Improving the Efficiency of Photodynamic Chemotherapy in Root Canals against Enterococcus faecalis In Vitro
Abstract
1. Introduction
2. Materials & Methods
2.1. Tooth Specimens
2.2. Infection of Specimens and Antibacterial Treatment
2.3. Scanning Electron Microscopy (SEM) Imaging of the Specimens
2.4. Statistical Analysis
3. Results
3.1. Antimicrobial Effect of the Treatments on Enterococcus Faecalis
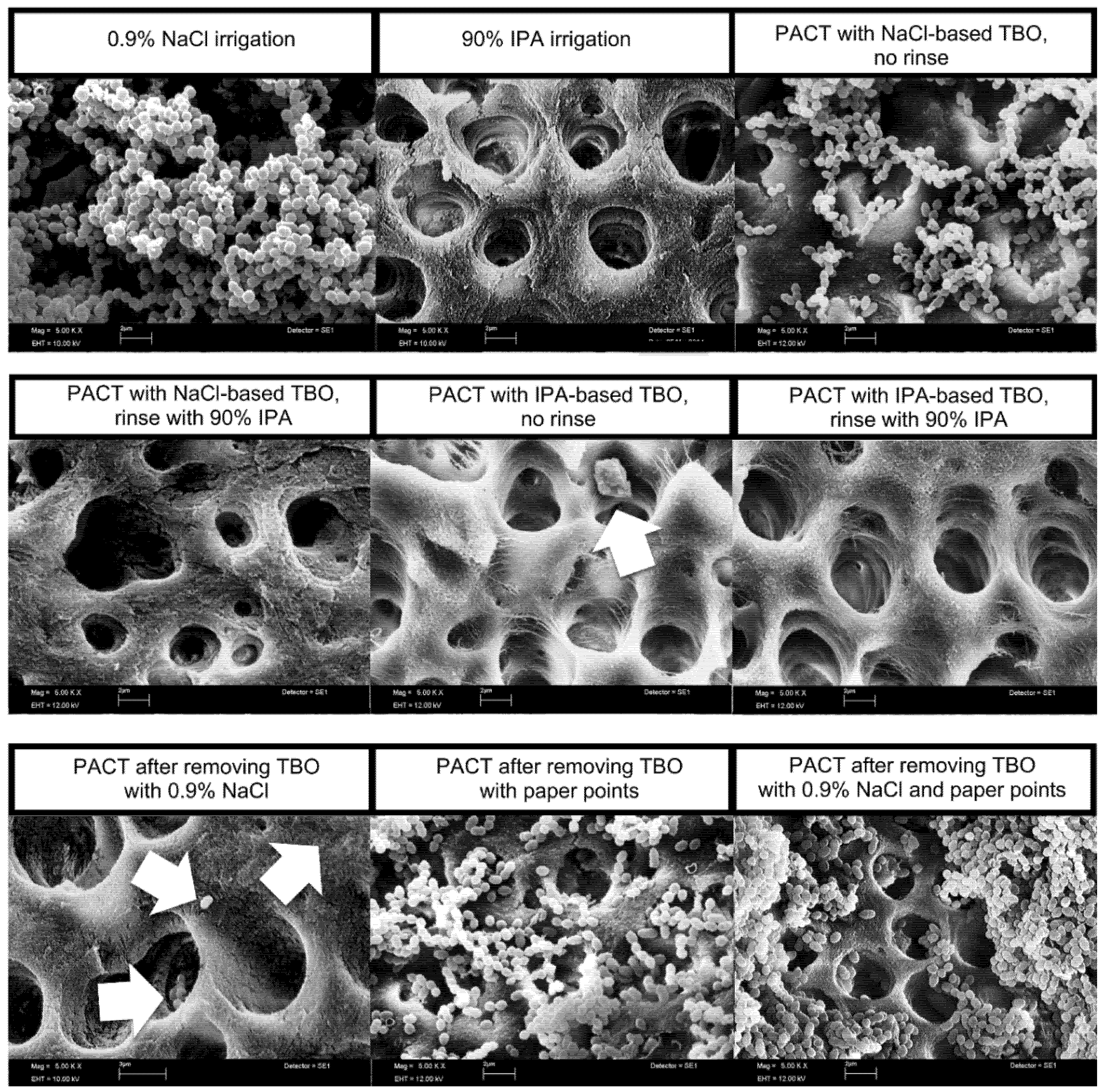
3.2. Scanning Electron Microscopic Evaluation of Root Canals after Antimicrobial Treatment
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siqueira, J.F., Jr.; Rocas, I.N. Clinical implications and microbiology of bacterial persistence after treatment procedures. J. Endod. 2008, 34, 1291–1301.e3. [Google Scholar] [CrossRef]
- Orstavik, D.; Haapasalo, M. Disinfection by endodontic irrigants and dressings of experimentally infected dentinal tubules. Endod. Dent. Traumatol. 1990, 6, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, L.L.; Vaishnavi, C. Endodontic microbiology. J. Conserv. Dent. 2010, 13, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Schirrmeister, J.F.; Liebenow, A.L.; Pelz, K.; Wittmer, A.; Serr, A.; Hellwig, E.; Al-Ahmad, A. New bacterial compositions in root-filled teeth with periradicular lesions. J. Endod. 2009, 35, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Tennert, C.; Fuhrmann, M.; Wittmer, A.; Karygianni, L.; Altenburger, M.J.; Pelz, K.; Hellwig, E.; Al-Ahmad, A. New bacterial composition in primary and persistent/secondary endodontic infections with respect to clinical and radiographic findings. J. Endod. 2014, 40, 670–677. [Google Scholar] [CrossRef]
- Rocas, I.N.; Siqueira, J.F., Jr.; Santos, K.R. Association of Enterococcus faecalis with different forms of periradicular diseases. J. Endod. 2004, 30, 315–320. [Google Scholar] [CrossRef]
- Gebel, J.; Gemein, S.; Kampf, G.; Pidot, S.J.; Buetti, N.; Exner, M. Isopropanol at 60% and at 70% are effective against ‘isopropanol-tolerant’ Enterococcus faecium. J. Hosp. Infect. 2019, 103, e88–e91. [Google Scholar] [CrossRef]
- Morton, H.E. The relationship of concentration and germicidal efficiency of ethyl alcohol. Ann. N. Y. Acad. Sci. 1950, 53, 191–196. [Google Scholar] [CrossRef]
- Presterl, E.; Suchomel, M.; Eder, M.; Reichmann, S.; Lassnigg, A.; Graninger, W.; Rotter, M. Effects of alcohols, povidone-iodine and hydrogen peroxide on biofilms of Staphylococcus epidermidis. J. Antimicrob. Chemother. 2007, 60, 417–420. [Google Scholar] [CrossRef]
- Rizzotti, L.; Rossi, F.; Torriani, S. Biocide and antibiotic resistance of Enterococcus faecalis and Enterococcus faecium isolated from the swine meat chain. Food Microbiol. 2016, 60, 160–164. [Google Scholar] [CrossRef]
- Fabricius, L.; Dahlen, G.; Sundqvist, G.; Happonen, R.P.; Moller, A.J. Influence of residual bacteria on periapical tissue healing after chemomechanical treatment and root filling of experimentally infected monkey teeth. Eur. J. Oral Sci. 2006, 114, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Waltimo, T.; Trope, M.; Haapasalo, M.; Orstavik, D. Clinical efficacy of treatment procedures in endodontic infection control and one year follow-up of periapical healing. J. Endod. 2005, 31, 863–866. [Google Scholar] [CrossRef] [PubMed]
- Eneide, C.; Castagnola, R.; Martini, C.; Grande, N.M.; Bugli, F.; Patini, R.; Cordaro, M.; Sanguinetti, M.; Olivi, G.; Isola, G.; et al. Antibiofilm Activity of Three Different Irrigation Techniques: An In Vitro Study. Antibiotics 2019, 8, 112. [Google Scholar] [CrossRef]
- Chavez de Paz, L.E.; Bergenholtz, G.; Svensater, G. The effects of antimicrobials on endodontic biofilm bacteria. J. Endod. 2010, 36, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Portenier, I.; Waltimo, T.; Orstavik, D.; Haapasalo, M. Killing of Enterococcus faecalis by MTAD and chlorhexidine digluconate with or without cetrimide in the presence or absence of dentine powder or BSA. J. Endod. 2006, 32, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Song, X.; Kristiansen, B.E.; Kjaereng, A.; Willems, R.J.; Eriksen, H.M.; Sundsfjord, A.; Sollid, J.E. Occurrence, population structure, and antimicrobial resistance of enterococci in marginal and apical periodontitis. J. Clin. Microbiol. 2009, 47, 2218–2225. [Google Scholar] [CrossRef] [PubMed]
- Cieplik, F.; Deng, D.; Crielaard, W.; Buchalla, W.; Hellwig, E.; Al-Ahmad, A.; Maisch, T. Antimicrobial photodynamic therapy—What we know and what we don’t. Crit. Rev. Microbiol. 2018, 44, 571–589. [Google Scholar] [CrossRef] [PubMed]
- Gursoy, H.; Ozcakir-Tomruk, C.; Tanalp, J.; Yilmaz, S. Photodynamic therapy in dentistry: A literature review. Clin. Oral Investig. 2012, 17, 1113–1125. [Google Scholar] [CrossRef] [PubMed]
- Konopka, K.; Goslinski, T. Photodynamic therapy in dentistry. J. Dent. Res. 2007, 86, 694–707. [Google Scholar] [CrossRef]
- Bago Juric, I.; Plecko, V.; Anic, I.; Plesko, S.; Jakovljevic, S.; Rocca, J.P.; Medioni, E. Antimicrobial efficacy of photodynamic therapy, Nd:YAG laser and QMiX solution against Enterococcus faecalis biofilm. Photodiagn. Photodyn. Ther. 2016, 13, 238–243. [Google Scholar] [CrossRef]
- Fimple, J.L.; Fontana, C.R.; Foschi, F.; Ruggiero, K.; Song, X.; Pagonis, T.C.; Tanner, A.C.; Kent, R.; Doukas, A.G.; Stashenko, P.P.; et al. Photodynamic treatment of endodontic polymicrobial infection in vitro. J. Endod. 2008, 34, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Rosa, R.A.D.; Santini, M.F.; Figueiredo, J.A.P.; Visioli, F.; Pereira, J.R.; Vivan, R.R.; Montagner, F.; So, M.V.R. Effectiveness of photodynamic therapy associated with irrigants over two biofilm models. Photodiagn. Photodyn. Ther. 2017, 20, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Guan, S.; Lu, H.; Zhao, C.; Chen, X.; Li, N.; Bai, Q.; Tian, Y.; Yu, Q. Evaluation of the bactericidal effect of Nd:YAG, Er:YAG, Er,Cr:YSGG laser radiation, and antimicrobial photodynamic therapy (aPDT) in experimentally infected root canals. Lasers Surg. Med. 2012, 44, 824–831. [Google Scholar] [CrossRef]
- Hecker, S.; Hiller, K.A.; Galler, K.M.; Erb, S.; Mader, T.; Schmalz, G. Establishment of an optimized ex vivo system for artificial root canal infection evaluated by use of sodium hypochlorite and the photodynamic therapy. Int. Endod. J. 2013, 46, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.R.; Mello, I.; Franco, G.C.; de Medeiros, J.M.; Dos Santos, S.S.; Habitante, S.M.; Lage-Marques, J.L.; Raldi, D.P. Effectiveness of photodynamic therapy against Enterococcus faecalis, with and without the use of an intracanal optical fiber: An in vitro study. Photomed. Laser Surg. 2011, 29, 803–808. [Google Scholar] [CrossRef]
- Rios, A.; He, J.; Glickman, G.N.; Spears, R.; Schneiderman, E.D.; Honeyman, A.L. Evaluation of photodynamic therapy using a light-emitting diode lamp against Enterococcus faecalis in extracted human teeth. J. Endod. 2011, 37, 856–859. [Google Scholar] [CrossRef]
- Souza, L.C.; Brito, P.R.; de Oliveira, J.C.; Alves, F.R.; Moreira, E.J.; Sampaio-Filho, H.R.; Rocas, I.N.; Siqueira, J.F., Jr. Photodynamic therapy with two different photosensitizers as a supplement to instrumentation/irrigation procedures in promoting intracanal reduction of Enterococcus faecalis. J. Endod. 2010, 36, 292–296. [Google Scholar] [CrossRef]
- Tennert, C.; Feldmann, K.; Haamann, E.; Al-Ahmad, A.; Follo, M.; Wrbas, K.T.; Hellwig, E.; Altenburger, M.J. Effect of photodynamic therapy (PDT) on Enterococcus faecalis biofilm in experimental primary and secondary endodontic infections. BMC Oral Health 2014, 14, 132. [Google Scholar] [CrossRef]
- Silva, L.A.; Novaes, A.B., Jr.; de Oliveira, R.R.; Nelson-Filho, P.; Santamaria, M., Jr.; Silva, R.A. Antimicrobial photodynamic therapy for the treatment of teeth with apical periodontitis: A histopathological evaluation. J. Endod. 2012, 38, 360–366. [Google Scholar] [CrossRef]
- Garcez, A.S.; Nunez, S.C.; Hamblim, M.R.; Suzuki, H.; Ribeiro, M.S. Photodynamic therapy associated with conventional endodontic treatment in patients with antibiotic-resistant microflora: A preliminary report. J. Endod. 2010, 36, 1463–1466. [Google Scholar] [CrossRef]
- Ghinzelli, G.C.; Souza, M.A.; Cecchin, D.; Farina, A.P.; Figueiredo, J.A. Influence of ultrasonic activation on photodynamic therapy over root canal system infected with Enterococcus faecalis—An in vitro study. Photodiagn. Photodyn. Ther. 2014, 11, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Tennert, C.; Drews, A.M.; Walther, V.; Altenburger, M.J.; Karygianni, L.; Wrbas, K.T.; Hellwig, E.; Al-Ahmad, A. Ultrasonic activation and chemical modification of photosensitizers enhances the effects of photodynamic therapy against Enterococcus faecalis root-canal isolates. Photodiagn. Photodyn. Ther. 2015, 12, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Senges, C.; Wrbas, K.T.; Altenburger, M.; Follo, M.; Spitzmuller, B.; Wittmer, A.; Hellwig, E.; Al-Ahmad, A. Bacterial and Candida albicans adhesion on different root canal filling materials and sealers. J. Endod. 2011, 37, 1247–1252. [Google Scholar] [CrossRef] [PubMed]
- De Melo, W.C.; Avci, P.; de Oliveira, M.N.; Gupta, A.; Vecchio, D.; Sadasivam, M.; Chandran, R.; Huang, Y.Y.; Yin, R.; Perussi, L.R.; et al. Photodynamic inactivation of biofilm: Taking a lightly colored approach to stubborn infection. Expert Rev. Anti Infect. Ther. 2013, 11, 669–693. [Google Scholar] [CrossRef] [PubMed]
- Zehnder, M. Root canal irrigants. J. Endod. 2006, 32, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Zanin, I.C.; Goncalves, R.B.; Junior, A.B.; Hope, C.K.; Pratten, J. Susceptibility of Streptococcus mutans biofilms to photodynamic therapy: An in vitro study. J. Antimicrob. Chemother. 2005, 56, 324–330. [Google Scholar] [CrossRef]
- Jungermann, G.B.; Burns, K.; Nandakumar, R.; Tolba, M.; Venezia, R.A.; Fouad, A.F. Antibiotic resistance in primary and persistent endodontic infections. J. Endod. 2011, 37, 1337–1344. [Google Scholar] [CrossRef]
- Ordinola-Zapata, R.; Bramante, C.M.; Garcia, R.B.; de Andrade, F.B.; Bernardineli, N.; de Moraes, I.G.; Duarte, M.A. The antimicrobial effect of new and conventional endodontic irrigants on intra-orally infected dentin. Acta Odontol. Scand. 2013, 71, 424–431. [Google Scholar] [CrossRef]
- Nair, P.N. On the causes of persistent apical periodontitis: A review. Int. Endod. J. 2006, 39, 249–281. [Google Scholar] [CrossRef]
- Siqueira, J.F., Jr. Aetiology of root canal treatment failure: Why well-treated teeth can fail. Int. Endod. J. 2001, 34, 1–10. [Google Scholar] [CrossRef]
- Hancock, H.H., III; Sigurdsson, A.; Trope, M.; Moiseiwitsch, J. Bacteria isolated after unsuccessful endodontic treatment in a North American population. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2001, 91, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Sundqvist, G.; Figdor, D.; Persson, S.; Sjogren, U. Microbiologic analysis of teeth with failed endodontic treatment and the outcome of conservative re-treatment. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 1998, 85, 86–93. [Google Scholar] [CrossRef]
- Sedgley, C.M.; Lennan, S.L.; Appelbe, O.K. Survival of Enterococcus faecalis in root canals ex vivo. Int. Endod. J. 2005, 38, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.C.; Hellwig, E.; Vespermann, R.; Wittmer, A.; Schmid, M.; Karygianni, L.; Al-Ahmad, A. Comprehensive analysis of secondary dental root canal infections: A combination of culture and culture-independent approaches reveals new insights. PLoS ONE 2012, 7, e49576. [Google Scholar] [CrossRef]
- Park, E.; Shen, Y.; Khakpour, M.; Haapasalo, M. Apical pressure and extent of irrigant flow beyond the needle tip during positive-pressure irrigation in an in vitro root canal model. J. Endod. 2013, 39, 511–515. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tennert, C.; Zinovieva, Y.; Shishkov, K.; Karygianni, L.; Altenburger, M.J.; Wierichs, R.J.; Al-Ahmad, A. Improving the Efficiency of Photodynamic Chemotherapy in Root Canals against Enterococcus faecalis In Vitro. Antibiotics 2020, 9, 543. https://doi.org/10.3390/antibiotics9090543
Tennert C, Zinovieva Y, Shishkov K, Karygianni L, Altenburger MJ, Wierichs RJ, Al-Ahmad A. Improving the Efficiency of Photodynamic Chemotherapy in Root Canals against Enterococcus faecalis In Vitro. Antibiotics. 2020; 9(9):543. https://doi.org/10.3390/antibiotics9090543
Chicago/Turabian StyleTennert, Christian, Yoana Zinovieva, Kalin Shishkov, Lamprini Karygianni, Makus Jörg Altenburger, Richard J Wierichs, and Ali Al-Ahmad. 2020. "Improving the Efficiency of Photodynamic Chemotherapy in Root Canals against Enterococcus faecalis In Vitro" Antibiotics 9, no. 9: 543. https://doi.org/10.3390/antibiotics9090543
APA StyleTennert, C., Zinovieva, Y., Shishkov, K., Karygianni, L., Altenburger, M. J., Wierichs, R. J., & Al-Ahmad, A. (2020). Improving the Efficiency of Photodynamic Chemotherapy in Root Canals against Enterococcus faecalis In Vitro. Antibiotics, 9(9), 543. https://doi.org/10.3390/antibiotics9090543




