Adhesion Effect on the Hyperfine Frequency Shift of an Alkali Metal Vapor Cell with Paraffin Coating Using Peak-Force Tapping AFM
Abstract
1. Introduction
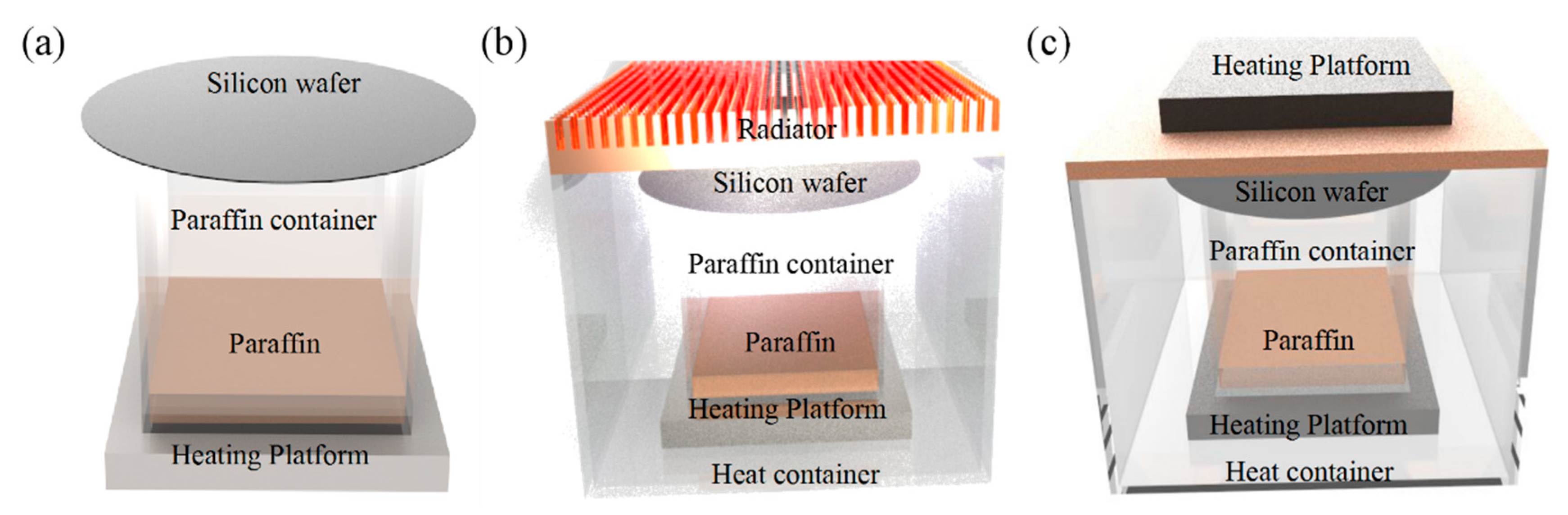
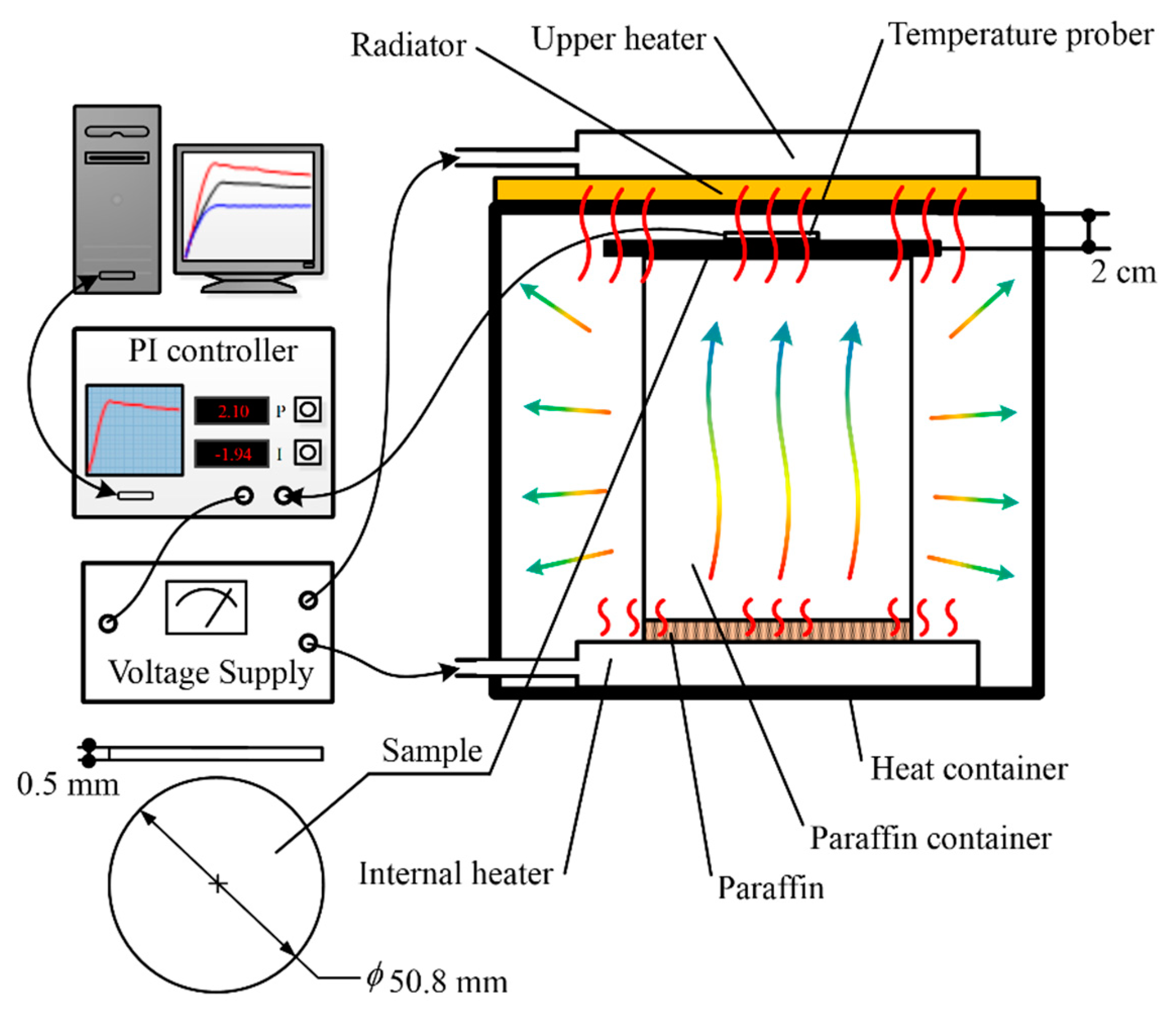
2. Experimental Details
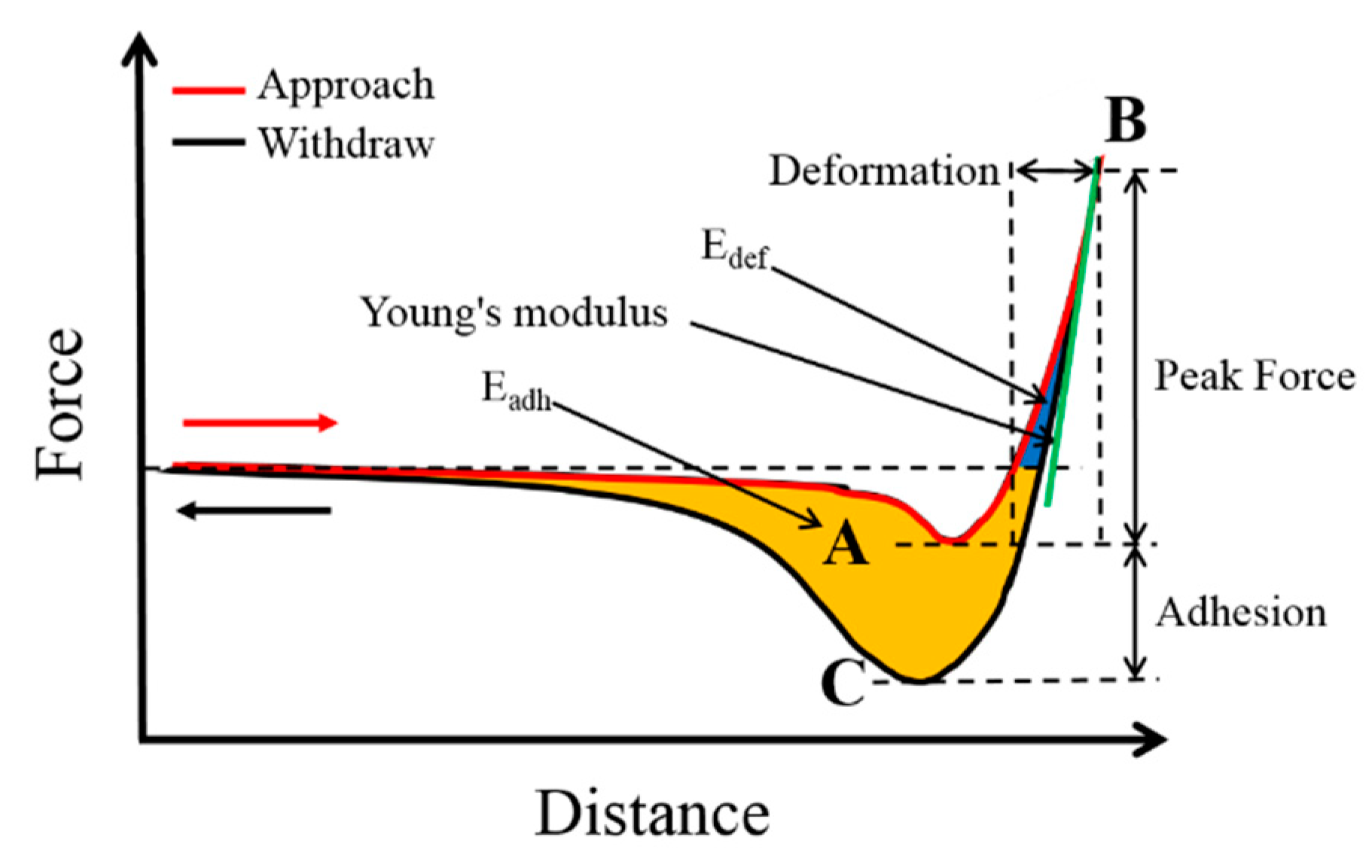
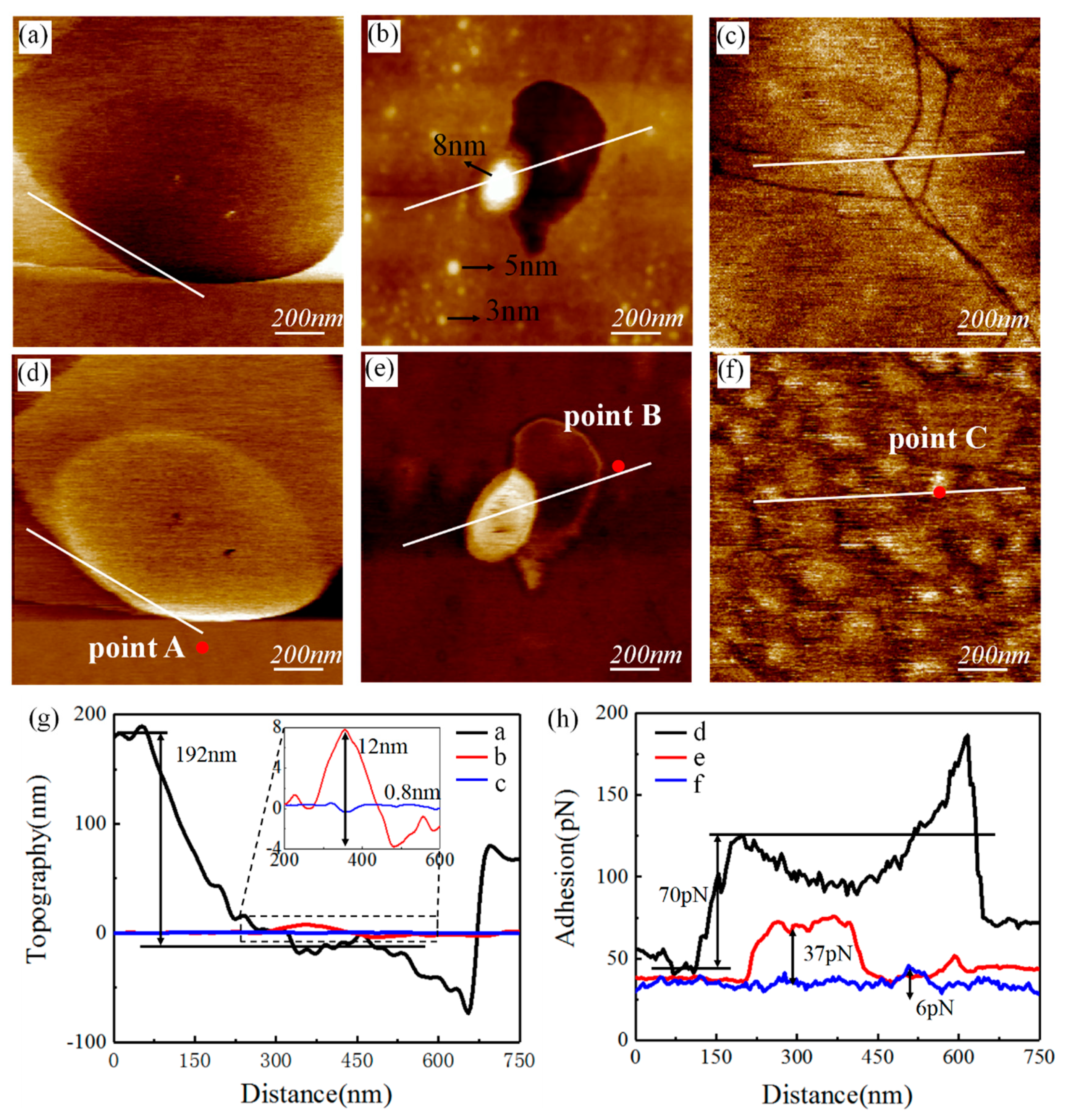
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rampulla, D.M.; Oncel, N.; Abelev, E.; Yi, Y.W.; Knappe, S.; Bernasek, S.L. Effects of organic film morphology on the formation of Rb clusters on surface coatings in alkali metal vapor cells. Appl. Phys. Lett. 2009, 94, 041116. [Google Scholar] [CrossRef]
- Budker, D.; Romalis, M. Optical magnetometry. Nat. Phys. 2007, 3, 227. [Google Scholar] [CrossRef]
- Budker, D.; Hollberg, L.; Kimball, D.F.; Kitching, J.; Pustelny, S.; Yashchuk, V.V. Investigation of microwave transitions and nonlinear magneto-optical rotation in anti-relaxation-coated cells. Phys. Rev. A 2005, 71, 012903. [Google Scholar] [CrossRef]
- Kuzmich, A.; Mandel, L.; Bigelow, N.P. Generation of Spin Squeezing via Continuous Quantum Nondemolition Measurement. Phys. Rev. Lett. 2000, 85, 1594–1597. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.F.; Schaden, M.; Wu, Z. Method for Measuring the Dwell Time of Spin-Polarized Rb Atoms on Coated Pyrex Glass Surfaces Using Light Shift. Phys. Rev. Lett. 2009, 103, 073201. [Google Scholar] [CrossRef]
- Ulanski, E.; Wu, Z. Measurement of dwell times of spin polarized rubidium atoms on octadecyltrichlorosilane- and paraffin-coated surfaces. Appl. Phys. Lett. 2011, 98, 201115. [Google Scholar] [CrossRef]
- Seltzer, S.J.; Rampulla, D.M.; Rivillon-Amy, S.; Chabal, Y.J.; Bernasek, S.L.; Romalis, M.V. Testing the effect of surface coatings on alkali atom polarization lifetimes. J. Appl. Phys. 2008, 104, 103116. [Google Scholar] [CrossRef]
- Klein, M.; Hohensee, M.; Nemiroski, A.; Xiao, Y.; Phillips, D.F.; Walsworth, R.L. Slow light in narrow paraffin-coated vapor cells. Appl. Phys. Lett. 2009, 95, 091102. [Google Scholar] [CrossRef]
- Breeze, S.R.; Lang, S.; Moudrakovski, I.; Ratcliffe, C.I.; Ripmeester, J.A.; Simard, B. Coatings for optical pumping cells and extending the lifetime of hyperpolarized xenon. J. Appl. Phys. 1999, 86, 4040. [Google Scholar] [CrossRef]
- Guzman, J.S.; Wojciechowski, A.; Stalnaker, J.E.; Tsigutkin, K.; Yashchuk, V.V.; Budker, D. Nonlinear magneto-optical rotation and Zeeman and hyperfine relaxation of potassium atoms in a paraffin-coated cell. Phys. Rev. A 2006, 74, 053415. [Google Scholar] [CrossRef]
- Dotto, M.E.R.; Ziglio, C.M.; Camargo, S.S., Jr. Toward a Direct Measurement of Paraffin Adhesion Forces. Energy Fuels 2008, 22, 3384–3389. [Google Scholar] [CrossRef]
- Dotto, M.E.R.; Camargo, S.S.; Ziglio, C.M. Study of Force Curves on Paraffin Deposits Using Atomic Force Microscopy. Energy Fuels 2007, 21, 1296–1300. [Google Scholar] [CrossRef]
- Dotto, M.E.R.; Martins, R.N.; Ferreira, M.; Camargo, S.S., Jr. Influence of hydrogenated amorphous carbon coatings on the formation of paraffin deposits. Surf. Coat. Technol. 2006, 200, 6479–6483. [Google Scholar] [CrossRef]
- Dong, H.F.; Yang, G. Comparison of Compensation Mechanism between an NMR Gyroscope and an SERF Gyroscope. IEEE Sens. J. 2017, 17, 4052–4055. [Google Scholar] [CrossRef]
- Alexandrov, E.B.; Balabas, M.V.; Budker, D.; English, D.; Kimball, D.F.; Li, C.-H.; Yashchuk, V.V. Light-induced desorption of alkali-metal atoms from paraffin coating. Phys. Rev. A 2002, 66, 042903. [Google Scholar] [CrossRef]
- Biedermann, G.; McGuinness, H.; Rakholia, A.; Jau, Y.-Y.; Wheeler, D.; Sterk, J.; Burns, G. Atom interferometry in a warm vapor. Phy. Rev. Lett. 2017, 118, 163601. [Google Scholar] [CrossRef] [PubMed]
- Li, W.H.; Balabas, M.; Peng, X.; Pustelny, S.; Wickenbrock, A.; Guo, H.; Budker, D. Characterization of high-temperature performance of cesium vapor cells with anti-relaxation coating. J. Appl. Phys. 2017, 121, 063104. [Google Scholar] [CrossRef]
- Bandia, T.; Affolderbach, C.; Mileti, G. Laser-pumped paraffin-coated cell rubidium frequency standard. J. Appl. Phys. 2012, 111, 124906. [Google Scholar] [CrossRef]
- Nasyrov, K.; Entin, V.; Nikolov, N.; Petrov, N.; Cartaleva, S. Simple method for characterization of anti-relaxation coating of optical cells. In Proceedings of the SPIE—The International Society for Optical Engineering, Sozopol, Bulgaria, 8 January 2015; p. 9447. [Google Scholar]
- Yi, Y.W.; Robinson, H.G.; Knappe, S.; Maclennan, J.E.; Jones, C.D.; Zhu, C.; Clark, N.A.; Kitching, J. Method for characterizing self-assembled monolayers as antirelaxation wall coatings for alkali vapor cells. J. Appl. Phys. 2008, 104, 023534. [Google Scholar] [CrossRef]
- Seltzer, S.J.; Michalak, D.J.; Donaldson, M.H.; Balabas, M.V.; Barber, S.K.; Bernasek, S.L.; Bouchiat, M.-A.; Hexemer, A.; Hibberd, A.M.; Kimball, D.F.J.; et al. Investigation of antirelaxation coatings for alkali-metal vapor cells using surface science techniques. J. Chem. Phys. 2010, 133, 144703. [Google Scholar] [CrossRef]
- Schiwek, S.; Heim, L.O.; Stark, R.W.; Dietz, C. Manipulation of polystyrene nanoparticles on a silicon wafer in the peak force tapping mode in water: pH-dependent friction and adhesion force. J. Appl. Phys. 2015, 17, 104303. [Google Scholar] [CrossRef]
- Smolyakova, G.; Pruvosta, S.; Cardosob, L.; Alonsob, B.; Belamiebc, E.; Duchet-Rumeau, J. AFM PeakForce QNM mode: Evidencing nanometre-scale mechanical properties of chitin-silica hybrid nanocomposites. Carbohydr. Polym. 2016, 151, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Żbik, M.; Horn, R.G.; Shawb, N. AFM study of paraffin wax surfaces. Colloids Surf. A Physicochem. Eng. Asp. 2006, 287, 139–146. [Google Scholar] [CrossRef]
- Lorenzoni, M.; Evangelio, L.; Verhaeghe, S.; Nicolet, C.; Navarro, C.; Pérez-Murano, F. Assessing the Local Nanomechanical Properties of Self-Assembled Block Copolymer Thin Films by Peak Force Tapping. Langmuir 2015, 31, 11630–11638. [Google Scholar] [CrossRef]
- Minne, S.C.; Hu, Y.; Hu, S.; Pittenger, B.; Su, C. NanoScale Quantitative Mechanical Property Mapping Using Peak Force Tapping Atomic Force Microscopy. Microsc. Microanal. 2010, 16, 464–465. [Google Scholar] [CrossRef][Green Version]
- Knittel, P.; Mizaikoff, B.; Kranz, C. Simultaneous Nanomechanical and Electrochemical Mapping: Combining Peak Force Tapping Atomic Force Microscopy with Scanning Electrochemical Microscopy. Anal. Chem. 2016, 88, 6174–6178. [Google Scholar] [CrossRef]
- Garcia, R.; Gómez, C.J.; Martinez, N.F.; Patil, S.; Dietz, C.; Magerle, R. Identification of Nanoscale Dissipation Processes by Dynamic Atomic Force Microscopy. Phys. Rev. Lett. 2006, 97, 016103. [Google Scholar] [CrossRef]
- Sikora, A.; Bednarz, L. Direct measurement and control of peak tapping forces in atomic force microscopy for improved height measurements. Meas. Sci. Technol. 2011, 22, 094005. [Google Scholar] [CrossRef]
- Young, T.J.; Monclus, M.A.; Burnett, T.L.; Broughton, W.R.; Ogin, S.L.; Smith, P.A. The use of the PeakForceTM quantitative nanomechanical mapping AFM-based method for high-resolution Young’s modulus measurement of polymers. Meas. Sci. Technol. 2011, 22, 125703. [Google Scholar] [CrossRef]
- Plomp, M.; van Enckevort, W.J.P.; van Hoof, P.J.C.M.; van de Streek, C.J. Morphology of and dislocation movement in n-C40H82 paraffin crystals grown from solution. J. Cryst. Growth 2003, 249, 600–613. [Google Scholar] [CrossRef]
- Balantekin, M.; Atalar, A. Power dissipation analysis in tapping-mode atomic force microscopy. Phys. Rev. B 2003, 67, 193404. [Google Scholar] [CrossRef]
- Bouchiat, M.A.; Brossel, J. Relaxation of Optically Pumped Rb Atoms on Paraffin-Coated Walls. Phys. Rev. 1966, 147, 41. [Google Scholar] [CrossRef]
- Stephens, M.; Rhodes, R.; Wieman, C. Study of wall coatings for vapor cell laser traps. J. Appl. Phys. 1994, 76, 3479–3488. [Google Scholar] [CrossRef]
- Corsini, E.P.; Karaulanov, T.; Balabas, M.; Budker, D. Hyperfine frequency shift and Zeeman relaxation in alkali-metal-vapor cells with antirelaxation alkene coating. Phys. Rev. A 2013, 87, 022901. [Google Scholar] [CrossRef]
- Peck, S.K.; Lane, N.; Ang, D.G.; Hunter, R.L. Using tensor light shifts to measure and cancel a cell’s quadrupolar frequency shift. Phys. Rev. A 2016, 93, 023426. [Google Scholar] [CrossRef]
- Romalis, M.V.; Lin, L. Surface nuclear spin relaxation of 199Hg. J. Chem. Phys. 2004, 120, 1511. [Google Scholar] [CrossRef]
- Nasyrov, K.; Gozzini, S.; Lucchesini, A.; Marinelli, C.; Gateva, S.; Cartaleva, S.; Marmugi, L. Antirelaxation coatings in coherent spectroscopy: Theoretical investigation and experimental test. Phys. Rev. A 2015, 92, 043803. [Google Scholar] [CrossRef]
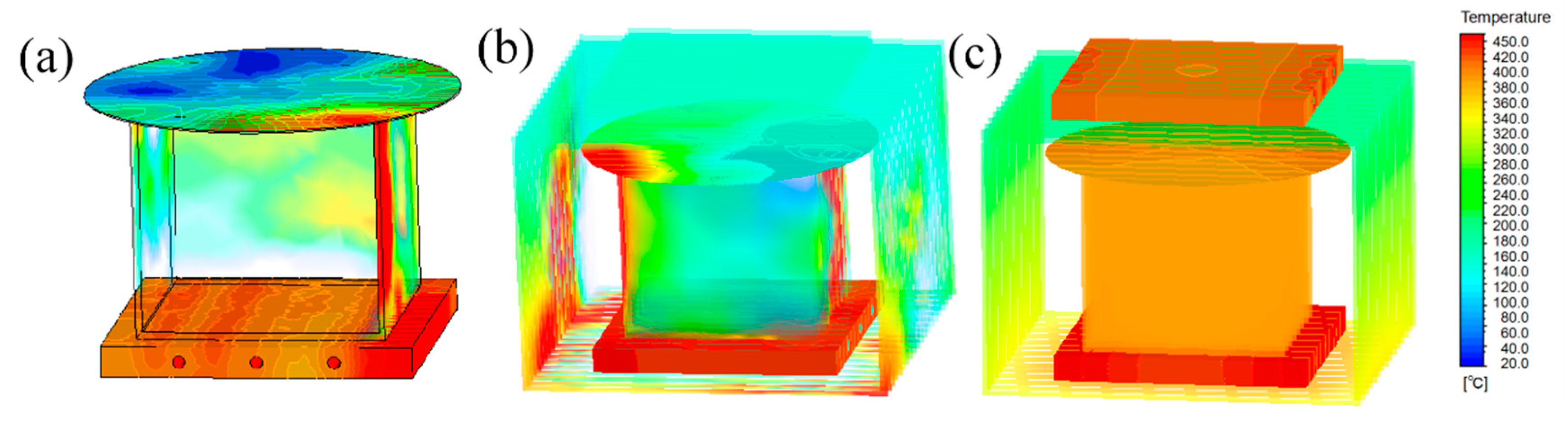
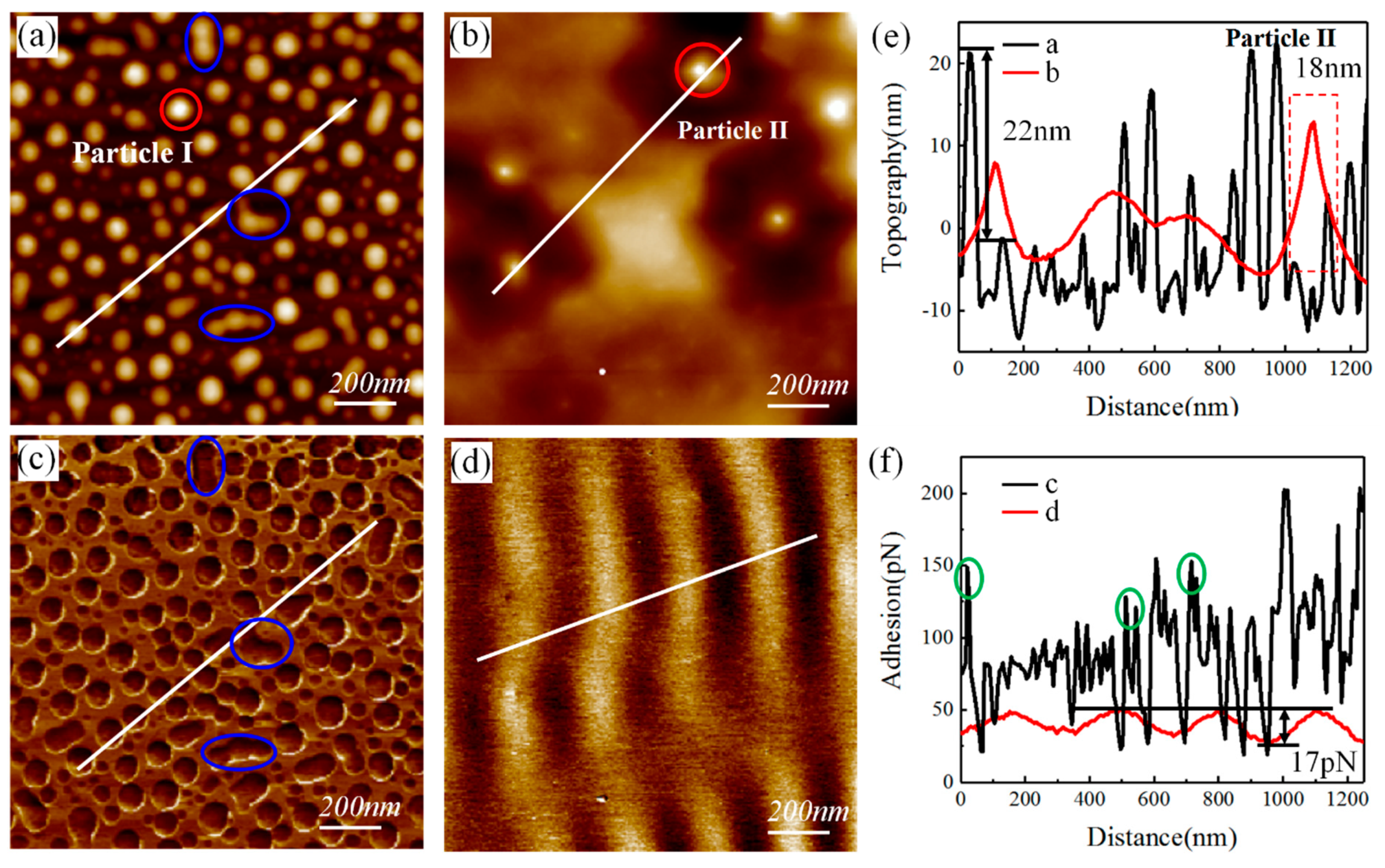
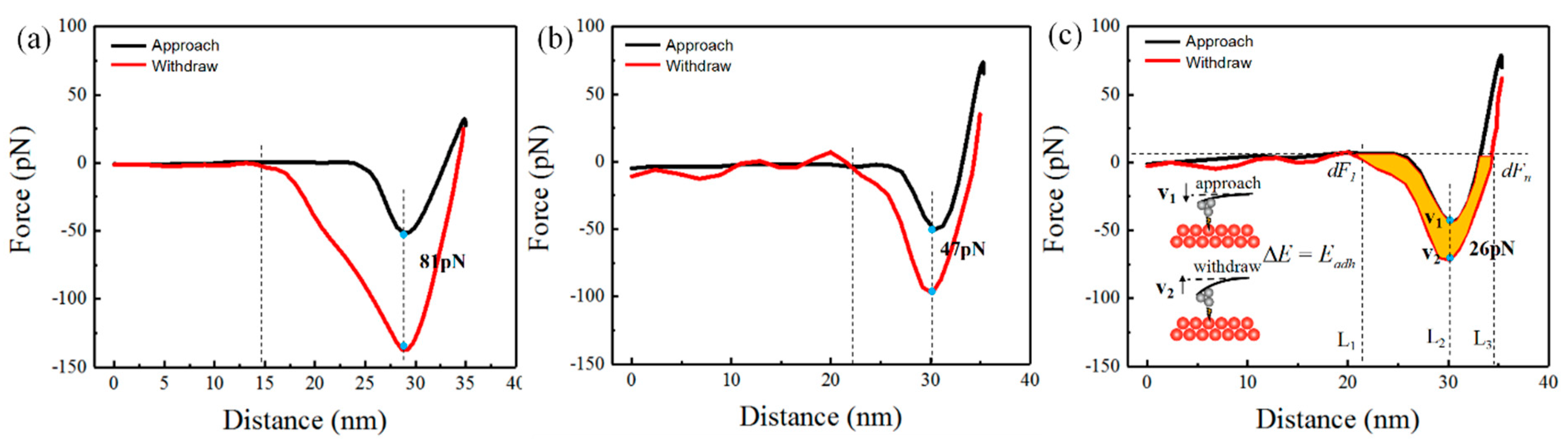
| TIR | TTV | Bow/Warp | Ra |
|---|---|---|---|
| <3 μm | <10 μm | <40 μm | <0.5 nm |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, J.; Ma, Z.; Wen, H.; Guo, H.; Tang, J.; Liu, J.; Li, Y.; Sugawara, Y. Adhesion Effect on the Hyperfine Frequency Shift of an Alkali Metal Vapor Cell with Paraffin Coating Using Peak-Force Tapping AFM. Coatings 2020, 10, 84. https://doi.org/10.3390/coatings10010084
Wei J, Ma Z, Wen H, Guo H, Tang J, Liu J, Li Y, Sugawara Y. Adhesion Effect on the Hyperfine Frequency Shift of an Alkali Metal Vapor Cell with Paraffin Coating Using Peak-Force Tapping AFM. Coatings. 2020; 10(1):84. https://doi.org/10.3390/coatings10010084
Chicago/Turabian StyleWei, Jiuyan, Zongmin Ma, Huanfei Wen, Hao Guo, Jun Tang, Jun Liu, Yanjun Li, and Yasuhiro Sugawara. 2020. "Adhesion Effect on the Hyperfine Frequency Shift of an Alkali Metal Vapor Cell with Paraffin Coating Using Peak-Force Tapping AFM" Coatings 10, no. 1: 84. https://doi.org/10.3390/coatings10010084
APA StyleWei, J., Ma, Z., Wen, H., Guo, H., Tang, J., Liu, J., Li, Y., & Sugawara, Y. (2020). Adhesion Effect on the Hyperfine Frequency Shift of an Alkali Metal Vapor Cell with Paraffin Coating Using Peak-Force Tapping AFM. Coatings, 10(1), 84. https://doi.org/10.3390/coatings10010084




