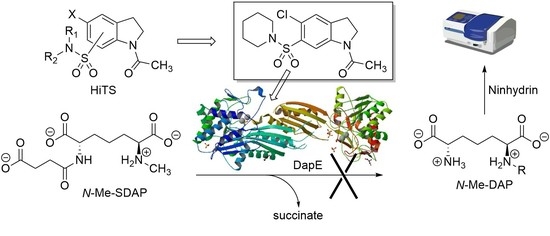
Indoline-6-Sulfonamide Inhibitors of the Bacterial Enzyme DapE
Abstract
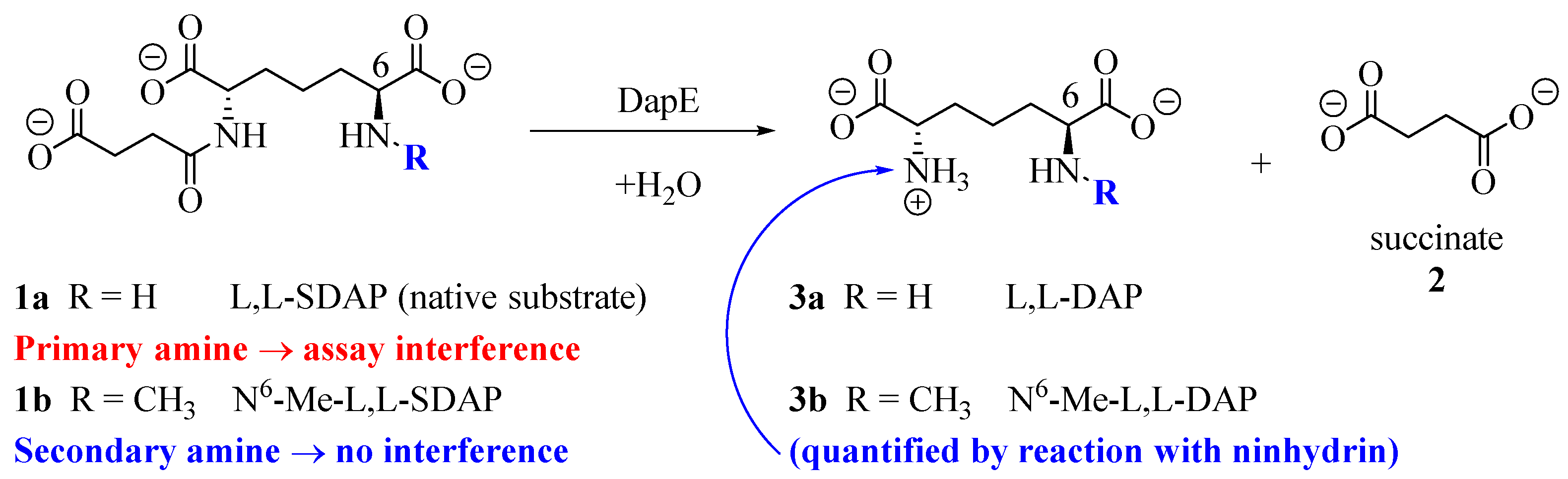
1. Introduction
2. Results
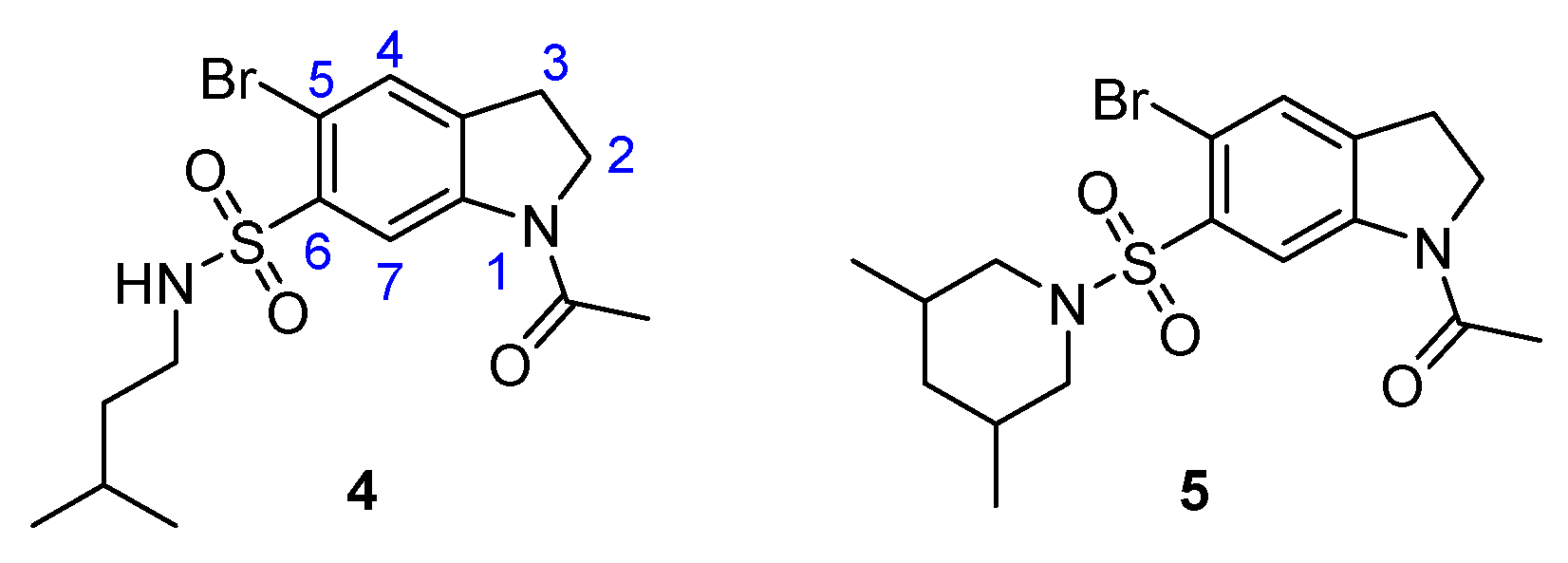
2.1. Overview and Regiochemistry
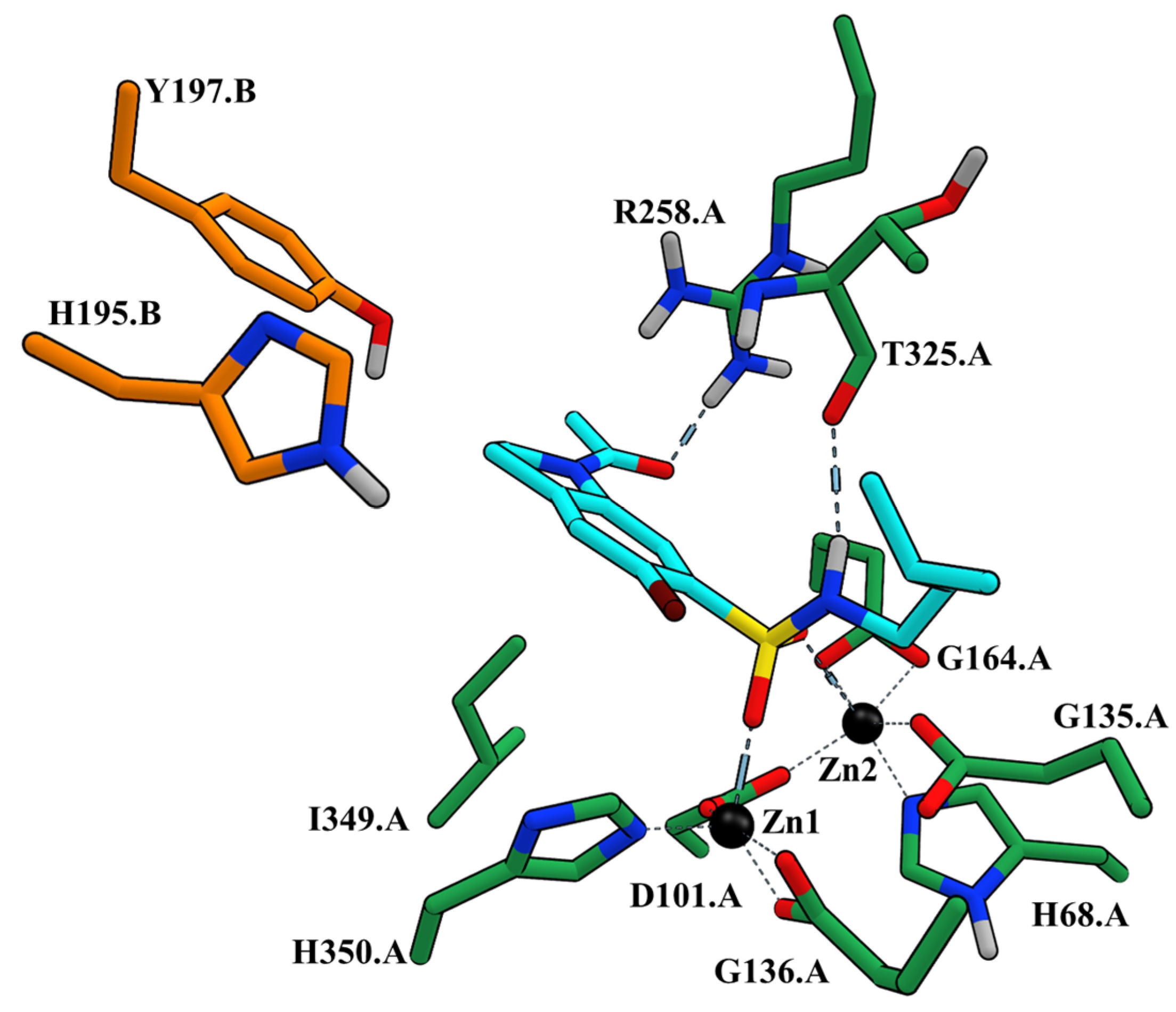
2.2. Molecular Docking Experiments
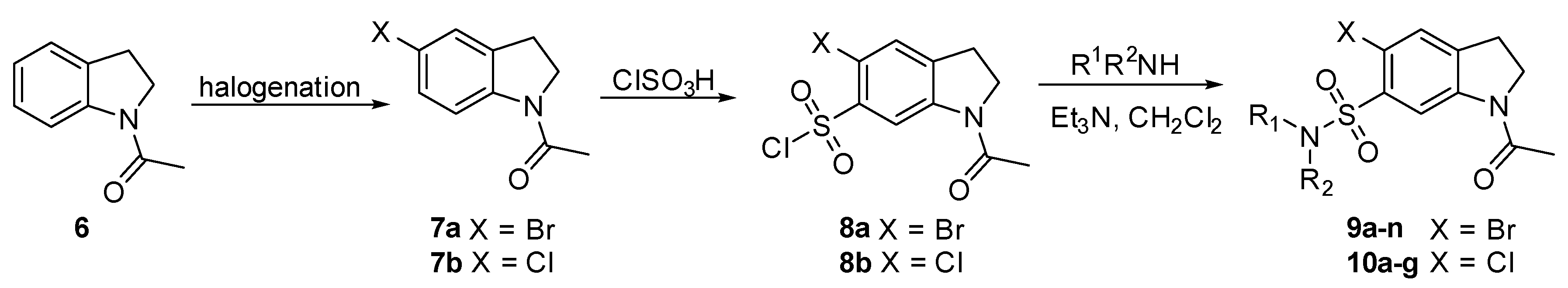
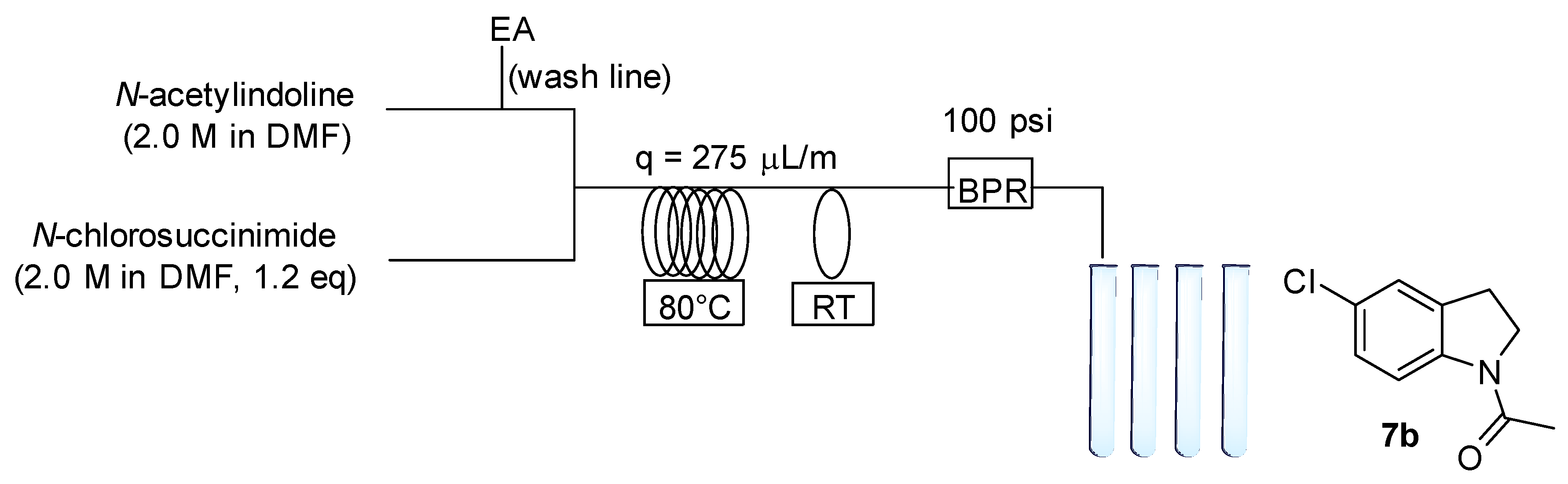
2.3. Chemistry
2.4. DapE Enzyme Inhibition and Structure–Activity Relationships
3. Summary
4. Materials and Methods
4.1. General Experimental Methods
4.2. Molecular Docking Protocol
4.3. DapE Enzyme Inhibition
4.4. Protein Expression and Purification
4.5. Synthetic Organic Chemistry
4.5.1. 1-Acetyl-5-Chloroindoline (7b)
4.5.2. 1-Acetyl 5-Bromoindoline-6-Sulfonyl Chloride (8a)
4.5.3. 1-Acetyl-5-Chloroindoline-6-Sulfonyl Chloride (8b)
4.5.4. General Procedure for the Synthesis of N-acetyl 5-Bromo-6-Sulfonamide Indolines (4, 9 and 10)
4.5.5. 1-Acetyl-5-Bromo-N-Isopentylindoline-6-Sulfonamide (4)
4.5.6. 1-Acetyl-5-Bromo-N-Isobutylindoline-6-Sulfonamide (9a)
4.5.7. 1-Acetyl-5-Bromo-N-Cyclohexylindoline-6-Sulfonamide (9b)
4.5.8. 1-Acetyl-N-Benzyl-5-Bromoindoline-6-Sulfonamide (9c)
4.5.9. 1-Acetyl-5-Bromo-N-(Tert-Butyl)Indoline-6-Sulfonamide (9d)
4.5.10. 1-Acetyl-5-Bromoindolin-6-(Sulfonyl Glycine Methyl Ester) (9e)
4.5.11. Methyl 3-((1-Acetyl-5-Bromoindoline)-6-Sulfonamido)Propanoate (9f)
4.5.12. Methyl ((1-Acetyl-5-Bromoindolin-6-yl)Sulfonyl)Valinate (9g)
4.5.13. Methyl ((1-Acetyl-5-Bromoindolin-6-yl)Sulfonyl)-L-Phenylalaninate (9h)
4.5.14. 1-Acetyl-5-Bromo-6-(Piperidin-1-Sulfonyl) Indoline (9i)
4.5.15. 1-Acetyl-5-Bromo-6-(Pyrrolidin-1-Sulfonyl) Indoline (9j)
4.5.16. 1-Acetyl-5-Bromo-6-(Indolin-1-Sulfonyl) Indoline (9k)
4.5.17. 1-Acetyl-5-Bromo-N,N-Dipropylindoline-6-Sulfonamide (9l)
4.5.18. 1-Acetyl-5-Bromo-N,N-bis(2-Methoxyethyl)Indoline-6-Sulfonamide (9m)
4.5.19. 1-Acetyl-5-Bromo-N,N-Diethylindoline-6-Sulfonamide (9n)
4.5.20. 1-Acetyl-5-Chloro-N-Isopentylindoline-6-Sulfonamide (10a)
4.5.21. 1-Acetyl-5-Chloro-N-Cyclohexylindoline-6-Sulfonamide (10b)
4.5.22. 1-(5-Chloro-6-(Piperidin-1-Ylsulfonyl)Indolin-1-yl)Ethan-1-One (10c)
4.5.23. 1-(5-Chloro-6-(Pyrrolidin-1-Ylsulfonyl)Indolin-1-yl)ethan-1-One (10d)
4.5.24. 1-Acetyl-5-Chloro-N,N-Dipropylindoline-6-Sulfonamide (10e)
4.5.25. 1-Acetyl-5-Chloro-N,N-bis(2-Methoxyethyl)Indoline-6-Sulfonamide (10f)
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Klevens, R.M.; Morrison, M.A.; Nadle, J.; Petit, S.; Gershrnan, K.; Ray, S.; Harrison, L.H.; Lynfield, R.; Dumyati, G.; Townes, J.M.; et al. Invasive methicillin-resistant staphylococcus aureus infections in the United States. JAMA 2007, 298, 1763–1771. [Google Scholar] [CrossRef] [PubMed]
- Howe, R.A.; Bowker, K.E.; Walsh, T.R.; Feest, T.G.; MacGowan, A.P. Vancomycin-resistant Staphylococcus aureus. Lancet 1998, 351, 602. [Google Scholar] [CrossRef]
- Hasenoehrl, E.J.; Sajorda, D.R.; Berney-Meyer, L.; Johnson, S.; Tufariello, J.M.; Fuhrer, T.; Cook, G.M.; Jacobs, W.R.; Berney, M. Derailing the aspartate pathway of Mycobacterium tuberculosis to eradicate persistent infection. Nat. Commun. 2019, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Tuberculosis Report 2019; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- World Health Organization. Antibacterial Agents in Clinical Development: An Analysis of the Antibacterial Clinical Development Pipeline, Including Tuberculosis; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Gillner, D.M.; Becker, D.P.; Holz, R.C. Lysine biosynthesis in bacteria: A metallodesuccinylase as a potential antimicrobial target. JBIC J. Biol. Inorg. Chem. 2013, 18, 155–163. [Google Scholar] [CrossRef]
- Scapin, G.; Blanchard, J.S. Enzymology of bacterial lysine biosynthesis. Adv. Enzymol. Relat. Areas Mol. Biol. 1998, 72, 279–324. [Google Scholar]
- Karita, M.; Etterbeek, M.L.; Forsyth, M.H.; Tummuru, M.K.R.; Blaser, M.J. Characterization of Helicobacter pylori dapE and construction of a conditionally lethal dapE mutant. Infect. Immun. 1997, 65, 4158–4164. [Google Scholar] [CrossRef]
- Pavelka, M.S., Jr.; Jacobs, W.R., Jr. Biosynthesis of diaminopimelate, the precursor of lysine and a component of peptidoglycan, is an essential function of Mycobacterium smegmatis. J. Bacteriol. 1996, 178, 6496–6507. [Google Scholar] [CrossRef]
- Heath, T.K.; Lutz, M.R., Jr.; Reidl, C.T.; Guzman, E.R.; Herbert, C.A.; Nocek, B.P.; Holz, R.C.; Olsen, K.W.; Ballicora, M.A.; Becker, D.P. Practical spectrophotometric assay for the dapE-encoded N-succinyl-l,l-diaminopimelic acid desuccinylase, a potential antibiotic target. PLoS ONE 2018, 13, e0196010. [Google Scholar] [CrossRef]
- Badger, J.; Sauder, J.; Adams, J.; Antonysamy, S.; Bain, K.; Bergseid, M.; Buchanan, S.; Buchanan, M.; Batiyenko, Y.; Christopher, J. Structural analysis of a set of proteins resulting from a bacterial genomics project. Proteins Struct. Funct. Bioinform. 2005, 60, 787–796. [Google Scholar] [CrossRef]
- Nocek, B.P.; Gillner, D.M.; Fan, Y.; Holz, R.C.; Joachimiak, A. Structural Basis for Catalysis by the Mono- and Dimetalated Forms of the dapE-Encoded N-succinyl-l,l-Diaminopimelic Acid Desuccinylase. J. Mol. Biol. 2010, 397, 617–626. [Google Scholar] [CrossRef]
- Starus, A.; Nocek, B.; Bennett, B.; Larrabee, J.A.; Shaw, D.L.; Sae-Lee, W.; Russo, M.T.; Gillner, D.M.; Makowska-Grzyska, M.; Joachimiak, A.; et al. Inhibition of the dapE-Encoded N-Succinyl-l,l-diaminopimelic Acid Desuccinylase from Neisseria meningitidis by L-Captopril. Biochemistry 2015, 54, 4834–4844. [Google Scholar] [CrossRef] [PubMed]
- Nocek, B.; Reidl, C.; Starus, A.; Heath, T.; Bienvenue, D.; Osipiuk, J.; Jedrzejczak, R.P.; Joachimiak, A.; Becker, D.P.; Holz, R.C. Structural Evidence for a Major Conformational Change Triggered by Substrate Binding in DapE Enzymes: Impact on the Catalytic Mechanism. Biochemistry 2018, 57, 574. [Google Scholar] [CrossRef] [PubMed]
- Reidl, C.; Majorek, K.A.; Dang, J.; Tran, D.; Jew, K.; Law, M.; Payne, Y.; Minor, W.; Becker, D.P.; Kuhn, M.L. Generating enzyme and radical-mediated bisubstrates as tools for investigating Gcn5-related N-acetyltransferases. FEBS Lett. 2017, 591, 2348–2361. [Google Scholar] [CrossRef]
- Gillner, D.; Armoush, N.; Holz, R.C.; Becker, D.P. Inhibitors of bacterial N-succinyl-l,l-diaminopimelic acid desuccinylase (DapE) and demonstration of in vitro antimicrobial activity. Bioorg. Med. Chem. Lett. 2009, 19, 6350–6352. [Google Scholar] [CrossRef]
- Mandal, R.S.; Das, S. In silico approach towards identification of potential inhibitors of Helicobacter pylori DapE. J. Biomol. Struct. Dyn. 2015, 33, 1460–1473. [Google Scholar] [CrossRef] [PubMed]
- Gerullis, H.; Wawroschek, F.; Köhne, C.; Ecke, T.H. Vinflunine in the treatment of advanced urothelial cancer: Clinical evidence and experience. Ther. Adv. Urol. 2017, 9, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Bolognesi, M.L.; Andrisano, V.; Bartolini, M.; Minarini, A.; Rosini, M.; Tumiatti, V.; Melchiorre, C. Hexahydrochromeno [4,3-b] pyrrole derivatives as acetylcholinesterase inhibitors. J. Med. Chem. 2001, 44, 105–109. [Google Scholar] [CrossRef]
- Kumar, B.; Kuhad, A.; Kuhad, A. Lumateperone: A new treatment approach for neuropsychiatric disorders. Drugs Today 2018, 54, 713–719. [Google Scholar] [CrossRef]
- Vanover, K.; Correll, C.; Dmitrienko, A.; Glass, S.; O’Gorman, C.; Saillard, J.; Weingart, M.; Mates, S.; Davis, R. 24. The Clinical Development of Lumateperone (ITI-007) for the Treatment of Schizophrenia. Schizophr. Bull. 2017, 43, S16. [Google Scholar] [CrossRef]
- Canale, V.; Rak, A.; Kotańska, M.; Knutelska, J.; Siwek, A.; Bednarski, M.; Nowiński, L.; Zygmunt, M.; Koczurkiewicz, P.; Pękala, E. Synthesis and Pharmacological Evaluation of Novel Silodosin-Based Arylsulfonamide Derivatives as α1A/α1D-Adrenergic Receptor Antagonist with Potential Uroselective Profile. Molecules 2018, 23, 2175. [Google Scholar] [CrossRef]
- Lesch, J.E. The first Miracle Drugs: How the Sulfa Drugs Transformed Medicine; Oxford University Press: New York, NY, USA, 2007. [Google Scholar]
- Li, J.J.; Corey, E.J. Drug Discovery: Practices, Processes, and Perspectives; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Smith, B.R.; Eastman, C.M.; Njardarson, J.T. Beyond C, H, O, and N! Analysis of the elemental composition of US FDA approved drug architectures: Miniperspective. J. Med. Chem. 2014, 57, 9764–9773. [Google Scholar] [CrossRef] [PubMed]
- Ilardi, E.A.; Vitaku, E.; Njardarson, J.T. Data-mining for sulfur and fluorine: An evaluation of pharmaceuticals to reveal opportunities for drug design and discovery: Miniperspective. J. Med. Chem. 2014, 57, 2832–2842. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.A.; Njardarson, J.T. Analysis of US FDA-approved drugs containing sulfur atoms. In Sulfur Chemistry; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–34. [Google Scholar]
- Fier, P.S.; Kim, S.; Maloney, K.M. Reductive Cleavage of Secondary Sulfonamides: Converting Terminal Functional Groups into Versatile Synthetic Handles. J. Am. Chem. Soc. 2019, 141, 18416–18420. [Google Scholar] [CrossRef] [PubMed]
- Borror, A.L.; Chinoporos, E.; Filosa, M.P.; Herchen, S.R.; Petersen, C.P.; Stern, C.A.; Onan, K.D. Regioselectivity of electrophilic aromatic substitution: Syntheses of 6- and 7-sulfamoylindolines and -indoles. J. Org. Chem. 1988, 53, 2047–2052. [Google Scholar] [CrossRef]
- Shalygina, E.E.; Kobylinskii, D.V.; Ivanovskii, S.A.; Balakin, K.V.; Dorogov, M.V.; Toporova, T.A. Synthesis and properties of 1-acylindolinesulfonamides. Izv. Vyssh. Uchebn. Zaved. Khim. Khim. Tekhnol. 2004, 47, 91–96. [Google Scholar]
- Pan, C.; Abdukader, A.; Han, J.; Cheng, Y.; Zhu, C. Ruthenium-Catalyzed C7 Amidation of Indoline C? H Bonds with Sulfonyl Azides. Chemistry 2014, 20, 3606–3609. [Google Scholar] [CrossRef]
- Gutmann, B.; Cantillo, D.; Kappe, C.O. Continuous-Flow Technology-A Tool for the Safe Manufacturing of Active Pharmaceutical Ingredients. Angew. Chem. Int. Ed. 2015, 54, 6688–6728. [Google Scholar] [CrossRef]
- Pelleter, J.; Renaud, F. Facile, Fast and Safe Process Development of Nitration and Bromination Reactions Using Continuous Flow Reactors. Org. Process Res. Dev. 2009, 13, 698–705. [Google Scholar] [CrossRef]
- Danheiser, R.L.; Renslo, A.R.; Amos, D.T.; Wright, G.T. Preparation of substituted pyridines via regiocontrolled [4 + 2]-cycloadditions of oximinosulfonates: Methyl 5-methylpyridine-2-carboxylate. Org. Synth. 2003, 80, 133–143. [Google Scholar]
- Dorogov, M.V.; Filimonov, S.I.; Kobylinsky, D.B.; Ivanovsky, S.A.; Korikov, P.V.; Soloviev, M.Y.; Khahina, M.Y.; Shalygina, E.E.; Kravchenko, D.V.; Ivachtchenko, A.V. A convenient synthesis of novel 3-(heterocyclylsulfonyl)propanoic acids and their amide derivatives. Synthesis 2004, 2004, 2999–3004. [Google Scholar] [CrossRef]
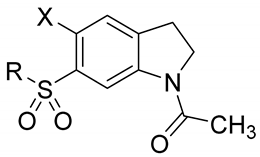
| 0 | X | R | MW | clogP | mp (°C) | IC50 (μM) or % Inhibition a |
|---|---|---|---|---|---|---|
| 4 | Br |  | 389.3 | 2.79 | 190–191 | 42% at 200 μM |
| 9a | Br | 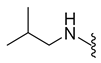 | 374.0 | 2.26 | 225.4–226.1 | 20% at 200 μM |
| 9b | Br | 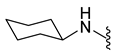 | 400.0 | 2.84 | 226–228 | 162 |
| 9c | Br |  | 408.0 | 2.57 | 205–208 | 56% at 200 μM |
| 9d | Br |  | 374.0 | 2.04 | 235.7–236.1 | 39% at 100 μM |
| 9e | Br |  | 390.0 | 0.79 | 179–182 | 17% at 200 μM |
| 9f | Br | 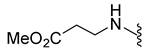 | 404.0 | 1.1 | 190–193 | 118 |
| 9g | Br |  | 432.0 | 2.0 | 190.9–191.7 | 82 |
| 9h | Br |  | 480.0 | 2.5 | 184.0–184.8 | 61% at 200 μM |
| 9i | Br | 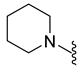 | 386.0 | 2.1 | 212–214 | 133 |
| 9j | Br | 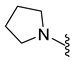 | 372.0 | 1.6 | 238–240 | 97 |
| 9k | Br |  | 420.0 | 2.6 | 215–218 | 86 |
| 9l | Br |  | 402.1 | 3.0 | 118–120 | 5% at 20 μM, insol. at 200 μM |
| 9m | Br | 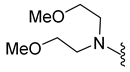 | 434.1 | 1.5 | 110–111 | 26% at 100 μM |
| 9n | Br | 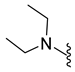 | 374.0 | 2.0 | 195–196 | 99 |
| 10a | Cl |  | 344.1 | 2.8 | 179.1–180.4 | 54 |
| 10b | Cl | 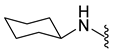 | 356.1 | 2.9 | 230.0–230.5 | 58% at 200 μM |
| 10c | Cl | 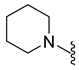 | 342.1 | 2.2 | 199.7–201.4 | 44 |
| 10d | Cl | 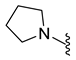 | 328.1 | 1.6 | 199.7–200.4 | 172 |
| 10e | Cl | 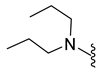 | 358.1 | 3.1 | 118–120 | 88 |
| 10f | Cl | 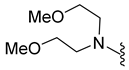 | 390.1 | 1.5 | 93.2–94.4 | >200 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reidl, C.T.; Heath, T.K.; Darwish, I.; Torrez, R.M.; Moore, M.; Gild, E.; Nocek, B.P.; Starus, A.; Holz, R.C.; Becker, D.P. Indoline-6-Sulfonamide Inhibitors of the Bacterial Enzyme DapE. Antibiotics 2020, 9, 595. https://doi.org/10.3390/antibiotics9090595
Reidl CT, Heath TK, Darwish I, Torrez RM, Moore M, Gild E, Nocek BP, Starus A, Holz RC, Becker DP. Indoline-6-Sulfonamide Inhibitors of the Bacterial Enzyme DapE. Antibiotics. 2020; 9(9):595. https://doi.org/10.3390/antibiotics9090595
Chicago/Turabian StyleReidl, Cory T., Tahirah K. Heath, Iman Darwish, Rachel M. Torrez, Maxwell Moore, Elliot Gild, Boguslaw P. Nocek, Anna Starus, Richard C. Holz, and Daniel P. Becker. 2020. "Indoline-6-Sulfonamide Inhibitors of the Bacterial Enzyme DapE" Antibiotics 9, no. 9: 595. https://doi.org/10.3390/antibiotics9090595
APA StyleReidl, C. T., Heath, T. K., Darwish, I., Torrez, R. M., Moore, M., Gild, E., Nocek, B. P., Starus, A., Holz, R. C., & Becker, D. P. (2020). Indoline-6-Sulfonamide Inhibitors of the Bacterial Enzyme DapE. Antibiotics, 9(9), 595. https://doi.org/10.3390/antibiotics9090595