Evaluation of Disulfiram Drug Combinations and Identification of Other More Effective Combinations against Stationary Phase Borrelia burgdorferi
Abstract
:1. Introduction
2. Results
2.1. MIC Testing and Relative Activity of DSF at 50 µM
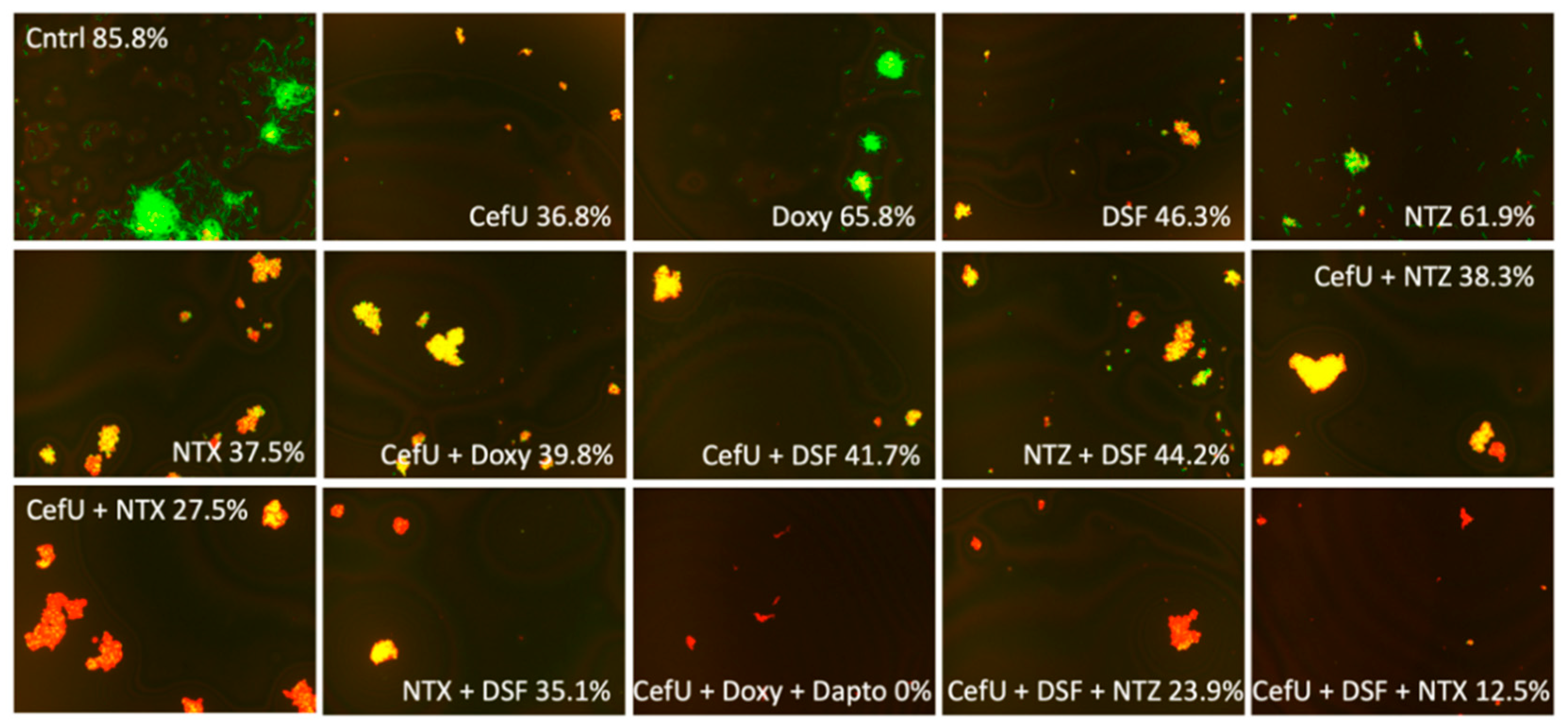
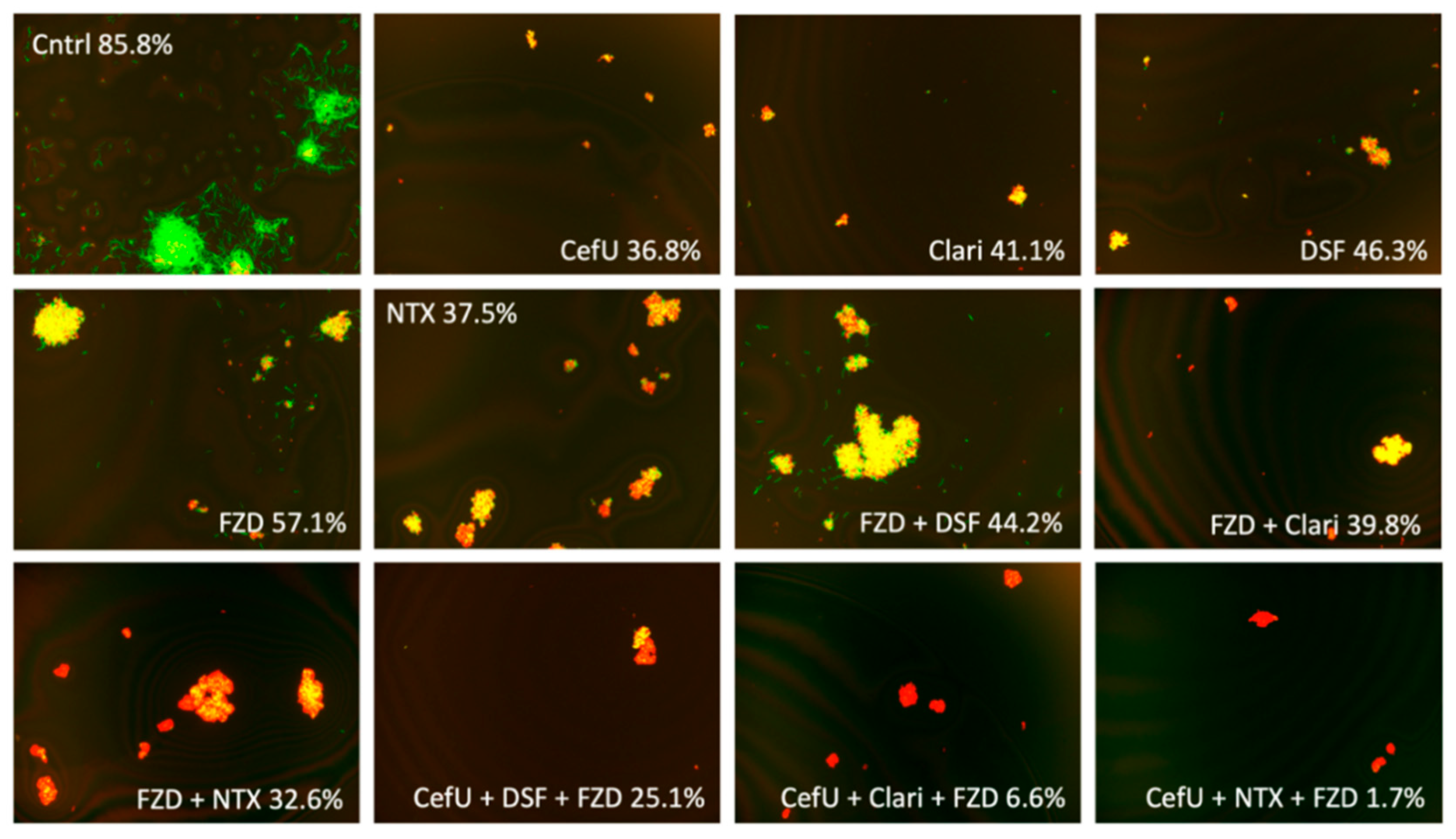
2.2. Activity against Stationary-Phase Culture at Standard Dose of 5 µg/mL and Cmax Concentration
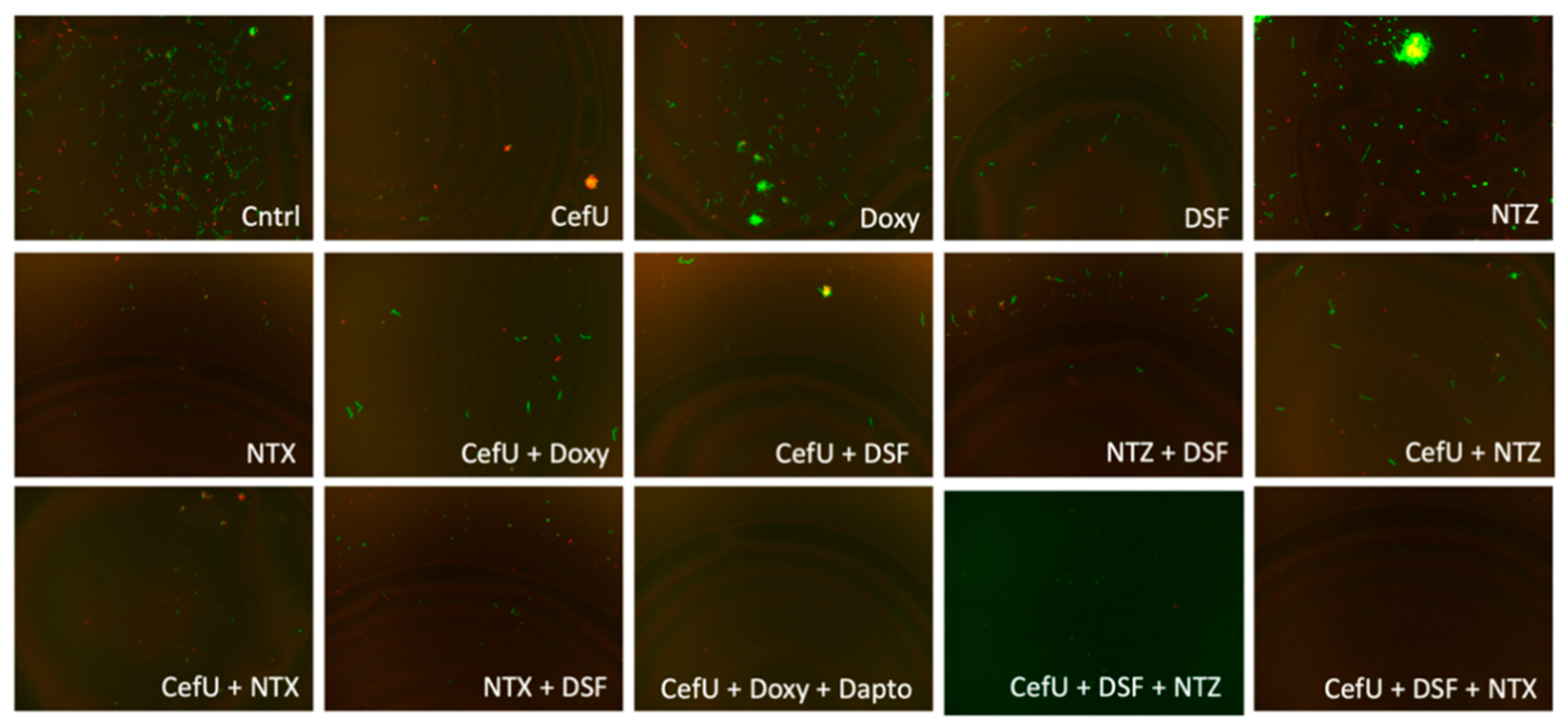
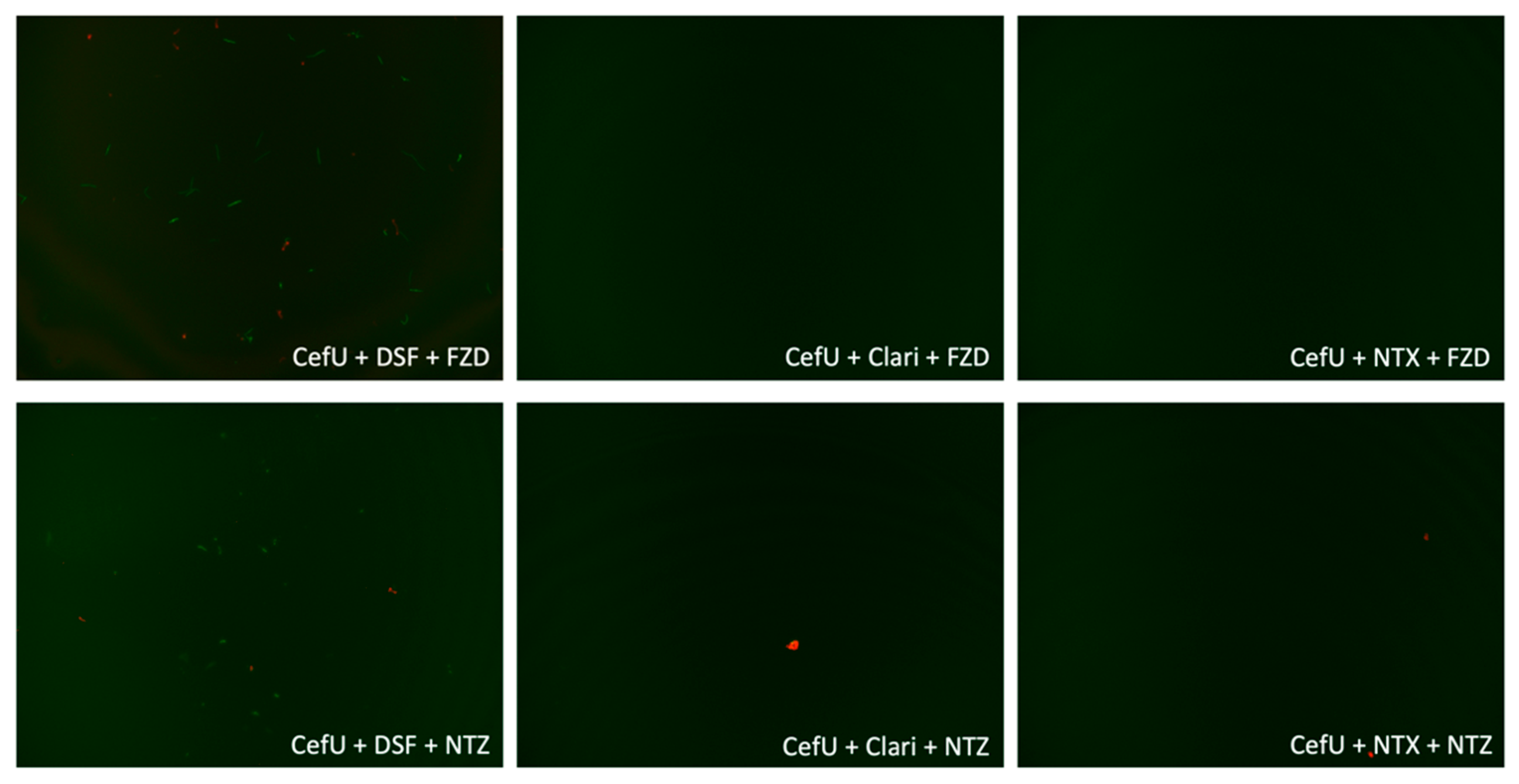
2.3. Subculture Study
3. Discussion
4. Materials and Methods
4.1. Strain, Media, and Culture Techniques
4.2. Drug Selection
4.3. Microscopy
4.4. Drug Susceptibility Testing
4.5. Subculture Study
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Borchers, A.T.; Keen, C.L.; Huntley, A.C.; Gershwin, M.E. Lyme disease: A rigorous review of diagnostic criteria and treatment. J. Autoimmun. 2015, 57, 82–115. [Google Scholar] [CrossRef] [PubMed]
- Aucott, J.N. Posttreatment Lyme disease syndrome. Infect. Dis. Clin. N. Am. 2015, 29, 309–323. [Google Scholar] [CrossRef]
- Eikeland, R.; Mygland, Å.; Herlofson, K.; Ljøstad, U. European neuroborreliosis: Quality of life 30 months after treatment. Acta Neurol. Scand. 2011, 124, 349–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berglund, J.; Stjernberg, L.; Ornstein, K.; Tykesson-Joelsson, K.; Walter, H. 5-y follow-up study of patients with Neuroborreliosis. Scand. J. Infect. Dis. 2002, 34, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC), Post-Treatment Lyme Disease Syndrome. Available online: https://www.cdc.gov/lyme/postlds/index.html (accessed on 19 April 2020).
- Bockenstedt, L.K.; Gonzalez, D.G.; Haberman, A.M.; Belperron, A.A. Spirochete antigens persist near cartilage after murine Lyme borreliosis therapy. J. Clin. Investig. 2012, 122, 2652–2660. [Google Scholar] [CrossRef] [PubMed]
- Embers, M.E.; Barthold, S.W.; Borda, J.T.; Bowers, L.; Doyle, L.; Hodzic, E.; Jacobs, M.B.; Hasenkampf, N.R.; Martin, D.S.; Narasimhan, S.; et al. Persistence of Borrelia burgdorferi in rhesus macaques following antibiotic treatment of disseminated infection. PLoS ONE 2012, 7, e29914. [Google Scholar]
- Hodžić, E.; Imai, D.; Feng, S.; Barthold, S.W. Resurgence of persisting non-cultivable Borrelia burgdorferi following antibiotic treatment in mice. PLoS ONE 2014, 9, e86907. [Google Scholar] [CrossRef] [Green Version]
- Straubinger, R.K.; Summers, B.A.; Chang, Y.F.; Appel, M.J. Persistence of Borrelia burgdorferi in experimentally infected dogs after antibiotic treatment. J. Clin. Microbiol. 1997, 35, 111–116. [Google Scholar] [CrossRef] [Green Version]
- Marques, A.; Telford, S.R.; Turk, S.-P.; Chung, E.; Williams, C.; Dardick, K.; Krause, P.J.; Brandeburg, C.; Crowder, C.D.; Carolan, H.E.; et al. Xenodiagnosis to detect Borrelia burgdorferi infection: A first-in-human study. Clin. Infect. Dis. 2014, 58, 937–945. [Google Scholar] [CrossRef]
- Feng, J.; Wang, T.; Shi, W.; Zhang, S.; Sullivan, D.; Auwaerter, P.G.; Zhang, Y. Identification of novel activity against Borrelia burgdorferi persisters using an FDA approved drug library. Emerg. Microbes Infect. 2014, 3, e49. [Google Scholar] [CrossRef]
- Feng, J.; Li, T.; Yee, R.; Yuan, Y.; Bai, C.; Cai, M.; Shi, W.; Embers, M.; Brayton, C.; Saeki, H.; et al. Stationary phase persister/biofilm microcolony of Borrelia burgdorferi causes more severe disease in a mouse model of Lyme arthritis: Implications for understanding persistence, Post-treatment Lyme Disease Syndrome (PTLDS), and treatment failure. Discov. Med. 2019, 27, 125–138. [Google Scholar] [PubMed]
- Feng, J.; Shi, W.; Zhang, S.; Zhang, Y. Persister mechanisms in Borrelia burgdorferi: Implications for improved intervention. Emerg. Microbes Infect. 2015, 4, e51. [Google Scholar] [PubMed] [Green Version]
- Feng, J.; Weitner, M.; Shi, W.; Zhang, S.; Sullivan, D.J.; Zhang, Y. Identification of additional anti-persister activity against Borrelia burgdorferi from an FDA drug library. Antibiotics 2015, 4, 397–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, J.; Shi, W.; Zhang, S.; Zhang, Y. Identification of new compounds with high activity against stationary phase Borrelia burgdorferi from the NCI compound collection. Emerg. Microbes Infect. 2015, 4, e31. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Auwaerter, P.G.; Zhang, Y. Drug Combinations against Borrelia burgdorferi persisters in vitro: Eradication achieved by using Daptomycin, Cefoperazone and Doxycycline. PLoS ONE 2015, 10, e0117207. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.; Shi, W.; Zhang, S.; Sullivan, D.; Auwaerter, P.G.; Zhang, Y. A drug combination screen identifies drugs active against amoxicillin-induced round bodies of in vitro Borrelia burgdorferi persisters from an FDA drug library. Front. Microbiol. 2016, 7, 743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, J.; Zhang, S.; Shi, W.; Zhang, Y. Ceftriaxone pulse dosing fails to eradicate biofilm-like microcolony B. burgdorferi persisters which are sterilized by daptomycin/ doxycycline/cefuroxime without pulse dosing. Front. Microbiol. 2016, 7, 1744. [Google Scholar] [CrossRef] [Green Version]
- Pothineni, V.R.; Wagh, D.; Babar, M.M.; Inayathullah, M.; Solow-Cordero, D.E.; Kim, K.-M.; Samineni, A.V.; Parekh, M.B.; Tayebi, L.; Rajadas, J. Identification of new drug candidates against Borrelia burgdorferi using high-throughput screening. Drug Des. Dev. Ther. 2016, 10, 1307–1322. [Google Scholar] [CrossRef] [Green Version]
- Long, T.E. Repurposing Thiram and Disulfiram as antibacterial agents for multidrug-resistant Staphylococcus aureus infections. Antimicrob. Agents Chemother. 2017, 61, e00898-17. [Google Scholar] [CrossRef] [Green Version]
- Potula, H.-H.S.; Shahryari, J.; Inayathullah, M.; Malkovskiy, A.V.; Kim, K.-M.; Rajadas, J. Repurposing disulfiram (Tetraethylthiuram Disulfide) as a potential drug candidate against Borrelia burgdorferi in vitro and in vivo. bioRxiv 2019, 842286. [Google Scholar] [CrossRef] [Green Version]
- Liegner, K. Disulfiram (Tetraethylthiuram Disulfide) in the treatment of Lyme disease and babesiosis: Report of experience in three cases. Antibiotics 2019, 8, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byrne, S.T.; Gu, P.; Zhou, J.; Denkin, S.M.; Chong, C.; Sullivan, D.; Liu, J.O.; Zhang, Y. Pyrrolidine Dithiocarbamate and Diethyldithiocarbamate are active against growing and nongrowing persister Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2007, 51, 4495–4497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaldívar-Machorro, V.J.; López-Ortiz, M.; Demare, P.; Regla, I.; Muñoz-Clares, R. The disulfiram metabolites S-methyl-N,N-diethyldithiocarbamoyl sulfoxide and S-methyl-N,N-diethylthiocarbamoyl sulfone irreversibly inactivate betaine aldehyde dehydrogenase from Pseudomonas aeruginosa, both in vitro and in situ, and arrest bacterial growth. Biochimie 2011, 93, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Wang, T.; Zhang, S.; Shi, W.; Zhang, Y. An optimized SYBR green I/PI assay for rapid viability assessment and antibiotic susceptibility testing for Borrelia burgdorferi. PLoS ONE 2014, 9, e111809. [Google Scholar] [CrossRef] [PubMed]
- Trautmann, A.; Gascan, H.; Ghozzi, R. Potential patient-reported toxicities with disulfiram treatment in late disseminated Lyme disease. Front. Med. 2020, 7, 133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dever, L.L.; Jorgensen, J.H.; Barbour, A.G. Comparative in vitro activities of clarithromycin, azithromycin, and erythromycin against Borrelia burgdorferi. Antimicrob. Agents Chemother. 1993, 37, 1704–1706. [Google Scholar] [CrossRef] [Green Version]
- Kwon, Y.-S.; Koh, W.-J.; Daley, C.L. Treatment of Mycobacterium avium complex pulmonary disease. Tuberc. Respir. Dis. 2019, 82, 15–26. [Google Scholar] [CrossRef]
- Cherdtrakulkiat, R.; Lawung, R.; Nabu, S.; Tantimavanich, S.; Sinthupoom, N.; Prachayasittikul, S.; Prachayasittikul, V. Nitroxoline: A potent antimicrobial agent against multidrug resistant Enterobacteriaceae. EXCLI J. 2019, 18, 445–453. [Google Scholar]
- Feng, J.; Leone, J.; Schweig, S.; Zhang, Y. Evaluation of natural and botanical medicines for activity against growing and non-growing forms of B. burgdorferi. Front. Med. 2020, 7, 6. [Google Scholar] [CrossRef] [Green Version]
- Bugyei, K.A.; Boye, G.L.; Addy, M.E. Clinical efficacy of a tea-bag formulation of Cryptolepis Sanguinolenta root in the treatment of acute uncomplicated falciparum malaria. Ghana Med. J. 2010, 44, 3–9. [Google Scholar] [CrossRef] [Green Version]
- Forkuo, A.D.; Ansah, C.; Pearson, D.; Gertsch, W.; Cirello, A.; Amaral, A.; Spear, J.; Wright, C.W.; Rynn, C. Identification of cryptolepine metabolites in rat and human hepatocytes and metabolism and pharmacokinetics of cryptolepine in Sprague Dawley rats. BMC Pharmacol. Toxicol. 2017, 18, 84. [Google Scholar] [CrossRef] [PubMed]
- Kresken, M.; Körber-Irrgang, B. In vitro activity of Nitroxoline against Escherichia coli urine isolates from outpatient departments in Germany. Antimicrob. Agents Chemother. 2014, 58, 7019–7020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wijma, R.A.; Huttner, A.; Koch, B.C.; Mouton, J.W.; Muller, A.E. Review of the pharmacokinetic properties of nitrofurantoin and nitroxoline. J. Antimicrob. Chemother. 2018, 73, 2916–2926. [Google Scholar] [CrossRef] [PubMed]
- Hof, H.; Hof, H.; Bertsch, D.; Bertsch, D.; Passek, D.; Passek, D.; Schwarz, R.; Schwarz, R. Nitroxolin-eine option zur antibiotischen Therapie von Harnwegsinfektionen. Urologe 2017, 56, 167–171. [Google Scholar] [CrossRef]
- Enkelmann, J.; Böhmer, M.; Fingerle, V.; Siffczyk, C.; Werber, D.; Littmann, M.; Merbecks, S.-S.; Helmeke, C.; Schroeder, S.; Hell, S.; et al. Incidence of notified Lyme borreliosis in Germany, 2013–2017. Sci. Rep. 2018, 8, 14976. [Google Scholar] [CrossRef]
- Mason, L.M.K.; Hovius, J.W.R. Investigating human dendritic cell immune responses to Borrelia burgdorferi. Methods Mol. Biol. 2018, 1690, 291–299. [Google Scholar] [CrossRef]
- Artemisinin. Available online: http://www.antimicrobe.org/drugpopup/artemisinin.htm (accessed on 19 April 2020).
- Cefuroxime. Available online: http://www.antimicrobe.org/drugpopup/Cefuroxime.htm (accessed on 19 April 2020).
- Clarithromycin. Available online: http://www.antimicrobe.org/drugpopup/clarithromycin.htm (accessed on 19 April 2020).
- Yawalkar, S.J.; Vischer, W. Lamprene (Clofazimine) in Leprosy. Lepr. Rev. 1979, 50, 135–144. [Google Scholar] [CrossRef]
- Daptomycin. Available online: http://antimicrobe.org/drugpopup/Daptomycin.pdf (accessed on 19 April 2020).
- Spillier, Q.; Vertommen, D.; Ravez, S.; Marteau, R.; Thémans, Q.; Corbet, C.; Feron, O.; Wouters, J.; Frédérick, R. Anti-alcohol abuse drug disulfiram inhibits human PHGDH via disruption of its active tetrameric form through a specific cysteine oxidation. Sci. Rep. 2019, 9, 4737. [Google Scholar] [CrossRef] [Green Version]
- Doxycycline. Available online: http://www.antimicrobe.org/drugpopup/Doxycycline.htm (accessed on 19 April 2020).
- Erythromycin. Available online: http://www.antimicrobe.org/drugpopup/Erythromycin.pdf (accessed on 19 April 2020).
- Calafatti, S.A.; Ortiz, R.A.M.; Deguer, M.; Martinez, M.; Pedrazzoli, J. Effect of acid secretion blockade by omeprazole on the relative bioavailability of orally administered furazolidone in healthy volunteers. Br. J. Clin. Pharmacol. 2001, 52, 205–209. [Google Scholar] [CrossRef] [Green Version]
- Linezolid and Other Oxazolidinones. Available online: http://www.antimicrobe.org/d13.asp (accessed on 19 April 2020).
- Food and Drug Administration (FDA) Prescribing Information for Alinia®. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2005/021818lbl.pdf (accessed on 19 April 2020).
- Bergogne-Berezin, E.; Berthelot, G.; Muller-Serieys, C. Present status of nitroxoline. Pathol. Biol. 1987, 35, 873–878. [Google Scholar]
- Rifabutin for Mycobacterial Infections. Available online: http://www.antimicrobe.org/drugpopup/Rifabutin-myco.htm (accessed on 19 April 2020).
- Feng, J.; Weitner, M.; Shi, W.; Zhang, S.; Zhang, Y. Eradication of biofilm-like microcolony structures of Borrelia burgdorferi by Daunomycin and Daptomycin but not Mitomycin C in combination with doxycycline and cefuroxime. Front. Microbiol. 2016, 7, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, J.; Shi, W.; Miklossy, J.; Tauxe, G.M.; McMeniman, C.J.; Zhang, Y. Identification of essential oils with strong activity against stationary phase Borrelia burgdorferi. Antibiotics 2018, 7, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Drug | MIC (µg/mL) | Drug | MIC (µg/mL) |
|---|---|---|---|
| Cefuroxime (CefU) | 0.15 | Daptomycin (Dapto) | >10.0 |
| Doxycycline (Doxy) | 0.08 | Disulfiram (DSF) | 0.3 |
| Amoxicillin (Amoxi) | 0.3 | Erythromycin (Ery) | <0.01 |
| Artemisinin (Arte) | 5.0 | Furazolidone (FZD) | 5.0 |
| Azithromycin (Azi) | 0.6 | Linezolid (LNZ) | 5.0 |
| Clarithromycin (Clari) | 0.04 | Nitazoxanide (NTZ) | 10.0 |
| Clofazimine (CFZ) | 5.0 | Nitroxoline (NTX) | 1.25 |
| Cryptolepine (Cry) | 0.6 | Rifabutin (Ribu) | 5.0 |
| Cntrl | CefU | Doxy | DSF | Arte | Clari | CFZ | Cry | Ery | FZD | LNZ | NTZ | NTX | Ribu | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 85.8 | 36.8 | 65.8 | 46.3 | 53.6 | 41.1 ns | 65.6 | 63.3 | 53.0 | 57.1 | 56.8 | 61.9 | 37.5 ns | 48.4 | |
| CefU | ––– | ––– | 39.8 | 41.7 | 33.7 | 25.9 * | 35.3 | 38.4 | 36.7 | 45.3 | 41.3 | 38.3 | 27.5 * | 37.7 |
| DSF | ––– | 41.7 | 46.3 | ––– | 41.0 | 32.3 | 44.9 | 42.8 | 39.8 | 37.7 | 44.7 | 44.2 | 35.1 | 39.1 |
| CefU + Dapto | ––– | ––– | 0.0 | ––– | ––– | ––– | ––– | ––– | ––– | ––– | ––– | ––– | ––– | ––– |
| Arte + DSF | ––– | ––– | ––– | ––– | ––– | ––– | ––– | ––– | ––– | ––– | ––– | ––– | ––– | ––– |
| CefU + DSF | ––– | ––– | ––– | ––– | ––– | ––– | ––– | 37.4 | ––– | 25.1 | ––– | 23.9 | 12.5 | ––– |
| Clari + DSF | ––– | ––– | ––– | ––– | ––– | ––– | ––– | ––– | ––– | ––– | 28.0 | ––– | 26.8 | ––– |
| CFZ + DSF | ––– | ––– | ––– | ––– | ––– | ––– | ––– | ––– | ––– | 38.6 | ––– | ––– | ––– | 29.2 |
| Cry + DSF | ––– | ––– | ––– | ––– | ––– | ––– | ––– | ––– | 36.2 | ––– | ––– | 33.3 | ––– | 32.6 |
| FZD + DSF | ––– | ––– | ––– | ––– | ––– | ––– | ––– | ––– | ––– | ––– | 28.1 | 32.2 | 24.0 | ––– |
| LNZ + DSF | ––– | ––– | ––– | ––– | ––– | ––– | ––– | ––– | ––– | ––– | ––– | 29.6 | 25.5 | ––– |
| NTZ + DSF | ––– | ––– | ––– | ––– | ––– | ––– | ––– | ––– | ––– | ––– | ––– | ––– | ––– | 30.0 |
| Drug | Cntrl | DSF | Clari | NTX |
|---|---|---|---|---|
| ––– | 85.8 | 46.3 | 41.1 | 37.5 |
| CefU | 36.8 | 41.7 | 25.9 | 27.5 |
| FZD | 57.1 | 37.7 | 34.2 | 21.4 |
| NTZ | 61.9 | 44.2 | 39.8 | 32.6 |
| CefU + FZD | 45.3 | 25.1 | 6.6 * | 1.7 * |
| CefU + NTZ | 38.3 | 23.9 | 5.0 * | 11.0 ns |
| Drug | Subculture Results | Drug | Subculture Results |
|---|---|---|---|
| Cntrl | +++ | FZD + Clari | +++ |
| CefU | +++ | FZD + NTX | +++ |
| Clari | +++ | NTZ + DSF | +++ |
| DSF | +++ | NTZ + Clari | +++ |
| Doxy | +++ | NTZ + NTX | +++ |
| FZD | +++ | NTX + DSF | +++ |
| NTZ | +++ | CefU + Doxy + Dapto | – – – |
| NTX | +++ | CefU + DSF + FZD | +++ |
| CefU + Doxy | +++ | CefU + Clari + FZD | – – – |
| CefU + Clari | +++ | CefU + NTX + FZD | – – – |
| CefU + DSF | +++ | CefU + DSF + NTZ | +++ |
| CefU + NTZ | +++ | CefU + Clari + NTZ | – – – |
| CefU + NTX | +++ | CefU + NTX + NTZ | – – – |
| FZD + DSF | +++ | CefU + DSF + NTX | – – – |
| Drug | Cmax Mean in µg/mL (Range) | Source |
|---|---|---|
| Arte | 0.43 | http://www.antimicrobe.org/ [38] |
| CefU | 8.9 (4.2–13.6) | http://www.antimicrobe.org/ [39] |
| Clari | 6.8 | http://www.antimicrobe.org/ [40] |
| CFZ | 1.1 (0.7–1.4) | Yawalkar and Vischer, 1979 [41] |
| Cry | 0.007–0.024 b | Forkuo et al., 2017 [32] |
| Dapto | 77.5 | http://www.antimicrobe.org/ [42] |
| DSF | 0.38 | Spillier et al., 2019 [43] |
| Doxy | 2.6 (1.5–3.6) | http://www.antimicrobe.org/ [44] |
| Ery | 3.0 | http://www.antimicrobe.org/ [45] |
| FZD | 0.3 | Calafatti, Ortiz, Deguer, Martinez, and Pedrazzoli, 2001 [46] |
| LNZ | 21.2 (15.4–27.0) | http://www.antimicrobe.org/ [47] |
| NTZ | 10.6 (8.6–12.6) | Food and Drug Administration, 2005 [48] |
| NTX | 5.6 (2.45–8.75) | Bergogne-Berezin, Berthelot, and Muller-Serieys, 1987 [49] |
| Ribu | 0.4 (0.2–0.6) | http://www.antimicrobe.org/ [50] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvarez-Manzo, H.S.; Zhang, Y.; Shi, W.; Zhang, Y. Evaluation of Disulfiram Drug Combinations and Identification of Other More Effective Combinations against Stationary Phase Borrelia burgdorferi. Antibiotics 2020, 9, 542. https://doi.org/10.3390/antibiotics9090542
Alvarez-Manzo HS, Zhang Y, Shi W, Zhang Y. Evaluation of Disulfiram Drug Combinations and Identification of Other More Effective Combinations against Stationary Phase Borrelia burgdorferi. Antibiotics. 2020; 9(9):542. https://doi.org/10.3390/antibiotics9090542
Chicago/Turabian StyleAlvarez-Manzo, Hector S., Yumin Zhang, Wanliang Shi, and Ying Zhang. 2020. "Evaluation of Disulfiram Drug Combinations and Identification of Other More Effective Combinations against Stationary Phase Borrelia burgdorferi" Antibiotics 9, no. 9: 542. https://doi.org/10.3390/antibiotics9090542





