Involvement of hpap2 and dgkA Genes in Colistin Resistance Mediated by mcr Determinants
Abstract
:1. Introduction
2. Results
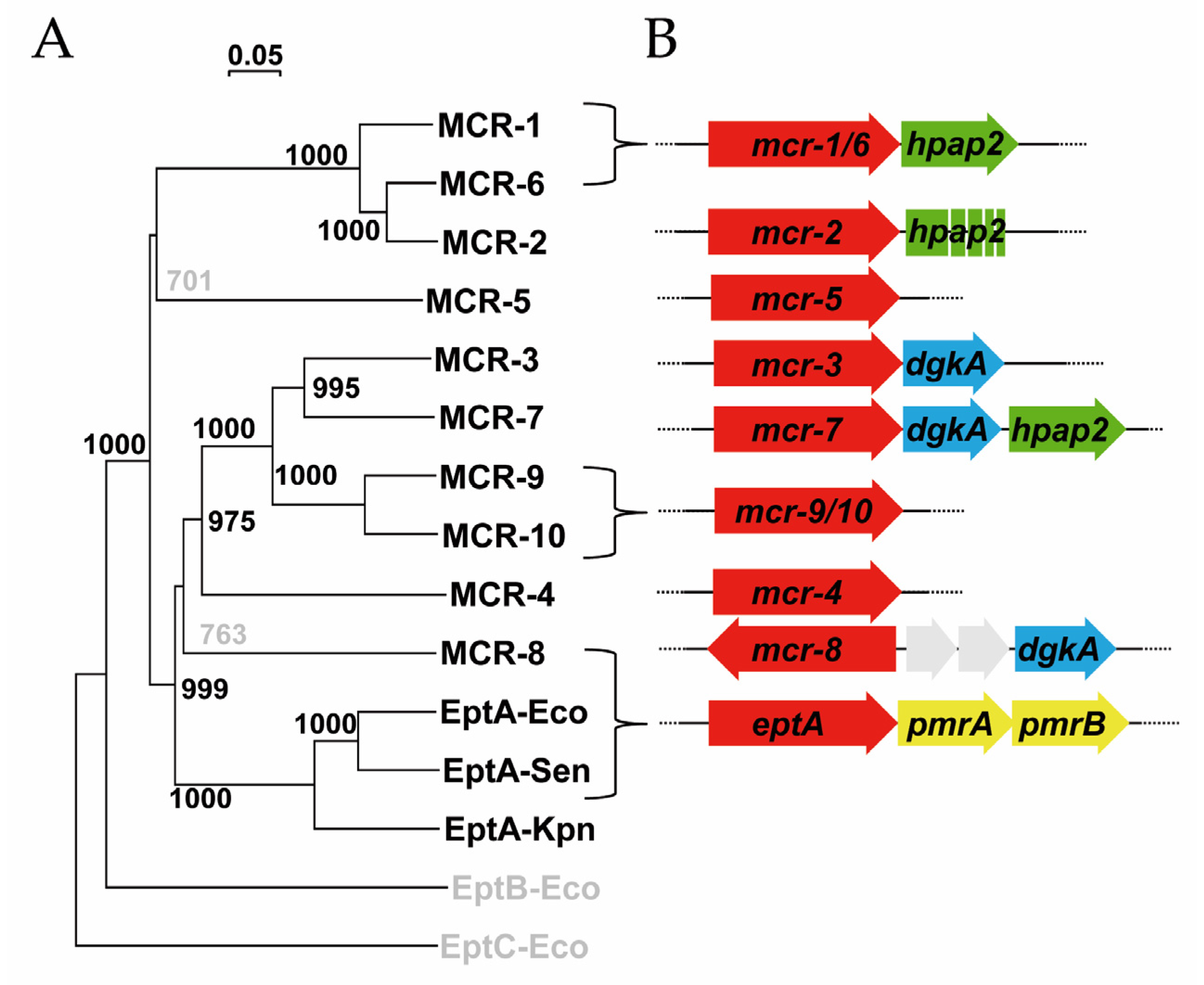
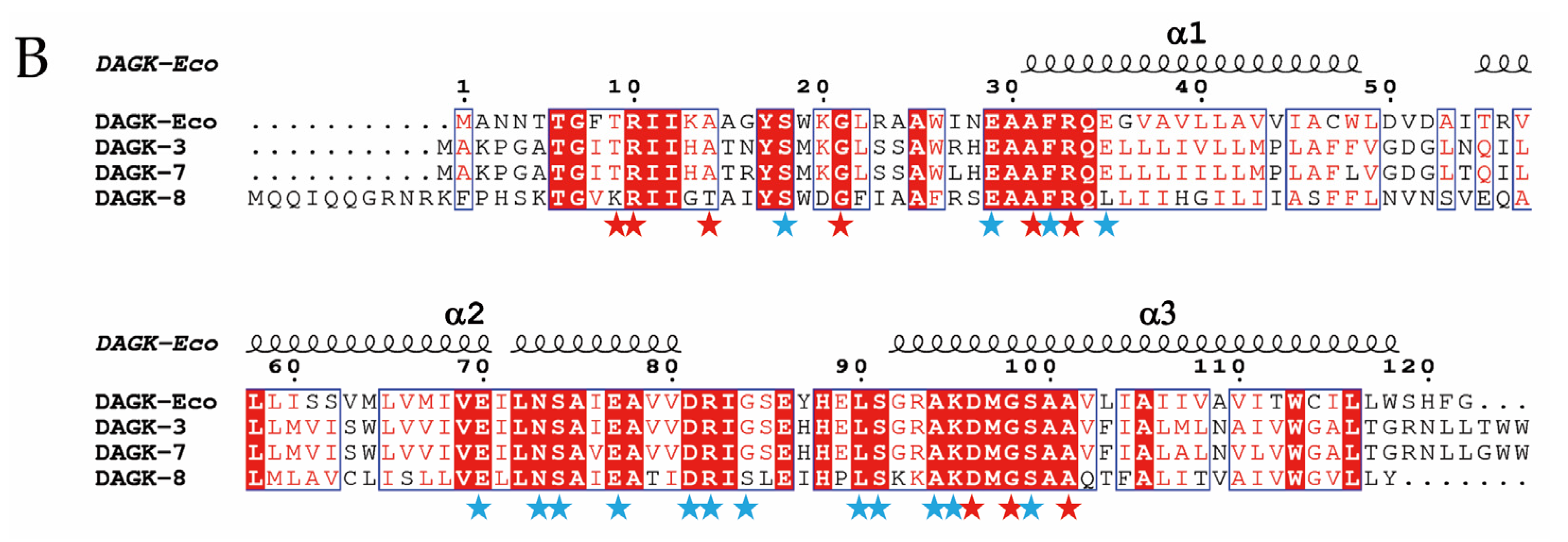
2.1. Genes Closely Linked to Colistin Resistance Determinants Are Widely Conserved among Members of the mcr/Epta Family
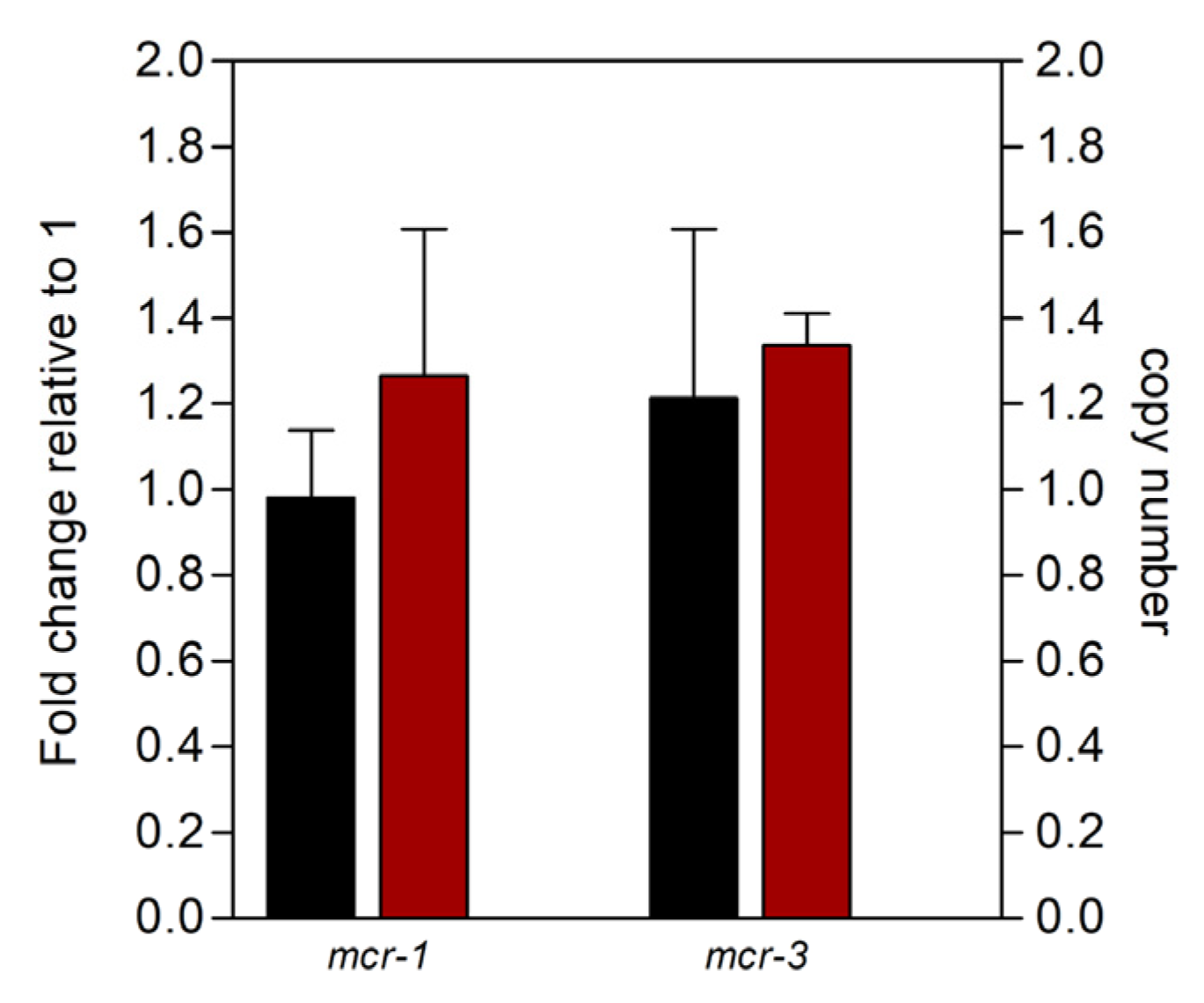
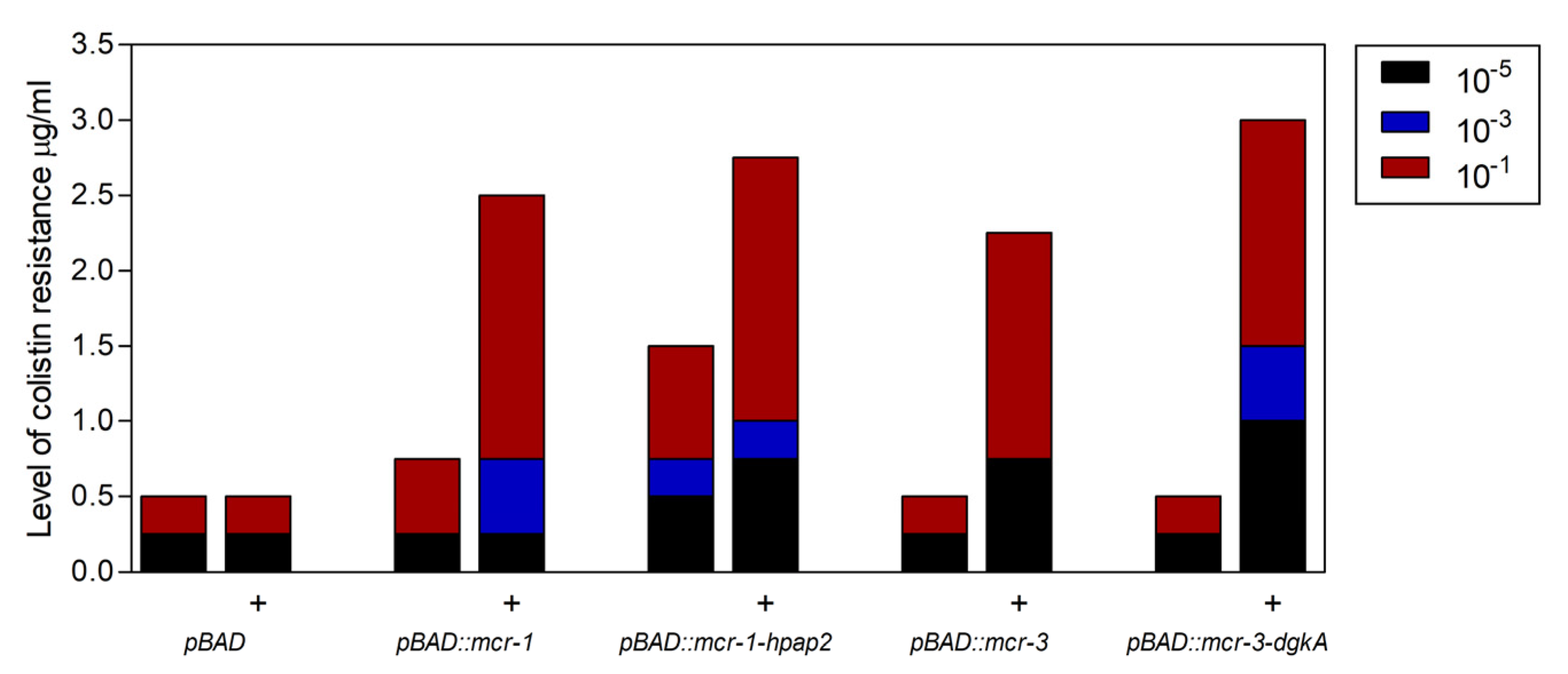
2.2. Functionality of Plasmids Carrying mcr Genetic Elements
2.3. Expression of hpap2 or dgkA Is Not Essential, Although It Slightly Increases Resistance to Colistin in E. coli
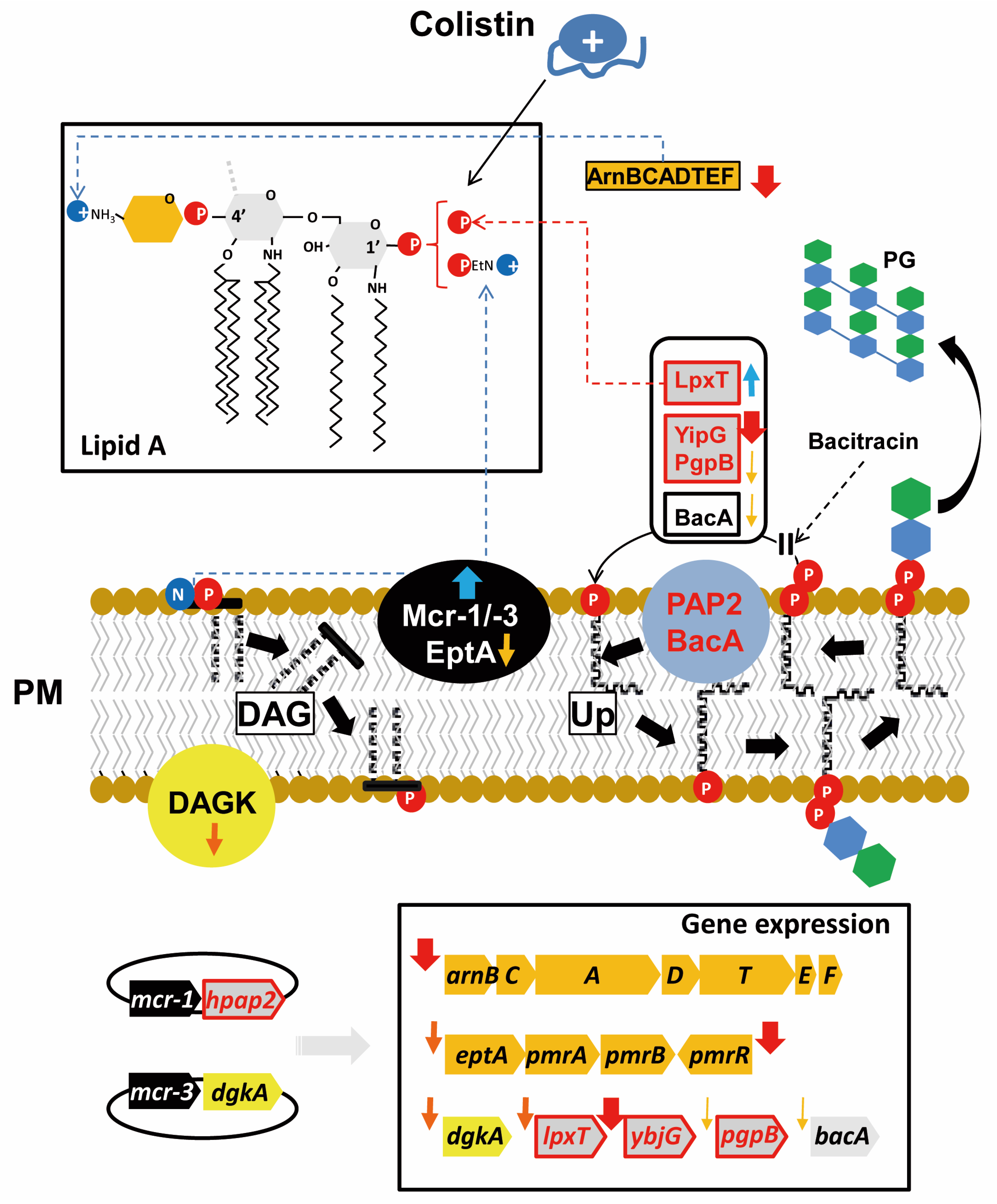
2.4. Expression of mcr Downregulates Genes for Phospholipid Metabolism Enzymes
2.5. Expression of mcr Genes, But Not hpap2, Confers Bacitracin Resistance
3. Discussion
4. Materials and Methods
4.1. Plasmid Constructs, Strains, and Growth Conditions
4.2. Quantitative PCR
4.3. Determination of Antibiotic Resistance
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, H.; Srinivas, S.; Xu, Y.; Wei, W.; Feng, Y. Genetic and Biochemical Mechanisms for Bacterial Lipid A Modifiers Associated with Polymyxin Resistance. Trends Biochem. Sci. 2019, 44, 973–988. [Google Scholar] [CrossRef] [PubMed]
- Raetz, C.R.H.; Reynolds, C.M.; Trent, M.S.; Bishop, R.E. Lipid A Modification Systems in Gram-Negative Bacteria. Annu. Rev. Biochem. 2007, 76, 295–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, A.; Chen, H.D.; Latifi, T.; Groisman, E.A. Reciprocal Control between a Bacterium’s Regulatory System and the Modification Status of Its Lipopolysaccharide. Mol. Cell 2012, 47, 897–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poirel, L.; Jayol, A.; Nordmanna, P. Polymyxins: Antibacterial activity, susceptibility testing, and resistance mechanisms encoded by plasmids or chromosomes. Clin. Microbiol. Rev. 2017, 30, 557–596. [Google Scholar] [CrossRef] [Green Version]
- Van Horn, W.D.; Sanders, C.R. Prokaryotic Diacylglycerol Kinase and Undecaprenol Kinase. Annu. Rev. Biophys. 2012, 41, 81–101. [Google Scholar] [CrossRef] [Green Version]
- Touzé, T.; Tran, A.X.; Hankins, J.V.; Mengin-Lecreulx, D.; Trent, M.S. Periplasmic phosphorylation of lipid A is linked to the synthesis of undecaprenyl phosphate. Mol. Microbiol. 2008, 67, 264–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manat, G.; Roure, S.; Auger, R.; Bouhss, A.; Barreteau, H.; Mengin-Lecreulx, D.; Touzé, T. Deciphering the metabolism of undecaprenyl-phosphate: The bacterial cell-wall unit carrier at the membrane frontier. Microb. Drug Res. 2014, 20, 199–214. [Google Scholar] [CrossRef] [Green Version]
- Anyanwu, M.U.; Jaja, I.F.; Nwobi, O.C. Occurrence and characteristics of mobile colistin resistance (Mcr) gene-containing isolates from the environment: A review. Int. J. Environ. Res. Public Health 2020, 17, 1028. [Google Scholar] [CrossRef] [Green Version]
- Zurfluh, K.; Kieffer, N.; Poirel, L.; Nordmann, P.; Stephan, R. Features of the mcr-1 cassette related to colistin resistance. Antimicrob. Agents Chemother. 2016, 60, 6438–6439. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.; Lee, J.Y.; Lim, S.K.; Ko, K.S. Intact pap2 downstream of mcr-1 appears to be required for colistin resistance. Diagn. Microbiol. Infect. Dis. 2020, 97, 114997. [Google Scholar] [CrossRef]
- Wang, C.; Feng, Y.; Liu, L.; Wei, L.; Kang, M.; Zong, Z. Identification of novel mobile colistin resistance gene mcr-10. Emerg. Microbes Infect. 2020, 9, 508–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hillis, D.M.; Bull, J.J. An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Syst. Biol. 1993, 42, 182–192. [Google Scholar] [CrossRef]
- Khedher, M.B.; Baron, S.A.; Riziki, T.; Ruimy, R.; Raoult, D.; Diene, S.M.; Rolain, J.-M. Massive analysis of 64,628 bacterial genomes to decipher water reservoir and origin of mobile colistin resistance genes: Is there another role for these enzymes? Sci. Rep. 2020, 10, 5970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kieffer, N.; Royer, G.; Decousser, J.W.; Bourrel, A.S.; Palmieri, M.; De La Rosa, J.M.O.; Jacquier, H.; Denamur, E.; Nordmann, P.; Poirel, L. Mcr-9, an Inducible Gene Encoding an Acquired Phosphoethanolamine Transferase in Escherichia coli, and Its Origin. Antimicrob. Agents Chemother. 2019, 63, e01866-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Q.; Li, M.; Spiller, O.B.; Andrey, D.O.; Hinchliffe, P.; Li, H.; MacLean, C.; Niumsup, P.; Powell, L.; Pritchard, M.; et al. Balancing mcr-1 expression and bacterial survival is a delicate equilibrium between essential cellular defence mechanisms. Nat. Commun. 2017, 8, 2054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrera, C.M.; Hankins, J.V.; Trent, M.S. Activation of PmrA inhibits LpxT-dependent phosphorylation of lipid A promoting resistance to antimicrobial peptides. Mol. Microbiol. 2010, 76, 1444–1460. [Google Scholar] [CrossRef]
- Xu, F.; Zeng, X.; Hinenoya, A.; Lin, J. MCR-1 Confers Cross-Resistance to Bacitracin, a Widely Used In-Feed Antibiotic. mSphere 2018, 3, e00411-18. [Google Scholar] [CrossRef] [Green Version]
- Carman, G.M.; Han, G.S. Roles of phosphatidate phosphatase enzymes in lipid metabolism. Trends Biochem. Sci. 2006, 31, 694–699. [Google Scholar] [CrossRef] [Green Version]
- Stukey, J.; Carman, G.M. Identification of a novel phosphatase sequence motif. Protein Sci. 2008, 6, 469–472. [Google Scholar] [CrossRef] [Green Version]
- El Ghachi, M.; Derbise, A.; Bouhss, A.; Mengin-Lecreulx, D. Identification of multiple genes encoding membrane proteins with undecaprenyl pyrophosphate phosphatase (UppP) activity in Escherichia coli. J. Biol. Chem. 2005, 280, 18689–18695. [Google Scholar] [CrossRef] [Green Version]
- Fan, J.; Jiang, D.; Zhao, Y.; Liu, J.; Zhang, X.C. Crystal structure of lipid phosphatase Escherichia coli phosphatidylglycerophosphate phosphatase B. Proc. Natl. Acad. Sci. USA 2014, 111, 7636–7640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Karbarz, M.J.; McGrath, S.C.; Cotter, R.J.; Raetz, C.R.H. MsbA transporter-dependent lipid A 1-dephosphorylation on the periplasmic surface of the inner membrane: Topography of Francisella novicida LpxE expressed in Escherichia coli. J. Biol. Chem. 2004, 279, 49470–49478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; McGrath, S.C.; Cotter, R.J.; Raetz, C.R.H. Expression cloning and periplasmic orientation of the Francisella novicida lipid A 4′-phosphatase LpxF. J. Biol. Chem. 2006, 281, 9321–9330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cullen, T.W.; Giles, D.K.; Wolf, L.N.; Ecobichon, C.; Boneca, I.G.; Trent, M.S. Helicobacter pylori versus the host: Remodeling of the bacterial outer membrane is required for survival in the gastric mucosa. PLoS Pathog. 2011, 7, e1002454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Benito, R.; Iglesias, M.R.; Quijada, N.M.; Campos, M.J.; Ugarte-Ruiz, M.; Hernández, M.; Pazos, C.; Rodríguez-Lázaro, D.; Garduño, E.; Domínguez, L.; et al. Escherichia coli ST167 carrying plasmid mobilisable mcr-1 and blaCTX-M-15 resistance determinants isolated from a human respiratory infection. Int. J. Antimicrob. Agents 2017, 50, 285–286. [Google Scholar] [CrossRef] [PubMed]
- Hernández, M.; Iglesias, M.R.; Rodríguez-Lázaro, D.; Gallardo, A.; Quijada, N.M.; Miguela-Villoldo, P.; Campos, M.J.; Píriz, S.; López-Orozco, G.; de Frutos, C.; et al. Co-occurrence of colistin-resistance genes mcr-1 and mcr-3 among multidrug-resistant Escherichia coli isolated from cattle, Spain, September 2015. Eurosurveillance 2017, 22, 30586. [Google Scholar] [CrossRef]
- Sambrook, J.; Green, M.R. Molecular Cloning: A Laboratory Manual, 4th ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2012; ISBN 9781936113415. [Google Scholar]
- Rieu, I.; Powers, S.J. Real-time quantitative RT-PCR: Design, calculations, and statistics. Plant. Cell 2009, 21, 1031–1033. [Google Scholar] [CrossRef] [Green Version]




| Locus | Sequence 5′–3′ 1 | Size 2 | E 3 | Corr. 4 | Slope 5 |
|---|---|---|---|---|---|
| mcr1c | F: AGTAGGAATTCATGATGCAGCATACTTCTG R: TAAGTCGACTGGAGTGTGCGGTGGGTTTGG | 1685 | - | - | - |
| mcr1pap2c | F: AGTAGGAATTCATGATGCAGCATACTTCTG R: AAAGTCGACTGAAAAAACCGTTCCGTAATA | 2493 | - | - | - |
| pap2c | F: CCATTGAATTCATGGGCGGTGGGGTGGG R: AAAGTCGACTGAAAAAACCGTTCCGTAATA | 820 | - | - | - |
| mcr3c | F: ATGGAGAATTCATGCCTTCCCTTATAAAAATA R: CCATGAAATACGTCGACAATAGTAATAAACCT | 1714 | - | - | - |
| mcr3dgkAc | F: ATGGAGAATTCATGCCTTCCCTTATAAAAATA R: AAAGGTCGACCTTATAATTAGCATCTATTGTT | 2233 | - | - | - |
| arnBq | F: ATTGGCAAGGGCGATGAA R: AGGCGTGACCATCAGCGTAT | 126 | 102.55 | 0.997 | −3.26 |
| bacAq | F: CCGCGTGCGCCGGGTCTT R: ACCCGGCCACAGCGCCAG | 84 | 103.5 | 0.999 | −3.24 |
| dgkAq | F: CTCAATAGCGCCATCGAAGC R: CGACGATAATGGCAATCAGCAC | 112 | 100.64 | 0.997 | −3.31 |
| eptAq | F: ACGGCAACGGCAGTTT R: CCGCTCGCTGAATGATATCCA | 112 | 95.1 | 0.996 | −3.44 |
| lpxTq | F: TGCTCTCTGTTCCCACGAAA R: ATAACGCCACATGAATGCC | 95 | 99.32 | 0.998 | −3.34 |
| mcr1q | F: CATCGCTCAAAGTATCCAGT R: ACCATGTAGATAGACACCGTTC | 115 | 97.307 | 0.999 | −3.39 |
| mcr3q | F: GACCGAGTACCTAACATCGAA R: CCTCGTCATAGCATGTGT | 76 | 101.6 | 0.999 | −3.28 |
| pgpBq | F: GGCCGCGTCGGCGAACGT R: GCAGCAGGCGGCTTCCCA | 75 | 101.0 | 0.998 | −3.30 |
| pmrRq | F: ATGAAAAACCGTGTTTATGA R: TCAGTACGTGGCAAACCA | 90 | 103.9 | 0.999 | −3.23 |
| recAq | F: GTATGATGAGCCAGGCGATGC R: GCGCGTTACCACCGGTAGTG | 138 | 100.38 | 1.0 | −3.31 |
| ybjGq | F: ACGCGACGCCAGACTCGG R: CACGGCCAGCAACGGCAC | 89 | 101.2 | 0.999 | −3.30 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallardo, A.; Ugarte-Ruiz, M.; Hernández, M.; Miguela-Villoldo, P.; Rodríguez-Lázaro, D.; Domínguez, L.; Quesada, A. Involvement of hpap2 and dgkA Genes in Colistin Resistance Mediated by mcr Determinants. Antibiotics 2020, 9, 531. https://doi.org/10.3390/antibiotics9090531
Gallardo A, Ugarte-Ruiz M, Hernández M, Miguela-Villoldo P, Rodríguez-Lázaro D, Domínguez L, Quesada A. Involvement of hpap2 and dgkA Genes in Colistin Resistance Mediated by mcr Determinants. Antibiotics. 2020; 9(9):531. https://doi.org/10.3390/antibiotics9090531
Chicago/Turabian StyleGallardo, Alejandro, María Ugarte-Ruiz, Marta Hernández, Pedro Miguela-Villoldo, David Rodríguez-Lázaro, Lucas Domínguez, and Alberto Quesada. 2020. "Involvement of hpap2 and dgkA Genes in Colistin Resistance Mediated by mcr Determinants" Antibiotics 9, no. 9: 531. https://doi.org/10.3390/antibiotics9090531
APA StyleGallardo, A., Ugarte-Ruiz, M., Hernández, M., Miguela-Villoldo, P., Rodríguez-Lázaro, D., Domínguez, L., & Quesada, A. (2020). Involvement of hpap2 and dgkA Genes in Colistin Resistance Mediated by mcr Determinants. Antibiotics, 9(9), 531. https://doi.org/10.3390/antibiotics9090531






