Antibiotic Tolerance of Staphylococcus aureus Biofilm in Periprosthetic Joint Infections and Antibiofilm Strategies
Abstract
:1. Role of Bacterial Biofilm in Prosthetic Joint Infections
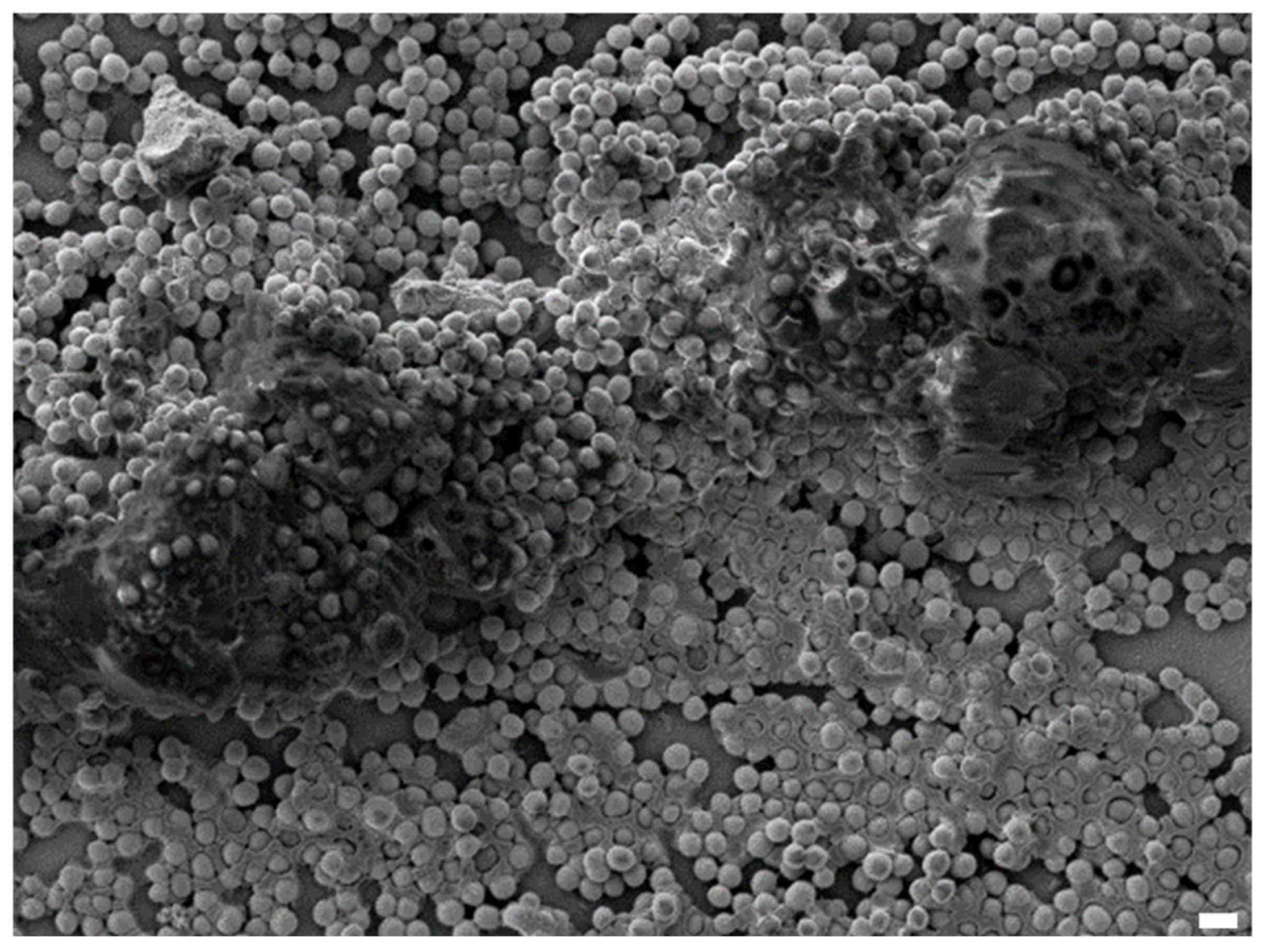
1.1. Definition of Biofilm
1.2. Biofilm and Clinical Impact
1.3. Periprosthetic Joint Infections and Biofilm
1.3.1. Periprosthetic Joint Infections: Definition and Clinical Impact
1.3.2. Patients’ Risk Factors
1.3.3. Clinical Signs
1.3.4. Current Strategies vs. Biofilm PJIs
2. Antibiotic Failure in PJI Due to S. aureus and Biofilm Role
2.1. Antibiotic Resistance
2.2. Antibiotic Tolerance
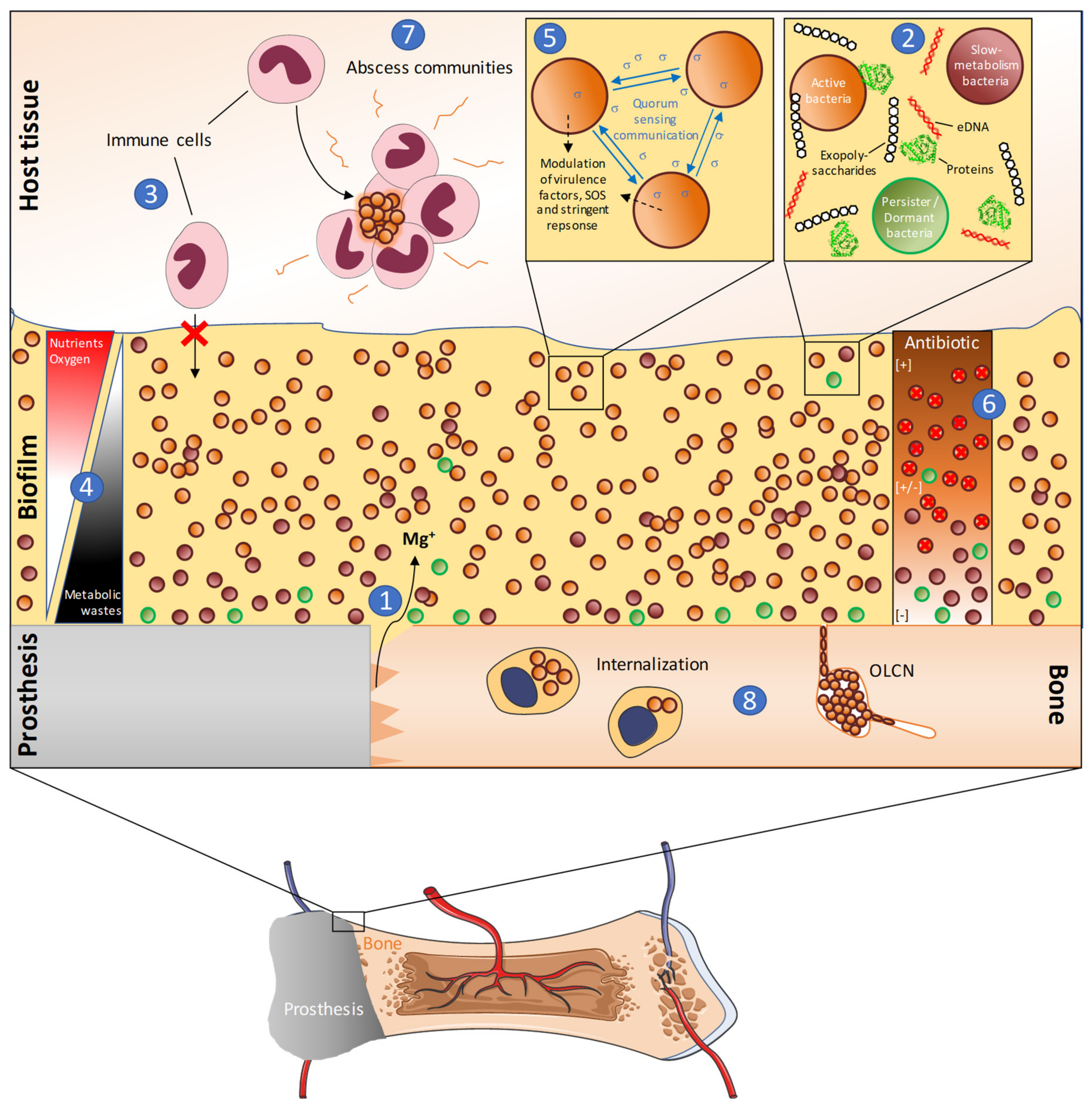
2.2.1. Matrix
2.2.2. Metabolism
2.3. The Persistence of PJI
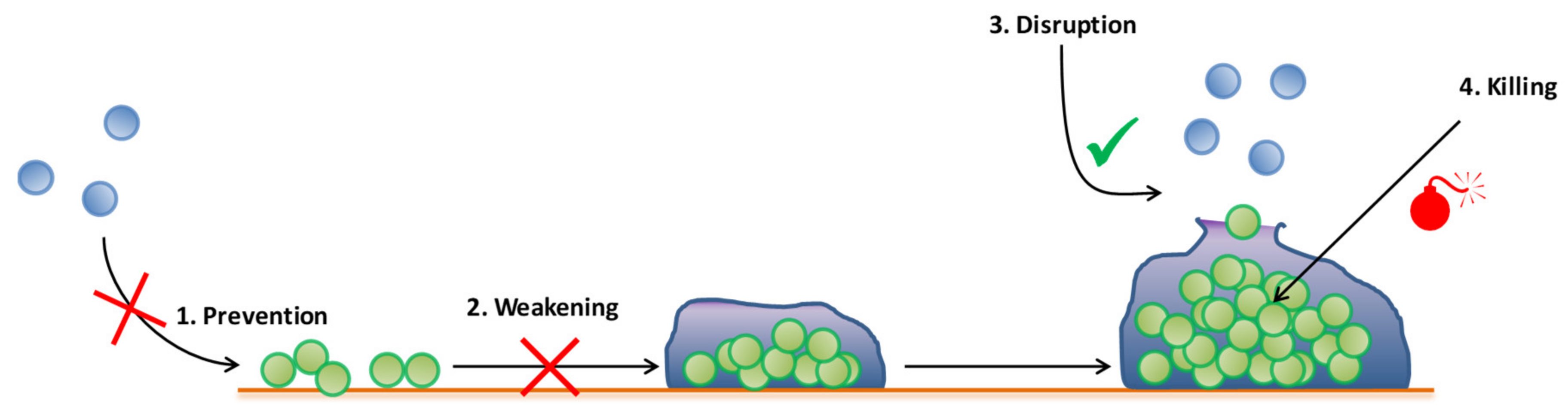
3. Emergent Antibiofilm Strategies in Periprosthetic Joint Infection
3.1. Antibiofilm Molecules
3.1.1. Antibiotics (Combination, Anti-MRSA)
3.1.2. Antimicrobial Peptides (AMPs)
3.2. Immune Modulation and Immunotherapy
3.3. Phage Therapy
3.4. Prosthesis Management by Coating Surfaces
4. Need for Adapted Models
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef] [Green Version]
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Reffuveille, F.; Josse, J.; Vallé, Q.; Gangloff, C.M.; Gangloff, S.C. Staphylococcus aureus Biofilms and their Impact on the Medical Field. Rise Virulence Antibiot. Resist. Staphylococcus Aureus 2017, 11, 187. [Google Scholar] [CrossRef] [Green Version]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.; Zhai, Z.; Gao, C. Adaptive antibacterial biomaterial surfaces and their applications. Mater. Today Bio 2019, 2, 100017. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M. Biofilms and device-associated infections. Emerg. Infect. Dis. 2001, 7, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Wingender, J. The biofilm matrix. Nature Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- García-Gareta, E.; Davidson, C.; Levin, A.; Coathup, M.J.; Blunn, G.W. Biofilm formation in total hip arthroplasty: Prevention and treatment. RSC Adv. 2016, 6, 80244–80261. [Google Scholar] [CrossRef] [Green Version]
- Olsen, I. Biofilm-specific antibiotic tolerance and resistance. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 877–886. [Google Scholar] [CrossRef]
- Crabbé, A.; Jensen, P.Ø.; Bjarnsholt, T.; Coenye, T. Antimicrobial Tolerance and Metabolic Adaptations in Microbial Biofilms. Trends Microbiol. 2019, 27, 850–863. [Google Scholar] [CrossRef]
- Peyrusson, F.; Varet, H.; Nguyen, T.K.; Legendre, R.; Sismeiro, O.; Coppée, J.-Y.; Wolz, C.; Tenson, T.; Van Bambeke, F. Intracellular Staphylococcus aureus persisters upon antibiotic exposure. Nat. Commun. 2020, 11, 2200. [Google Scholar] [CrossRef] [PubMed]
- Loss, G.; Simões, P.M.; Valour, F.; Cortês, M.F.; Gonzaga, L.; Bergot, M.; Trouillet-Assant, S.; Josse, J.; Diot, A.; Ricci, E.; et al. Staphylococcus aureus Small Colony Variants (SCVs): News From a Chronic Prosthetic Joint Infection. Front. Cell Infect. Microbiol. 2019, 9, 363. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Mendis, N.; Trigui, H.; Oliver, J.D.; Faucher, S.P. The importance of the viable but non-culturable state in human bacterial pathogens. Front. Microbiol. 2014, 5, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foster, T.J.; Geoghegan, J.A.; Ganesh, V.K.; Höök, M. Adhesion, invasion and evasion: The many functions of the surface proteins of Staphylococcus aureus. Nat. Rev. Microbiol. 2014, 12, 49–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waters, E.M.; Rowe, S.E.; O’Gara, J.P.; Conlon, B.P. Convergence of Staphylococcus aureus Persister and Biofilm Research: Can Biofilms Be Defined as Communities of Adherent Persister Cells? PLoS Pathog. 2016, 12, e1006012. [Google Scholar] [CrossRef] [PubMed]
- Pasquaroli, S.; Zandri, G.; Vignaroli, C.; Vuotto, C.; Donelli, G.; Biavasco, F. Antibiotic pressure can induce the viable but non-culturable state in Staphylococcus aureus growing in biofilms. J. Antimicrob. Chemother. 2013, 68, 1812–1817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mangiaterra, G.; Cedraro, N.; Vaiasicca, S.; Citterio, B.; Galeazzi, R.; Laudadio, E.; Mobbili, G.; Minnelli, C.; Bizzaro, D.; Biavasco, F. Role of Tobramycin in the Induction and Maintenance of Viable but Non-Culturable Pseudomonas aeruginosa in an In Vitro Biofilm Model. Antibiotics 2020, 9, 399. [Google Scholar] [CrossRef]
- Foster, T.J. Antibiotic resistance in Staphylococcus aureus. Current status and future prospects. FEMS Microbiol. Rev. 2017, 41, 430–449. [Google Scholar] [CrossRef]
- Tashiro, Y.; Eida, H.; Ishii, S.; Futamata, H.; Okabe, S. Generation of Small Colony Variants in Biofilms by Escherichia coli Harboring a Conjugative F Plasmid. Microbes Environ. 2017, 32, 40–46. [Google Scholar] [CrossRef] [Green Version]
- Savage, V.J.; Chopra, I.; O’Neill, A.J. Staphylococcus aureus biofilms promote horizontal transfer of antibiotic resistance. Antimicrob. Agents Chemother. 2013, 57, 1968–1970. [Google Scholar] [CrossRef] [Green Version]
- Moormeier, D.E.; Bayles, K.W. Staphylococcus aureus biofilm: A complex developmental organism. Mol. Microbiol. 2017, 104, 365–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Høiby, N.; Ciofu, O.; Johansen, H.K.; Song, Z.; Moser, C.; Jensen, P.Ø.; Molin, S.; Givskov, M.; Tolker-Nielsen, T.; Bjarnsholt, T. The clinical impact of bacterial biofilms. Int. J. Oral Sci. 2011, 3, 55–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seebach, E.; Kubatzky, K.F. Chronic Implant-Related Bone Infections—Can Immune Modulation be a Therapeutic Strategy? Front. Immunol. 2019, 10, 1724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muthukrishnan, G.; Masters, E.A.; Daiss, J.L.; Schwarz, E.M. Mechanisms of immune evasion and bone tissue colonization that make Staphylococcus aureus the primary pathogen in osteomyelitis. Curr. Osteoporos. Rep. 2019, 17, 395–404. [Google Scholar] [CrossRef]
- Kurtz, S.; Ong, K.; Lau, E.; Mowat, F.; Halpern, M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. Jbjs 2007, 89, 780–785. [Google Scholar] [CrossRef]
- Tande, A.J.; Patel, R. Prosthetic joint infection. Clin. Microbiol. Rev. 2014, 27, 302–345. [Google Scholar] [CrossRef] [Green Version]
- Masters, E.A.; Trombetta, R.P.; de Mesy Bentley, K.L.; Boyce, B.F.; Gill, A.L.; Gill, S.R.; Nishitani, K.; Ishikawa, M.; Morita, Y.; Ito, H.; et al. Evolving concepts in bone infection: Redefining “biofilm”, “acute vs. chronic osteomyelitis”, “the immune proteome” and “local antibiotic therapy”. Bone Res. 2019, 7, 20. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Renz, N.; Trampuz, A. Management of periprosthetic joint infection. Hip Pelvis 2018, 30, 138–146. [Google Scholar] [CrossRef]
- Josse, J.; Valour, F.; Maali, Y.; Diot, A.; Batailler, C.; Ferry, T.; Laurent, F. Interaction between staphylococcal biofilm and bone: How does the presence of biofilm promote prosthesis loosening? Front. Microbiol. 2019, 10, 1602. [Google Scholar] [CrossRef]
- Wildeman, P.; Tevell, S.; Eriksson, C.; Lagos, A.C.; Söderquist, B.; Stenmark, B. Genomic characterization and outcome of prosthetic joint infections caused by Staphylococcus aureus. Sci. Rep. 2020, 10, 5938. [Google Scholar] [CrossRef] [Green Version]
- Shoji, M.M.; Chen, A.F. Biofilms in periprosthetic joint infections: A review of diagnostic modalities, current treatments, and future directions. J. Knee Surg. 2020, 33, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Renz, N.; Mudrovcic, S.; Perka, C.; Trampuz, A. Orthopedic implant-associated infections caused by Cutibacterium spp.—A remaining diagnostic challenge. PLoS ONE 2018, 13, e0202639. [Google Scholar] [CrossRef] [PubMed]
- Aynardi, M.C.; Plöger, M.M.; Walley, K.C.; Arena, C.B. What is the definition of acute and chronic periprosthetic joint infection (PJI) of Total Ankle Arthroplasty (TAA)? Foot Ankle Int. 2019, 40, 19S–21S. [Google Scholar] [CrossRef] [PubMed]
- Jacqueline, C.; Caillon, J. Impact of bacterial biofilm on the treatment of prosthetic joint infections. J. Antimicrob. Chemother. 2014, 69 (Suppl. S1), i37–i40. [Google Scholar] [CrossRef] [Green Version]
- Löffler, B.; Tuchscherr, L.; Niemann, S.; Peters, G. Staphylococcus aureus persistence in non-professional phagocytes. Int. J. Med. Microbiol. 2014, 304, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Josse, J.; Velard, F.; Gangloff, S.C. Staphylococcus aureus vs. Osteoblast: Relationship and Consequences in Osteomyelitis. Front. Cell Infect. Microbiol. 2015, 5, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krauss, J.L.; Roper, P.M.; Ballard, A.; Shih, C.-C.; Fitzpatrick, J.A.J.; Cassat, J.E.; Ng, P.Y.; Pavlos, N.J.; Veis, D.J. Staphylococcus aureus infects osteoclasts and replicates intracellularly. MBio 2019, 10, e02447-19. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Wijenayaka, A.R.; Solomon, L.B.; Pederson, S.M.; Findlay, D.M.; Kidd, S.P.; Atkins, G.J. Novel Insights into Staphylococcus aureus deep bone infections: The involvement of osteocytes. MBio 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Surewaard, B.G.J.; Deniset, J.F.; Zemp, F.J.; Amrein, M.; Otto, M.; Conly, J.; Omri, A.; Yates, R.M.; Kubes, P. Identification and treatment of the Staphylococcus aureus reservoir in vivo. J. Exp. Med. 2016, 213, 1141–1151. [Google Scholar] [CrossRef] [Green Version]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef] [Green Version]
- Wright, J.A.; Nair, S.P. Interaction of staphylococci with bone. Int. J. Med. Microbiol. 2010, 300, 193–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alamanda, V.K.; Springer, B.D. The prevention of infection: 12 modifiable risk factors. Bone Joint J. 2019, 101-B, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Fisichella, L.; Fenga, D.; Rosa, M.A. Surgical Site Infection in orthopaedic surgery: Correlation between age, diabetes, smoke and surgical risk. Folia Med. 2014, 56, 259–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuiper, J.W.; Willink, R.T.; Moojen, D.J.F.; van den Bekerom, M.P.; Colen, S. Treatment of acute periprosthetic infections with prosthesis retention: Review of current concepts. World J. Orthop. 2014, 5, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Al Mohajer, M.; Darouiche, R.O. The expanding horizon of prosthetic joint infections. J. Appl. Biomater. Funct. Mater. 2014, 12, 1–12. [Google Scholar] [CrossRef]
- Nelson, G.N.; Davis, D.E.; Namdari, S. Outcomes in the treatment of periprosthetic joint infection after shoulder arthroplasty: A systematic review. J. Shoulder Elb. Surg. 2016, 25, 1337–1345. [Google Scholar] [CrossRef]
- Morrell, A.T.; Golladay, G.J.; Kates, S.L. Surgical selection criteria compliance is associated with a lower risk of periprosthetic joint infection in total hip arthroplasty. Arthroplast. Today 2019, 5, 521–524. [Google Scholar] [CrossRef] [Green Version]
- Nair, R.; Schweizer, M.L.; Singh, N. Septic Arthritis and Prosthetic Joint Infections in older adults. Infect. Dis. Clin. N. Am. 2017, 31, 715–729. [Google Scholar] [CrossRef]
- Zimmerli, W.; Sendi, P. Role of Rifampin against staphylococcal biofilm infections in vitro, in animal models, and in orthopedic-device-related infections. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef] [Green Version]
- Tande, A.J.; Gomez-Urena, E.O.; Berbari, E.F.; Osmon, D.R. Management of Prosthetic Joint Infection. Infect. Dis. Clin. N. Am. 2017, 31, 237–252. [Google Scholar] [CrossRef]
- Dufour, S.; Piroth, L.; Chirouze, C.; Tattevin, P.; Becker, A.; Braquet, P.; Ferry, T.; Duval, X.; Le Moing, V. VIRSTA/AEPEI study group Staphylococcus aureus bloodstream infection in patients with Prosthetic Joints in the prospective VIRSTA cohort study: Frequency and time of occurrence of periprosthetic joint infection. Open Forum. Infect. Dis. 2019, 6, ofz515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rakow, A.; Perka, C.; Trampuz, A.; Renz, N. Origin and characteristics of haematogenous periprosthetic joint infection. Clin. Microbiol. Infect. 2019, 25, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Sun, X.; Zeng, Y.; Feng, W.; Li, J.; Zeng, J.; Zeng, Y. Can nasal Staphylococcus aureus screening and decolonization prior to elective total joint arthroplasty reduce surgical site and prosthesis-related infections? A systematic review and meta-analysis. J. Orthop. Surg. Res. 2020, 15, 60. [Google Scholar] [CrossRef] [PubMed]
- Romero-Palacios, A.; Petruccelli, D.; Main, C.; Winemaker, M.; de Beer, J.; Mertz, D. Screening for and decolonization of Staphylococcus aureus carriers before total joint replacement is associated with lower S aureus prosthetic joint infection rates. Am. J. Infect. Control 2020, 48, 534–537. [Google Scholar] [CrossRef]
- Kapadia, B.H.; Berg, R.A.; Daley, J.A.; Fritz, J.; Bhave, A.; Mont, M.A. Periprosthetic joint infection. Lancet 2016, 387, 386–394. [Google Scholar] [CrossRef]
- Flurin, L.; Greenwood-Quaintance, K.E.; Patel, R. Microbiology of polymicrobial prosthetic joint infection. Diagn. Microbiol. Infect. Dis. 2019, 94, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, B.F.; Muthukrishnan, G.; Masters, E.; Ninomiya, M.; Lee, C.C.; Schwarz, E.M. Staphylococcus aureus evasion of host immunity in the setting of Prosthetic Joint Infection: Biofilm and beyond. Curr. Rev. Musculoskelet. Med. 2018, 11, 389–400. [Google Scholar] [CrossRef]
- Vanhommerig, E.; Moons, P.; Pirici, D.; Lammens, C.; Hernalsteens, J.-P.; De Greve, H.; Kumar-Singh, S.; Goossens, H.; Malhotra-Kumar, S. Comparison of biofilm formation between major clonal lineages of methicillin resistant Staphylococcus aureus. PLoS ONE 2014, 9, e104561. [Google Scholar] [CrossRef]
- Tan, X.; Yang, D.; Yang, G.; Chen, J.; Dong, W.; Shi, J.; Jia, A. The investigation of inhibiting quorum sensing and methicillin-resistant Staphylococcus aureus biofilm formation from Liriodendron hybrid. Pak. J. Pharm. Sci. 2015, 28, 903–908. [Google Scholar] [PubMed]
- Pozzi, C.; Waters, E.M.; Rudkin, J.K.; Schaeffer, C.R.; Lohan, A.J.; Tong, P.; Loftus, B.J.; Pier, G.B.; Fey, P.D.; Massey, R.C.; et al. Methicillin resistance alters the biofilm phenotype and attenuates virulence in Staphylococcus aureus device-associated infections. PLoS Pathog. 2012, 8, e1002626. [Google Scholar] [CrossRef]
- Stewart, P.S. Antimicrobial tolerance in biofilms. Microb. Biofilms 2015, 3, 269–285. [Google Scholar] [CrossRef] [Green Version]
- Lima-E-Silva, A.A.; Silva-Filho, R.G.; Fernandes, H.M.Z.; Saramago, C.S.M.; Viana, A.S.; Souza, M.J.; Nogueira, E.M. Sub-Inhibitory concentrations of rifampicin strongly stimulated biofilm production in S. aureus. Open Microbiol. J. 2017, 11, 142–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thabit, A.K.; Fatani, D.F.; Bamakhrama, M.S.; Barnawi, O.A.; Basudan, L.O.; Alhejaili, S.F. Antibiotic penetration into bone and joints: An updated review. Int. J. Infect. Dis. 2019, 81, 128–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abad, L.; Josse, J.; Tasse, J.; Lustig, S.; Ferry, T.; Diot, A.; Laurent, F.; Valour, F. Antibiofilm and intraosteoblastic activities of rifamycins against Staphylococcus aureus: Promising in vitro profile of rifabutin. J. Antimicrob. Chemother. 2020, 75, 1466–1473. [Google Scholar] [CrossRef]
- Recommandations. Available online: https://www.infectiologie.com/fr/recommandations.html (accessed on 16 July 2020).
- Brauner, A.; Fridman, O.; Gefen, O.; Balaban, N.Q. Distinguishing between resistance, tolerance and persistence to antibiotic treatment. Nat. Rev. Microbiol. 2016, 14, 320–330. [Google Scholar] [CrossRef]
- AMR Review Home Page. Available online: https://amr-review.org/ (accessed on 16 July 2020).
- Bowler, P.G. Antibiotic resistance and biofilm tolerance: A combined threat in the treatment of chronic infections. J. Wound Care 2018, 27, 273–277. [Google Scholar] [CrossRef]
- Mah, T.-F. Biofilm-specific antibiotic resistance. Future Microbiol. 2012, 7, 1061–1072. [Google Scholar] [CrossRef] [Green Version]
- Montanaro, L.; Poggi, A.; Visai, L.; Ravaioli, S.; Campoccia, D.; Speziale, P.; Arciola, C.R. Extracellular DNA in biofilms. Int. J. Artif. Organs. 2011, 34, 824–831. [Google Scholar] [CrossRef]
- Olsen, I.; Tribble, G.D.; Fiehn, N.-E.; Wang, B.-Y. Bacterial sex in dental plaque. J. Oral Microbiol. 2013, 5, 20736. [Google Scholar] [CrossRef]
- Ryder, V.J.; Chopra, I.; O’Neill, A.J. Increased mutability of Staphylococci in biofilms as a consequence of oxidative stress. PLoS ONE 2012, 7, e47695. [Google Scholar] [CrossRef] [Green Version]
- Alav, I.; Sutton, J.M.; Rahman, K.M. Role of bacterial efflux pumps in biofilm formation. J. Antimicrob. Chemother. 2018, 73, 2003–2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jolivet-Gougeon, A.; Bonnaure-Mallet, M. Biofilms as a mechanism of bacterial resistance. Drug Discov. Today Technol. 2014, 11, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh, S.; Ganjloo, S.; Pourmand, M.R.; Mashhadi, R.; Ghazvini, K. Epidemiology of efflux pumps genes mediating resistance among Staphylococcus aureus; A systematic review. Microb. Pathog. 2020, 139, 103850. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, K.K.; Goldmann, D.A.; Pier, G.B. Use of confocal microscopy to analyze the rate of vancomycin penetration through Staphylococcus aureus biofilms. Antimicrob. Agents Chemother. 2005, 49, 2467–2473. [Google Scholar] [CrossRef] [Green Version]
- Mulcahy, H.; Charron-Mazenod, L.; Lewenza, S. Extracellular DNA chelates cations and induces antibiotic resistance in Pseudomonas aeruginosa biofilms. PLoS Pathog. 2008, 4, e1000213. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, J.B.; Izano, E.A.; Gopal, P.; Karwacki, M.T.; Kim, S.; Bose, J.L.; Bayles, K.W.; Horswill, A.R. Low levels of β-lactam antibiotics induce extracellular DNA release and biofilm formation in Staphylococcus aureus. MBio 2012, 3, e00198-12. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, L.C.N.; Diniz, R.C.; Lima, I.M.d.S.F.; dos Santos, C.I.; SilvaAlves, M.; de Souza, L.I.O.; de Souza Monteiro, A. SOS Response and Staphylococcus aureus: Implications for Drug Development. Rise Virulence Antibiot. Resist. 2017, 95. [Google Scholar] [CrossRef] [Green Version]
- Bernier, S.P.; Lebeaux, D.; DeFrancesco, A.S.; Valomon, A.; Soubigou, G.; Coppée, J.-Y.; Ghigo, J.-M.; Beloin, C. Starvation, together with the SOS response, mediates high biofilm-specific tolerance to the fluoroquinolone ofloxacin. PLoS Genet. 2013, 9, e1003144. [Google Scholar] [CrossRef] [Green Version]
- Nagel, M.; Reuter, T.; Jansen, A.; Szekat, C.; Bierbaum, G. Influence of ciprofloxacin and vancomycin on mutation rate and transposition of IS256 in Staphylococcus aureus. Int. J. Med. Microbiol. 2011, 301, 229–236. [Google Scholar] [CrossRef]
- Rowe, S.E.; Wagner, N.J.; Li, L.; Beam, J.E.; Wilkinson, A.D.; Radlinski, L.C.; Zhang, Q.; Miao, E.A.; Conlon, B.P. Reactive oxygen species induce antibiotic tolerance during systemic Staphylococcus aureus infection. Nat. Microbiol. 2020, 5, 282–290. [Google Scholar] [CrossRef]
- Vestergaard, M.; Paulander, W.; Ingmer, H. Activation of the SOS response increases the frequency of small colony variants. BMC Res. Notes 2015, 8, 749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urish, K.L.; DeMuth, P.W.; Kwan, B.W.; Craft, D.W.; Ma, D.; Haider, H.; Tuan, R.S.; Wood, T.K.; Davis, C.M. Antibiotic-tolerant Staphylococcus aureus biofilm persists on arthroplasty materials. Clin. Orthop. Relat. Res. 2016, 474, 1649–1656. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Mandell, J.B.; Donegan, N.P.; Cheung, A.L.; Ma, W.; Rothenberger, S.; Shanks, R.M.Q.; Richardson, A.R.; Urish, K.L. The Toxin-Antitoxin MazEF drives Staphylococcus aureus biofilm formation, antibiotic tolerance, and chronic infection. MBio 2019, 10, e01658-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harms, A.; Brodersen, D.E.; Mitarai, N.; Gerdes, K. Toxins, targets, and triggers: An overview of toxin-antitoxin biology. Mol. Cell 2018, 70, 768–784. [Google Scholar] [CrossRef] [Green Version]
- Amato, S.M.; Fazen, C.H.; Henry, T.C.; Mok, W.W.K.; Orman, M.A.; Sandvik, E.L.; Volzing, K.G.; Brynildsen, M.P. The role of metabolism in bacterial persistence. Front. Microbiol. 2014, 5, 70. [Google Scholar] [CrossRef] [Green Version]
- Haney, E.F.; Mansour, S.C.; Hancock, R.E.W. Antimicrobial peptides: An introduction. Methods Mol. Biol. 2017, 1548, 3–22. [Google Scholar] [CrossRef]
- Aedo, S.; Tomasz, A. Role of the stringent stress response in the antibiotic resistance phenotype of Methicillin-Resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2016, 60, 2311–2317. [Google Scholar] [CrossRef] [Green Version]
- Geiger, T.; Kästle, B.; Gratani, F.L.; Goerke, C.; Wolz, C. Two small (p)ppGpp synthases in Staphylococcus aureus mediate tolerance against cell envelope stress conditions. J. Bacteriol. 2014, 196, 894–902. [Google Scholar] [CrossRef] [Green Version]
- Heim, C.E.; West, S.C.; Ali, H.; Kielian, T. Heterogeneity of Ly6G+ Ly6C+ myeloid-derived suppressor cell infiltrates during Staphylococcus aureus biofilm infection. Infect. Immun. 2018, 86, e00684-18. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.K.; Argudín, M.A.; Deplano, A.; Nhung, P.H.; Nguyen, H.A.; Tulkens, P.M.; Dodemont, M.; Van Bambeke, F. Antibiotic resistance, biofilm formation, and intracellular survival as possible determinants of persistent or recurrent infections by Staphylococcus aureus in a Vietnamese Tertiary Hospital: Focus on bacterial response to moxifloxacin. Microb. Drug Resist. 2019, 26, 537–544. [Google Scholar] [CrossRef]
- Stewart, P.S.; Franklin, M.J. Physiological heterogeneity in biofilms. Nat. Rev. Microbiol. 2008, 6, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Tasse, J.; Croisier, D.; Badel-Berchoux, S.; Chavanet, P.; Bernardi, T.; Provot, C.; Laurent, F. Preliminary results of a new antibiotic susceptibility test against biofilm installation in device-associated infections: The Antibiofilmogram®. Pathog. Dis. 2016, 74, ftw057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tasse, J.; Trouillet-Assant, S.; Josse, J.; Martins-Simões, P.; Valour, F.; Langlois-Jacques, C.; Badel-Berchoux, S.; Provot, C.; Bernardi, T.; Ferry, T.; et al. Association between biofilm formation phenotype and clonal lineage in Staphylococcus aureus strains from bone and joint infections. PLoS ONE 2018, 13, e0200064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reffuveille, F. Antibiofilm Peptide Development for Clinical and Industrial Applications. Available online: https://www.semanticscholar.org/paper/Antibiofilm-Peptide-Development-for-Clinical-and-Reffuveille/7c31834b92c1323c8ce857a3309296cfe8d7493b (accessed on 17 July 2020).
- Akturk, E.; Oliveira, H.; Santos, S.B.; Costa, S.; Kuyumcu, S.; Melo, L.D.R.; Azeredo, J. Synergistic action of phage and antibiotics: Parameters to enhance the killing efficacy against mono and dual-species biofilms. Antibiotics 2019, 8, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandell, J.B.; Orr, S.; Koch, J.; Nourie, B.; Ma, D.; Bonar, D.D.; Shah, N.; Urish, K.L. Large variations in clinical antibiotic activity against Staphylococcus aureus biofilms of periprosthetic joint infection isolates. J. Orthop. Res. 2019, 37, 1604–1609. [Google Scholar] [CrossRef]
- Pletzer, D.; Mansour, S.C.; Hancock, R.E.W. Synergy between conventional antibiotics and anti-biofilm peptides in a murine, sub-cutaneous abscess model caused by recalcitrant ESKAPE pathogens. PLoS Pathog. 2018, 14, e1007084. [Google Scholar] [CrossRef]
- Vergidis, P.; Rouse, M.S.; Euba, G.; Karau, M.J.; Schmidt, S.M.; Mandrekar, J.N.; Steckelberg, J.M.; Patel, R. Treatment with linezolid or vancomycin in combination with rifampin is effective in an animal model of methicillin-resistant Staphylococcus aureus foreign body osteomyelitis. Antimicrob. Agents Chemother. 2011, 55, 1182–1186. [Google Scholar] [CrossRef] [Green Version]
- Taha, M.; Abdelbary, H.; Ross, F.P.; Carli, A.V. New Innovations in the treatment of PJI and biofilms-clinical and preclinical topics. Curr. Rev. Musculoskelet. Med. 2018, 11, 380–388. [Google Scholar] [CrossRef]
- Dusane, D.H.; Kyrouac, D.; Petersen, I.; Bushrow, L.; Calhoun, J.H.; Granger, J.F.; Phieffer, L.S.; Stoodley, P. Targeting intracellular Staphylococcus aureus to lower recurrence of orthopaedic infection. J. Orthop. Res. 2018, 36, 1086–1092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hancock, R.E.W. Collateral damage. Nat. Biotechnol. 2014, 32, 66–68. [Google Scholar] [CrossRef]
- Meng, S.; Xu, H.; Wang, F. Research advances of antimicrobial peptides and applications in food industry and agriculture. Curr. Protein Pept. Sci. 2010, 11, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Colagiorgi, A.; Festa, R.; Di Ciccio, P.A.; Gogliettino, M.; Balestrieri, M.; Palmieri, G.; Anastasio, A.; Ianieri, A. Rapid biofilm eradication of the antimicrobial peptide 1018-K6 against Staphylococcus aureus: A new potential tool to fight bacterial biofilms. Food Control 2020, 107, 106815. [Google Scholar] [CrossRef]
- Grassi, L.; Maisetta, G.; Esin, S.; Batoni, G. Combination strategies to enhance the efficacy of antimicrobial peptides against bacterial biofilms. Front. Microbiol. 2017, 8, 2409. [Google Scholar] [CrossRef]
- Dickey, J.; Perrot, V. Adjunct phage treatment enhances the effectiveness of low antibiotic concentration against Staphylococcus aureus biofilms in vitro. PLoS ONE 2019, 14, e0209390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumaran, D.; Taha, M.; Yi, Q.; Ramirez-Arcos, S.; Diallo, J.-S.; Carli, A.; Abdelbary, H. Does treatment order matter? investigating the ability of bacteriophage to augment antibiotic activity against Staphylococcus aureus biofilms. Front. Microbiol. 2018, 9, 127. [Google Scholar] [CrossRef] [Green Version]
- Kolenda, C.; Josse, J.; Medina, M.; Fevre, C.; Lustig, S.; Ferry, T.; Laurent, F. Evaluation of the activity of a combination of three bacteriophages alone or in association with antibiotics on Staphylococcus aureus embedded in biofilm or internalized in osteoblasts. Antimicrob. Agents Chemother. 2020, 64. [Google Scholar] [CrossRef]
- Levack, A.E.; Cyphert, E.L.; Bostrom, M.P.; Hernandez, C.J.; von Recum, H.A.; Carli, A.V. Current options and emerging biomaterials for periprosthetic joint infection. Curr. Rheumatol Rep. 2018, 20, 33. [Google Scholar] [CrossRef]
- Biomaterials; Elsevier: Amsterdam, The Netherlands, 2020. Available online: https://www.journals.elsevier.com/biomaterials (accessed on 16 July 2020).
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.-F.; Alarcon, E.I. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 2018, 4, e01067. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Yang, S.; Wang, Y.; Yu, Z.; Ao, H.; Zhang, H.; Qin, L.; Guillaume, O.; Eglin, D.; Richards, R.G.; et al. Anti-infective efficacy, cytocompatibility and biocompatibility of a 3D-printed osteoconductive composite scaffold functionalized with quaternized chitosan. Acta Biomater. 2016, 46, 112–128. [Google Scholar] [CrossRef]
- Chu, L.; Yang, Y.; Yang, S.; Fan, Q.; Yu, Z.; Hu, X.-L.; James, T.D.; He, X.-P.; Tang, T. Preferential colonization of osteoblasts over co-cultured bacteria on a bifunctional biomaterial surface. Front. Microbiol. 2018, 9, 2219. [Google Scholar] [CrossRef]
- Zahar, A.; Kocsis, G.; Citak, M.; Puskás, G.; Domahidy, M.; Hajdú, M.; Antal, I.; Szendrői, M. Use of antibiotic-impregnated bone grafts in a rabbit osteomyelitis model. Technol. Health Care 2017, 25, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Fleming, D.; Rumbaugh, K.P. Approaches to dispersing medical biofilms. Microorganisms 2017, 5, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Onsea, J.; Wagemans, J.; Pirnay, J.P.; Di Luca, M.; Gonzalez-Moreno, M.; Lavigne, R.; Trampuz, A.; Moriarty, T.F.; Metsemakers, W.-J. Bacteriophage therapy as a treatment strategy for orthopaedic-device-related infections: Where do we stand? Eur. Cell Mater. 2020, 39, 193–210. [Google Scholar] [CrossRef] [PubMed]
- Davidson, D.J.; Spratt, D.; Liddle, A.D. Implant materials and prosthetic joint infection: The battle with the biofilm. EFORT Open Rev. 2019, 4, 633–639. [Google Scholar] [CrossRef]
- Czerwinski, F. Magnesium Alloys: Corrosion and Surface Treatments; BoD—Books on Demand; Natural Resources Canada: Ottawa, QC, Canada, 2011; ISBN 978-953-307-972-1. [Google Scholar]
- Bjarnsholt, T. The role of bacterial biofilms in chronic infections. APMIS Suppl. 2013, 121, 1–51. [Google Scholar] [CrossRef]
- Reffuveille, F.; Josse, J.; Velard, F.; Lamret, F.; Varin-Simon, J.; Dubus, M.; Haney, E.F.; Hancock, R.E.W.; Mongaret, C.; Gangloff, S.C. Bone environment influences irreversible adhesion of a Methicillin-Susceptible Staphylococcus aureus strain. Front. Microbiol. 2018, 9, 2865. [Google Scholar] [CrossRef] [Green Version]
- Hall, C.W.; Mah, T.-F. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol. Rev. 2017, 41, 276–301. [Google Scholar] [CrossRef]
| Strategies | Details | References |
|---|---|---|
| Antibiotic | Novel antibiotics and combination therapy | [31,57,101] |
| Other molecules | Antimicrobial peptides | [101] |
| Enzymes | [31,57,116] | |
| Immunotherapy | Monoclonal antibodies | [31,57] |
| Viruses | Bacteriophage therapy | [31,57,101,117] |
| Implant management | Coatings and surface modifications | [5,110,112,118] |
| Nanoparticle | Loaded or with passive passive effects | [31,57,101,110,112] |
| Electrical and electromagnetic methods | - | [101,112] |
| Ultrasound | - | [31,112] |
| Photodynamic therapy | - | [31,101,112] |
| Plasma | Non-thermal plasma | [101] |
| Targets | Dormant state bacteria | [31] |
| Disruption of biofilm | [112] | |
| Other | Hydrogels | [110] |
| Cyclodextrin-based drug delivery | ||
| Titanium nanotube arrays |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamret, F.; Colin, M.; Mongaret, C.; Gangloff, S.C.; Reffuveille, F. Antibiotic Tolerance of Staphylococcus aureus Biofilm in Periprosthetic Joint Infections and Antibiofilm Strategies. Antibiotics 2020, 9, 547. https://doi.org/10.3390/antibiotics9090547
Lamret F, Colin M, Mongaret C, Gangloff SC, Reffuveille F. Antibiotic Tolerance of Staphylococcus aureus Biofilm in Periprosthetic Joint Infections and Antibiofilm Strategies. Antibiotics. 2020; 9(9):547. https://doi.org/10.3390/antibiotics9090547
Chicago/Turabian StyleLamret, Fabien, Marius Colin, Céline Mongaret, Sophie C. Gangloff, and Fany Reffuveille. 2020. "Antibiotic Tolerance of Staphylococcus aureus Biofilm in Periprosthetic Joint Infections and Antibiofilm Strategies" Antibiotics 9, no. 9: 547. https://doi.org/10.3390/antibiotics9090547
APA StyleLamret, F., Colin, M., Mongaret, C., Gangloff, S. C., & Reffuveille, F. (2020). Antibiotic Tolerance of Staphylococcus aureus Biofilm in Periprosthetic Joint Infections and Antibiofilm Strategies. Antibiotics, 9(9), 547. https://doi.org/10.3390/antibiotics9090547





