Vancomycin-Resistant Enterococci (VRE) in Nigeria: The First Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Results
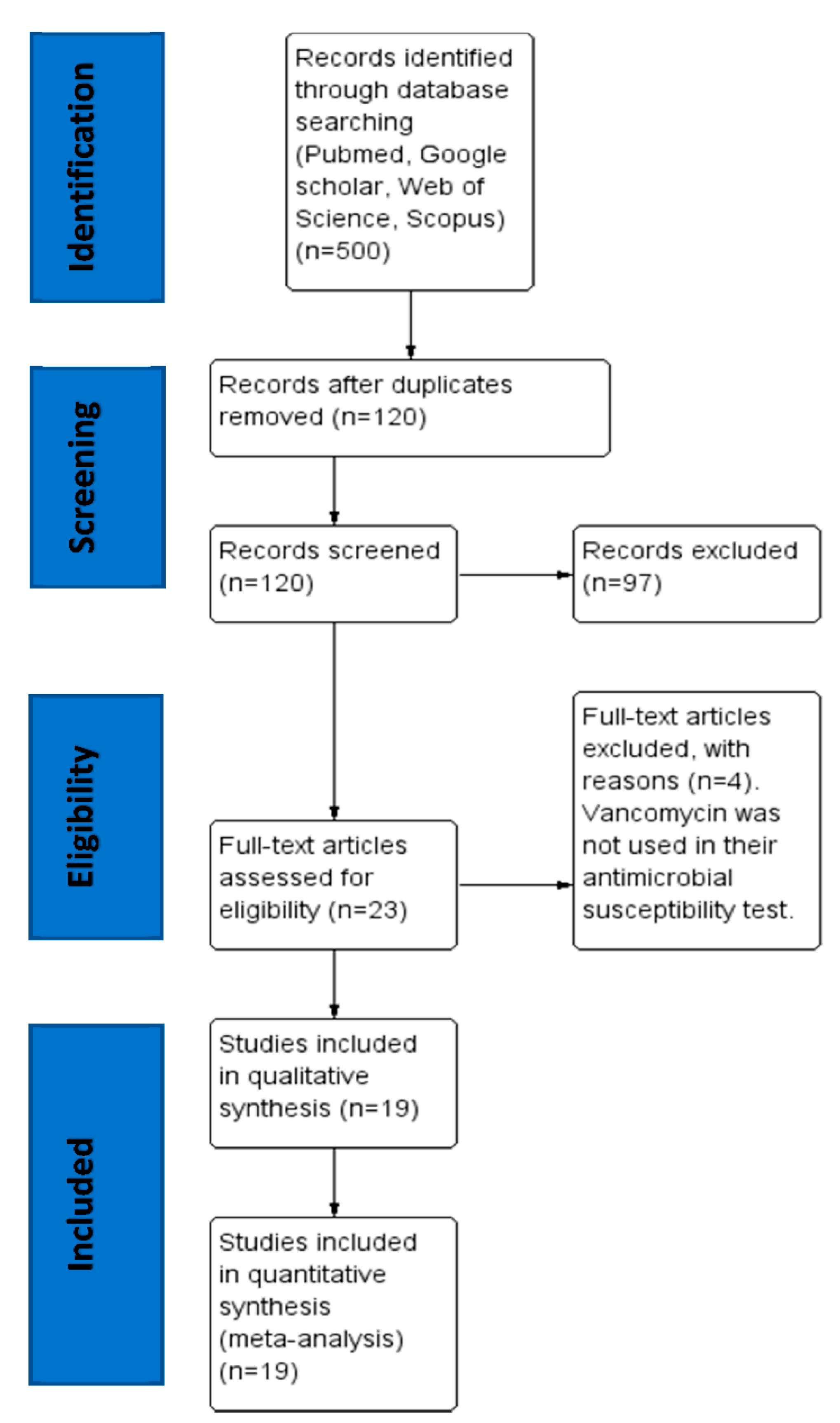
2.1. Search Results and Eligible Studies
2.2. Characteristics of the Eligible Studies
2.3. The Pooled Prevalence of VRE
2.4. Subgroup Meta-Analysis
2.5. Meta-Regression
3. Discussion
4. Materials and Methods
4.1. Study Design and Protocol
4.2. Literature Review
4.3. Inclusion and Exclusion Criteria for Studies
4.4. Data Extraction
4.5. Data Analysis
4.6. Bias and Heterogeneity Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wada, Y.; Harun, A.B.; Yean, C.Y.; Zaidah, A.R.; Wada, Y. Vancomycin-Resistant Enterococcus: Issues in Human Health, Animal Health, Resistant Mechanisms and the Malaysian Paradox. Adv. Anim. Veter-Sci. 2019, 7, 1021–1034. [Google Scholar] [CrossRef]
- Iseppi, R.; Di Cerbo, A.; Messi, P.; Sabia, C. Antibiotic Resistance and Virulence Traits in Vancomycin-Resistant Enterococci (VRE) and Extended-Spectrum β-Lactamase/AmpC-producing (ESBL/AmpC) Enterobacteriaceae from Humans and Pets. Antibiotics 2020, 9, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adapa, S.; Naramala, S.; Boken, D.; Moreno, A.; Konala, V.M. Peritonitis from Anaerobic Gram-positive Cocci Likely Due to Translocation of Bacteria from Gut in a Patient Undergoing Peritoneal Dialysis. Cureus 2019, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Argudín, M. Ángeles; Deplano, A.; Meghraoui, A.; Dodémont, M.; Heinrichs, A.; Denis, O.; Nonhoff, C.; Roisin, S. Bacteria from Animals as a Pool of Antimicrobial Resistance Genes. Antibiotics 2017, 6, 12. [Google Scholar] [CrossRef]
- Rostkowska, O.M.; Kuthan, R.; Burban, A.; Salińska, J.; Ciebiera, M.; Mlynarczyk, G.; Durlik, M. Analysis of Susceptibility to Selected Antibiotics in Klebsiella pneumoniae, Escherichia coli, Enterococcus faecalis and Enterococcus faecium Causing Urinary Tract Infections in Kidney Transplant Recipients over 8 Years: Single-Center Study. Antibiotics 2020, 9, 284. [Google Scholar] [CrossRef]
- Folliero, V.; Caputo, P.; Della Rocca, M.T.; Chianese, A.; Galdiero, M.; Iovene, M.R.; Hay, C.; Franci, G.; Galdiero, M. Prevalence and Antimicrobial Susceptibility Patterns of Bacterial Pathogens in Urinary Tract Infections in University Hospital of Campania “Luigi Vanvitelli” between 2017 and 2018. Antibiotics 2020, 9, 215. [Google Scholar] [CrossRef]
- Banik, A.; Halder, S.K.; Ghosh, C.; Mondal, K.C. Enterococcal Infections, Drug Resistance, and Application of Nanotechnology; Springer Science and Business Media LLC: New York, NY, USA, 2020; pp. 417–445. [Google Scholar]
- Bhardwaj, S.B. Enterococci: An Important Nosocomial Pathogen. In Pathogenic Bacteria [Working Title]; IntechOpen: Rijeka, Croatia, 2019. [Google Scholar]
- Bender, J.K.; Cattoir, V.; Hegstad, K.; Sadowy, E.; Coque, T.M.; Westh, H.; Hammerum, A.M.; Schaffer, K.; Burns, K.; Murchan, S.; et al. Update on prevalence and mechanisms of resistance to linezolid, tigecycline and daptomycin in enterococci in Europe: Towards a common nomenclature. Drug Resist. Updat. 2018, 40, 25–39. [Google Scholar] [CrossRef]
- Wada, Y.H.; Harun, A.; Yean, C.; Nasir, N.M.; Zaidah, A.R.; Wada, Y. Vancomycin-resistant enterococcus, obesity and antibiotics: Is there a possible link? Obes. Med. 2020, 18, 100226. [Google Scholar] [CrossRef]
- Freitas, A.R.; Tedim, A.P.; Coque, T.M.; Jensen, L.; Novais, C.; Peixe, L.; Sánchez-Valenzuela, A.; Sundsfjord, A.; Hegstad, K.; Werner, G.; et al. Multilevel population genetic analysis ofvanAandvanB Enterococcus faeciumcausing nosocomial outbreaks in 27 countries (1986–2012). J. Antimicrob. Chemother. 2016, 71, 3351–3366. [Google Scholar] [CrossRef] [Green Version]
- Hansen, T.A.; Pedersen, M.S.; Nielsen, L.G.; Ma, C.M.G.; Søes, L.M.; Worning, P.; Østergaard, C.; Westh, H.; Pinholt, M.; Schoenning, K. Emergence of a vancomycin-variable Enterococcus faecium ST1421 strain containing a deletion in vanX. J. Antimicrob. Chemother. 2018, 73, 2936–2940. [Google Scholar] [CrossRef]
- Lee, T.; Pang, S.; Abraham, S.; Coombs, G.W. Molecular characterization and evolution of the first outbreak of vancomycin-resistant Enterococcus faecium in Western Australia. Int. J. Antimicrob. Agents 2019, 53, 814–819. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.J.; Vu, B.N.; Seddon, A.N.; Hodgson, H.A.; Wang, S.K. Treatment Considerations for CNS Infections Caused by Vancomycin-Resistant Enterococcus faecium: A Focused Review of Linezolid and Daptomycin. Ann. Pharmacother. 2020, 106002802093251. [Google Scholar] [CrossRef]
- Ross, J.L.; Rankin, S.; Marshik, P.; Mercier, R.-C.; Brett, M.; Walraven, C.J. Antimicrobial Stewardship Intervention and Feedback to Infectious Disease Specialists: A Case Study in High-Dose Daptomycin. Antibiotics 2015, 4, 309–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dagnaw, A.M.; Genet, C.; Andualem, T. Prevalence of Vancomycin resistant enterococci (VRE) in Ethiopia: A systematic review and meta-analysis. BMC Infect. Dis. 2020, 20, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Olawale, K.; Fadiora, S.; Taiwo, S. Prevalence of hospital acquired enterococci infections in two primary-care hospitals in Osogbo, Southwestern Nigeria. Afr. J. Infect. Dis. 2011, 5, 40–46. [Google Scholar] [CrossRef]
- Oyedeji, O.; Olutiola, P.O.; Owolabi, K.D.; Adeojo, K.A. Multiresistant faecal indicator bacteria in stream and well waters of Ile-ife city, southwestern Nigeria: Public health implications. J. Public Health Epidemiol. 2011. Available online: https://academicjournals.org/journal/JPHE/article-abstract/5C2D8F51666 (accessed on 22 March 2020).
- Olawale, A.K.; Akinro, E.B.; Olawale, A.O.; Olakunle, T.P. Transmission of Antibiotic-Resistant Enterococcus faecalis through Currency Notes. Am. J. Biol. Life Sci. 2014, 2, 162–165. Available online: http://www.openscienceonline.com/journal/archive2?journalId704&paperId1078 (accessed on 22 March 2020).
- Olawale, A.; Salako, R.; Famurewa, O. Antibiotic-Resistant Enterococcus faecalis Isolated from Food Canteens in Osun States, Nigeria. Br. Microbiol. Res. J. 2015, 6, 196–206. [Google Scholar] [CrossRef]
- Ayeni, F.A.; Odumosu, B.T.; Oluseyi, A.E.; Ruppitsch, W. Identification and prevalence of tetracycline resistance in enterococci isolated from poultry in Ilishan, Ogun State, Nigeria. J. Pharm. Bioallied Sci. 2016, 8, 69. [Google Scholar] [CrossRef]
- Ekuma, A.E.; Oduyebo, O.O.; Efunshile, A.M.; Konig, B. Surveillance for Vancomycin Resistant Enterococci in a Tertiary Institution in South Western Nigeria. Afr. J. Infect. Dis. 2016, 10, 121–126. [Google Scholar] [CrossRef] [Green Version]
- Adesida, S.A.; Ezenta, C.C.; Adagbada, A.O.; Aladesokan, A.A.; Coker, A.O. Carriage of Multidrug Resistant Enterococcus Faecium and Enterococcus Faecalis among Apparently Healthy Humans. Afr. J. Infect. Dis. 2017, 11, 83–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- David, O.M.; Imonitie, K.; Osuntoyinbo, R.T.; Olawale, A.K. Virulence Factors and Beta-Lactamase Production among Vancomycin-Resistant Enterococcus Faecalis Isolated from Clinical Samples and Hospital Environment. Int. J. Biol. Res. 2017, 5, 1–5. [Google Scholar] [CrossRef]
- Oguntoyinbo, F.A.; Okueso, O. Prevalence, distribution and antibiotic resistance pattern among enterococci species in two traditional fermented dairy foods. Ann. Microbiol. 2012, 63, 755–761. [Google Scholar] [CrossRef]
- Ndubuisi, J.C.; Olonitola, O.S.; Olayinka, A.T.; Jatau, E.D.; Iregbu, K.C. Prevalence and antibiotics susceptibility profile of Enterococcus spp. Isolated from some hospitals in Abuja, Nigeria. Afr. J. Clin. Exp. Microbiol. 2017, 18, 154. [Google Scholar] [CrossRef] [Green Version]
- Shettima, S.A.; Iregbu, K.C. Antimicrobial Resistance Pattern of Enterococci Isolated from Stool Samples in a Tertiary Hospital in Nigeria. Ann. Trop. Pathol. 2019, 10, 126. [Google Scholar] [CrossRef]
- Anyanwu, M.U.; Obetta, T.U. Prevalence and Antibiogram of Generic Enterococci in Ready-to-Slaughter Beef Cattle. Not. Sci. Boil. 2015, 7, 390–399. [Google Scholar] [CrossRef]
- Nsofor, C.A. High Antibiotic Resistance Pattern Observed in Bacterial Isolates from a Tertiary Hospital in South East Nigeria. Int. J. Res. Pharm. Biosci. 2016, 3, 1–6. [Google Scholar]
- Anyanwu, M.U.; Okorie-Kanu, O.J.; Ogugua, A.J.; Ezenduka, E.V.; Anidebe, C.O. Occurrence, Antibiogram and Vancomycin Resistance of Generic Enterococci in Horses in Nigeria. Revue Méd. Vét. 2019, 170, 46–52. [Google Scholar]
- Foka, F.E.T.; Yah, C.S.; Bissong, M.E.A. Physico-Chemical Properties and Microbiological Quality of Borehole Water in Four Crowded Areas of Benin City, Nigeria, During Rainfalls. Shiraz E-Med. J. 2018, 19. [Google Scholar] [CrossRef] [Green Version]
- Abasiubong, V.N.; Ikon, G.M.; Amadi, C.P. Enterococcus Isolates in Clinical Samples from In-Patients in Uyo, Nigeria. Int. J. Life Sci. Res. 2019, 7, 353–358. [Google Scholar]
- Igbinosa, E.O.; Beshiru, A. Antimicrobial Resistance, Virulence Determinants, and Biofilm Formation of Enterococcus Species from Ready-to-Eat Seafood. Front. Microbiol. 2019, 10, 728. [Google Scholar] [CrossRef] [PubMed]
- Igbinosa, I.H.; Raje, O.C. Characterization of Enterococcus species isolated from abattoir environment in Benin city, Nigeria. Ife J. Sci. 2020, 21, 81. [Google Scholar] [CrossRef] [Green Version]
- Enenya, R.P.; Yakubu, S.E.; Ado, S.A.; Ella, E.E.; Igwe, J.C. Antibiotic Resistance Profile of Enterococcus species Isolated from Drinking Water Sources in Zaria, Kaduna State, Nigeria. J. Trop. Biosci. 2017, 12, 20–25. Available online: https://www.researchgate.net/profile/Rufus_Enenya/publication/325688794_Antibiotic_Resistance_Profile_of_Enterococcus_species_Isolated_from_Drinking_Water_Sources_in_Zaria_Kaduna_State_Nigeria/links/5b1e5cf745851587f29feef1/Antibiotic-Resistance-Profile-of-Enterococcus-species-Isolated-from-Drinking-Water-Sources-in-Zaria-Kaduna-State-Nigeria.pdf (accessed on 23 March 2020).
- Federal Ministries of Agriculture, Environment and Health. In National Action Plan for Antimicrobial Resistance 2017–2022; 2017. Available online: https://ncdc.gov.ng/themes/common/docs/protocols/77_1511368219.pdf (accessed on 8 August 2020).
- Wada, Y.; Harun, A.B.; Chan, Y.Y.; Mohamad Nasir, N.S.; Zaidah, A.R. Prevalence of Vancomycin resistant enterococcus in Malaysia: A meta-analysis and systematic review. Int. J. Infect. Dis. 2020. Accepted Manuscript. [Google Scholar]
- Ali, S.; Alemayehu, M.A.; Dagnew, M.; Gebrecherkos, T. Vancomycin-Resistant Enterococci and Its Associated Risk Factors among HIV-Positive and -Negative Clients Attending Dessie Referral Hospital, Northeast Ethiopia. Int. J. Microbiol. 2018, 2018, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Moghimbeigi, A.; Moghimbeygi, M.; Dousti, M.; Kiani, F.; Sayehmiri, F.; Sadeghifard, N.; Nazari, A. Prevalence of vancomycin resistance among isolates of enterococci in Iran: A systematic review and meta-analysis. Adolesc. Health Med. Ther. 2018, 9, 177–188. [Google Scholar] [CrossRef] [Green Version]
- Alevizakos, M.; Gaitanidis, A.; Nasioudis, D.; Tori, K.; Flokas, M.E.; Mylonakis, E. Colonization With Vancomycin-Resistant Enterococci and Risk for Bloodstream Infection Among Patients with Malignancy: A Systematic Review and Meta-Analysis. Open Forum Infect. Dis. 2016, 4. [Google Scholar] [CrossRef] [Green Version]
- Kramer, T.; Remschmidt, C.; Werner, S.; Behnke, M.; Schwab, F.; Werner, G.; Gastmeier, P.; Leistner, R. The importance of adjusting for enterococcus species when assessing the burden of vancomycin resistance: A cohort study including over 1000 cases of enterococcal bloodstream infections. Antimicrob. Resist. Infect. Control 2018, 7, 133. [Google Scholar] [CrossRef]
- Emaneini, M.; Hosseinkhani, F.; Jabalameli, F.; Nasiri, M.J.; Dadashi, M.; Pouriran, R.; Beigverdi, R. Prevalence of vancomycin-resistant Enterococcus in Iran: A systematic review and meta-analysis. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 1387–1392. [Google Scholar] [CrossRef]
- Toner, L.; Papa, N.; Aliyu, S.H.; Dev, H.; Lawrentschuk, N.; Al-Hayek, S. Vancomycin resistant enterococci in urine cultures: Antibiotic susceptibility trends over a decade at a tertiary hospital in the United Kingdom. Investig. Clin. Urol. 2016, 57, 129–134. [Google Scholar] [CrossRef]
- Yang, K.-S.; Fong, Y.-T.; Lee, H.-Y.; Kurup, A.; Koh, T.-H.; Koh, D.; Lim, M.-K. Predictors of vancomycin-resistant enterococcus (VRE) carriage in the first major VRE outbreak in Singapore. Ann. Acad. Med. Singap. 2007, 36, 379–383. [Google Scholar]
- Barger, M.; Blodget, E.; Pena, S.; Mack, W.J.; Fong, T.-L. VRE in cirrhotic patients. BMC Infect. Dis. 2019, 19, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Camins, B.C.; Farley, M.M.; Jernigan, J.J.; Ray, S.M.; Steinberg, J.P.; Blumberg, H.M. A Population-Based Investigation of Invasive Vancomycin-ResistantEnterococcusInfection in Metropolitan Atlanta, Georgia, and Predictors of Mortality. Infect. Control Hosp. Epidemiol. 2007, 28, 983–991. [Google Scholar] [CrossRef]
- Jakovljevic, M.; Al Ahdab, S.; Jurisevic, M.; Mouselli, S. Antibiotic Resistance in Syria: A Local Problem Turns Into a Global Threat. Front. Public Health 2018, 6, 212. [Google Scholar] [CrossRef] [Green Version]
- Zhussupova, G.; Skvirskaya, G.; Reshetnikov, V.A.; Dragojević-Simić, V.; Rančić, N.; Utepova, D.; Jakovljevic, M. The Evaluation of Antibiotic Consumption at the Inpatient Level in Kazakhstan from 2011 to 2018. Antibiotics 2020, 9, 57. [Google Scholar] [CrossRef] [Green Version]
- Robertson, J.; Iwamoto, K.; Hoxha, I.; Ghazaryan, L.; Abilova, V.; Cvijanovic, A.; Pyshnik, H.; Darakhvelidze, M.; Makalkina, L.; Jakupi, A.; et al. Antimicrobial Medicines Consumption in Eastern Europeand Central Asia–An Updated Cross-National Study and Assessment of QuantitativeMetrics for Policy Action. Front. Pharmacol. 2019, 9. [Google Scholar] [CrossRef] [Green Version]
- Jakovljevic, M.; Timofeyev, Y.; Ranabhat, C.L.; Fernandes, P.O.; Teixeira, J.P.; Rancic, N.; Reshetnikov, V. Real GDP growth rates and healthcare spending – comparison between the G7 and the EM7 countries. Glob. Health 2020, 16, 1–13. [Google Scholar] [CrossRef]
- Shokoohizadeh, L.; Mobarez, A.M.; Zali, M.R.; Ranjbar, R.; Alebouyeh, M.; Sakinc, T.; Ali, L. High frequency distribution of heterogeneous vancomycin resistant Enterococcous faecium (VREfm) in Iranian hospitals. Diagn. Pathol. 2013, 8, 163. [Google Scholar] [CrossRef] [Green Version]
- Adams, D.J.; Eberly, M.D.; Goudie, A.; Nylund, C.M. Rising Vancomycin-Resistant Enterococcus Infections in Hospitalized Children in the United States. Hosp. Pediatr. 2016, 6, 404–411. [Google Scholar] [CrossRef] [Green Version]
- Leclercq, R.; Derlot, E.; Duval, J.; Courvalin, P. Plasmid-Mediated Resistance to Vancomycin and Teicoplanin in Enterococcus Faecium. N. Engl. J. Med. 1988, 319, 157–161. [Google Scholar] [CrossRef]
- Uttley, A.; Collins, C.; Naidoo, J.; George, R. vancomycin-resistant enterococci. Lancet 1988, 331, 57–58. [Google Scholar] [CrossRef]
- Werner, G.; Coque, T.M.; Hammerum, A.M.; Hope, R.; Hryniewicz, W.; Johnson, A.; Klare, I.; Kristinsson, K.G.; Leclercq, R.; Lester, C.H.; et al. Emergence and spread of vancomycin resistance among enterococci in Europe. Eurosurveillance 2008, 13, 19046. [Google Scholar]
- Levitus, M.; Rewane, A.; Perera, T.B. Vancomycin-Resistant Enterococci (VRE). In StatPearls 2020 Treasure Island (FL); StatPearls Publishing: Florida, FL, USA, 2020. [Google Scholar]
- Arias, C.A.; Murray, B.E. The rise of the Enterococcus: Beyond vancomycin resistance. Nat. Rev. Genet. 2012, 10, 266–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, E.; Hicks, L.; Ali, I.; Salzman, E.; Wang, J.; Snitkin, E.; Gibson, K.; Cassone, M.; Mody, L.; Foxman, B. Epidemiology of Vancomycin-Resistant Enterococcus faecium and Enterococcus faecalis Colonization in Nursing Facilities. Open Forum Infect. Dis. 2020, 7, ofz553. [Google Scholar] [CrossRef]
- Treitman, A.N.; Yarnold, P.R.; Warren, J.; Noskin, G.A. Emerging Incidence of Enterococcus faecium among Hospital Isolates (1993 to 2002). J. Clin. Microbiol. 2005, 43, 462–463. [Google Scholar] [CrossRef] [Green Version]
- Deshpande, L.M.; Fritsche, T.R.; Moet, G.J.; Biedenbach, D.J.; Jones, R.N. Antimicrobial resistance and molecular epidemiology of vancomycin-resistant enterococci from North America and Europe: A report from the SENTRY antimicrobial surveillance program. Diagn. Microbiol. Infect. Dis. 2007, 58, 163–170. [Google Scholar] [CrossRef]
- Hidron, A.I.; Edwards, J.R.; Patel, J.; Horan, T.C.; Sievert, D.M.; Pollock, D.A.; Fridkin, S.K.; Participating National Healthcare Safety Network Facilities. NHSN annual update: Antimicrobial-resistant pathogens associated with healthcare-associated infections: Annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect. Control Hosp. Epidemiol. 2008, 29, 996–1011. [Google Scholar] [CrossRef] [Green Version]
- Jakovljevic, M.; Gerdtham, U.-G.; McDaid, D.; Ogura, S.; Varavikova, E.; Merrick, J.; Adany, R.; Okunade, A.; Getzen, T.E. Comparative financing analysis and political economy of noncommunicable diseases. J. Med. Econ. 2019, 22, 722–727. [Google Scholar] [CrossRef]
- Cattoir, V.; Giard, J.-C. Antibiotic resistance inEnterococcus faeciumclinical isolates. Expert Rev. Anti-Infect. Ther. 2014, 12, 239–248. [Google Scholar] [CrossRef]
- Hollenbeck, B.L.; Rice, L.B. Intrinsic and acquired resistance mechanisms in enterococcus. Virulence 2012, 3, 421–569. [Google Scholar] [CrossRef] [Green Version]
- Top, J.; Willems, R.J.; Bonten, M. Emergence of CC17Enterococcus faecium: From commensal to hospital-adapted pathogen. FEMS Immunol. Med. Microbiol. 2008, 52, 297–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madu Tella, C. Insecurity in Northern Nigeria: Causes, Consequences and Resolutions. Int. J. Peace Confl. Stud. 2015, 2, 23–36. [Google Scholar]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.; the PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 349, g7647. [Google Scholar] [CrossRef] [Green Version]
- George, B.J.; Aban, I.B. An application of meta-analysis based on DerSimonian and Laird method. J. Nucl. Cardiol. 2015, 23, 690–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Wallace, B.C.; Dahabreh, I.J.; Trikalinos, T.A.; Lau, J.; Trow, P.; Schmid, C.H. Closing the Gap between Methodologists and End-Users:Ras a Computational Back-End. J. Stat. Softw. 2012, 49, 1–15. [Google Scholar] [CrossRef] [Green Version]




| Author, Publication Year | Study Year | Study Area | Isolate Sources | Sample Size | Number Positive | Prevalence (%) | Detection Method |
|---|---|---|---|---|---|---|---|
| Olawale et al., 2011 [17] | 2009 | South-West | Clinical specimens | 7 | 3 | 42.9 | Disc diffusion |
| Oyedeji et al., 2011 [18] | 2010 | South-West | Environmental | 78 | 14 | 18 | Agar dilution |
| Oguntoyinbo & Okueso, 2013 [25] | 2012 | North-Central | Environmental | 95 | 32 | 33.7 | Disc diffusion |
| Olawale et al., 2014 [19] | 2012 | South-West | Environmental | 246 | 10 | 4.1 | Disc diffusion |
| Anyanwu & Obetta, 2015 [28] | 2015 | South-East | Animal | 75 | 5 | 6.7 | Disc diffusion |
| Olawale et al., 2015 [20] | 2013 | South-West | Environmental | 658 | 77 | 11.7 | Disc diffusion |
| Ayeni et al., 2016 [21] | 2015 | South-West | Animal | 60 | 39 | 65 | Disc diffusion |
| Nsofor et al., 2016 [29] | 2016 | South-East | Clinical | 34 | 7 | 20.59 | Disc diffusion |
| Ekuma et al., 2016 [22] | 2013 | South-West | Clinical | 319 | 13 | 4.07 | E test |
| Adesida et al., 2017 [23] | 2017 | South-West | Clinical | 65 | 9 | 13.85 | Disc diffusion |
| David et al., 2017 [24] | 2017 | South-West | Clinical | 69 | 27 | 39.13 | Disc diffusion |
| Enenya et al., 2017 [35] | 2014 | North-West | Environmental | 16 | 4 | 25 | Disc diffusion |
| Ndubuisi et al., 2017 [26] | 2017 | North-Central | Clinical | 102 | 34 | 33.3 | Disc diffusion |
| Foka et al., 2018 [31] | 2018 | South-South | Environmental | 9 | 8 | 88.9 | Disc diffusion |
| Abasiubong et al., 2019 [32] | 2018 | South-South | Clinical | 19 | 13 | 68.4 | Disc diffusion |
| Anyanwu et al., 2019 [30] | 2018 | South-East | Animal | 30 | 7 | 23.3 | Disc diffusion |
| Igbinosa & Beshiru., 2019 [33] | 2018 | South-South | Animal | 59 | 22 | 37.3 | Disc diffusion |
| Igbinosa & Raje, 2019 [34] | 2017 | South-South | Environmental | 64 | 23 | 35.9 | Disc diffusion |
| Shettima & Iregbu, 2019 [27] | 2015 | North-Central | Clinical | 545 | 6 | 1.1 | VRE Chromogenic agar |
| Author, Publication Year | E. faecium | E. faecalis | E. gallinarum | E. casseliflavus | E. mundti | E. hirae | E. dispar | Total |
|---|---|---|---|---|---|---|---|---|
| Olawale et al., 2011 [17] | 1 | 2 | - | - | - | - | - | 3 |
| Olawale et al., 2014 [19] | - | 10 | - | - | - | - | - | 10 |
| Olawale et al., 2015 [20] | - | 77 | - | - | - | - | - | 77 |
| Nsofor et al., 2016 [29] | 4 | 3 | - | - | - | - | - | 7 |
| Ekuma et al., 2016 [22] | 3 | - | 9 | 1 | - | - | - | 13 |
| Adesida et al., 2017 [23] | 6 | 3 | - | - | - | - | - | 9 |
| David et al., 2017 [24] | - | 27 | - | - | - | - | - | 27 |
| Enenya et al., 2017 [35] | 2 | - | 1 | 1 | - | - | - | 4 |
| Ndubuisi et al., 2017 [26] | 12 | 10 | 1 | - | 9 | 1 | 1 | 34 |
| Igbinosa & Beshiru., 2019 [33] | 13 | 8 | 1 | - | - | - | - | 22 |
| Igbinosa & Raje, 2019 [34] | 7 | 8 | - | 2 | - | 3 | 3 | 23 |
| Shettima & Iregbu, 2019 [27] | 3 | - | 2 | 1 | - | - | - | 6 |
| 51 (21.7%) | 148 (62.98%) | 14 (5.96%) | 5 (2.13%) | 9 (3.83%) | 4 (1.70%) | 4 (1.70%) | 235 |
| Study Region | Number of Studies | Prevalence (%) | 95% CI | I2 (%) | Q | Heterogeneity Test | |
|---|---|---|---|---|---|---|---|
| DF | p | ||||||
| South-West | 8 | 20.7 | 13.1–28.2 | 95.5 | 155.404 | 7 | <0.001 |
| North-Central | 3 | 22.4 | −3.6–48.3 | 97.81 | 91.227 | 2 | <0.001 |
| South-East | 3 | 10.2 | 2.7–17.8 | 51.15 | 4.094 | 2 | 0.129 |
| North-West | 1 | 25.0 | 3.8–46.2 | NA | - | - | - |
| South-South | 4 | 56.2 | 33.5–79.0 | 88.37 | 25.802 | 3 | <0.001 |
| Overall | 19 | 25.3 | 19.8–30.8 | 96.26 | 480.667 | 18 | <0.001 |
| Isolate Source | Number of Studies | Prevalence (%) | 95% CI | I2 (%) | Q | Heterogeneity Test | |
|---|---|---|---|---|---|---|---|
| DF | p | ||||||
| Clinical | 8 | 19.9 | 12.5–27.2 | 195.18 | 145.362 | 7 | <0.001 |
| Environmental | 7 | 27.2 | 17.3–37.2 | 95.33 | 128.519 | 6 | <0.001 |
| Animal | 4 | 32.9 | 5.1–60.7 | 96.37 | 82.535 | 3 | <0.001 |
| Overall | 19 | 25.3 | 19.8–30.8 | 96.26 | 480.667 | 18 | <0.001 |
| Detection Method | Number of Studies | Prevalence (%) | 95% CI | I2 (%) | Q | Heterogeneity Test | |
|---|---|---|---|---|---|---|---|
| DF | p | ||||||
| Disc diffusion | 15 | 33.8 | 24.3–43.4 | 93.84 | 227.406 | 14 | <0.001 |
| Disc diffusion | 1 | 4.1 | 1.6–6.5 | - | - | - | - |
| Agar dilution | 1 | 17.9 | 9.4–26.5 | - | - | - | - |
| E test | 1 | 1.9 | 1.9–6.2 | - | - | - | - |
| VRE chromogenic agar | 1 | 1.1 | 1.1–0.2 | - | - | - | - |
| Overall | 19 | 25.3 | 19.8–30.8 | 96.26 | 480.667 | 18 | <0.001 |
| Study Period | Number of Studies | Prevalence (%) | 95% CI | I2 (%) | Q | Heterogeneity Test | |
|---|---|---|---|---|---|---|---|
| DF | p | ||||||
| 2009 | 1 | 42.9 | 6.2–79.5 | - | - | - | - |
| 2010 | 1 | 17.9 | 9.4–26.5 | - | - | - | - |
| 2012 | 2 | 18.5 | −10.4–47.5 | 97.14 | 34.954 | 1 | <0.001 |
| 2013 | 2 | 7.9 | 4.0–15.3 | 95.19 | 20.806 | 1 | <0.001 |
| 2014 | 1 | 25.0 | 3.8–46.2 | - | - | - | - |
| 2015 | 3 | 23.1 | 0.7–45.5 | 98.19 | 110.342 | 2 | <0.001 |
| 2016 | 1 | 8.3 | −0.7–17.4 | - | - | - | - |
| 2017 | 4 | 30.2 | 18.0–42.3 | 82.92 | 17.560 | 3 | <0.001 |
| 2018 | 4 | 53.6 | 26.5–80.7 | 90.54 | 31.711 | 3 | <0.001 |
| Overall | 19 | 25.3 | 19.8–30.8 | 96.26 | 480.667 | 18 | <0.001 |
| Variable | Coefficient | p-Value | 95% CI |
|---|---|---|---|
| Study area | |||
| South-West | Reference | ||
| North-Central | 0.175 | 0.005 | 5.2–29.8 |
| North-West | −1.044 | <0.001 | −142.1–−66.7 |
| South-East | −0.533 | <0.001 | −65.9–−40.7 |
| South-South | −0.286 | 0.003 | −47.7–−9.4 |
| Isolates source | |||
| Clinical | Reference | ||
| Animal | 0.273 | 0.188 | −13.3–67.9 |
| Environmental | 0.865 | <0.001 | 38.4–134.6 |
| Detection method | |||
| Disc diffusion | Reference | ||
| Agar dilution | −1.114 | <0.001 | −143.7–−79.2 |
| E test | 0.371 | <0.001 | 16.7–57.5 |
| VRE chromogenic agar | −0.917 | <0.001 | −113.9–−69.6 |
| Study period | |||
| 2009 | Reference | ||
| 2010 | −1.132 | <0.001 | −147.8–−78.6 |
| 2012 | −1.177 | <0.001 | −148.9–−86.5 |
| 2013 | −0.093 | 0.219 | −24.1–55.0 |
| 2014 | −0.230 | 0.041 | −45.1–−0.9 |
| 2015 | −0.688 | <0.001 | −87.9–−49.6 |
| Constant | 0.429 | 0.022 | 6.2–79.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wada, Y.; Harun, A.B.; Yean, C.Y.; Zaidah, A.R. Vancomycin-Resistant Enterococci (VRE) in Nigeria: The First Systematic Review and Meta-Analysis. Antibiotics 2020, 9, 565. https://doi.org/10.3390/antibiotics9090565
Wada Y, Harun AB, Yean CY, Zaidah AR. Vancomycin-Resistant Enterococci (VRE) in Nigeria: The First Systematic Review and Meta-Analysis. Antibiotics. 2020; 9(9):565. https://doi.org/10.3390/antibiotics9090565
Chicago/Turabian StyleWada, Yusuf, Azian Binti Harun, Chan Yean Yean, and Abdul Rahman Zaidah. 2020. "Vancomycin-Resistant Enterococci (VRE) in Nigeria: The First Systematic Review and Meta-Analysis" Antibiotics 9, no. 9: 565. https://doi.org/10.3390/antibiotics9090565
APA StyleWada, Y., Harun, A. B., Yean, C. Y., & Zaidah, A. R. (2020). Vancomycin-Resistant Enterococci (VRE) in Nigeria: The First Systematic Review and Meta-Analysis. Antibiotics, 9(9), 565. https://doi.org/10.3390/antibiotics9090565






