Novel Procedure for Brochantite Based Pigment Production and Its Immobilization for Restoration of Historical Copper Objects
Abstract
:1. Introduction
2. Experimental
2.1. Small Volume Experiments
2.2. Large Volume Experiments
2.3. Pigment Immobilization
3. Results and Discussion
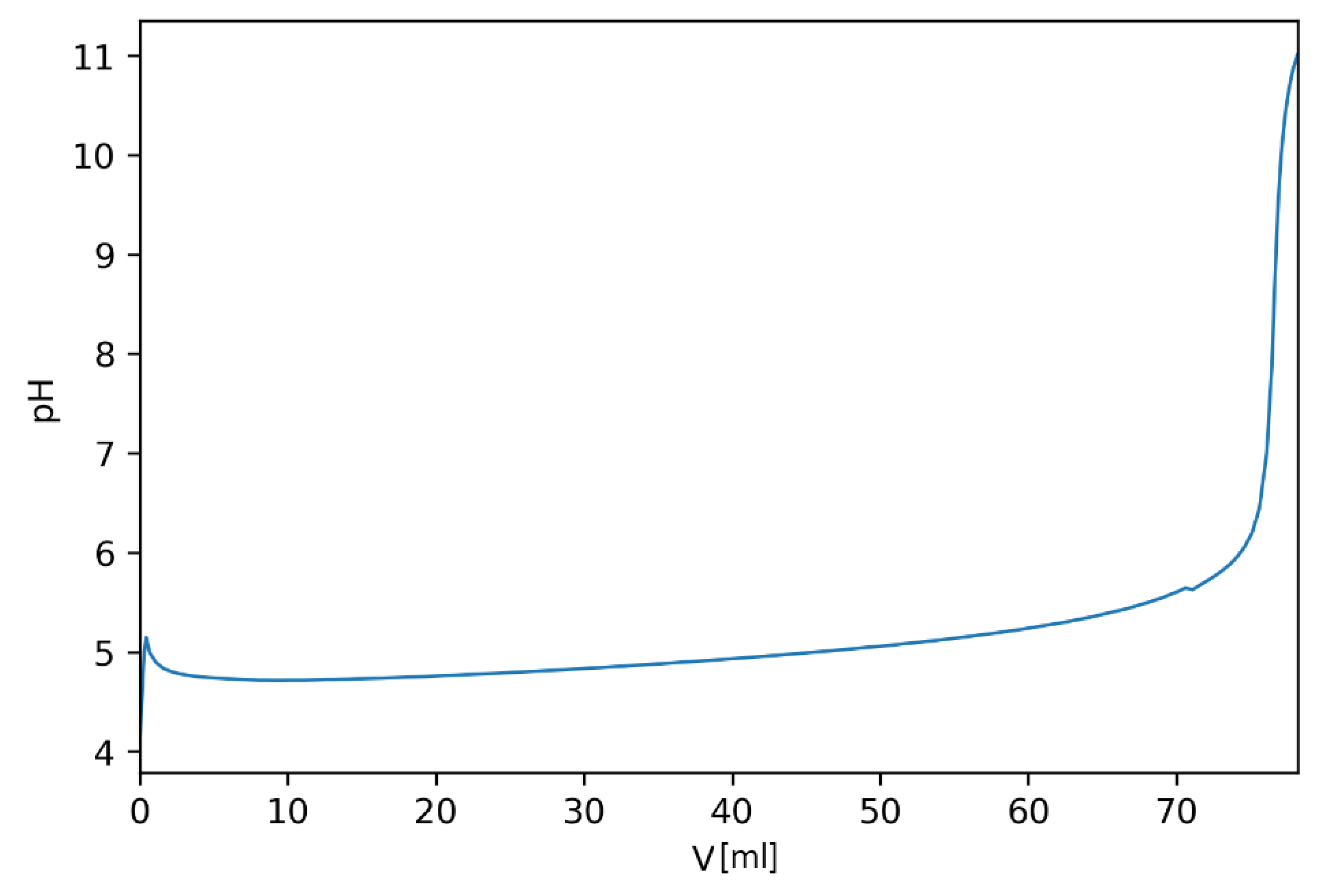
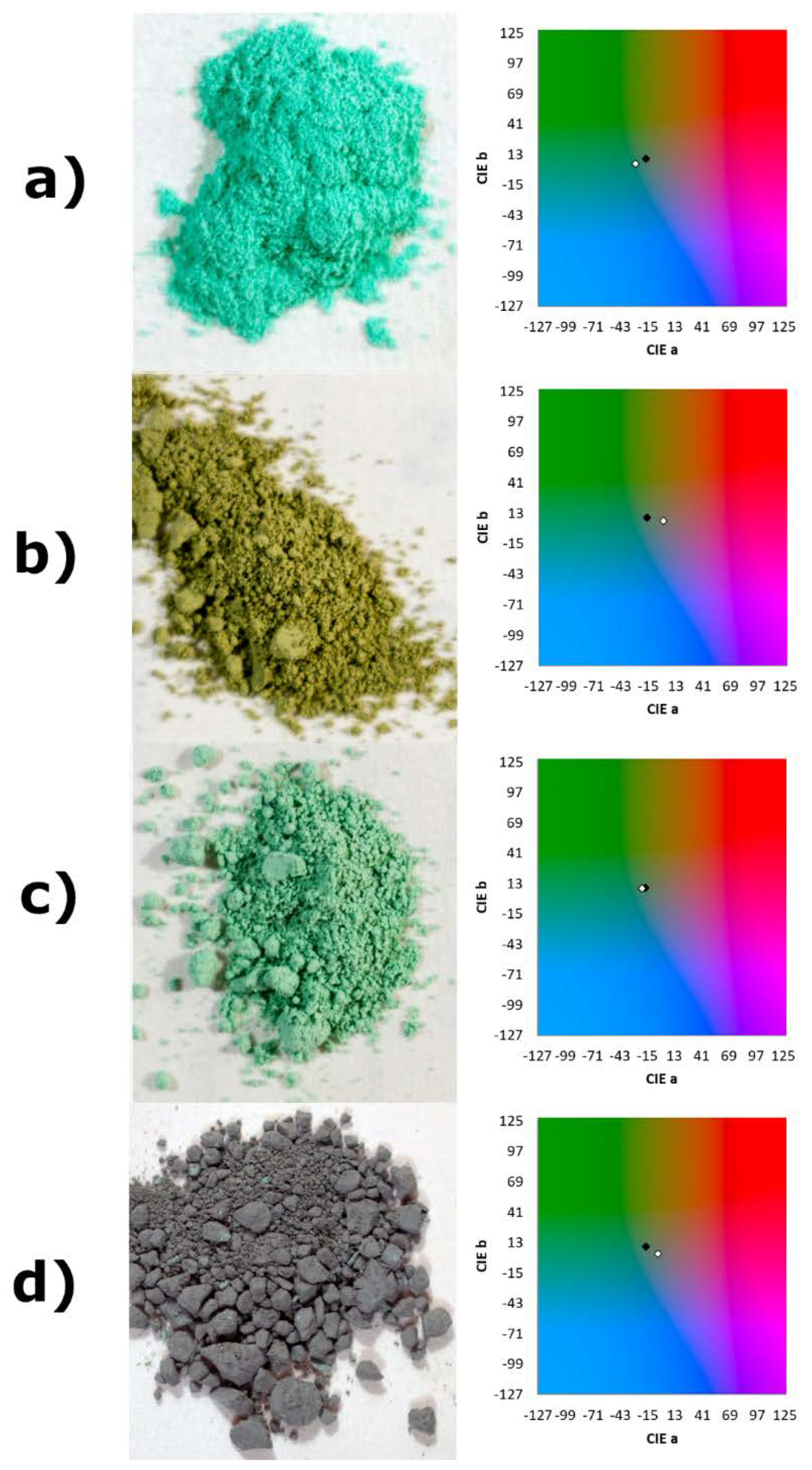
3.1. Small Volume Experiments
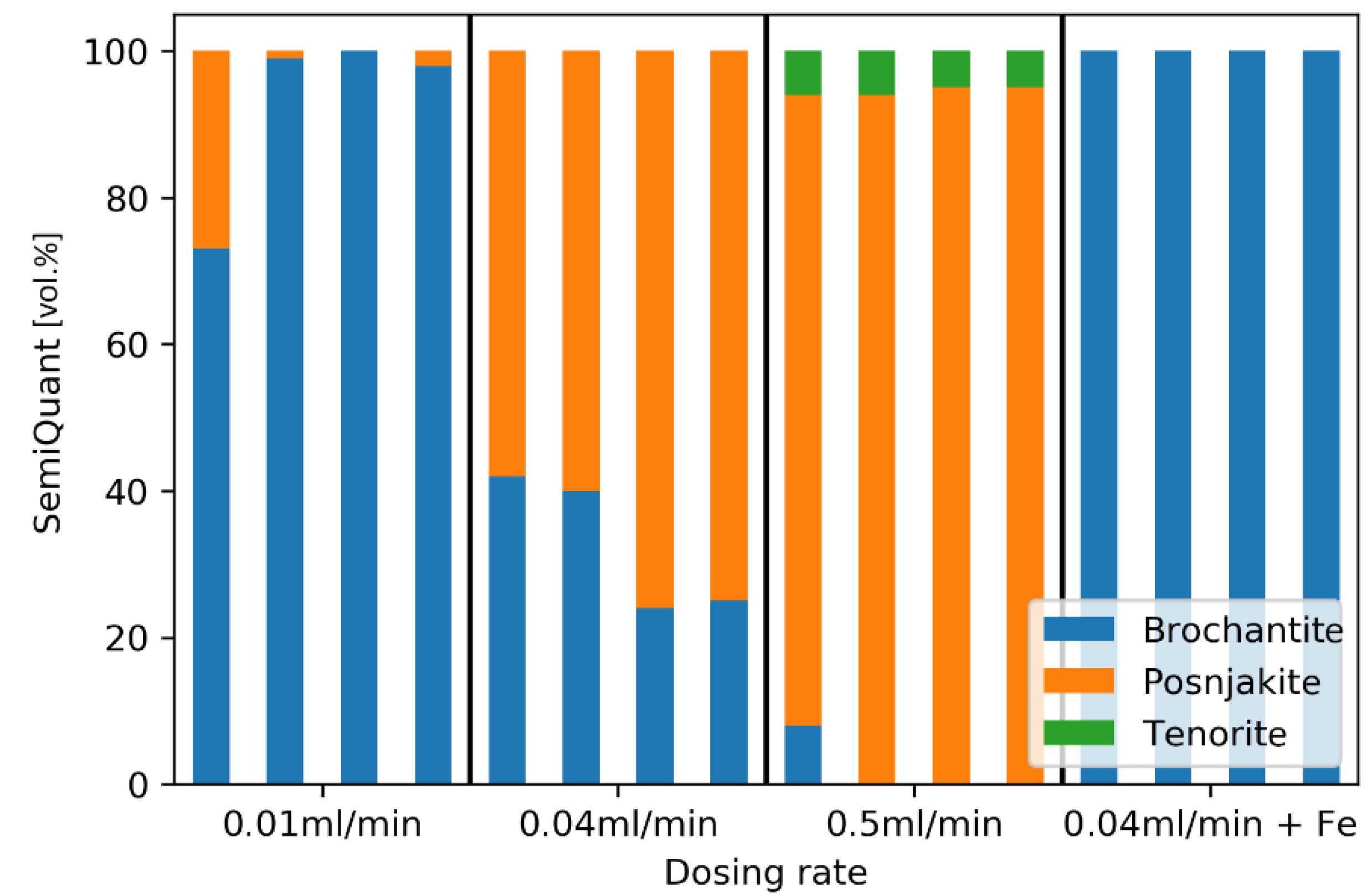
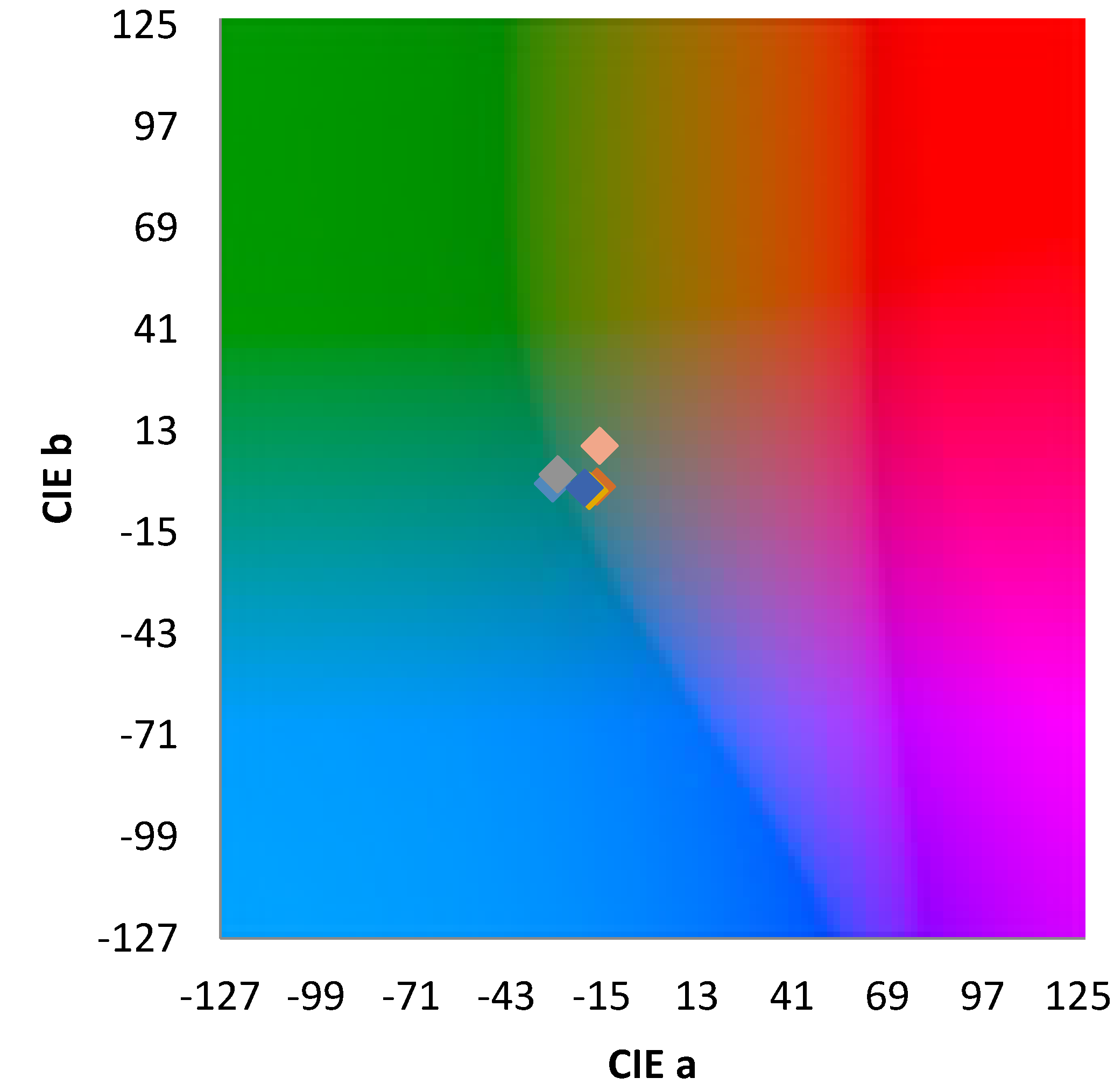
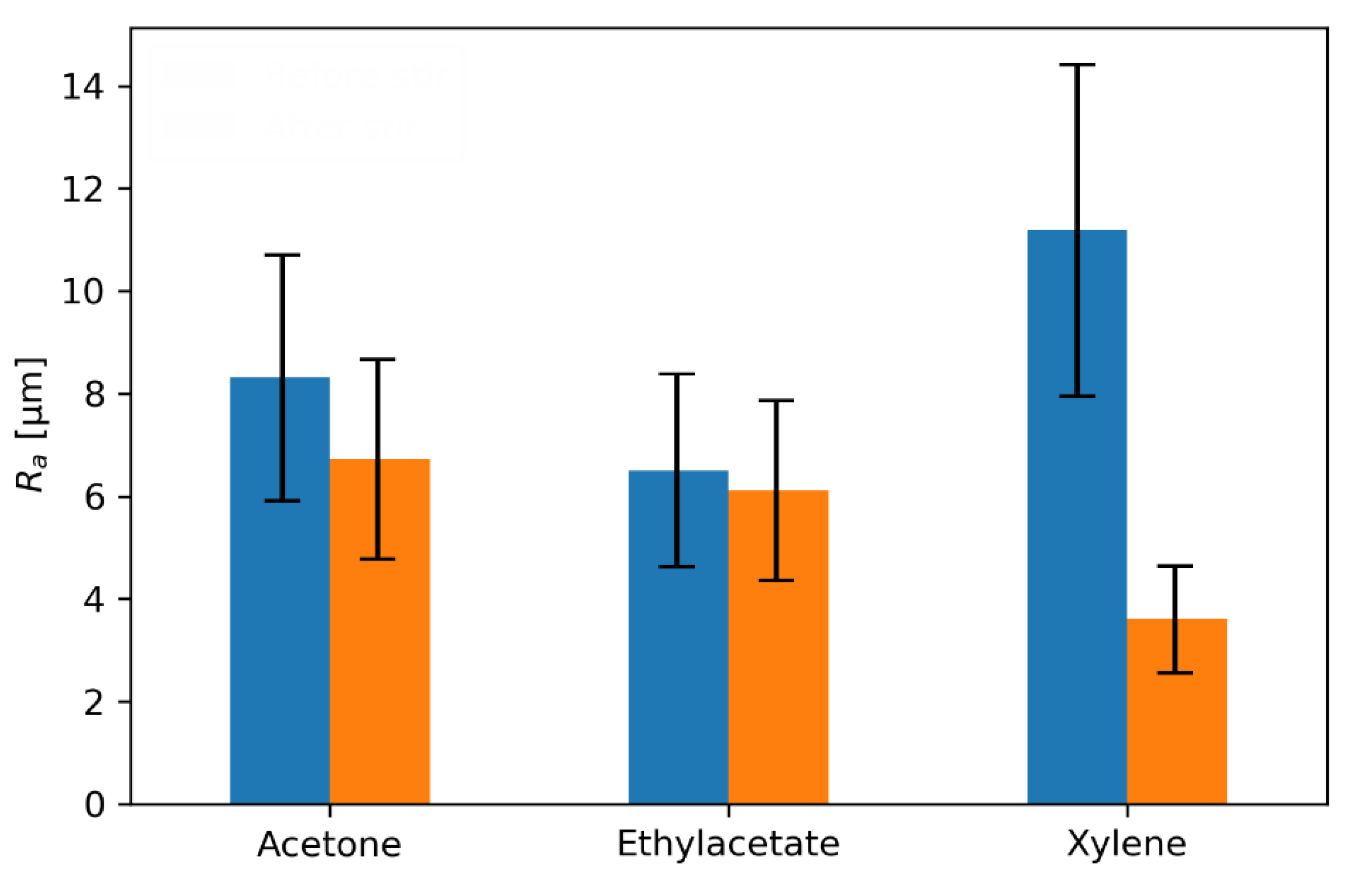
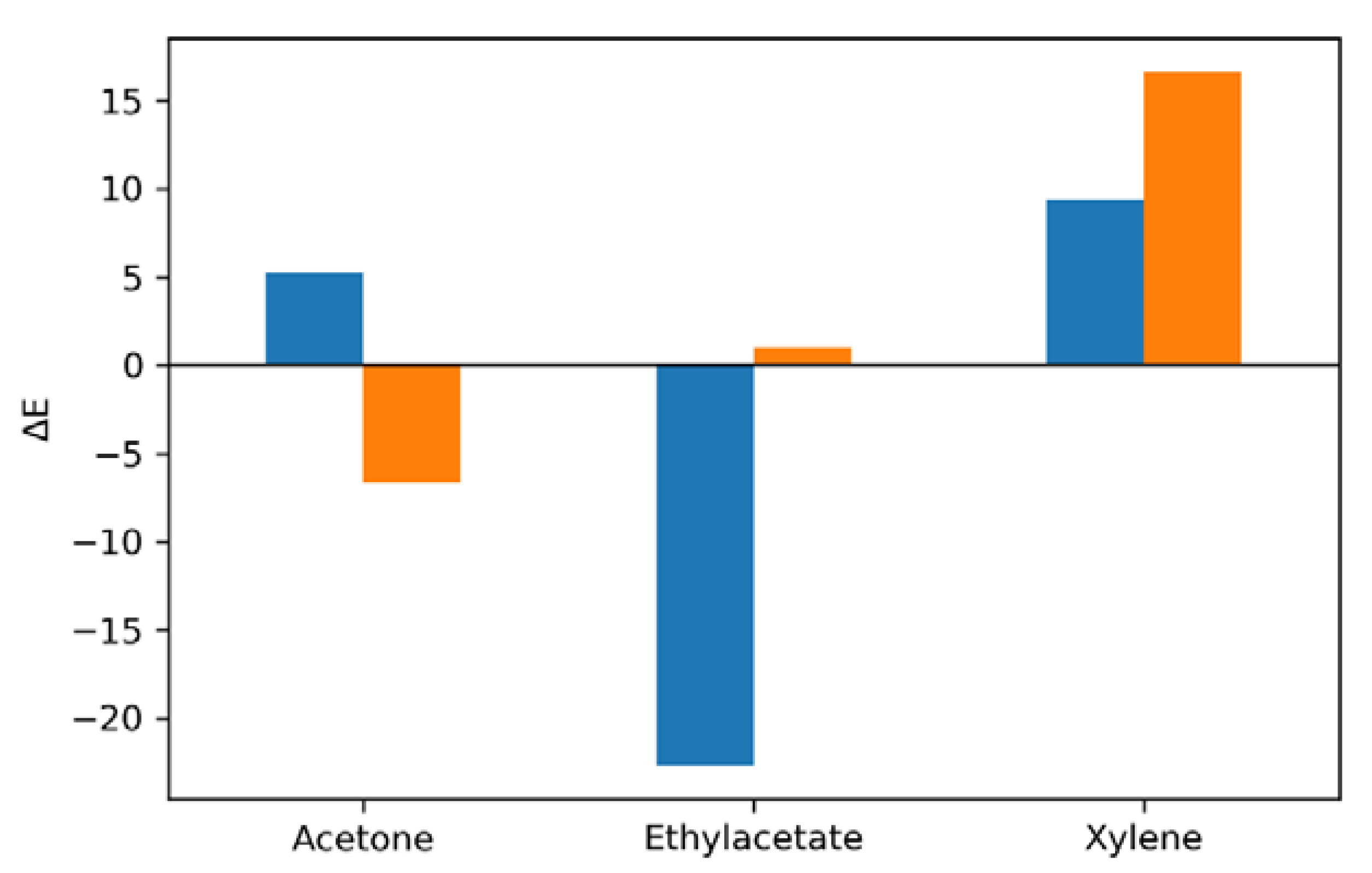
3.2. Large Volume Experiments
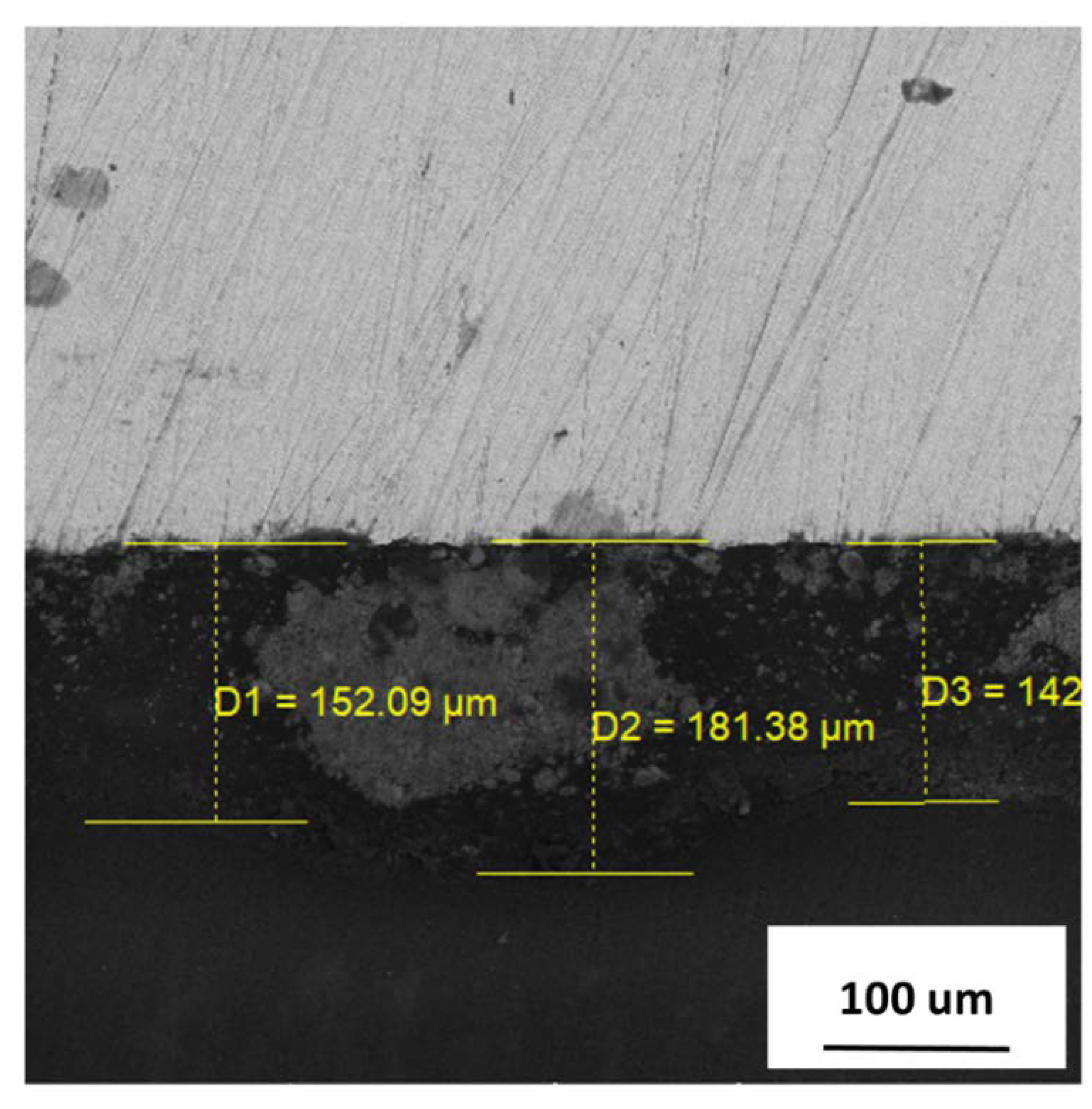
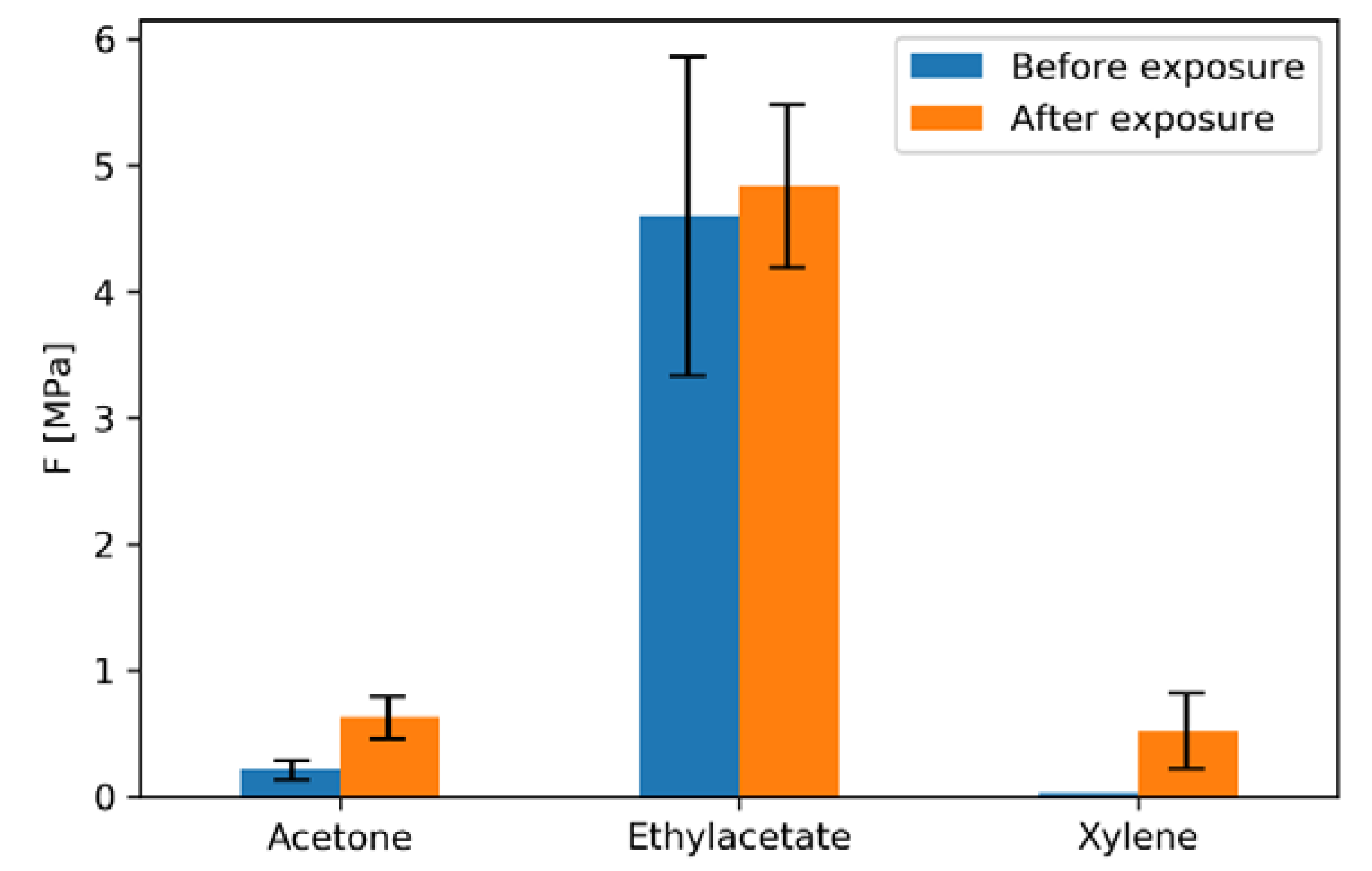
3.3. Pigment Immobilization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Krätschmer, A.; Wallinder, I.O.; Leygraf, C. The evolution of outdoor copper patina. Corros. Sci. 2002, 44, 425–450. [Google Scholar] [CrossRef]
- Livingstone, R.A. Influence of the environment on the patina of the Statue of Liberty. Environ. Sci. Technol. 1991, 25, 8. [Google Scholar] [CrossRef]
- Franey, J.P.; Davis, M.E. Metallographic studies of the copper patina formed in the atmosphere. Corros. Sci. 1987, 27, 659–668. [Google Scholar] [CrossRef]
- Hernández, R.d.P.B.; Aoki, I.V.; Tribollet, B.; de Melo, H.G. Electrochemical impedance spectroscopy investigation of the electrochemical behaviour of copper coated with artificial patina layers and submitted to wet and dry cycles. Electrochim. Acta 2011, 56, 2801–2814. [Google Scholar] [CrossRef]
- López, J.L.; Veleva, L.; López-Sauri, D.A. Multifractal Detrended Analysis of the Corrosion Potential Fluctuations During Copper Patina formation on Its First Stages in Sea Water. Int. J. Electrochem. Sci. 2014, 9, 1637–1649. [Google Scholar]
- Nassau, K.; Miller, A.E.; Graedel, T.E. The reaction of simulated rain with copper, copper patina, and some copper compounds. Corros. Sci. 1987, 27, 703–719. [Google Scholar] [CrossRef]
- Robbiola, L.; Rahmouni, K.; Chiavari, C.; Martini, C.; Prandstraller, D.; Texier, A.; Takenouti, H.; Vermaut, P. New insight into the nature and propertiesof pale gren surfaces of outdoor bronze monuments. Appl. Phys. A 2008, 92, 8. [Google Scholar] [CrossRef]
- Salnick, A.; Faubel, W.; Klewe-Nebenius, H.; Vendl, A.; Ache, H.J. Photothermal studies of copper patina formed in the atmosphere. Corros. Sci. 1995, 37, 741–767. [Google Scholar] [CrossRef]
- Mennucci, M.M.; Sanchez-Moreno, M.; Aoki, I.V.; Bernard, M.C.; de Melo, H.G.; Joiret, S.; Vivier, V. Local electrochemical investigation of copper patina. J. Solid State Electrochem. 2011, 16, 109–116. [Google Scholar] [CrossRef]
- Hayez, V.; Segato, T.; Hubin, A.; Terryn, H. Study of copper nitrate-based patinas. J. Raman Spectrosc. 2006, 37, 1211–1220. [Google Scholar] [CrossRef]
- Marušić, K.; Otmačić-Ćurković, H.; Horvat-Kurbegović, Š.; Takenouti, H.; Stupnišek-Lisac, E. Comparative studies of chemical and electrochemical preparation of artificial bronze patinas and their protection by corrosion inhibitor. Electrochim. Acta 2009, 54, 7106–7113. [Google Scholar] [CrossRef]
- Graedel, T.E.; Nassau, K.; Franey, J.P. Copper patinas formed in the atmosphere—I. Introduction. Corros. Sci. 1987, 27, 639–657. [Google Scholar] [CrossRef]
- Palmer, D.A.; Bénézeth, P. Solubility of Copper Oxides in Water and Steam.pdf. In Proceedings of the 14th International Conference on the Properties of Water and Steam, Kyoto, Japan, 29 August–3 September 2004; pp. 491–496. [Google Scholar]
- Núñez, L.; Reguera, E.; Corvo, F.; González, E.; Vazquez, C. Corrosion of copper in seawater and its aerosols in a tropical island. Corros. Sci. 2005, 47, 461–484. [Google Scholar] [CrossRef]
- Noli, F.; Misaelides, P.; Pavlidou, E.; Kokkoris, M. Investigation of copper patinas using ion beam analysis and scanning electron microscopy. Surf. Interface Anal. 2005, 37, 288–293. [Google Scholar] [CrossRef]
- Watanabe, M.; Toyoda, E.; Handa, T.; Ichino, T.; Kuwaki, N.; Higashi, Y.; Tanaka, T. Evolution of patinas on copper exposed in a suburban area. Corros. Sci. 2007, 49, 766–780. [Google Scholar] [CrossRef]
- Watanabe, M.; Higashi, Y.; Tanaka, T. Differences between corrosion products formed on copper exposed in Tokyo in summer and winter. Corros. Sci. 2003, 45, 1439–1453. [Google Scholar] [CrossRef]
- Veleva, L.; Farro, W. Influence of seawater and its aerosols on copper patina composition. Appl. Surf. Sci. 2012, 258, 10072–10076. [Google Scholar] [CrossRef]
- Fitzgerald, K.P.; Nairn, J.; Atrens, A. The chemistry of copper patination. Corros. Sci. 1998, 40, 2029–2050. [Google Scholar] [CrossRef]
- Kreislová, K.; Geiplova, H.; Skořepová, I.; Skořepa, J.; Majtás, D. Up-dated maps of atmospheric corrosivity for Czech Republic. KOM 2015, 59, 81–86. [Google Scholar]
- Kreislová, K.; Geiplová, H.; Barták, Z.; Majtás, D. Atmospheric corrosion models. KOM 2017, 61, 59–66. [Google Scholar] [CrossRef] [Green Version]
- Bureš, R.; Klajmon, M.; Fojt, J.; Rak, P.; Jílková, K.; Stoulil, J. Artificial Patination of Copper and Copper Alloys in Wet Atmosphere with Increased Content of SO2. Coatings 2019, 9, 837. [Google Scholar] [CrossRef] [Green Version]
- Stoulil, J.; Šedá, P.; Anisová, M.; Fencl, Z.; Novák, P.; Děd, J. Defects of copper patina. KOM 2015, 59, 87–90. [Google Scholar]
- Rak, P.; Bureš, R. Copper and copper patina. KOM 2017, 61, 118–122. [Google Scholar] [CrossRef] [Green Version]
- Atrens, A. Artificial Patina. Patent EP0797631A1, 21 April 1995. [Google Scholar]
- Di Carlo, G.; Giuliani, C.; Riccucci, C.; Pascucci, M.; Messina, E.; Fierro, G.; Lavorgna, M.; Ingo, G.M. Artificial patina formation onto copper-based alloys: Chloride and sulphate induced corrosion processes. Appl. Surf. Sci. 2017, 421, 120–127. [Google Scholar] [CrossRef]
- Gervais, J. Methode for Forming Artificially and Rapidly Patina on Copper, Products Thereof and Solutions Therefor. U.S. Patent 5,160,381A, 26 June 1991. [Google Scholar]
- Hokozano, T.; Tanaka, S. Artificially Patinated Copper Materail. U.S. Patent 6,063,480A, 24 June 1998. [Google Scholar]
- Hoffman, G. CIELab Color Space. Available online: http://docs-hoffmann.de/cielab03022003.pdf (accessed on 14 September 2020).


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rak, P.; Fink, D.; Bureš, R.; Stoulil, J. Novel Procedure for Brochantite Based Pigment Production and Its Immobilization for Restoration of Historical Copper Objects. Coatings 2020, 10, 972. https://doi.org/10.3390/coatings10100972
Rak P, Fink D, Bureš R, Stoulil J. Novel Procedure for Brochantite Based Pigment Production and Its Immobilization for Restoration of Historical Copper Objects. Coatings. 2020; 10(10):972. https://doi.org/10.3390/coatings10100972
Chicago/Turabian StyleRak, Pavol, Dominika Fink, Richard Bureš, and Jan Stoulil. 2020. "Novel Procedure for Brochantite Based Pigment Production and Its Immobilization for Restoration of Historical Copper Objects" Coatings 10, no. 10: 972. https://doi.org/10.3390/coatings10100972
APA StyleRak, P., Fink, D., Bureš, R., & Stoulil, J. (2020). Novel Procedure for Brochantite Based Pigment Production and Its Immobilization for Restoration of Historical Copper Objects. Coatings, 10(10), 972. https://doi.org/10.3390/coatings10100972




