.; Carvalho, Y.R. Effect of DLC films with and without silver nanoparticles deposited on titanium alloy. Braz. Dent. J. 2019, 30, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wu, H.; Wang, F.; Kaewmanee, R.; Pan, Y.; Wang, D.; Qu, P.; Wang, Z.; Hu, G.; Zhao, J.; et al. A hierarchical nanostructural coating of amorphous silicon nitride on polyetheretherketone with antibacterial activity and promoting responses of rBMSCs for orthopedic applications. J. Mater. Chem. B 2019, 7, 6035–6047. [Google Scholar] [CrossRef] [PubMed]
- Hickey, D.J.; Lorman, B.; Fedder, I.L. Improved response of osteoprogenitor cells to titanium plasma-sprayed PEEK surfaces. Colloids Surf. B Biointerfaces 2019, 175, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Beigi, M.H.; Safaie, N.; Nasr-Esfahani, M.H.; Kiani, A. 3D titania nanofiber-like webs induced by plasma ionization: A new direction for bioreactivity and osteoinductivity enhancement of biomaterials. Sci. Rep. 2019, 9, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.Z.; Chen, C.Z.; Yu, H.J.; Zhang, S.J. Application of magnetron sputtering for producing bioactive ceramic coatings on implant materials. Bull. Mater. Sci. 2008, 31, 877–884. [Google Scholar] [CrossRef]
- Juhasz, J.A.; Best, S.M. Surface modification of biomaterials by calcium phosphate deposition. In Surface Modification of Biomaterials: Methods Analysis and Applications; Williams, R., Ed.; Woodhead Publishing Series in Biomaterials: Liverpool, UK, 2011; pp. 143–169. ISBN 9781845696405. [Google Scholar]
- Hwang, C.; Park, S.; Kang, I.G.; Kim, H.E.; Han, C.M. Tantalum-coated polylactic acid fibrous membranes for guided bone regeneration. Mater. Sci. Eng. C 2020, 115, 111112. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Li, J.; Zhu, C.; Zhang, Q.; Yu, J.; Wang, J.; Wang, Q.; Tang, J.; Zhou, H.; Shen, H. Advanced antibacterial activity of biocompatible tantalum nanofilm via enhanced local innate immunity. Acta Biomater. 2019, 89, 403–418. [Google Scholar] [CrossRef]
- Milan, P.B.; Khamseh, S.; Zarrintaj, P.; Ramezanzadeh, B.; Badawi, M.; Morisset, S.; Vahabi, H.; Saeb, M.R.; Mozafari, M. Copper-enriched diamond-like carbon coatings promote regeneration at the bone–implant interface. Heliyon 2020, 6, e03798. [Google Scholar] [CrossRef]
- Tolde, Z.; Starý, V.; Cvrček, L.; Vandrovcová, M.; Remsa, J.; Daniš, S.; Krčil, J.; Bačáková, L.; Špatenka, P. Growth of a TiNb adhesion interlayer for bioactive coatings. Mater. Sci. Eng. C 2017, 80, 652–658. [Google Scholar] [CrossRef]
- Mistry, S.; Roy, S.; Jyoti Maitra, N.; Roy, R.; Datta, S.; Chanda, A.; Sarkar, S. Safety and efficacy of additive and subtractive surface modification of Ti6Al4V endosseous implant in goat bone. J. Mech. Behav. Biomed. Mater. 2016, 57, 69–87. [Google Scholar] [CrossRef]
- Hannink, G.; Arts, J.J.C. Bioresorbability, porosity and mechanical strength of bone substitutes: What is optimal for bone regeneration? Injury 2011, 42, S22–S25. [Google Scholar] [CrossRef] [PubMed]
- Annabi, N.; Nichol, J.W.; Zhong, X.; Ji, C.; Koshy, S.; Khademhosseini, A.; Dehghani, F. Controlling the porosity and microarchitecture of hydrogels for tissue engineering. Tissue Eng. Part B Rev. 2010, 16, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-I.; Wang, Y. Cell responses to surface and architecture of tissue engineering scaffolds. In Regenerative Medicine and Tissue Engineering—Cells and Biomaterials; Eberli, D., Ed.; InTechOpen: Zagreb, Croatia, 2011; pp. 569–588. ISBN 978-953-307-663-8. [Google Scholar]
- Wang, Q.; Zhou, P.; Liu, S.; Attarilar, S.; Ma, R.L.W.; Zhong, Y.; Wang, L. Multi-scale surface treatments of titanium implants for rapid osseointegration: A review. Nanomaterials 2020, 10, 1244. [Google Scholar] [CrossRef]
- Zahran, R.; Rosales Leal, J.I.; Rodríguez Valverde, M.A.; Cabrerizo Vílchez, M.A. Effect of hydrofluoric acid etching time on titanium topography, chemistry, wettability, and cell adhesion. PLoS ONE 2016, 11, e0165296. [Google Scholar] [CrossRef] [PubMed]
- Jemat, A.; Ghazali, M.J.; Razali, M.; Otsuka, Y. Surface modifications and their effects on titanium dental implants. Biomed. Res. Int. 2015, 2015, 791725. [Google Scholar] [CrossRef] [PubMed]
- Shemtov-Yona, K.; Rittel, D.; Dorogoy, A. Mechanical assessment of grit blasting surface treatments of dental implants. J. Mech. Behav. Biomed. Mater. 2014, 39, 375–390. [Google Scholar] [CrossRef]
- Minagar, S.; Berndt, C.C.; Wang, J.; Ivanova, E.; Wen, C. A review of the application of anodization for the fabrication of nanotubes on metal implant surfaces. Acta Biomater. 2012, 8, 2875–2888. [Google Scholar] [CrossRef]
- De Tullio, I.; Berardini, M.; Di Iorio, D.; Perfetti, F.; Perfetti, G. Comparative evaluation among laser-treated, machined, and sandblasted/acid-etched implant surfaces: An in vivo histologic analysis on sheep. Int. J. Implant Dent. 2020, 6, 4–11. [Google Scholar] [CrossRef]
- Riveiro, A.; Maçon, A.L.B.; del Val, J.; Comesaña, R.; Pou, J. Laser surface texturing of polymers for biomedical applications. Front. Phys. 2018, 6, 16. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Wang, C.; Chen, F.; Wang, X.; Lin, K. A comparative study of the osteogenic performance between the hierarchical micro/submicro-textured 3D-printed Ti6Al4V surface and the SLA surface. Bioact. Mater. 2020, 5, 9–16. [Google Scholar] [CrossRef]
- He, W.; Yin, X.; Xie, L.; Liu, Z.; Li, J.; Zou, S.; Chen, J. Enhancing osseointegration of titanium implants through large-grit sandblasting combined with micro-arc oxidation surface modification. J. Mater. Sci. Mater. Med. 2019, 30, 73. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Li, S.; Cui, Y.; Tao, A.; Wang, C.; Li, H.; Zhang, L.; Yu, H.; Jiang, J.; Li, C. Comparison of the osteoblastic activity of low elastic modulus Ti-24Nb-4Zr-8Sn alloy and pure titanium modified by physical and chemical methods. Mater. Sci. Eng. C 2020, 113, 111018. [Google Scholar] [CrossRef] [PubMed]
- Kunrath, M.; dos Santos, R.; de Oliveira, S.; Hubler, R.; Sesterheim, P.; Teixeira, E. Osteoblastic cell behavior and early bacterial adhesion on macro-, micro-, and nanostructured titanium surfaces for biomedical implant applications. Int. J. Oral Maxillofac. Implants 2020, 35, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Yang, G.; Zhang, W.; Hu, J. Investigating the effect of picosecond laser texturing on microstructure and biofunctionalization of titanium alloy. J. Mater. Process. Technol. 2018, 255, 129–136. [Google Scholar] [CrossRef]
- Wu, Y.; Tang, H.; Liu, L.; He, Q.; Zhao, L.; Huang, Z.; Yang, J.; Cao, C.; Chen, J.; Wang, A. Biomimetic titanium implant coated with extracellular matrix enhances and accelerates osteogenesis. Nanomedicine 2020, 15, 1779–1793. [Google Scholar] [CrossRef]
- Keceli, H.G.; Bayram, C.; Celik, E.; Ercan, N.; Demirbilek, M.; Nohutcu, R.M. Dual delivery of platelet-derived growth factor and bone morphogenetic factor-6 on titanium surface to enhance the early period of implant osseointegration. J. Periodontal Res. 2020, 55, 694–704. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Carinci, F.; Orsini, T.; Valbonetti, L.; Qorri, E.; Bignozzi, C.A.; Lorusso, F. Titanium implants coated with a bifunctional molecule with antimicrobic activity: A rabbit study. Materials (Basel) 2020, 13, 3613. [Google Scholar] [CrossRef]
- Feletto, L.; Bengazi, F.; Urbizo Velez, J.J.; Ferri, M.; Favero, R.; Botticelli, D. Bone healing at collagenated bicortically installed implants: An experimental study in rabbits. Oral Maxillofac. Surg. 2020. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Z. Involvement of FAK/P38 signaling pathways in mediating the enhanced osteogenesis induced by nano-graphene oxide modification on titanium implant surface. Int. J. Nanomed. 2020, 15, 4659–4676. [Google Scholar] [CrossRef]
- Choi, J.Y.; Kim, S.; Jo, S.B.; Kang, H.K.; Jung, S.Y.; Kim, S.W.; Min, B.M.; Yeo, I.S.L. A laminin-211-derived bioactive peptide promotes the osseointegration of a sandblasted, large-grit, acid-etched titanium implant. J. Biomed. Mater. Res. Part A 2020, 108, 1214–1222. [Google Scholar] [CrossRef]
- Ko, K.A.; Kim, S.; Choi, S.H.; Lee, J.S. Randomized controlled clinical trial on calcium phosphate coated and conventional SLA surface implants: 1-year study on survival rate and marginal bone level. Clin. Implant Dent. Relat. Res. 2019, 21, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Lorusso, F.; Orsini, T.; Morra, M.; Iviglia, G.; Valbonetti, L. Biomimetic surfaces coated with covalently immobilized collagen type I: An x-ray photoelectron spectroscopy, atomic force microscopy, micro-CT and histomorphometrical study in rabbits. Int. J. Mol. Sci. 2019, 20, 724. [Google Scholar] [CrossRef] [PubMed]
- Roest, R.; Latella, B.A.; Heness, G.; Ben-Nissan, B. Adhesion of sol-gel derived hydroxyapatite nanocoatings on anodised pure titanium and titanium (Ti6Al4V) alloy substrates. Surf. Coat. Technol. 2011, 205, 3520–3529. [Google Scholar] [CrossRef]
- Van Hooreweder, B.; Apers, Y.; Lietaert, K.; Kruth, J.P. Improving the fatigue performance of porous metallic biomaterials produced by Selective Laser Melting. Acta Biomater. 2017, 47, 193–202. [Google Scholar] [CrossRef] [PubMed]

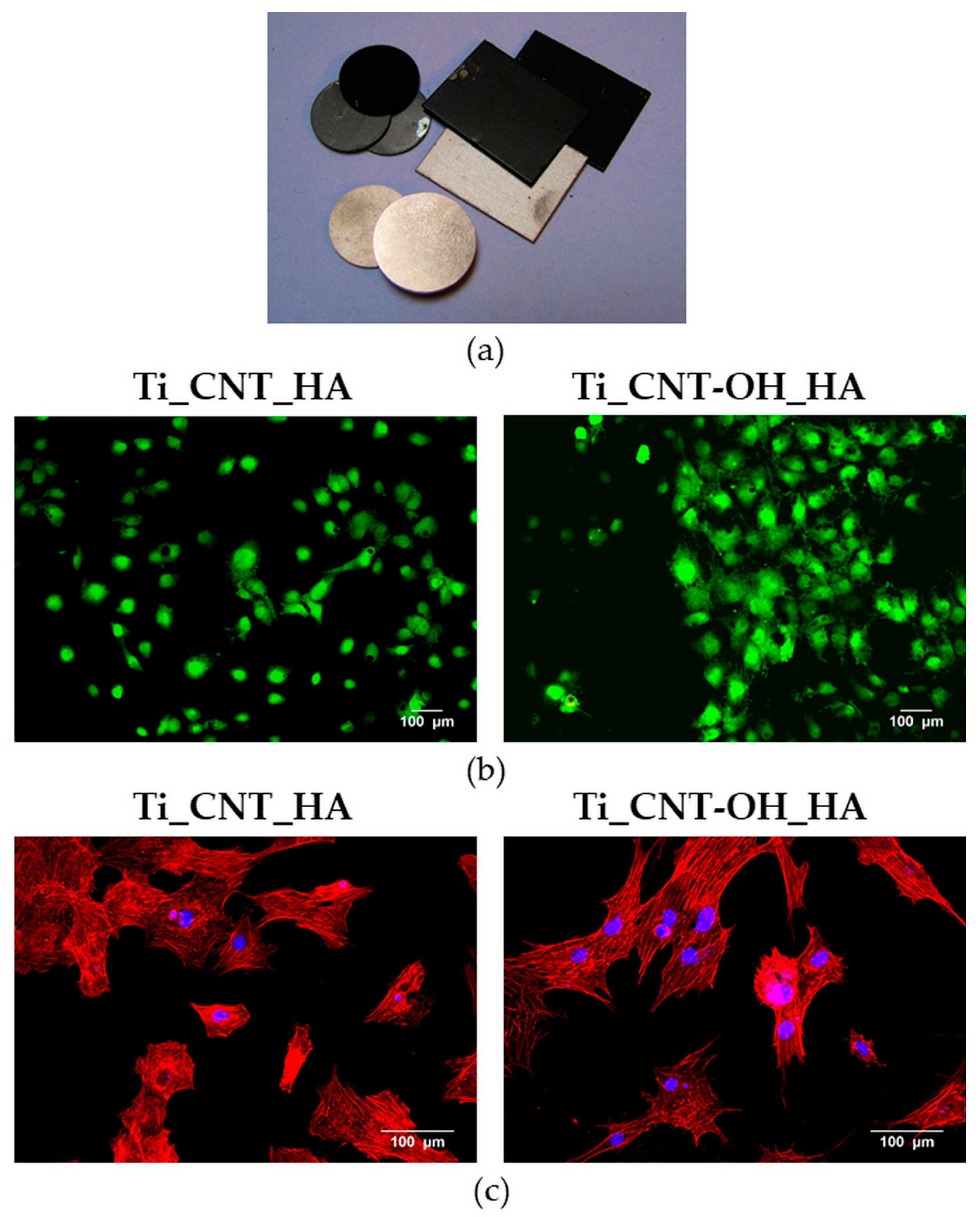
| Coating Material | Type of Biomaterial | Experimental Model | Impact of Surface Coating on Biological Properties of Biomaterial | Limitations | Ref. |
|---|---|---|---|---|---|
| HA | Titanium | In vivo (rat model) | Supported new bone formation in vivo | Not provided | [37] |
| HA and Si-doped HA | Titanium | In vitro (mouse calvarial preosteoblast cell line—MC3T3-E1 and HUVECs cell line) | Supported cell spreading and osteogenic differentiation | Not provided | [39] |
| Oxidized multi-walled carbon nanotubes | Titanium | In vitro (human fetal osteoblast cell line—hFOB 1.19) | Highly conductive and non-toxic character | Inhibited cell growth and proliferation | [59] |
| Multi-walled carbon nanotubes functionalized with OH groups | Titanium | In vitro (human fetal osteoblast cell line—hFOB 1.19) | Nanorough topography supporting cell attachment, spreading and growth | Not provided | [60] |
| Calcium phosphate (CaP)/Ag | Titanium | In vitro (human osteosarcoma cell line—Saos-2) | Non-toxic character, supported cell attachment and spreading | Cytotoxic character of microsized silver phosphate particles (unlike nanoparticles) | [41] |
| TiO2/ZnO | Titanium | In vitro (human osteosarcoma cell line—Saos-2) | Enhanced cell adhesion and osteogenic differentiation | Not provided | [43] |
| Zeolitic imidazolate framework-8 film | Titanium | In vitro (human osteosarcoma cell line—MG-63) | Enhanced bALP activity, superior expression of genes for bALP, Runx2, and increased ECM mineralization | Cytotoxic character of microsized zeolitic imidazolate framework-8 film (unlike nanosized film) | [53] |
| HA/chitosan/gentamicin | Titanium | In vitro (mice fibroblast cell line—L929 and human lung fibroblast cell line—MRC-5) | Non-cytotoxicity | Risk of the development of antibiotic resistance | [65] |
| Tricalcium phosphate (TCP)/silk/vancomycin | Titanium | In vitro (mouse calvarial preosteoblast cell line—MC3T3-E1) | Enhanced cell attachment, spreading, proliferation, and ECM mineralization | Risk of the development of antibiotic resistance | [67] |
| HA/tannic acid | Titanium modified by TiO2 nanotube arrays | In vitro (mouse calvarial preosteoblast cell line—MC3T3-E1) | Improved cell adhesion and proliferation | Not provided | [66] |
| HA | Titanium alloy Ti–6Al–4V, Stainless steel | In vivo (canine model) | Induced bone formation and proved good osseointegration in vivo | Not provided | [38] |
| Si-doped HA and Sr-doped HA | Titanium alloy Ti–6Al–4V | In vivo (sheep model) | Increased osseointegration | Not provided | [40] |
| Glass-ceramics | Titanium alloy Ti–6Al–4V | In vitro (human osteosarcoma cell line—Saos-2) | Supported cell spreading and proliferation | Disordered/random surface topography | [42] |
| BaTiO3 | Titanium alloy Ti–6Al–4V | In vitro (rabbit BMDSCs) and in vivo (rabbit model) | Supported cell adhesion, proliferation, and osteogenic differentiation in vitro; increased new bone formation in vivo | Decreased compressive strength | [46] |
| Fe3O4/polydopamine | Titanium alloy Ti–6Al–4V | In vitro (human BMDSCs) and in vivo (rabbit model) | Supported cell attachment, proliferation and osteogenic differentiation in vitro; accelerated new bone formation in vivo | Not provided | [45] |
| Polycrystalline diamond | Titanium alloy Ti–6Al–4V | In vitro (Chinese hamster ovarian cell line—CHO) | Enhanced cell growth in vitro | Not provided | [55] |
| HA and HA/collagen | Titanium alloy Ti–6Al–4V | In vitro (mouse calvarial preosteoblast cell line—MC3T3-E1) | Improved cell adhesion and proliferation | Decreased mechanical properties | [64] |
| poly(methyl methacrylate) (PMMA)/SiO2 | Titanium alloy Ti–6Al–4V | In vitro (human fetal osteoblast cell line—hFOB 1.19) | Enhanced cell proliferation | Decreased surface roughness | [68] |
| CaP/PLA | Tantalum | In vitro (human osteosarcoma cell line—MG-63) and in vivo (rabbit model) | Supported cell attachment and spreading in vitro; accelerated new bone formation in vivo | Not provided | [69] |
| Gellatin/HA | BaTiO3-based scaffold | In vitro (human osteosarcoma cell line—MG-63) | Supported cell adhesion and proliferation | Not provided | [48] |
| Zeolite/Ag | Stainless steel | In vitro (rabbit BMDSCs) | Non-cytotoxicity | Slightly decreased cell adhesion and proliferation | [52] |
| Boron nitride (BN) | Stainless steel wire | In vivo (rat model) | Accelerated fracture healing by increase in bone volume/tissue volume ratio and bone surface values, increased bALP levels | Decreased osteocalcin levels | [63] |
| Ultra-nanocrystalline diamond | Silicon microchips | In vivo (mouse model) | Reduced foreign-body response in vivo | Not provided | [56] |
| SiO2/P2O5/CaO/MgO/Na2O | Silicon | In vitro (human fibroblast cell line—BJ) | Non-cytotoxicity | Not provided | [44] |
| Carbon nanotubes | Glass | In vitro (rat BMDSCs) | Promoted expression of osteogenic markers and ECM mineralization | Unaffected ECM mineralization on multi-walled carbon nanotubes coating (unlike single-walled carbon nanotubes coating) | [58] |
| Amorphous carbon | β-TCP | In vitro (rat BMDSCs) and in vivo (rat model) | Enhanced cell adhesion, proliferation, and bALP activity; supported new bone formation in vivo | Decreased surface roughness | [54] |
| Carbon nanotubes | PCL nanofibers | In vitro (human BMDSCs) and in vivo (rat model) | Supported cell adhesion and bone formation in vitro; enhanced ECM synthesis and new bone formation in vivo | Slightly reduced elastic modulus | [57] |
| Graphene oxide coating | Alginate-based scaffold | In vitro (human ADSCs) | Supported cell adhesion, proliferation, and osteogenic differentiation | Not provided | [61] |
| Coating Material | Type of Biomaterial | Experimental Model | Impact of Surface Coating on Biological Properties of Biomaterial | Limitations | Ref. |
|---|---|---|---|---|---|
| Albumin | Titanium | In vitro (mouse calvarial preosteoblast cell line—MC3T3-E1) | Increased cell viability | Not provided | [76] |
| Whey protein isolate fibril | Glass | In vitro (human BMDSCs) | Promoted cell spreading and osteogenic differentiation | Not provided | [72] |
| Hyaluronic acid/collagen and chondroitin sulfate/collagen | Glass | In vitro (murine embryonic fibroblast cell line—C3H10T1/2) | Promoted cell adhesion and osteogenic differentiation | Not provided | [73] |
| PLGA | β-TCP | In vitro (canine mesenchymal stem cells and canine endothelial progenitor cells) | Increased cell attachment and proliferation, enhanced osteogenic differentiation | Decreased total porosity | [78] |
| Dopamine/gelatin/rhBMP-2 | β-TCP/Mg–Zn | In vitro (rat BMDSCs) and in vivo (rabbit model) | Supported cell proliferation and osteogenic differentiation in vitro; enhanced new bone formation in vivo | Not provided | [74] |
| Gelatin/mucic acid | PLA | In vitro (murine embryonic fibroblast cell line—C3H10T1/2) | Supported cell adhesion, osteogenic differentiation, and ECM mineralization | Decreased mechanical properties and increased degradation degree | [77] |
| PLGA/aspirin | Ti-polydopamine | In vitro (rat BMDSCs) and in vivo (rat model) | Increased cell proliferation, induced osteogenic differentiation, anti-inflammatory effect in vitro; improved osseointegration in vivo | Not provided | [79] |
| Simvastatin/chitosan | HA-coated polyethylene terephthalate | In vitro (mouse calvarial preosteoblast cell line—MC3T3-E1) and in vivo (rat model) | Induced osteogenic differentiation in vitro; enhanced osseointegration in vivo | Not provided | [80] |
| Coating Material and Plasma Technique | Type of Biomaterial | Experimental Model | Impact of Surface Coating on Biological Properties of Biomaterial | Ref. |
|---|---|---|---|---|
| Air-plasma spraying treatment and strontium-doped CaP coating | Titanium | In vitro (rabbit BMDSCs) | Enhanced cell adhesion, proliferation, osteogenic differentiation and ECM mineralization | [90] |
| Ion-assisted plasma polymerization treatment and BMP-2 coating | Titanium | In vitro (human BMDSCs and mouse macrophage reporter cell line—RAW Blue) and in vivo (rat model) | Promoted spreading, proliferation, and osteogenic differentiation of stem cells in vitro, supported adhesion and viability of macrophages without promoting NF-κB activation in vitro. Promoted new bone formation in vivo | [105] |
| Tantalum-doped HA coating deposited by atmospheric plasma spraying | Titanium | In vitro (rat BMDSCs) | Enhanced cell adhesion, spreading, proliferation, osteogenic differentiation, and ECM mineralization | [92] |
| Titanium carbide coating deposited by ion plating plasma assisted | Titanium | In vitro (human osteosarcoma cell line—Saos-2 and human primary osteoblasts) | Enhanced cell adhesion, spreading, and proliferation | [93] |
| Coating composed of 60% graphitic carbon, 25% titanium oxides and 15% titanium carbide deposited by ion plating plasma assisted | Titanium | In vivo (rabbit model) | Improved osseointegration | [94] |
| Zinc ions containing coating deposited by plasma electrolytic oxidation | Titanium | In vivo (rabbit model) | Enhanced osseointegration and new bone formation | [96] |
| Magnesium-doped titanium dioxide coating deposited by plasma electrolytic oxidation | Titanium | In vitro (mouse calvarial preosteoblast cell line—MC3T3-E1) and in vivo (rabbit model) | Promoted cell adhesion, proliferation, and osteogenic differentiation in vitro; enhanced osseointegration in vivo | [97] |
| Silicon-doped HA coating deposited by atmospheric plasma spraying | Titanium alloy Ti–6Al–4V | In vitro (rabbit BMDSCs) and in vivo (rabbit model) | Improved cell proliferation, osteogenic and angiogenic differentiation, hindered osteoclastogenesis in vitro; promoted new bone formation and osseointegration in vivo | [91] |
| Zinc-doped HA coating deposited by liquid precursor plasma spraying | Titanium alloy Ti–6Al–4V | In vitro (rat BMDSCs and mouse osteoclast-like cell precursor cell—RAW 264.7 cell line) | Moderately promoted osteogenic differentiation of BMDSCs and hindered osteoclastic activity at early stages | [101] |
| ZnO-, SiO2-, Ag2O-doped HA coating deposited by inductively coupled radio-frequency plasma spraying system | Titanium alloy Ti–6Al–4V | In vivo (rat model) | Enhanced new bone formation, osseointegration, and bone mineralization | [103] |
| MgO-, Ag2O-doped HA coating deposited by plasma spray process | Titanium alloy Ti–6Al–4V | In vitro (primary human osteoblasts) | Slightly positive effect on cell proliferation and osteogenic differentiation | [104] |
| Diamond-like carbon and diamond-like carbon with silver nanoparticles coatings deposited by plasma-enhanced chemical vapor deposition | Titanium alloy Ti–6Al–4V | In vivo (rabbit model) | Enhanced osseointegration | [106] |
| Hydrogenated black TiO2 coating deposited by inductively coupled radio-frequency plasma spraying system | Titanium alloy Ti–6Al–4V | In vitro (rat BMDSCs) | Improved cell adhesion, proliferation, and differentiation | [102] |
| Calcium and phosphorus ions containing coating deposited by plasma electrolytic oxidation | Titanium alloy Ti–6Al–4V | In vivo (rat model) | Enhanced osseointegration and new bone formation | [95] |
| Calcium ions containing coating deposited by plasma electrolytic oxidation | Titanium alloy Ti–13Nb–13Zr alloy | In vitro (human BMDSCs) | Enhanced cell adhesion, proliferation, and osteogenic differentiation | [100] |
| Bilayer coating containing HA nanorods and MgO with HA/Mg(OH)2 deposited by plasma electrolytic oxidation | Magnesium | In vitro (rabbit BMDSCs) and in vivo (rabbit model) | Enhanced ECM mineralization in vitro; promoted osseointegration in vivo | [98] |
| Calcium, phosphorus, and silicon or fluorine ions containing coating deposited by plasma electrolytic oxidation | Magnesium 0.8 wt.% calcium alloy | In vitro (mouse calvarial preosteoblast cell line—MC3T3-E1) | Supported cell growth, collagen secretion, and ECM mineralization | [99] |
| Nano fibrous titania coating deposited by high intensity laser-induced reverse transfer | Glass | In vitro (human BMDSCs) | Promoted cell spreading, proliferation, differentiation, and ECM mineralization | [109] |
| Silicon nitride coating deposited by inductively coupled plasma-enhanced chemical vapor deposition | PEEK | In vitro (rat BMDSCs) | Promoted cell spreading, proliferation, and osteogenic differentiation | [107] |
| Titanium coating deposited by plasma spray process | PEEK | In vitro (human fetal osteoblast cells—hFOB 1.19) | Promoted cell proliferation and ECM mineralization | [108] |
| Subtractive Modification Method | Type of Biomaterial | Experimental Model | Impact of Surface Coating on Biological Properties of Biomaterial | Limitations | Ref. |
|---|---|---|---|---|---|
| Acid etching and anodizing | Titanium | In vitro (rat ADSCs) | Promoted cell adhesion | Not provided | [130] |
| SLA or laser-treatment | Titanium | In vivo (sheep model) | Increased osseointegration | Not provided | [125] |
| SLA and micro-arc oxidation | Titanium | In vitro (rat BMDSCs) and in vivo (dog model) | Enhanced cell adhesion, proliferation, and osteogenic differentiation in vitro; promoted osseointegration in vivo | Not provided | [128] |
| SLA or anodizing | Titanium and titanium alloy Ti–24Nb–4Zr–8Sn | In vitro (human BMDSCs) | Only anodizing increased ECM mineralization | SLA and anodizing decreased cell proliferation | [129] |
| SLA | Titanium alloy Ti–6Al–4V | In vitro (rat BMDSCs) and in vivo (rat model) | Enhanced cell adhesion, proliferation, and osteogenic differentiation in vitro; increased osseointegration in vivo | Not provided | [127] |
| Picosecond laser | Titanium alloy Ti–6Al–4V | In vitro (rat BMDSCs) | Supported cell adhesion | Not provided | [131] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazimierczak, P.; Przekora, A. Osteoconductive and Osteoinductive Surface Modifications of Biomaterials for Bone Regeneration: A Concise Review. Coatings 2020, 10, 971. https://doi.org/10.3390/coatings10100971
Kazimierczak P, Przekora A. Osteoconductive and Osteoinductive Surface Modifications of Biomaterials for Bone Regeneration: A Concise Review. Coatings. 2020; 10(10):971. https://doi.org/10.3390/coatings10100971
Chicago/Turabian StyleKazimierczak, Paulina, and Agata Przekora. 2020. "Osteoconductive and Osteoinductive Surface Modifications of Biomaterials for Bone Regeneration: A Concise Review" Coatings 10, no. 10: 971. https://doi.org/10.3390/coatings10100971
APA StyleKazimierczak, P., & Przekora, A. (2020). Osteoconductive and Osteoinductive Surface Modifications of Biomaterials for Bone Regeneration: A Concise Review. Coatings, 10(10), 971. https://doi.org/10.3390/coatings10100971