1. Introduction
With the development of infrared technology, low infrared emissive coatings, which conceal the object from infrared detection due to their high infrared reflectivity properties, have become a new field of concern [
1]. Infrared detection is widely used in military, medical, and engineering fields for its convenience and high accuracy [
2]. In recent years, many researchers have conducted extensive work on infrared thermal stealth coatings. Liang et al. [
3] studied the preparation and characterization of low infrared emissive color coatings based on nanometer pigment. Ji et al. [
4] studied the vanadium dioxide nanopowders with tunable emissivity for adaptive infrared camouflage in both thermal atmospheric windows. Qiao et al. [
5] prepared the multifunctional poly (melamine-urea-formaldehyde)/graphene microcapsules via in situ polymerization and studied the low infrared emissivity and high thermal conductivity of the MUF@graphene composites. Zhao et al. [
6] prepared high-temperature-resistant coatings with low infrared emissivity by using polysiloxane resin and flake aluminum as the adhesive and pigment, respectively. Feng et al. [
7] studied infrared normal spectral emissivity on quasi-periodic microstructured silicon. The above researchers focused on the tunable emissivity and high temperature resistance of infrared coatings, but less on the comprehensive properties of corrosion resistance, chromatic distortion, and gloss. Low infrared emissive coatings can achieve infrared stealth without changing the shape of military targets under existing conditions, and have been widely used in stealth technology [
8]. In addition to low emissivity, infrared emissive coating also requires environmental protection, good corrosion resistance, low gloss, low brightness, in order to facilitate visible light compatibility [
9].
With the enhancement of national awareness of environmental protection [
10], waterborne coatings as the new environment-friendly type of coatings, began to be advocated by the public [
11]. At the moment, the waterborne coatings have found wide application in wood [
12], metallic materials, and walls, etc. [
13]. The term waterborne coatings refers to coatings using water as solvent or dispersing medium [
14]. Waterborne acrylic based coatings use cheap and easily available water as the solvent and water evaporation does not produce any toxic atmospheres. This not only reduces the pollution to the atmosphere and improves the working environment, but also saves a lot of resources [
15]. However, the corrosion resistance, hardness, and impact resistance of waterborne coatings are poor [
16], and the gloss is high, which limits the application of waterborne coatings for low infrared emissivity.
To a certain extent, several fillers such as Cu powder [
17], silane coupling agent [
18], Al powder [
19], CeO
2 powder [
20], can enhance corrosion resistance, hydrophobic, mechanical properties, or wear resistance of coatings. In particular, Al powder can be used as filler in waterborne coatings to prepare coatings with low infrared emissivity and high gloss, due to its silver color, high brightness, and good adherence [
21]. However, high gloss limits the application of aluminum powder for compatibility with visible light. Stringent safety measures are required to prevent dust explosions and corrosion of aluminum, which can release hydrogen gas [
22]. The Al powder used in this paper was modified with oleic acid. Furthermore, the nano-silica concentrate slurry (nano-silica slurry) can reduce the gloss of coatings [
23]. For this reason, it would be an ideal method to control interfacial interaction between the filler and the polymer by using nano-silica slurry to improve the corrosion resistance properties and mechanical properties in low infrared emissive coatings.
In this paper, orthogonal experiments were carried out to optimize the parameters of infrared emissive waterborne coating, such as performance formula, curing time, etc. Emphasis was laid on changing drying time. On the premise of meeting the two indices of the emissivity and gloss, the effects of infrared emissivity, chromatic distortion, gloss, mechanical properties, and corrosion resistance of waterborne coating were explored to establish a foundation for application of low infrared emissive coatings in engineering.
3. Results and Discussion
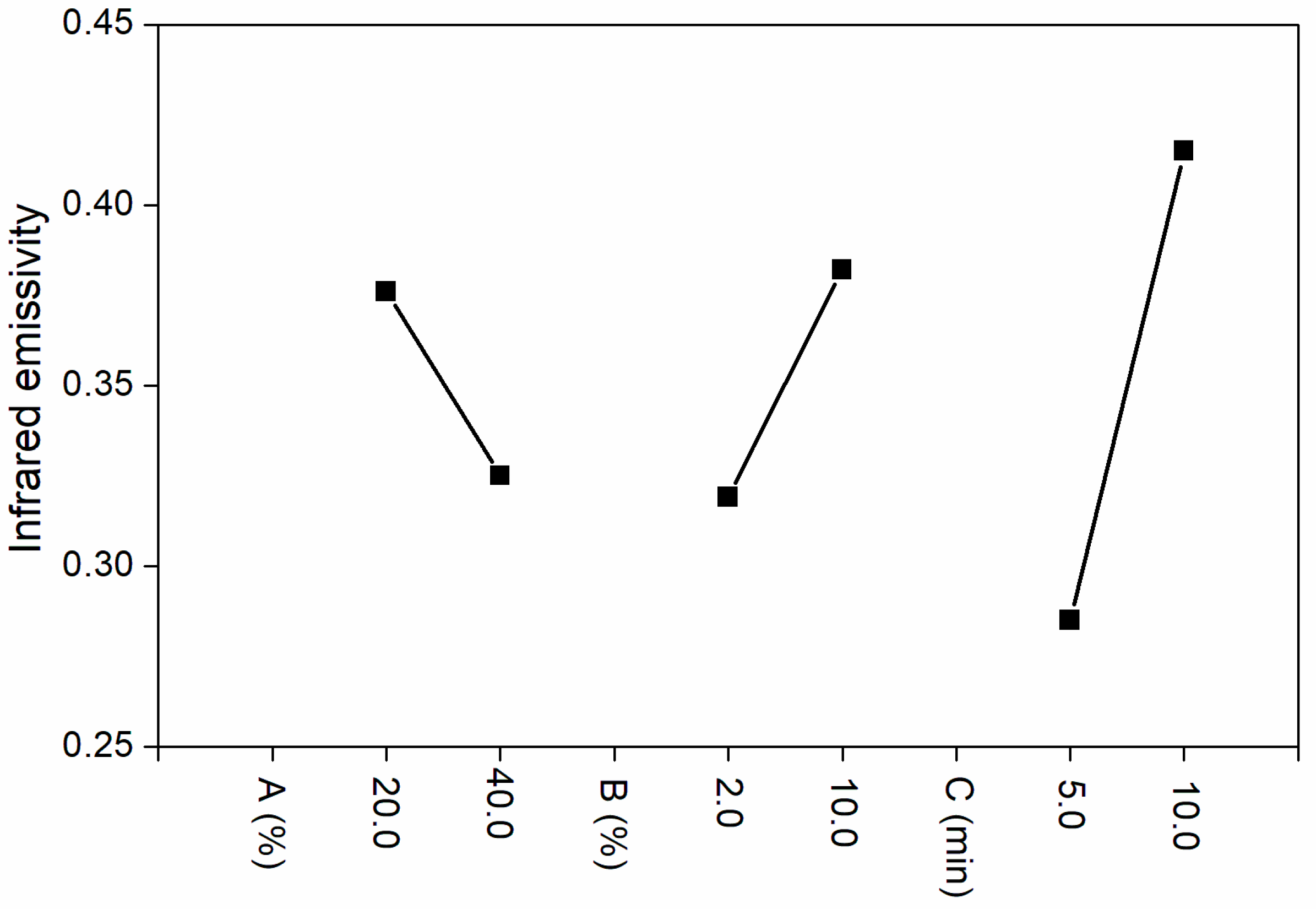
From the analysis of range and variance in
Table 3 and the influence factors of orthogonal experiment in
Figure 1, the influence of drying time on infrared emissivity of coatings was more obvious than that of Al powder concentration and nano-silica slurry concentration. When the nano-silica slurry was 2.0%, the infrared emissivity was low. F-test (
Table 4) showed that the three factors had no significant effect. According to
Table 3, the optimum level combination was as follows: The concentration of Al powder was 40.0%, the concentration of nano-silica slurry was 2.0%, and the drying time was 5.0 min. Al powder concentration was too low which would lead to higher emissivity of coatings. Due to the strong reflectivity and gloss of Al powder, high concentration of Al powder will lead to higher gloss of coatings, which is not conducive to compatibility with visible light [
31]. Therefore, 30.0% of Al powder was selected for comprehensive performance, and the concentration of Al powder was fixed to 30.0% in the following independent experiments. The optical, mechanical, chromatic distortion, and corrosion resistance of coatings were also studied.
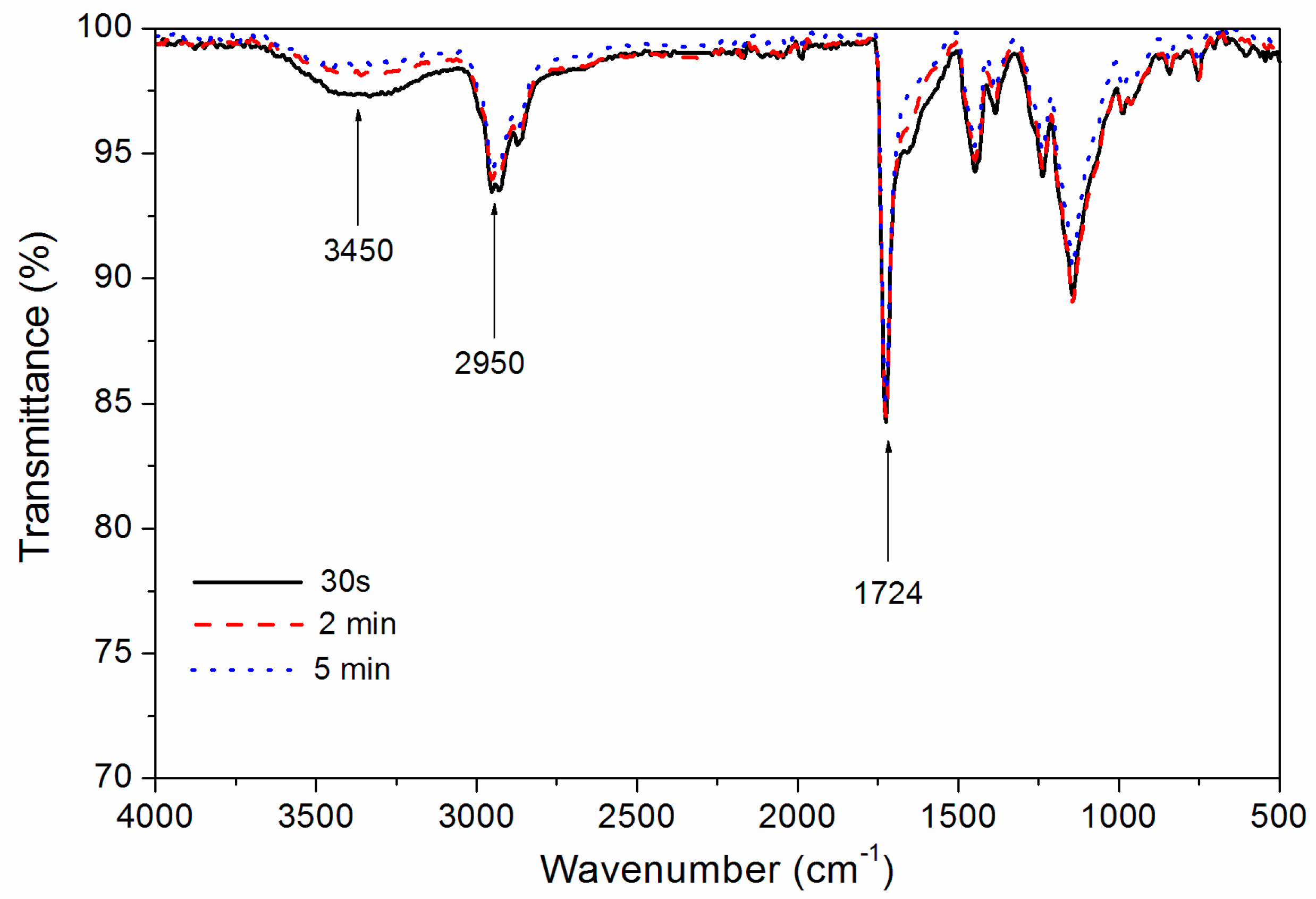
Figure 2 shows the influence of drying time on the infrared spectra of coatings. The absorption peak of wave number 3450 cm
−1 is –OH stretching vibration peak. With the prolongation of drying time, the –OH stretching vibration peak weakened, which indicated that the solvent water evaporated gradually. The peaks at 2950 cm
−1 are the asymmetric and symmetric stretching vibration peaks of CH
3 and CH
2 in waterborne acrylic coatings. The peaks at 1724 cm
−1 belongs to C=O absorption of carboxyl in waterborne acrylic coatings. As shown in
Figure 2, when the drying time was 30 s, 2.0 and 5.0 min, the infrared peaks of the coatings were basically similar. This showed that the drying time had little influence on the infrared peak of the coating and did not affect the composition of the polymer in the coating.
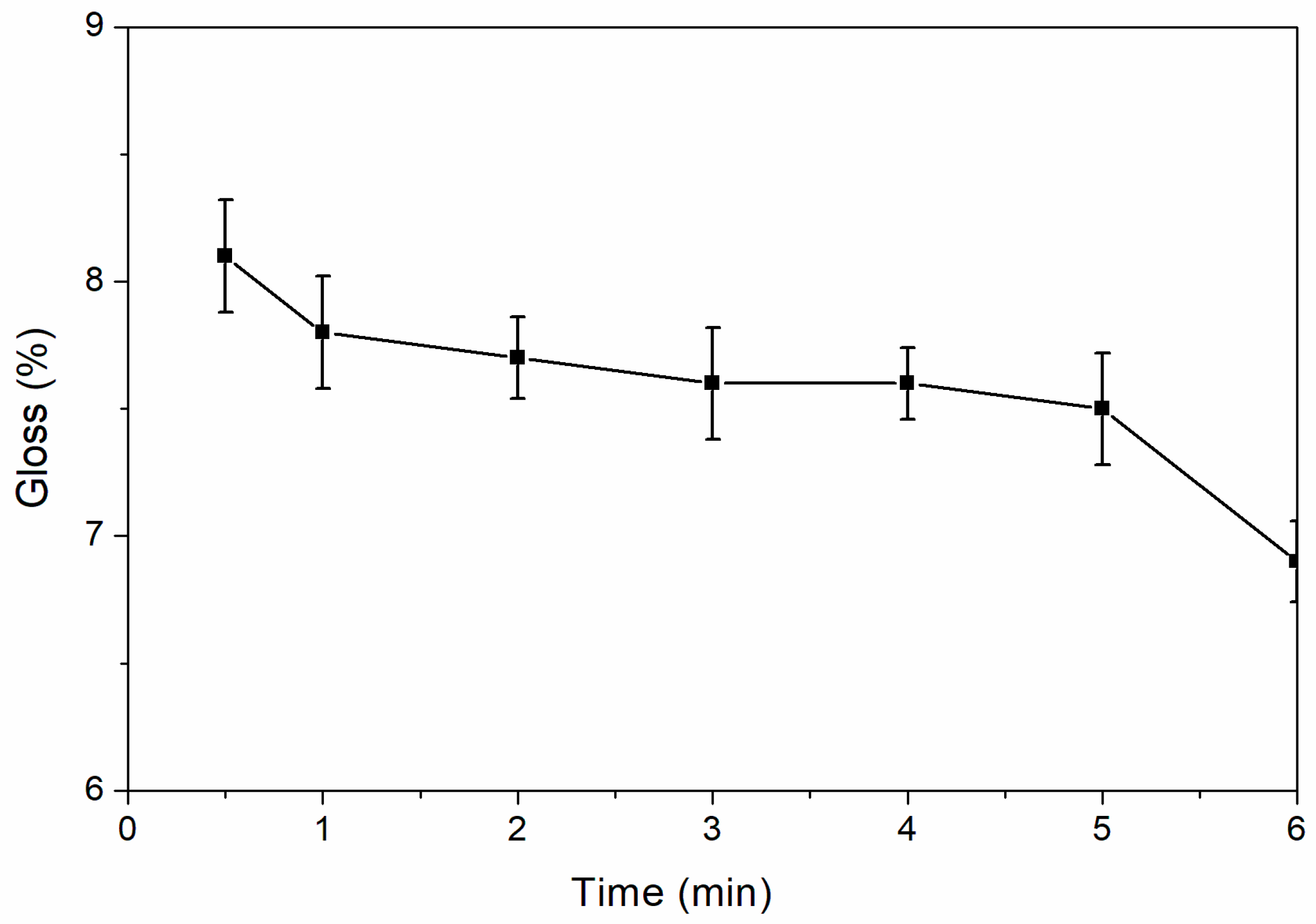
The effect of drying time on the gloss of waterborne curing coatings is shown in
Figure 3. The gloss of coating decreased with the increase of drying time. When the time was prolonged from 0.5 to 6.0 min, the gloss of coatings decreased from 8.1% to 6.9%, but the overall gloss remained low. For the low infrared emissive coating, the lower the gloss, the better the coating. When the drying time was 6.0 min, the gloss of was the lowest, which was 6.9%. The chemical structure of waterborne acrylic resin and the corresponding chemical reaction are shown in Equation (1). The moisture in the waterborne coating evaporated gradually, the degree of curing and crosslinking of the coating strengthened according to Equation (1), the reflectivity of the coating to visible light weakened gradually, and the gloss of coating was gradually decreased. The effect of nano-silica slurry on the gloss of coating is shown in
Table 5. The sample 12# was coated without nano-silica slurry, and its gloss was higher than that of sample 6# with nano-silica slurry, which indicated that nano-silica slurry can reduce the gloss of coatings. High gloss is not conducive to compatibility with visible light, so the low gloss obtained by adding nano-silica slurry was better.
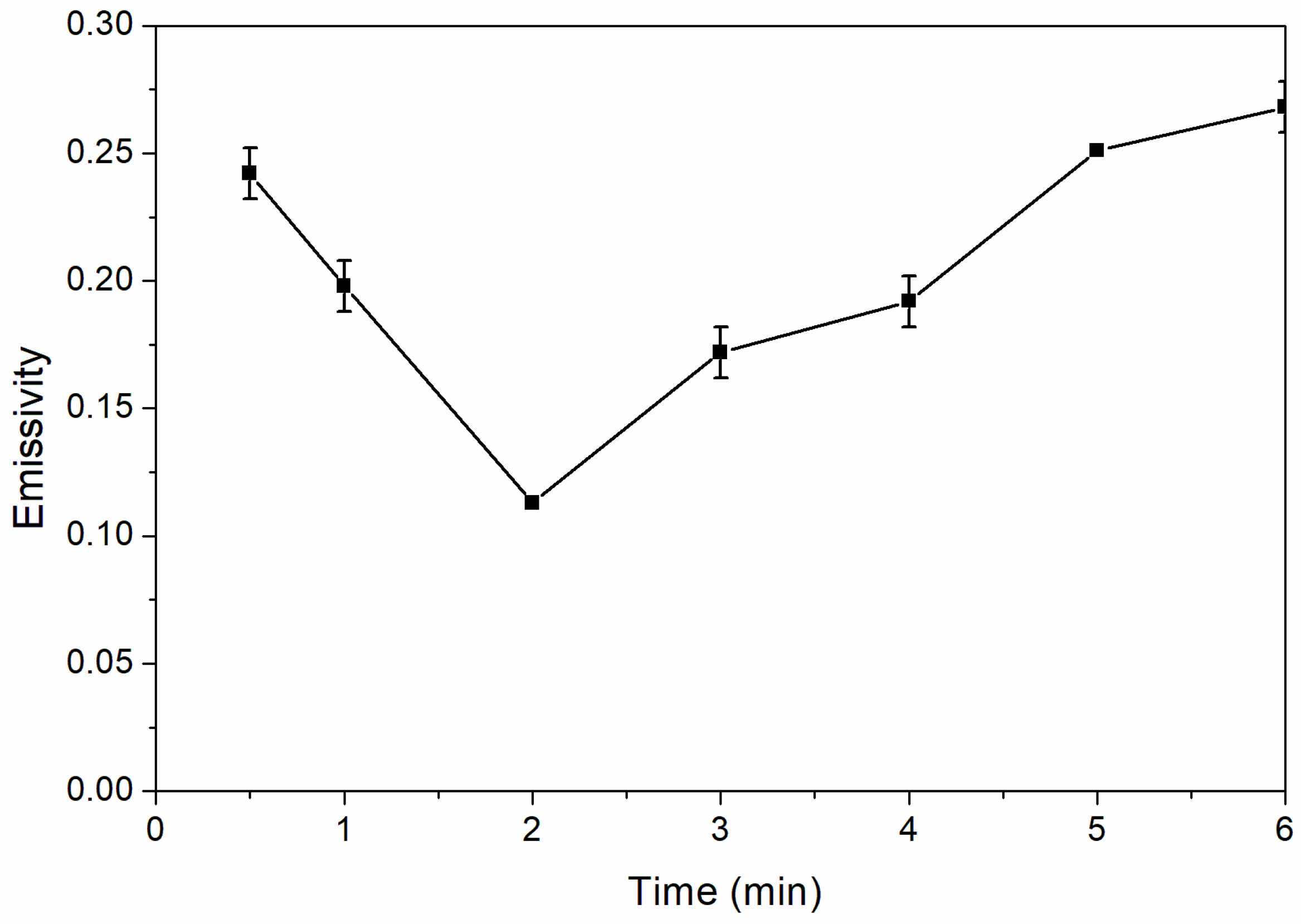
Figure 4 shows the influence of drying time on infrared emissivity of waterborne coatings. The infrared emissivity of waterborne coatings tends to be U-shape with the increase of drying time in
Figure 4. When the time increased from 0.5 to 2.0 min, the infrared emissivity of waterborne coatings decreased from 0.242 to 0.113. As the drying time continued to increase from 2.0 to 6.0 min, the infrared emissivity of waterborne coatings increased slowly from 0.113 to 0.268. Therefore, when the drying time was 2.0 min, the waterborne coating had the lowest infrared emissivity. In the period of 0.5 to 2.0 min, the addition of nano-silica slurry helped to cure the coating, reduced the thickness of the surface resin, weakened its absorption of far infrared ray, and decreased the emissivity of coating. However, after 2.0 min, continuous heating may lead to the oxidation of Al powder in the coating, so the infrared emissivity of coating began to increase. The infrared emissivity of coatings dried for 2.0 min was better.
Chromatic distortion is the value of color when polychromatic light is used as a light source.
L,
a*,
b* represent the black and white, red and green, yellow and blue values of Al substrates, respectively.
L’,
a*’,
b*’ represent the black-white, red-green, yellow-blue values of the coated substrate surface, respectively. After subtraction, the brightness difference Δ
L, the red-green index difference Δ
a*, the yellow-blue index difference Δ
b*, and the color difference Δ
E can be calculated based on Equation (2):
The results of the effect of drying time on the chromatic distortion and color difference Δ
E of coatings are shown in
Table 6. The color difference Δ
E and the brightness
L’ of waterborne coatings decreased gradually with the increase of drying time and the variation range was small. When the drying time increased from 0.5 to 6.0 min, the
L’ value of waterborne coatings decreased from 80.8 to 78.8. As the time went on, the polymer in the coating was continuously cross-linked to form a network, which was equivalent to increasing the concentration of the coating to some extent, that was, reducing the
L’ value [
32].
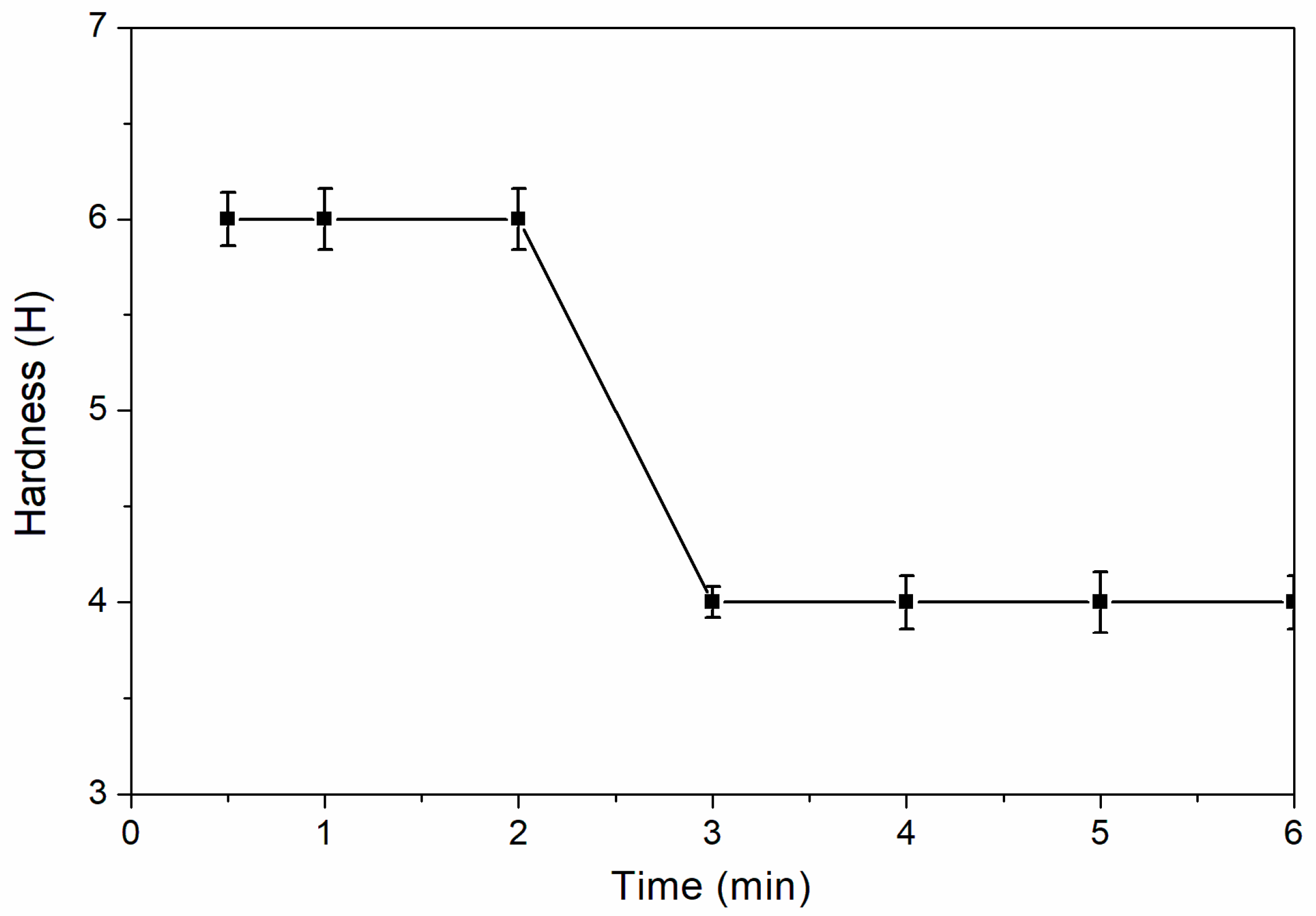
The influence of drying time on the hardness of coating is shown in
Figure 5. When the drying time is 0.5–2.0 min, the hardness of coating was the highest, which was 6H. When the drying time was over 2.0 min, the hardness of coating decreased to 4H. This is supposed to be the degree of polymer crosslinking of the coatings that was appropriate in the period of 0.5–2.0 min. When the drying time was 0.5–6.0 min, the adhesion level of the coating was better, which was level 0 (
Figure 6). This is supposed to be the drying time of 0.5–6.0 min that had no effect on the adhesion level.
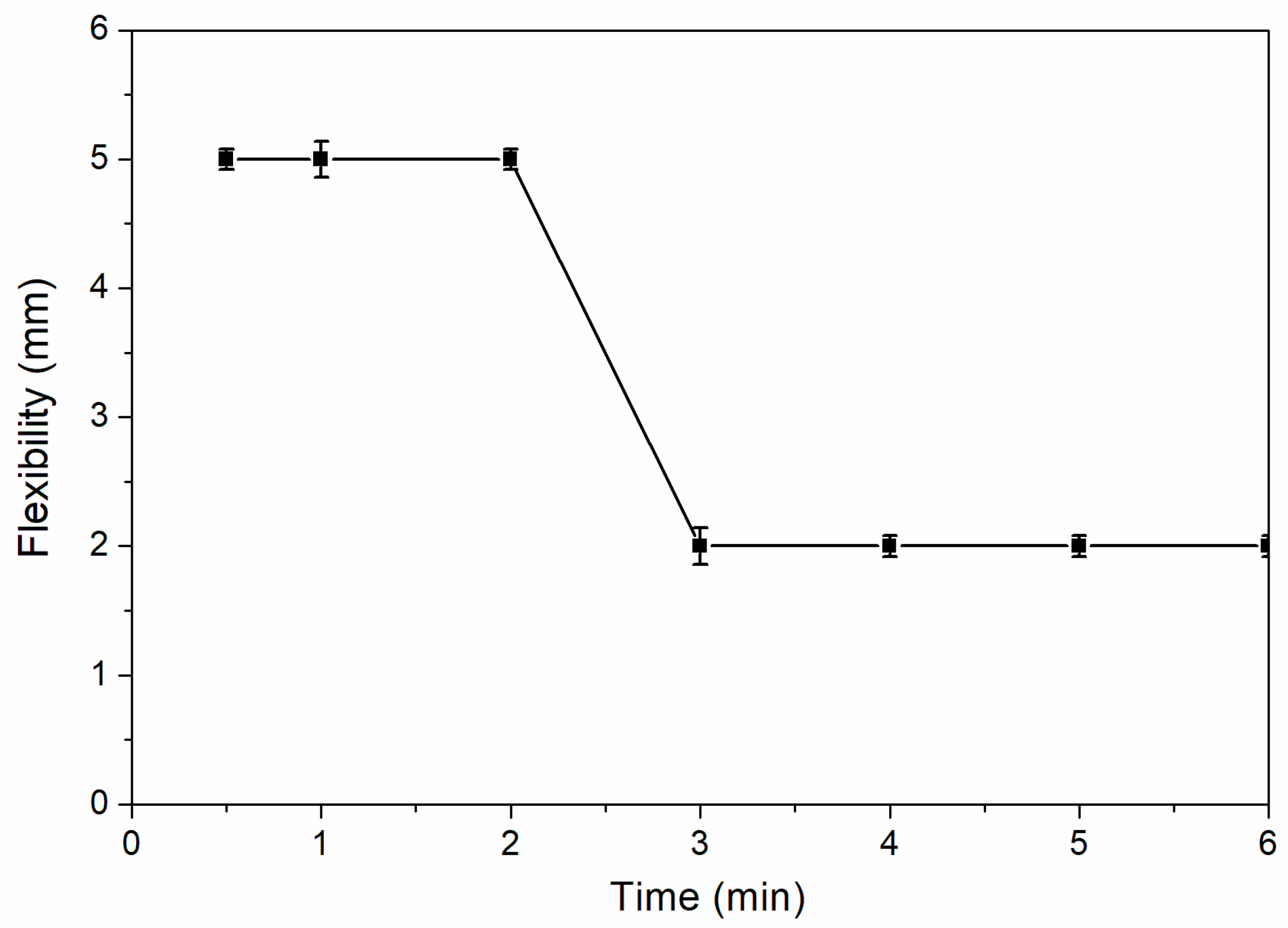
Figure 7 shows the influence of drying time on flexibility of coatings. The flexibility of coatings decreased from 5.0 to 2.0 mm.
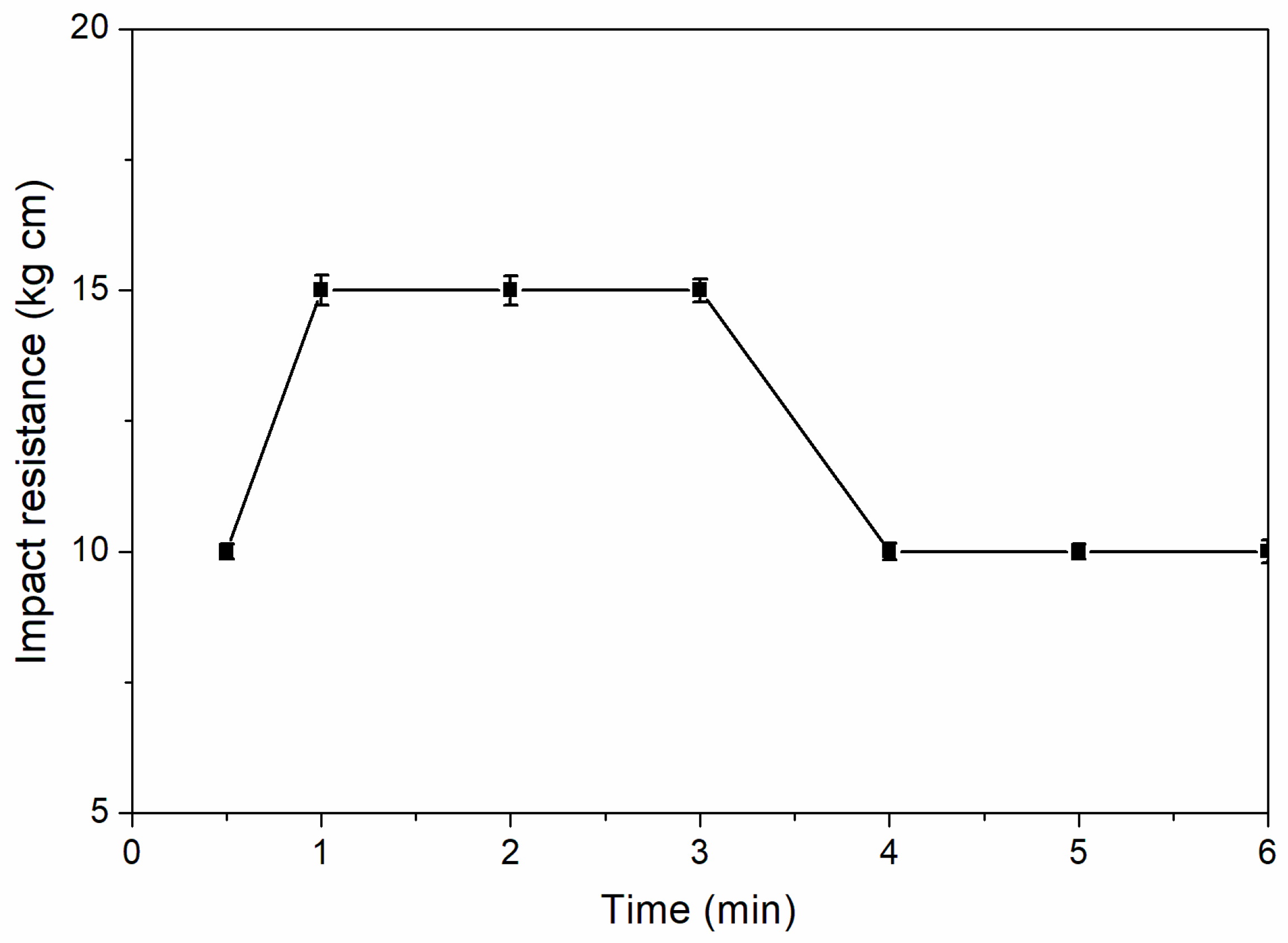
Figure 8 shows the influence of coating drying time on impact resistance. According to
Figure 8, when the drying time was 1.0–3.0 min, the impact resistance of the coating was better, which was 15.0 kg∙cm. The impact resistance of coating decreased with drying time prolonging. The reason was that the coating polymer had a suitable degree of crosslinking during the drying period of 1.0–3.0 min.
The colorimetric tests, mechanical, and optical properties showed that coatings had the better properties at 1.0 and 2.0 min. Consequently, the corrosion resistance of two coatings was tested to further explore the changes of their corrosion resistance. The electrochemical polarization curves are shown in
Figure 9, and the corrosion parameters obtained from
Figure 9 are shown in
Table 7. The porosity of coating plays a significance role in corrosion resistance. The porosity (P) of aluminum/waterborne acrylic coating can be calculated according to Equation (3):
In Equation (3),
RPS and
RPC are polarization resistance of the substrate and coated substrate, respectively. Δ
Ecorr is the corrosion potential difference between the coating and aluminum substrate, and β
as is the anode constant of aluminum substrate. In
Table 7,
Ecorr is the corrosion potential,
Icorr is the corrosion current density,
RP is the polarization resistance, and β
a and β
c are anode constant and cathode constant, respectively.
Table 7 showed that when the drying time was 1.0 min, the waterborne coating had lower
RPC, higher
Icorr, and higher porosity. The low porosity can decrease the NaCl solution penetration. Therefore, compared with 1.0 min, when the drying time was 2.0 min, the coating had better corrosion resistance. From
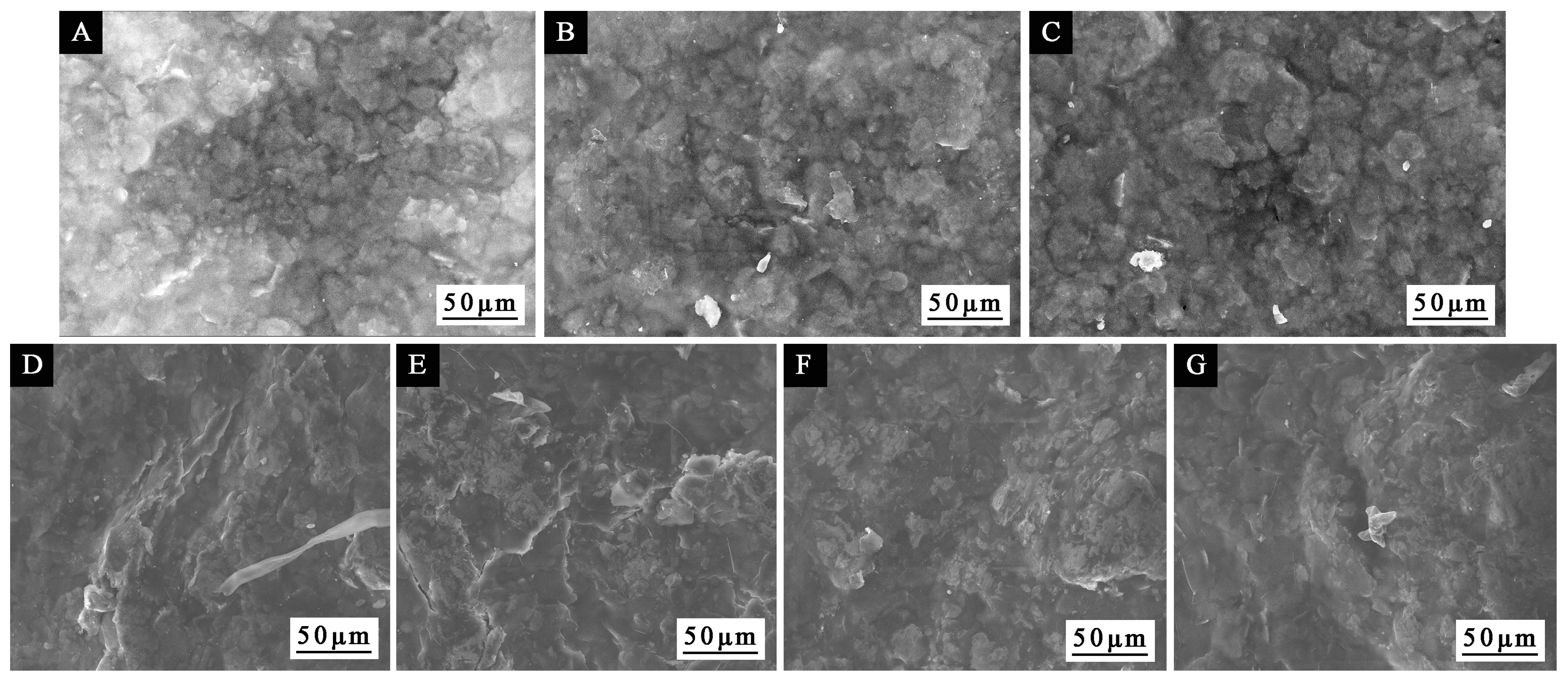
Figure 10, it can be seen that the morphology of the coating after drying at different times had little change. Compared with other drying times, when the drying time was 1.0 and 2.0 min, the distribution of Al powder in the coating was more uniform, and the spacing between particles was smaller.
The performance of waterborne acrylic based coating was compared with that of the PU based coating [
33,
34,
35] and epoxy-lacquer based coatings [
36], as shown in
Table 8. Compared with other coatings, the infrared emissivity of aluminum/waterborne acrylic coating can reach a low value of about 0.1. The gloss of aluminum/waterborne acrylic coating is lower than that of PU-based coatings, which is similar to that of epoxy-lacquer based coatings. The adhesion of aluminum/waterborne acrylic coating is the highest, which is level 0. The porosity (
p) of the aluminum/waterborne acrylic coating is the largest, which is 0.3. The impact resistance and corrosion resistance of low infrared emissive aluminum/waterborne acrylic coating are worse than low infrared emissive PU/(ball-milled Ag-Cu) [
33], PU/Al coatings [
35] and epoxy-lacquer/Al [
36].