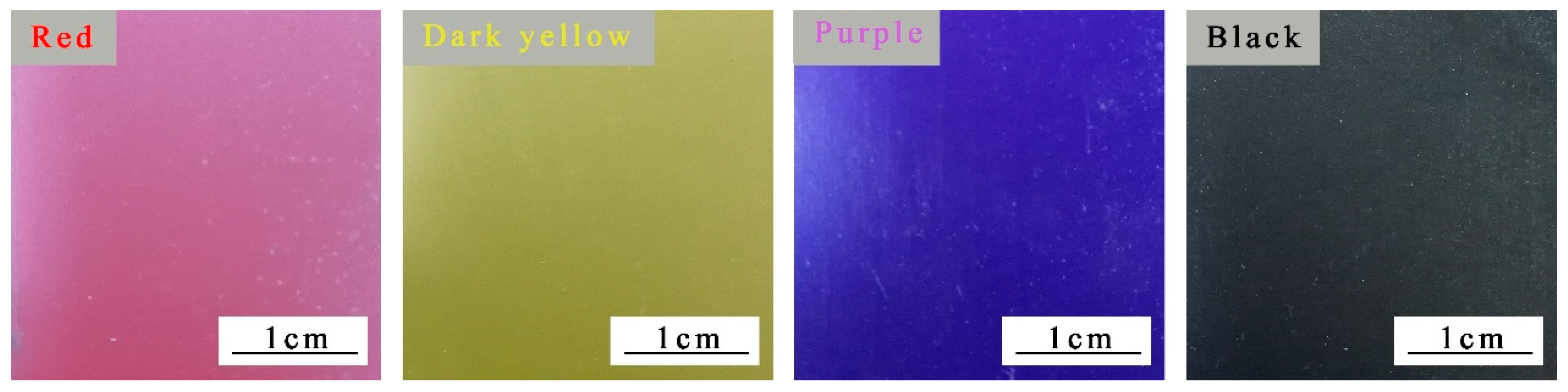
3.3. Influence of Color Paste on Properties of Coatings
The effect of different color pastes on the properties of the coating is shown in
Table 4. As shown in
Table 4, compared with dark yellow and purple pastes, red and black pastes could make fluorine coatings possess lower luster. The gloss of black coating was the lowest. This is because the coatings with red and black pastes had a stronger ability to absorb and emit light and a weaker ability to reflect light, and therefore to present a lower gloss [
26].
Different color pastes have different effects on the hardness of coatings. The hardness of red, dark yellow, purple coating and the coating without paste was 6H, while the hardness of black coating was the lowest (6B). This is because black paste is mainly composed of carbon black particles. Compared with the other three pastes, carbon black particles are more loose affecting the compactness of the coating [
27]. For low infrared emissivity coatings, an important role is to protect the covering, so the hardness cannot be too low [
28]. It can be seen that the hardness of the coating added with black paste does not meet the basic hardness index (2H) [
29]. For black coatings, in order to ensure that the coating can meet basic hardness index, the hardness can be enhanced by adding modifiers [
30].
Table 4 indicated that the adhesion of the coating without paste was the best. The dark yellow coating had better adhesion and the black coating had the worst adhesion. This is because from a microscopic point of view, the dark yellow coating is more uniform and denser, and the components are easy to mix evenly, while the interaction between carbon black particles of black paste and other components of coating is less, so it is difficult to mix with them uniformly [
31]. The influence of different color pastes on impact resistance of the coating was different. The black coating had the lowest impact resistance, while the dark yellow coating and the coating without paste had the highest. This is because the dark yellow coating and the coating without paste has high adhesion. Good adhesion can ensure good impact resistance. The adhesion of coatings with black pastes is relatively small, and it is easy to fall off when impacted [
32].
As can be seen in
Table 4, the roughness of dark yellow and purple coatings was lower, and the surface was smoother. The roughness of black coatings was the highest. This may be because the smaller particle size of the dark yellow and purple pastes, which can fill the gap between the aluminum powder in the coating and increase the compactness of the coating. The carbon black particles of the black paste aggregate together to form large particles, thus increasing the roughness [
33].
Table 5 is the effect of four kinds of color pastes on the chromatic aberration of coatings. The color difference refers to the difference of the overall brightness and hue of the coating [
34].
L,
a and
b represent the brightness, red-green and yellow-blue value of different color coating respectively.
L’,
a’ and
b’ represent these values of the substrate itself. The difference
ΔL,
Δa and
Δb are obtained by subtracting them, which are respectively expressed as lightness difference, red-green index difference and yellow-blue index difference. Thus, the color difference
ΔE can be obtained according to the formula [
35].
According to
Table 5, the effect of different color pastes on color difference of the coatings was different. The color difference of purple paste was the smallest compared with that of red, dark yellow and black pastes. At the same time, the L value of purple coating was the lowest, which made the coating darker. The other three coatings had larger L value and were brighter.
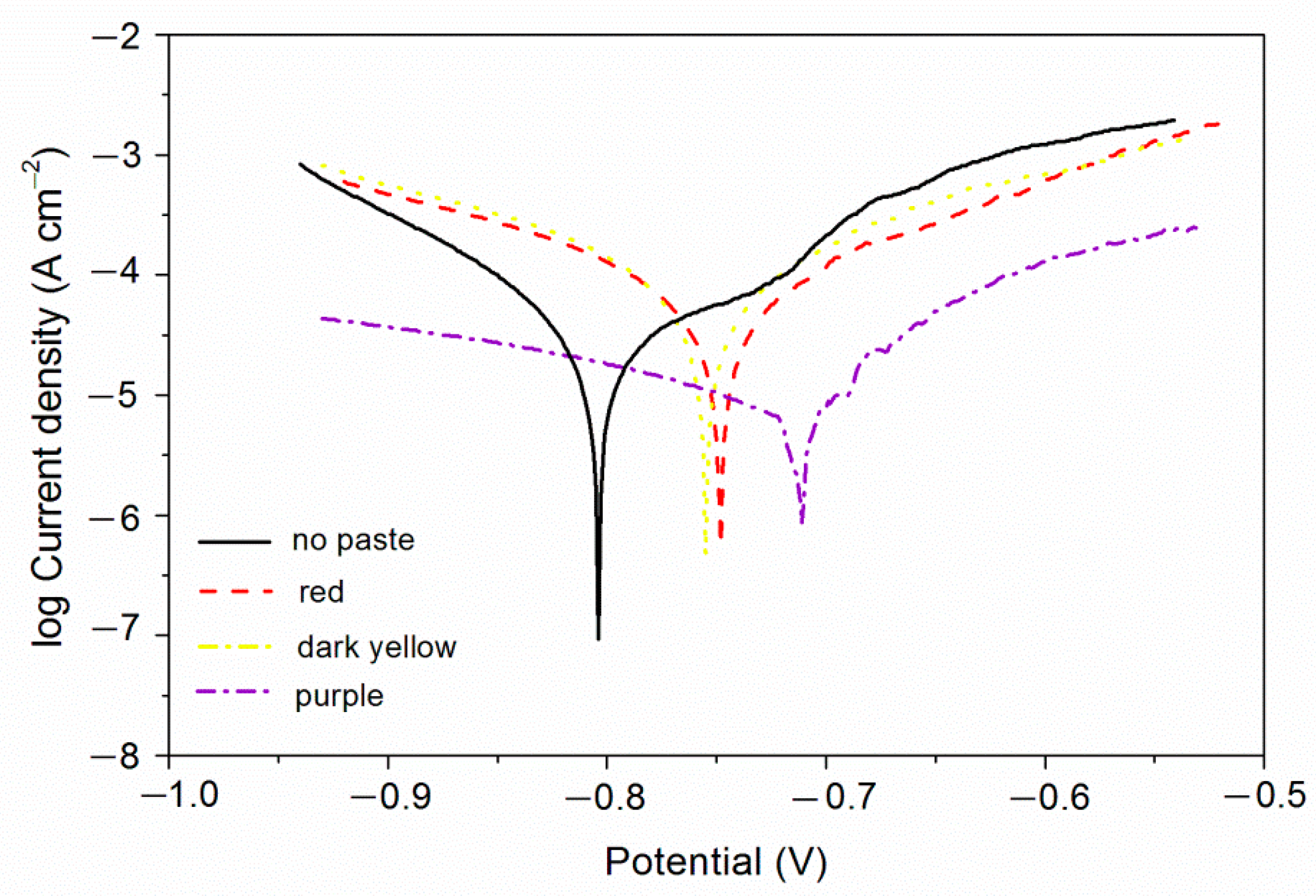
From the above study, it can be found that the coatings with purple, red, and dark yellow pastes have better comprehensive performance. Therefore, the electrochemical corrosion resistance of these three coatings was tested. The test results are shown in
Figure 4 and
Table 6. The
βa and
βc are respectively the anode Tafel slope and the cathode Tafel slope [
36]. The unit is mv/dec, mv refers to the voltage, and dec refers to the logarithmic current. The higher the potential
Ecorr, the greater the resistance
Rp, and the smaller the current
Icorr and the better the corrosion resistance.
The salt water resistance of coatings with four color pastes immersed in 3.5% NaCl solution for 7 days was tested to observe the change of surface condition after a certain corrosion time. The loss of light, discoloration, and foaming density in the actual corrosion process of the paint film were evaluated. When the loss of light was <3%, 4–15%, 16–30%, 31–50%, 51–80% and >80%, respectively, the degree of loss of light of coatings were at the 0, 1, 2, 3, 4, and 5 level, respectively. When the change of color difference before and after salt water resistance was <1.5, 1.6–3.0, 3.1–6.0, 6.1–9.0, 9.1–12.0 and >12.0, respectively, the degree of loss of light of coatings was non-discoloration, very slight discoloration, slight discoloration, obvious discoloration, severe discoloration and complete discoloration, and the grades were 0, 1, 2, 3, 4 and 5 level, respectively. The test results are shown in
Table 7 and
Table 8, and
Figure 5.
As can be seen in
Table 7, the coatings had no obvious loss of light. The loss of light grade was 0 or 1, indicating that fluorine resin coatings with different color pastes had good corrosion resistance. As can be seen in
Table 8, there was no obvious discoloration of the purple coating. The discoloration grade was 0, indicating that the purple coating had better corrosion resistance, which was consistent with the results of electrochemical testing (
Figure 4). Under the same experimental conditions, the dark yellow coating and the black coating changed color obviously, and the color change grade was at level 3 or above.
As can be seen in
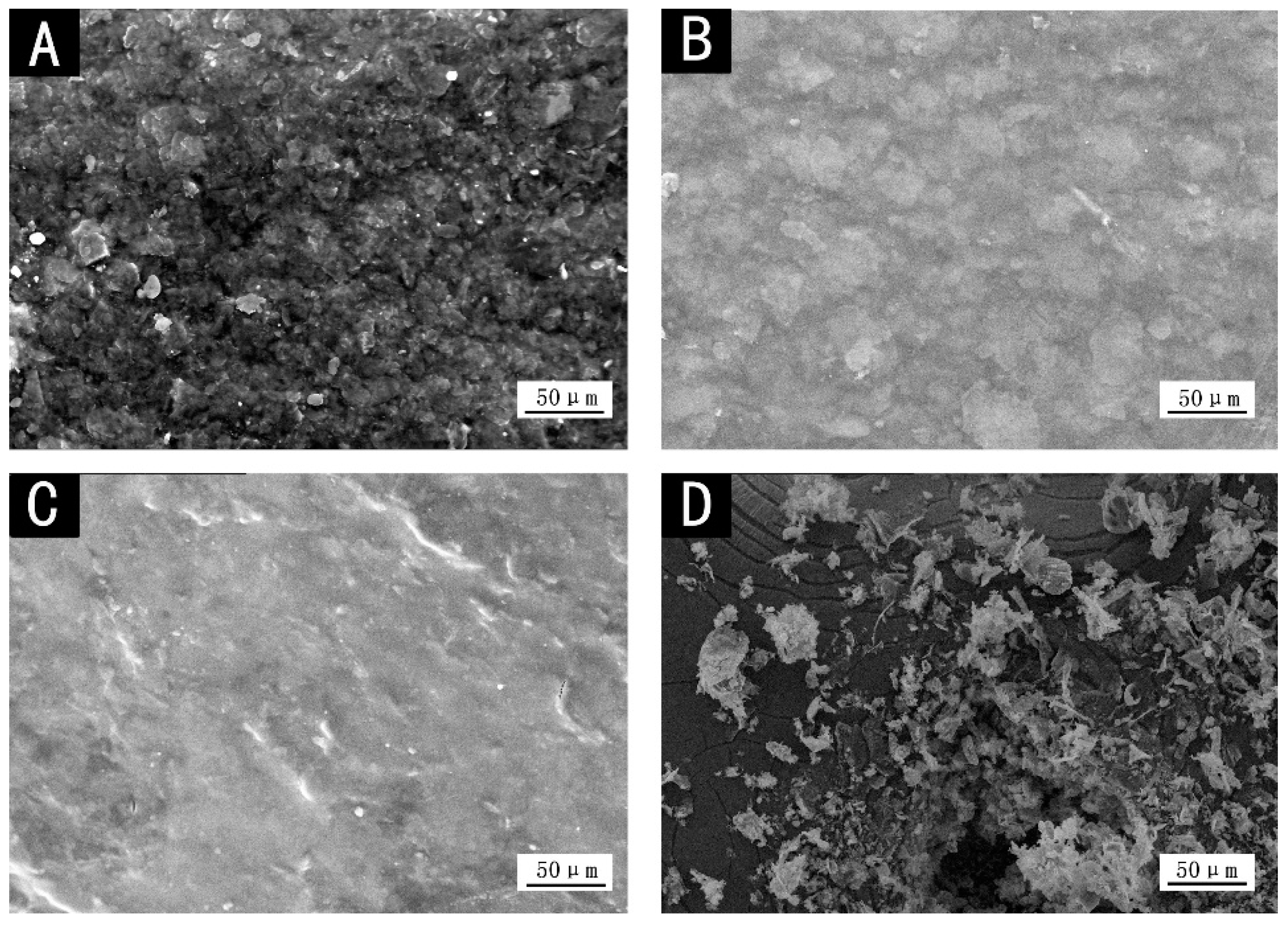
Figure 5, the coating was destroyed after 7 days of 3.5% NaCl solution immersion for the corrosion resistance test, and coatings with color pastes had a certain degree of foaming. The foaming density of dark yellow and purple coatings was lower, and black coating had a small amount of foaming, while red foaming was more serious. The compact coating and little pores (
Figure 3B,C) can prevent the NaCl solution from entering, improve the corrosion resistance of the coating, and not easily cause the phenomenon of light loss, discoloration, and bubbling.
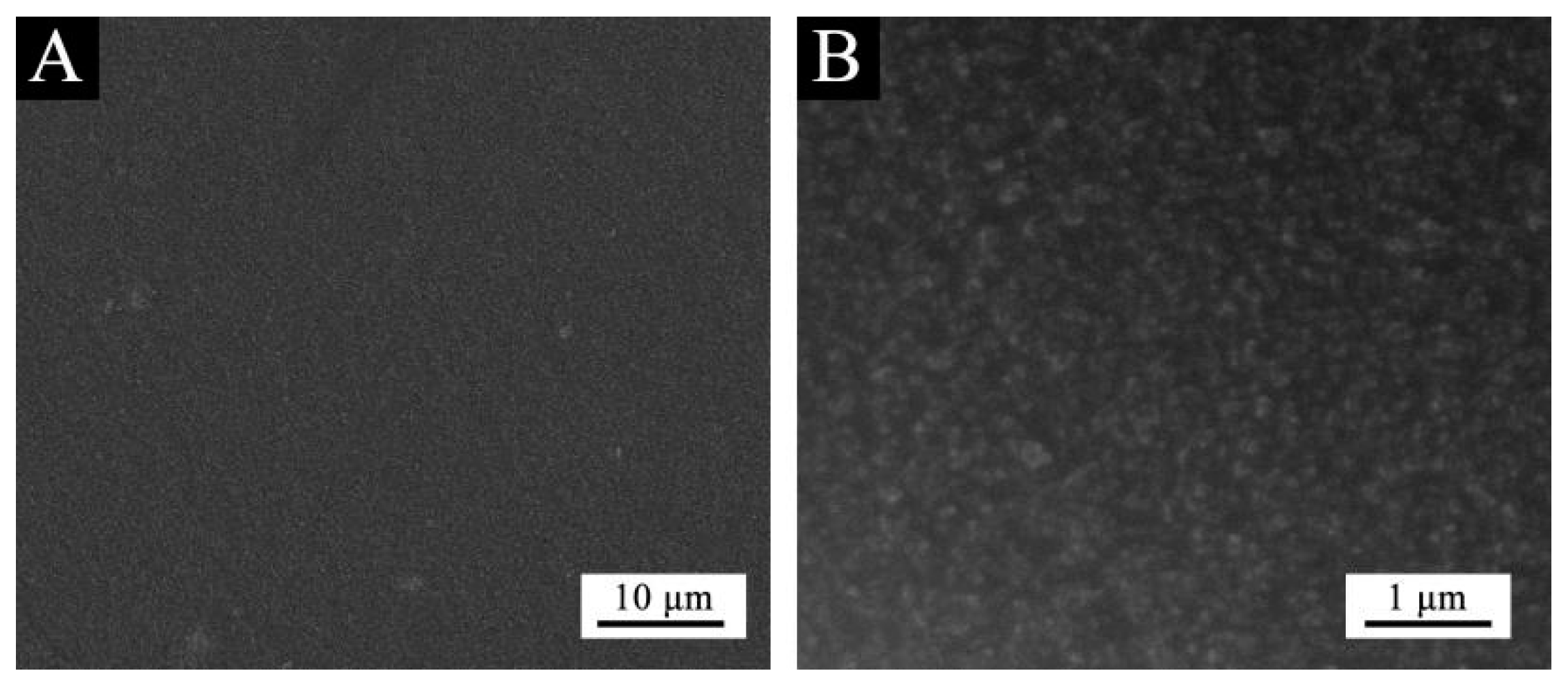
Based on the above analysis, it can be concluded that purple 8005 can be used to prepare low infrared emissivity fluorine resin coatings with better performance. The structure of purple 8005 paste was characterized. SEM image of purple 8005 is shown in
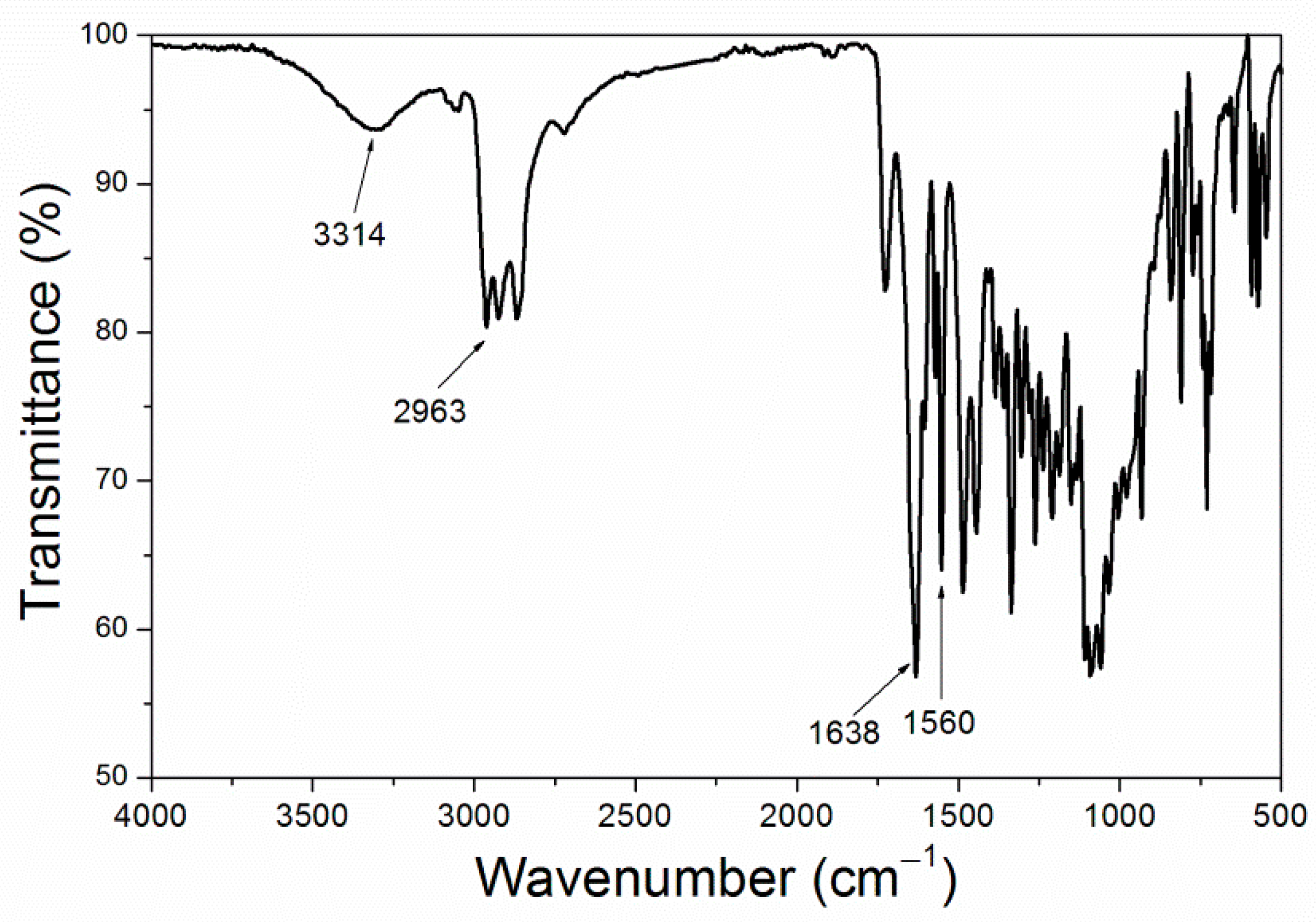
Figure 6, and the infrared spectrum is shown in
Figure 7.
Figure 6 showed that the size of purple 8005 paste was small. As can be seen in
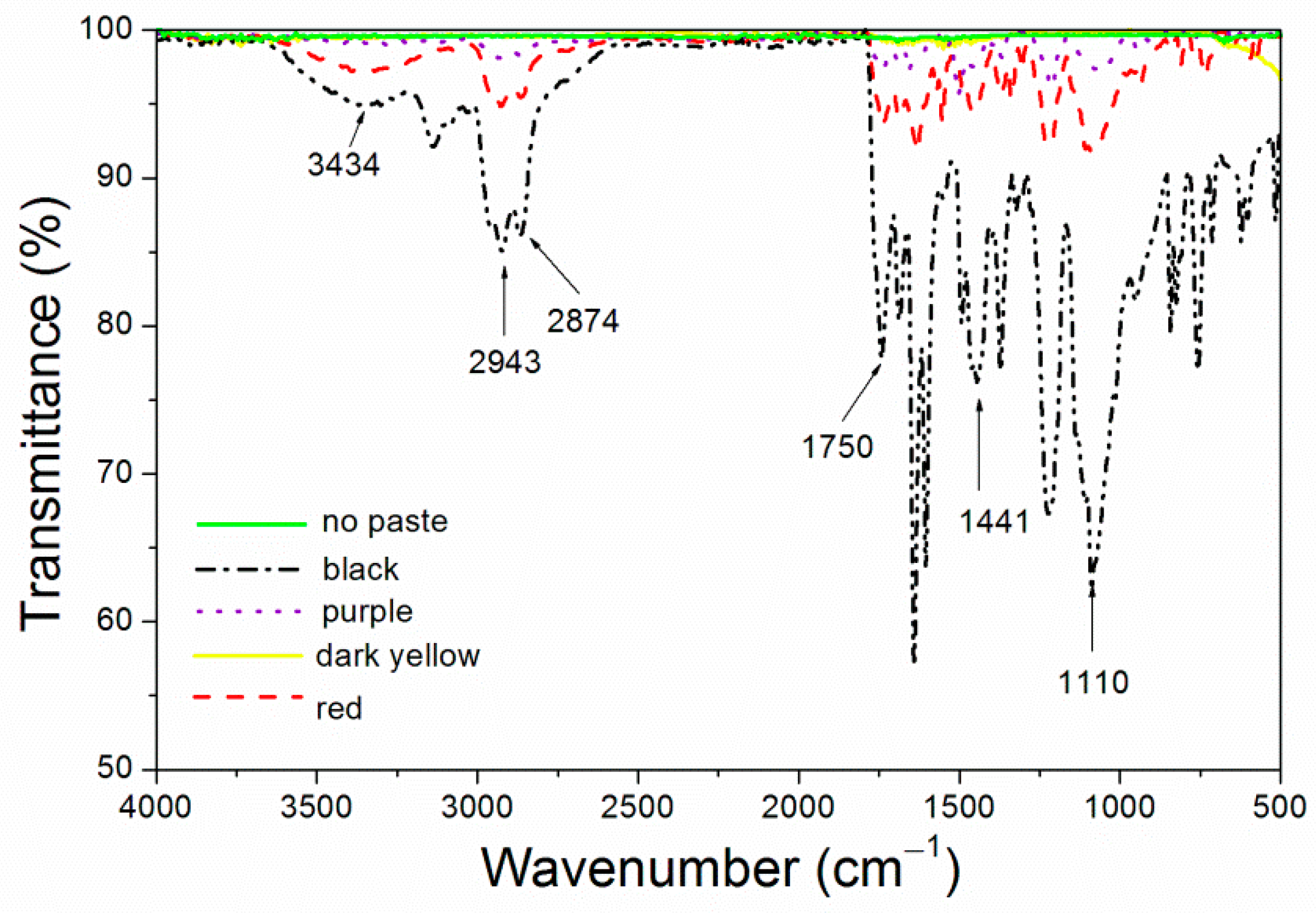
Figure 7, purple 8005 paste contains abundant organic functional groups [
37]. 3314 cm
−1, 2963 cm
−1, 1638 cm
−1 and 1560 cm
−1 corresponded to the characteristic absorption peaks of N-H, C-H, C=C and C-N respectively, so they were easy to disperse in fluorine resin coatings and had good compatibility with fluorine resin coatings.